Cell Extracts Derived from Cypress and Cedar Show Antiviral Activity against Enveloped Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. CEs Derived from C. obtusa and C. japonica
2.2. GC-MS Analysis
2.3. Preparation of Bacteriophages phi6 and MS2 Stock Solutions
2.4. Evaluation of the Antiviral Activity of CEs through Exposure Experiment
2.5. Statistical Analysis
3. Results
3.1. Chemical Composition of CEs
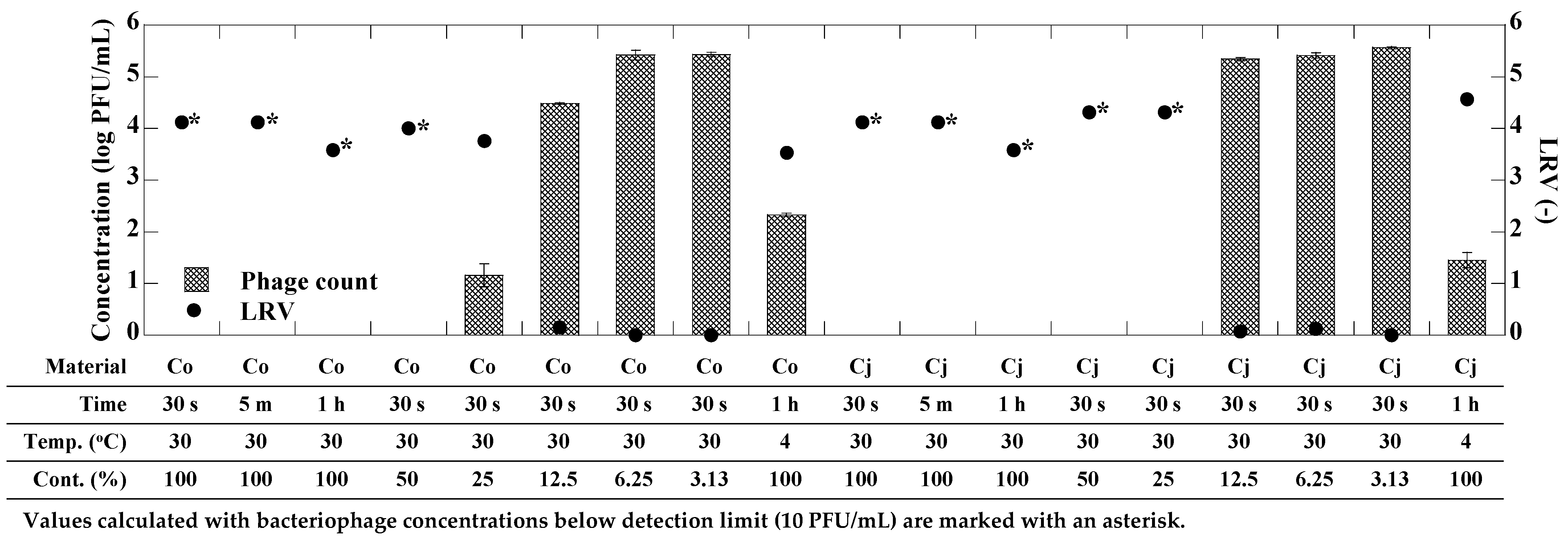
3.2. Antiviral Activity of CEs against phi6 Bacteriophage
3.3. Antiviral Activity of CEs against MS2 Bacteriophage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arruda, F.; Rosa, J.S.; Rodrigues, A.; Oliveira, L.; Lima, A.; Barroso, J.G.; Lima, E. Essential Oil Variability of Azorean Cryptomeriajaponica Leaves under Different Distillation Methods, Part 1: Color, Yield and Chemical Composition Analysis. Appl. Sci. 2022, 12, 452. [Google Scholar] [CrossRef]
- Luna, E.C.; Luna, I.S.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; De Moura, R.O.; De Araújo, R.S.A.; Monteiro, K.L.C.; De Aquino, T.M.; Ribeiro, F.F.; et al. Active Essential Oils and Their Components in Use against Neglected Diseases and Arboviruses. Oxid. Med. Cell. Longev. 2019, 2019, 6587150. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J. Plant-Microbe Interactions and Secondary Metabolites with Antibacterial, Antifungal and Antiviral Properties. Funct. Biotechnol. Plant Second. Metab. Second Ed. 2010, 39, 214–347. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; Volume 8, p. 804. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Chalchat, J.C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and Quantitative Variation in Monoterpene Co-Occurrence and Composition in the Essential Oil of Thymus vulgaris Chemotypes. J. Chem. Ecol. 2003, 29, 859–880. [Google Scholar] [CrossRef]
- Caputi, L.; Aprea, E. Use of Terpenoids as Natural Flavouring Compounds in Food Industry. Recent Pat. Food. Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef]
- Djilani, A.; Dicko, A.; Djilani, A.; Dicko, A. The Therapeutic Benefits of Essential Oils. In Nutrition, Well-Being and Health; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Spinozzi, E.; Pavela, R.; Bonacucina, G.; Perinelli, D.R.; Cespi, M.; Petrelli, R.; Cappellacci, L.; Fiorini, D.; Scortichini, S.; Garzoli, S.; et al. Spilanthol-Rich Essential Oil Obtained by Microwave-Assisted Extraction from Acmella oleracea (L.) R.K. Jansen and Its Nanoemulsion: Insecticidal, Cytotoxic and Anti-Inflammatory Activities. Ind. Crops Prod. 2021, 172, 114027. [Google Scholar] [CrossRef]
- Spinozzi, E.; Ferrati, M.; Cappellacci, L.; Caselli, A.; Perinelli, D.R.; Bonacucina, G.; Maggi, F.; Strzemski, M.; Petrelli, R.; Pavela, R.; et al. Carlina acaulis L. (Asteraceae): Biology, Phytochemistry, and Application as a Promising Source of Effective Green Insecticides and Acaricides. Ind. Crops Prod. 2023, 192, 116076. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Ikeda, N.Y.; Miano, A.C.; Saldaña, E.; Moreno, A.M.; Stashenko, E.; Contreras-Castillo, C.J.; Da Gloria, E.M. Unraveling the Selective Antibacterial Activity and Chemical Composition of Citrus Essential Oils. Sci. Rep. 2019, 9, 17719. [Google Scholar] [CrossRef]
- Saleem, M.; Saeed, M.T. Potential Application of Waste Fruit Peels (Orange, Yellow Lemon and Banana) as Wide Range Natural Antimicrobial Agent. J. King Saud Univ.-Sci. 2020, 32, 805–810. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal Effects of Melaleuca alternifolia (Tea Tree) Oil and Its Components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2004, 53, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Z.; Cheng, A.X.; Sun, L.M.; Lou, H.X. Effect of Plagiochin E, an Antifungal Macrocyclic Bis(Bibenzyl), on Cell Wall Chitin Synthesis in Candida albicans. Acta Pharmacol. Sin. 2008, 29, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Gavanji, S.; Sayedipour, S.S.; Larki, B.; Bakhtari, A. Antiviral Activity of Some Plant Oils against Herpes Simplex Virus Type 1 in Vero Cell Culture. J. Acute Med. 2015, 5, 62–68. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Hudson, J. Anti-Influenza Virus Activity of Essential Oils and Vapors. Am. J. Essent. Oils Nat. Prod. 2014, 2, 47–53. [Google Scholar]
- Wnorowska, S.; Targowska-Duda, K.; Kurzepa, J.; Wnorowski, A.; Strzemski, M. Carlina Oxide Inhibits the Interaction of SARS-CoV-2 S Glycoprotein with Angiotensin-Converting Enzyme 2. Ind. Crops Prod. 2022, 187, 115338. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds—A Scientific Approach. Planta Med. 2021, 88, 587–603. [Google Scholar] [CrossRef]
- Shibato, J.; Takenoya, F.; Hirabayashi, T.; Kimura, A.; Iwasaki, Y.; Toyoda, Y.; Hori, M.; Tamogami, S.; Rakwal, R.; Shioda, S. Towards Identification of Bioactive Compounds in Cold Vacuum Extracted Double Cherry Blossom (Gosen-Sakura) Leaves. Plant Signal. Behav. 2019, 14, e1644594. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Bashir, S.; Mohamed, A.E.; Sharaf, O.A.; Nabil, R.; Su, Y.; Abdelkhalek, A.; Behiry, S.I. Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium Campestre. Separations 2023, 10, 362. [Google Scholar] [CrossRef]
- Alagarasu, K.; Patil, P.; Kaushik, M.; Chowdhury, D.; Joshi, R.K.; Hegde, H.V.; Kakade, M.B.; Hoti, S.L.; Cherian, S.; Parashar, D. In Vitro Antiviral Activity of Potential Medicinal Plant Extracts against Dengue and Chikungunya Viruses. Front. Cell. Infect. Microbiol. 2022, 12, 866452. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Forestry Agency Annual Report on Forest and Forestry in Japan. Available online: https://www.maff.go.jp/e/data/publish/attach/pdf/index-96.pdf (accessed on 22 June 2023).
- Cheng, S.S.; Lin, H.Y.; Chang, S.T. Chemical Composition and Antifungal Activity of Essential Oils from Different Tissues of Japanese Cedar (Cryptomeria japonica). J. Agric. Food Chem. 2005, 53, 614–619. [Google Scholar] [CrossRef]
- Hong, E.J.; Na, K.J.; Choi, I.G.; Choi, K.C.; Jeung, E.B. Antibacterial and Antifungal Effects of Essential Oils from Coniferous Trees. Biol. Pharm. Bull. 2004, 27, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Choi, W.S.; Kang, H.Y.; Gwak, K.S.; Lee, G.S.; Jeung, E.B.; Choi, I.G. Inhibitory Effect of the Essential Oil from Chamaecyparis obtusa on the Growth of Food-Borne Pathogens. J. Microbiol. 2010, 48, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yamasaki, F. A Practical Manual for Handling Bacteriophages. MAFF Microorg. Genet. Resour. Man. 2019, 42, 1–12. [Google Scholar]
- Miyaoka, Y.; Kabir, M.H.; Hasan, M.A.; Yamaguchi, M.; Shoham, D.; Murakami, H.; Takehara, K. Virucidal Activity of Slightly Acidic Hypochlorous Acid Water toward Influenza Virus and Coronavirus with Tests Simulating Practical Usage. Virus Res. 2021, 297, 198383. [Google Scholar] [CrossRef]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Mechanisms of Antiviral Action of Plant Antimicrobials against Murine Norovirus. Appl. Environ. Microbiol. 2014, 80, 4898–4910. [Google Scholar] [CrossRef]
- Selvarani, V.; James, H. The Activity of Cedar Leaf Oil Vapor against Respiratory Viruses: Practical Applications. J. Appl. Pharm. Sci. 2013, 3, 011–015. [Google Scholar] [CrossRef]
- Usachev, E.V.; Pyankov, O.V.; Usacheva, O.V.; Agranovski, I.E. Antiviral Activity of Tea Tree and Eucalyptus Oil Aerosol and Vapour. J. Aerosol Sci. 2013, 59, 22–30. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Moiteiro, C.; Rodrigues, M.C.S.M.; Almeida, A.J.R.M. Essential Oil Composition from Cryptomeria japonica D.Don Grown in Azores: Biomass Valorization From Forest Management. Nat. Prod. Commun. 2021, 16, 1934578X211038431. [Google Scholar] [CrossRef]
- Raha, S.; Kim, S.M.; Lee, H.J.; Lee, S.J.; Heo, J.D.; Saralamma, V.V.G.; Ha, S.E.; Kim, E.H.; Mun, S.P.; Kim, G.S. Essential Oil from Korean Chamaecyparis obtusa Leaf Ameliorates Respiratory Activity in Sprague-Dawley Rats and Exhibits Protection from NF-ΚB-Induced Inflammation in WI38 Fibroblast Cells. Int. J. Mol. Med. 2019, 43, 393. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker, D.L.; Han, S.W.; Cheong, G.W. Chemical Composition and Antimicrobial Activity of Chamaecyparis obtusa Leaf Essential Oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef]
- Cha, J.D.; Jeong, M.R.; Jeong, S.I.; Moon, S.E.; Kil, B.S.; Yun, S.I.; Lee, K.Y.; Song, Y.H. Chemical Composition and Antimicrobial Activity of the Essential Oil of Cryptomeria japonica. Phytother. Res. 2007, 21, 295–299. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Utegenova, G.A.; Pallister, K.B.; Kushnarenko, S.V.; Özek, G.; Özek, T.; Abidkulova, K.T.; Kirpotina, L.N.; Schepetkin, I.A.; Quinn, M.T.; Voyich, J.M. Chemical Composition and Antibacterial Activity of Essential Oils from Ferula L. Species against Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 1679. [Google Scholar] [CrossRef]
- Vaičiulytė, V.; Ložienė, K.; Švedienė, J.; Raudonienė, V.; Paškevičius, A. α-Terpinyl Acetate: Occurrence in Essential Oils Bearing Thymus Pulegioides, Phytotoxicity, and Antimicrobial Effects. Molecules 2021, 26, 1065. [Google Scholar] [CrossRef]
- Garozzo, A.; Timpanaro, R.; Bisignano, B.; Furneri, P.M.; Bisignano, G.; Castro, A. In Vitro Antiviral Activity of Melaleuca Alternifolia Essential Oil. Lett. Appl. Microbiol. 2009, 49, 806–808. [Google Scholar] [CrossRef]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-Ol, the Main Component of the Essential Oil of Melaleuca alternifolia (Tea Tree Oil), Suppresses Inflammatory Mediator Production by Activated Human Monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef]
- Li, X.; Duan, S.; Chu, C.; Xu, J.; Zeng, G.; Lam, A.K.Y.; Zhou, J.; Yin, Y.; Fang, D.; Reynolds, M.J.; et al. Melaleuca alternifolia Concentrate Inhibits in Vitro Entry of Influenza Virus into Host Cells. Molecules 2013, 18, 9550–9566. [Google Scholar] [CrossRef] [PubMed]
- Pliego Zamora, A.; Edmonds, J.H.; Reynolds, M.J.; Khromykh, A.A.; Ralph, S.J. The in Vitro and in Vivo Antiviral Properties of Combined Monoterpene Alcohols against West Nile Virus Infection. Virology 2016, 495, 18–32. [Google Scholar] [CrossRef] [PubMed]

| No. | Components | Retention Time (min) | C. obtusa | C. japonica | ||
|---|---|---|---|---|---|---|
| SI a (-) | Relative Content (%) | SI (-) | Relative Content (%) | |||
| 1 | α-Pinene | 4.27 | 943 | 3.5 | 954 | 15.9 |
| 2 | α-Thujene | 4.36 | 816 | 0.4 | 851 | 0.6 |
| 3 | (-)-β-Pinene | 5.63 | - | - | 866 | 0.5 |
| 4 | (1R)-α-Pinene | 6.41 | - | - | 890 | 1.1 |
| 5 | Myrcene | 6.71 | 937 | 5.3 | 902 | 1.6 |
| 6 | Terpinolene | 6.97 | 919 | 2.0 | 905 | 1.2 |
| 7 | Limonene | 7.32 | 931 | 7.1 | 917 | 1.9 |
| 8 | β-Phellandrene | 7.49 | 850 | 0.5 | 846 | 0.5 |
| 9 | Isoamyl alcohol | 7.62 | - | - | 832 | 0.6 |
| 10 | γ-Terpinene | 8.19 | 946 | 6.7 | 931 | 3.0 |
| 11 | p-Cymene | 8.62 | 868 | 0.4 | 911 | 0.8 |
| 12 | Terpinolene | 8.85 | 942 | 2.3 | 907 | 0.8 |
| 13 | 1-Hexanol | 10.16 | 878 | 0.9 | - | - |
| 14 | cis-3-Hexen-1-ol | 10.68 | 935 | 5.0 | 922 | 1.6 |
| 15 | 1-Octen-3-ol | 11.75 | 930 | 2.1 | 891 | 0.6 |
| 16 | Linalol | 13.27 | 896 | 1.0 | 849 | 0.5 |
| 17 | trans-p-Menth-2-en-1-ol | 13.56 | 817 | 0.4 | 883 | 0.6 |
| 18 | α-Himachalene | 13.6 | - | - | 848 | 0.2 |
| 19 | Bornyl acetate | 13.8 | 949 | 9.7 | 923 | 1.7 |
| 20 | Isobornyl acetate | 13.88 | 864 | 0.6 | - | - |
| 21 | Isocaryophillene | 14.02 | 882 | 0.6 | - | - |
| 22 | Cedrene | 14.03 | - | - | 827 | 0.2 |
| 23 | Terpinen-4-ol | 14.15 | 930 | 18.0 | 933 | 48.0 |
| 24 | Thujopsene | 14.4 | 936 | 3.3 | 914 | 1.4 |
| 25 | Cadina-3,5-diene | 14.64 | 868 | 0.7 | - | - |
| 26 | cis-Muurola-4(15),5-diene | 15.12 | 906 | 1.2 | - | - |
| 27 | α-Terpinyl acetate | 15.5 | 939 | 10.1 | 921 | 2.3 |
| 28 | (-)-Borneol | 15.6 | 939 | 2.0 | 876 | 0.6 |
| 29 | γ-Muurolene | 15.69 | 870 | 1.1 | 857 | 0.5 |
| 30 | α-Muurolene | 15.88 | 900 | 0.4 | 848 | 0.2 |
| 31 | α-Longipinene | 16.05 | 886 | 0.4 | 836 | 0.2 |
| 32 | δ-Cadinene | 16.34 | 917 | 2.1 | 904 | 1.1 |
| 33 | Elemol | 20.52 | 919 | 0.8 | 925 | 1.1 |
| 34 | Cedrol | 21.02 | 821 | 0.3 | 821 | 0.3 |
| 35 | γ-Eudesmol | 21.59 | 893 | 0.6 | 893 | 0.7 |
| 36 | τ-Muurolol | 21.79 | - | - | 833 | 0.3 |
| 37 | α-Eudesmol | 22.2 | 868 | 0.5 | 889 | 0.6 |
| 38 | β-Eudesmol | 22.3 | 882 | 0.8 | 893 | 0.9 |
| Identified componetns (%) | 90.7 | 92.1 | ||||
| Others (%) | 9.3 | 7.9 | ||||
| Total (%) | 100 | 100 | ||||
| Oils | Tea Tree Oil | Tea Tree Oil | MAC a | CMA b | C. obtusa | C. japonica | |
|---|---|---|---|---|---|---|---|
| Relative contents (%) | terpinen-4-ol | 41.6 | 36.71 | 56–58 | 60.0–64.0 | 18.0 | 48.0 |
| γ-terpinene | 21.5 | 22.20 | 20.65 | 0.5–1.0 | 6.7 | 3.0 | |
| α-terpinene | 10.0 | 10.10 | 9.8 | ||||
| p-Cymene | 1.8 | 2.52 | 8.0–14.0 | 0.4 | 0.8 | ||
| α-terpineol | 3.1 | 2.74 | 5.0–7.0 | ||||
| δ-Cadinene | 1.0 | 4.0–6.0 | 2.1 | 1.1 | |||
| α-Terpinyl acetate | 10.1 | 2.3 | |||||
| Bornyl acetate | 9.7 | 1.7 | |||||
| Limonene | 0.9 | 7.1 | 1.9 | ||||
| α-Pinene | 2.4 | 3.5 | 15.9 | ||||
| Reference | [43] | [42] | [44] | [45] | this study | this study | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, T.; Inagaki, A.; Hatta, T.; Moroishi, S.; Kawanishi, K.; Itoh, Y.; Maehana, S.; Amarasiri, M.; Sei, K. Cell Extracts Derived from Cypress and Cedar Show Antiviral Activity against Enveloped Viruses. Microorganisms 2024, 12, 1813. https://doi.org/10.3390/microorganisms12091813
Furukawa T, Inagaki A, Hatta T, Moroishi S, Kawanishi K, Itoh Y, Maehana S, Amarasiri M, Sei K. Cell Extracts Derived from Cypress and Cedar Show Antiviral Activity against Enveloped Viruses. Microorganisms. 2024; 12(9):1813. https://doi.org/10.3390/microorganisms12091813
Chicago/Turabian StyleFurukawa, Takashi, Ayumu Inagaki, Takeshi Hatta, Suzuha Moroishi, Katsuki Kawanishi, Yuki Itoh, Shotaro Maehana, Mohan Amarasiri, and Kazunari Sei. 2024. "Cell Extracts Derived from Cypress and Cedar Show Antiviral Activity against Enveloped Viruses" Microorganisms 12, no. 9: 1813. https://doi.org/10.3390/microorganisms12091813







