Bioremediation of Polycyclic Aromatic Hydrocarbons by Means of Bacteria and Bacterial Enzymes
Abstract
:1. Introduction
2. Sources of PAHs
3. Toxicity of PAHs
4. Physicochemical Methods of Remediation
4.1. Physical Approaches
4.2. Thermal Approaches
4.3. Chemical Approaches
5. Bioremediation
6. Bacterial Bioremediation of PAHs
6.1. Individual Bacterial Strains
6.2. Consortia of Microorganisms
6.3. Enzymatic Degradation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, M.; Chen, D.; Parales, R.E.; Jiang, J. Oxygenases as Powerful Weapons in the Microbial Degradation of Pesticides. Annu. Rev. Microbiol. 2022, 76, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Jie, H.; Khan, I.; Alharthi, M.; Zafar, M.W.; Saeed, A. Sustainable Energy Policy, Socio-Economic Development, and Ecological Footprint: The Economic Significance of Natural Resources, Population Growth, and Industrial Development. Util. Policy 2023, 81, 101490. [Google Scholar] [CrossRef]
- Malawska, M.; Wiłkomirski, B. An Analysis of Soil and Plant (Taraxacum Officinale) Contamination with Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in The Area of The Railway Junction Iława Główna, Poland. Water Air Soil Pollut. 2001, 127, 339–349. [Google Scholar] [CrossRef]
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. 21—Environmental Pollution: Causes, Effects, and the Remedies. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. ISBN 978-0-12-819001-2. [Google Scholar]
- Varjani, S.J.; Gnansounou, E.; Pandey, A. Comprehensive Review on Toxicity of Persistent Organic Pollutants from Petroleum Refinery Waste and Their Degradation by Microorganisms. Chemosphere 2017, 188, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, R.T.; Johnson, A.C.; Edyvean, R.G.J. Biotechnology in the Petroleum Industry: An Overview. Int. Biodeterior. Biodegrad. 2014, 86, 225–237. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial Degradation of Petroleum Hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Salimnezhad, A.; Soltani-Jigheh, H.; Soorki, A.A. Effects of Oil Contamination and Bioremediation on Geotechnical Properties of Highly Plastic Clayey Soil. J. Rock Mech. Geotech. Eng. 2021, 13, 653–670. [Google Scholar] [CrossRef]
- Ausuri, J.; Vitale, G.A.; Coppola, D.; Palma Esposito, F.; Buonocore, C.; de Pascale, D. Assessment of the Degradation Potential and Genomic Insights towards Phenanthrene by Dietzia psychralcaliphila JI1D. Microorganisms 2021, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.L.; Nine, M.J.; Hassan, K.; Tung, T.T.; Tran, D.N.H.; Losic, D. Graphene-Based Sorbents for Multipollutants Removal in Water: A Review of Recent Progress. Adv. Funct. Mater. 2021, 31, 2007356. [Google Scholar] [CrossRef]
- John, R.C.; Essien, J.P.; Akpan, S.B.; Okpokwasili, G.C. Polycyclic Aromatic Hydrocarbon-Degrading Bacteria from Aviation Fuel Spill Site at Ibeno, Nigeria. Bull. Environ. Contam. Toxicol. 2012, 88, 1014–1019. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Lee, B.K. Sources, Distribution and Toxicity of Polyaromatic Hydrocarbons (PAHs) in Particulate Matter. In Air Pollution; Vanda, V., Ed.; IntechOpen: Rijeka, Croatia, 2010; pp. 99–122. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation Aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Lemaire, J.; Buès, M.; Kabeche, T.; Hanna, K.; Simonnot, M.-O. Oxidant Selection to Treat an Aged PAH Contaminated Soil by in Situ Chemical Oxidation. J. Environ. Chem. Eng. 2013, 1, 1261–1268. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Priority Pollutant List. Available online: www.epa.gov/sites/default/files/2015-09/documents/priority-pollutant-list-epa.pdf (accessed on 24 May 2024).
- Substance Priority List|ATSDR. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 24 May 2024).
- Evangelista, N.N.; Micheletto, M.C.; Kava, E.; Mendes, L.F.S.; Costa-Filho, A.J. Biomolecular Condensates of Chlorocatechol 1,2-Dioxygenase as Prototypes of Enzymatic Microreactors for the Degradation of Polycyclic Aromatic Hydrocarbons. Int. J. Biol. Macromol. 2024, 270, 132294. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Singh, O.V.; Jain, R.K. Polycyclic Aromatic Hydrocarbons: Environmental Pollution and Bioremediation. Trends Biotechnol. 2002, 20, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Levels of Polycyclic Aromatic Hydrocarbons in the Water and Sediment of Buffalo River Estuary, South Africa and Their Health Risk Assessment. Arch. Environ. Contam. Toxicol. 2019, 76, 657–669. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation Approaches for Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Soils: Technological Constraints, Emerging Trends and Future Directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Zhang, T.; Fang, H.H.-P. Bacteria-Mediated PAH Degradation in Soil and Sediment. Appl. Microbiol. Biotechnol. 2011, 89, 1357–1371. [Google Scholar] [CrossRef]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of Soils Contaminated with Polycyclic Aromatic Hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.; Tripathi, A.; Patel, H.; Rudakiya, D.; Gupte, S. Bioremediation of Polycyclic Aromatic Hydrocarbon (PAHs): A Perspective. Open Biotechnol. J. 2016, 10, 363–378. [Google Scholar] [CrossRef]
- Sakshi; Singh, S.K.; Haritash, A.K. Polycyclic Aromatic Hydrocarbons: Soil Pollution and Remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar] [CrossRef]
- Fetzner, S. Bacterial Degradation of Pyridine, Indole, Quinoline, and Their Derivatives under Different Redox Conditions. Appl. Microbiol. Biotechnol. 1998, 49, 237–250. [Google Scholar] [CrossRef]
- Fuchs, G. Anaerobic Metabolism of Aromatic Compounds. Ann. N. Y. Acad. Sci. 2008, 1125, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, G.; Boll, M.; Heider, J. Microbial Degradation of Aromatic Compounds—From One Strategy to Four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Qureshi, A.; Purohit, H.J. Aromatic Compounds and Biofilms: Regulation and Interlinking of Metabolic Pathways in Bacteria. In Microbial Metabolism of Xenobiotic Compounds; Arora, P.K., Ed.; Springer: Singapore, 2019; pp. 145–164. ISBN 978-981-13-7462-3. [Google Scholar]
- Nagy, K.K.; Takács, K.; Németh, I.; Varga, B.; Grolmusz, V.; Molnár, M.; Vértessy, B.G. Novel Enzymes for Biodegradation of Polycyclic Aromatic Hydrocarbons Identified by Metagenomics and Functional Analysis in Short-Term Soil Microcosm Experiments. Sci. Rep. 2024, 14, 11608. [Google Scholar] [CrossRef]
- Copley, S.D. Evolution of Efficient Pathways for Degradation of Anthropogenic Chemicals. Nat. Chem. Biol. 2009, 5, 559–566. [Google Scholar] [CrossRef]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating Pesticide Degradation in the Environment: Blind Spots and Emerging Opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef]
- Acevedo, F.; Pizzul, L.; Castillo, M.D.; González, M.E.; Cea, M.; Gianfreda, L.; Diez, M.C. Degradation of Polycyclic Aromatic Hydrocarbons by Free and Nanoclay-Immobilized Manganese Peroxidase from Anthracophyllum discolor. Chemosphere 2010, 80, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Eibes, G.; Arca-Ramos, A.; Feijoo, G.; Lema, J.M.; Moreira, M.T. Enzymatic Technologies for Remediation of Hydrophobic Organic Pollutants in Soil. Appl. Microbiol. Biotechnol. 2015, 99, 8815–8829. [Google Scholar] [CrossRef]
- Villaverde, J.; Láiz, L.; Lara-Moreno, A.; González-Pimentel, J.L.; Morillo, E. Bioaugmentation of PAH-Contaminated Soils with Novel Specific Degrader Strains Isolated from a Contaminated Industrial Site. Effect of Hydroxypropyl-β-Cyclodextrin as PAH Bioavailability Enhancer. Front. Microbiol. 2019, 10, 2588. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive Review of Polycyclic Aromatic Hydrocarbons in Water Sources, Their Effects and Treatments. Sci. Total. Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of Environmental Exposure to Polycyclic Aromatic Hydrocarbons: A Review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Callcott, D. Partitioning and Physical Chemical Properties of PAHs. In PAHs and Related Compounds: Chemistry; Neilson, A.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 325–345. ISBN 978-3-540-49697-7. [Google Scholar]
- Patra, J.; Lu, S.-X.; Kao, J.-C.; Yu, B.-R.; Chen, Y.-T.; Su, Y.-S.; Wu, T.-Y.; Bresser, D.; Hsieh, C.-T.; Lo, Y.-C.; et al. Engineering of Aromatic Naphthalene and Solvent Molecules to Optimize Chemical Prelithiation for Lithium-Ion Batteries. Adv. Sci. 2024, 11, e2309155. [Google Scholar] [CrossRef]
- Rocha, A.C.; Palma, C. Source Identification of Polycyclic Aromatic Hydrocarbons in Soil Sediments: Application of Different Methods. Sci. Total Environ. 2019, 652, 1077–1089. [Google Scholar] [CrossRef]
- Marris, C.R.; Kompella, S.N.; Miller, M.R.; Incardona, J.P.; Brette, F.; Hancox, J.C.; Sørhus, E.; Shiels, H.A. Polyaromatic Hydrocarbons in Pollution: A Heart-Breaking Matter. J. Physiol. 2020, 598, 227–247. [Google Scholar] [CrossRef]
- Varjani, S.J.; Rana, D.P.; Jain, A.K.; Bateja, S.; Upasani, V.N. Synergistic Ex-Situ Biodegradation of Crude Oil by Halotolerant Bacterial Consortium of Indigenous Strains Isolated from on Shore Sites of Gujarat, India. Int. Biodeterior. Biodegrad. 2015, 103, 116–124. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Zhang, R.; Yu, X.-Z.; Qian, P.-Y.; Wong, M.H. Bacterial Communities in PAH Contaminated Soils at an Electronic-Waste Processing Center in China. Ecotoxicology 2010, 19, 96–104. [Google Scholar] [CrossRef]
- Desforges, J.-P.W.; Sonne, C.; Levin, M.; Siebert, U.; De Guise, S.; Dietz, R. Immunotoxic Effects of Environmental Pollutants in Marine Mammals. Environ. Int. 2016, 86, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.V.; Balaam, J.; Barnard, N.; Dyer, R.; Jones, C.; Lavender, J.; McHugh, M. Characterisation of Potentially Genotoxic Compounds in Sediments Collected from United Kingdom Estuaries. Chemosphere 2002, 49, 247–258. [Google Scholar] [CrossRef]
- Haverinen, J.; Badr, A.; Korajoki, H.; Hassinen, M.; Vornanen, M. Dual Effect of Polyaromatic Hydrocarbons on Sarco(Endo)Plasmic Reticulum Calcium ATPase (SERCA) Activity of a Teleost Fish (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 276, 109785. [Google Scholar] [CrossRef]
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Cancer Risk from Occupational and Environmental Exposure to Polycyclic Aromatic Hydrocarbons. Cancer Causes Control. 1997, 8, 444–472. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Xie, Q.; Jiang, B.; Li, J.; Xie, Y.; Ji, W. Evaluating the Kinetics and Molecular Mechanism for Biomimetic Metabolic Activation of PAHs by Surface-Enhanced Raman Scattering Spectroscopy. Anal. Chem. 2024, 96, 10365–10372. [Google Scholar] [CrossRef]
- McFadyen, M.C.E.; Melvin, W.T.; Murray, G.I. Cytochrome P450 Enzymes: Novel Options for Cancer Therapeutics. Mol. Cancer Ther. 2004, 3, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Gitipour, S.; Sorial, G.A.; Ghasemi, S.; Bazyari, M. Treatment Technologies for PAH-Contaminated Sites: A Critical Review. Environ. Monit. Assess. 2018, 190, 546. [Google Scholar] [CrossRef]
- Drouin, M.; Parravicini, G.; Nasser, S.; Moulin, P. Membrane Separation Used as Treatment of Alkaline Wastewater from a Maritime Scrubber Unit. Membranes 2022, 12, 968. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Hang, X.; Zhao, S.; Wan, Y. Removal of Polycyclic Aromatic Hydrocarbons by Nanofiltration Membranes: Rejection and Fouling Mechanisms. J. Membr. Sci. 2019, 582, 264–273. [Google Scholar] [CrossRef]
- Smol, M.; Włodarczyk-Makuła, M.; Mielczarek, K.; Bohdziewicz, J.; Włóka, D. The Use of Reverse Osmosis in the Removal of PAHs from Municipal Landfill Leachate. Polycycl. Aromat. Compd. 2016, 36, 20–39. [Google Scholar] [CrossRef]
- Gan, X.; Teng, Y.; Ren, W.; Ma, J.; Christie, P.; Luo, Y. Optimization of Ex-Situ Washing Removal of Polycyclic Aromatic Hydrocarbons from a Contaminated Soil Using Nano-Sulfonated Graphene. Pedosphere 2017, 27, 527–536. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Polycyclic Aromatic Hydrocarbons (PAHs) Removal by Sorption: A Review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of Persulfate (PS) and Peroxymonosulfate (PMS) and Application for the Degradation of Emerging Contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Jackulin, F.; Senthil Kumar, P.; Chitra, B.; Karthick, S.; Rangasamy, G. A Review on Recent Advancements in the Treatment of Polyaromatic Hydrocarbons (PAHs) Using Sulfate Radicals Based Advanced Oxidation Process. Environ. Res. 2024, 253, 119124. [Google Scholar] [CrossRef]
- Suara, M.A.; Ganiyu, S.O.; Paul, S.; Stafford, J.L.; Gamal El-Din, M. Solar-Activated Zinc Oxide Photocatalytic Treatment of Real Oil Sands Process Water: Effect of Treatment Parameters on Naphthenic Acids, Polyaromatic Hydrocarbons and Acute Toxicity Removal. Sci. Total Environ. 2022, 819, 153029. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, J.; Sheng, G.; Fu, J.; Peng, P. Photocatalytic Reactions of Pyrene at TiO2/Water Interfaces. Chemosphere 2003, 50, 111–119. [Google Scholar] [CrossRef]
- Abbasi, M.; Aziz, R.; Rafiq, M.T.; Bacha, A.U.R.; Ullah, Z.; Ghaffar, A.; Mustafa, G.; Nabi, I.; Hayat, M.T. Efficient Performance of InP and InP/ZnS Quantum Dots for Photocatalytic Degradation of Toxic Aquatic Pollutants. Environ. Sci. Pollut. Res. Int. 2024, 31, 19986–20000. [Google Scholar] [CrossRef]
- Tripathi, V.; Gaur, V.K.; Kaur, I.; Srivastava, P.K.; Manickam, N. Unlocking Bioremediation Potential for Site Restoration: A Comprehensive Approach for Crude Oil Degradation in Agricultural Soil and Phytotoxicity Assessment. J. Environ. Manag. 2024, 355, 120508. [Google Scholar] [CrossRef]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative Bioremediation of Heavy Metals and Petroleum Hydrocarbons Co-Contaminated Soil by Natural Attenuation, Phytoremediation, Bioaugmentation and Bioaugmentation-Assisted Phytoremediation. Sci. Total Environ. 2016, 563–564, 693–703. [Google Scholar] [CrossRef]
- Goveas, L.C.; Selvaraj, R.; Vinayagam, R.; Sajankila, S.P.; Pugazhendhi, A. Biodegradation of Benzo(a)Pyrene by Pseudomonas Strains, Isolated from Petroleum Refinery Effluent: Degradation, Inhibition Kinetics and Metabolic Pathway. Chemosphere 2023, 321, 138066. [Google Scholar] [CrossRef]
- Mehetre, G.T.; Dastager, S.G.; Dharne, M.S. Biodegradation of Mixed Polycyclic Aromatic Hydrocarbons by Pure and Mixed Cultures of Biosurfactant Producing Thermophilic and Thermo-Tolerant Bacteria. Sci. Total Environ. 2019, 679, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Bharadvaja, N. Chapter 6—Enzymatic Bioremediation: A Smart Tool to Fight Environmental Pollutants. In Smart Bioremediation Technologies; Bhatt, P., Ed.; Academic Press: Cambridge MA, USA, 2019; pp. 99–118. ISBN 978-0-12-818307-6. [Google Scholar]
- Koshlaf, E.; Ball, A.S. Soil Bioremediation Approaches for Petroleum Hydrocarbon Polluted Environments. AIMS Microbiol. 2017, 3, 25–49. [Google Scholar] [CrossRef]
- Abioye, O.P. Biological Remediation of Hydrocarbon and Heavy Metals Contaminated Soil. In Soil Contamination; Pascucci, S., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 127–142. [Google Scholar] [CrossRef]
- Mohan, S.V.; Kisa, T.; Ohkuma, T.; Kanaly, R.A.; Shimizu, Y. Bioremediation Technologies for Treatment of PAH-Contaminated Soil and Strategies to Enhance Process Efficiency. Rev. Environ. Sci. Bio/Technol. 2006, 5, 347–374. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Košnář, Z.; Mercl, F.; Aranda, E.; Tlustoš, P. A Comparative Study to Evaluate Natural Attenuation, Mycoaugmentation, Phytoremediation, and Microbial-Assisted Phytoremediation Strategies for the Bioremediation of an Aged PAH-Polluted Soil. Ecotoxicol. Environ. Saf. 2018, 147, 165–174. [Google Scholar] [CrossRef]
- Iqbal, S.; Ummara, U.; Noreen, S.; Akhter, M.S.; Jaleel, F.; Jabeen, S.; Naz, N.; Wahid, A.; Alotaibi, M.O.; Nour, M.M.; et al. Enhancing Systematic Tolerance in Bermuda Grass (Cynodon dactylon L.) through Amplified alkB Gene Expression and Bacterial-Driven Hydrocarbon Degradation. Environ. Sci. Pollut. Res. Int. 2024, 31, 19871–19885. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Pandey, P.; Bhargava, B.; Sharma, S.; Kumar, V.; Sharma, K.D. Bioremediation of Polyaromatic Hydrocarbons (PAHs) Using Rhizosphere Technology. Braz. J. Microbiol. 2015, 46, 7–21. [Google Scholar] [CrossRef]
- Moreno, B.; Cañizares, R.; Macci, C.; Doni, S.; Masciandaro, G.; Benitez, E. Molecular Tools to Understand the Bioremediation Effect of Plants and Earthworms on Contaminated Marine Sediments. J. Hazard. Mater. 2015, 300, 398–405. [Google Scholar] [CrossRef]
- Sinha, R.K.; Bharambe, G.; Ryan, D. Converting Wasteland into Wonderland by Earthworms—A Low-Cost Nature’s Technology for Soil Remediation: A Case Study of Vermiremediation of PAHs Contaminated Soil. Environmentalist 2008, 28, 466–475. [Google Scholar] [CrossRef]
- Rorat, A.; Wloka, D.; Grobelak, A.; Grosser, A.; Sosnecka, A.; Milczarek, M.; Jelonek, P.; Vandenbulcke, F.; Kacprzak, M. Vermiremediation of Polycyclic Aromatic Hydrocarbons and Heavy Metals in Sewage Sludge Composting Process. J. Environ. Manag. 2017, 187, 347–353. [Google Scholar] [CrossRef]
- Hamad, A.A.; Moubasher, H.A.; Moustafa, Y.M.; Mohamed, N.H.; Abd-El Rhim, E.H. Petroleum Hydrocarbon Bioremediation Using Native Fungal Isolates and Consortia. Sci. World J. 2021, 2021, 6641533. [Google Scholar] [CrossRef] [PubMed]
- Ide-Pérez, M.R.; Fernández-López, M.G.; Sánchez-Reyes, A.; Leija, A.; Batista-García, R.A.; Folch-Mallol, J.L.; Sánchez-Carbente, M.D.R. Aromatic Hydrocarbon Removal by Novel Extremotolerant Exophiala and Rhodotorula Spp. from an Oil Polluted Site in Mexico. J. Fungi 2020, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Karaçay, H.A.; Shahi, A.; Gökçe, S.; Ince, B.; Ince, O. Aerobic and Anaerobic Fungal Metabolism and Omics Insights for Increasing Polycyclic Aromatic Hydrocarbons Biodegradation. Fungal Biol. Rev. 2017, 31, 61–72. [Google Scholar] [CrossRef]
- Evans, W.C.; Fernley, H.N.; Griffiths, E. Oxidative Metabolism of Phenanthrene and Anthracene by Soil Pseudomonads. The Ring-Fission Mechanism. Biochem. J. 1965, 95, 819–831. [Google Scholar] [CrossRef]
- Kumari, S.; Regar, R.K.; Manickam, N. Improved Polycyclic Aromatic Hydrocarbon Degradation in a Crude Oil by Individual and a Consortium of Bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef]
- Lily, M.K.; Bahuguna, A.; Dangwal, K.; Garg, V. Degradation of Benzo [a] Pyrene by a Novel Strain Bacillus Subtilis BMT4i (MTCC 9447). Braz. J. Microbiol. 2009, 40, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Swaathy, S.; Kavitha, V.; Pravin, A.S.; Mandal, A.B.; Gnanamani, A. Microbial Surfactant Mediated Degradation of Anthracene in Aqueous Phase by Marine Bacillus Licheniformis MTCC 5514. Biotechnol. Rep. 2014, 4, 161–170. [Google Scholar] [CrossRef]
- Emelyanova, E.V.; Solyanikova, I.P. Evaluation of Phenol-Degradation Activity of Rhodococcus Opacus 1CP Using Immobilized and Intact Cells. Int. J. Environ. Sci. Technol. 2020, 17, 2279–2294. [Google Scholar] [CrossRef]
- Shumkova, E.S.; Solyanikova, I.P.; Plotnikova, E.G.; Golovleva, L.A. Phenol Degradation by Rhodococcus Opacus Strain 1G. Appl. Biochem. Microbiol. 2009, 45, 43–49. [Google Scholar] [CrossRef]
- Muneeswari, R.; Iyappan, S.; Swathi, K.; Sudheesh, K.; Sekaran, G.; Ramani, K. Genomic Characterization of Enterobacter Xiangfangensis STP-3: Application to Real Time Petroleum Oil Sludge Bioremediation. Microbiol. Res. 2021, 253, 126882. [Google Scholar] [CrossRef]
- Wang, X.; Cai, T.; Wen, W.; Ai, J.; Ai, J.; Zhang, Z.; Zhu, L.; George, S.C. Surfactin for Enhanced Removal of Aromatic Hydrocarbons during Biodegradation of Crude Oil. Fuel 2020, 267, 117272. [Google Scholar] [CrossRef]
- Lazzem, A.; Lekired, A.; Ouzari, H.-I.; Landoulsi, A.; Chatti, A.; El May, A. Isolation and Characterization of a Newly Chrysene-Degrading Achromobacter aegrifaciens. Int. Microbiol. 2024, 27, 857–869. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Tian, W.-J.; Liu, Q.; Yu, H.-B.; Jin, X.; Zhao, Y.-G.; Zhou, Y.-H.; Feng, G. Enhanced Biodegradation of Pyrene and Indeno(1,2,3-Cd)Pyrene Using Bacteria Immobilized in Cinder Beads in Estuarine Wetlands. Mar. Pollut. Bull. 2016, 102, 128–133. [Google Scholar] [CrossRef]
- Ren, L.; Jia, Y.; Zhang, R.; Lin, Z.; Zhen, Z.; Hu, H.; Yan, Y. Insight into Metabolic Versatility of an Aromatic Compounds-Degrading Arthrobacter Sp. YC-RL1. Front. Microbiol. 2018, 9, 2438. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Devpura, N.; Jain, K.; Madamwar, D. Degradation of Chrysene by Enriched Bacterial Consortium. Front. Microbiol. 2018, 9, 1333. [Google Scholar] [CrossRef]
- Abdelhaleem, H.A.R.; Zein, H.S.; Azeiz, A.; Sharaf, A.N.; Abdelhadi, A.A. Identification and Characterization of Novel Bacterial Polyaromatic Hydrocarbon-Degrading Enzymes as Potential Tools for Cleaning up Hydrocarbon Pollutants from Different Environmental Sources. Environ. Toxicol. Pharmacol. 2019, 67, 108–116. [Google Scholar] [CrossRef]
- Kotoky, R.; Singha, L.P.; Pandey, P. Draft Genome Sequence of Polyaromatic Hydrocarbon-Degrading Bacterium Bacillus Subtilis SR1, Which Has Plant Growth-Promoting Attributes. Genome Announc. 2017, 5, e01339-17. [Google Scholar] [CrossRef]
- Cauduro, G.P.; Leal, A.L.; Marmitt, M.; de Ávila, L.G.; Kern, G.; Quadros, P.D.; Mahenthiralingam, E.; Valiati, V.H. New Benzo(a)Pyrene-Degrading Strains of the Burkholderia Cepacia Complex Prospected from Activated Sludge in a Petrochemical Wastewater Treatment Plant. Environ. Monit. Assess. 2021, 193, 163. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, H.; Lee, A.H.; Kwon, B.-O.; Khim, J.S.; Yim, U.H.; Kim, B.S.; Kim, J.-J. Microbial Community Composition and PAHs Removal Potential of Indigenous Bacteria in Oil Contaminated Sediment of Taean Coast, Korea. Environ. Pollut. 2018, 234, 503–512. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Lee, Y.B.; Naidu, R. Polyaromatic Hydrocarbon (PAH) Degradation Potential of a New Acid Tolerant, Diazotrophic P-Solubilizing and Heavy Metal Resistant Bacterium Cupriavidus Sp. MTS-7 Isolated from Long-Term Mixed Contaminated Soil. Chemosphere 2016, 162, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Okoye, C.O.; Gao, L.; Jiang, H.; Wu, Y.; Wang, Y.; Li, X.; Jiang, J. Whole-Genome Sequence Analysis Reveals Phenanthrene and Pyrene Degradation Pathways in Newly Isolated Bacteria Klebsiella michiganensis EF4 and Klebsiella oxytoca ETN19. Microbiol. Res. 2023, 273, 127410. [Google Scholar] [CrossRef]
- Rajkumari, J.; Choudhury, Y.; Bhattacharjee, K.; Pandey, P. Rhizodegradation of Pyrene by a Non-Pathogenic Klebsiella pneumoniae Isolate Applied with Tagetes erecta L. and Changes in the Rhizobacterial Community. Front. Microbiol. 2021, 12, 593023. [Google Scholar] [CrossRef]
- Rajkumari, J.; Paikhomba Singha, L.; Pandey, P. Genomic Insights of Aromatic Hydrocarbon Degrading Klebsiella pneumoniae AWD5 with Plant Growth Promoting Attributes: A Paradigm of Soil Isolate with Elements of Biodegradation. 3 Biotech 2018, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, X.; Zhang, Z.; Li, Y.; Zhang, Y.; Wang, C.; Fan, W. Mechanism of Phenanthrene Degradation by the Halophilic Pelagerythrobacter Sp. N7. Chemosphere 2024, 350, 141175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, W.; Yin, X.; Wang, X.; Liu, Z.; Liu, Q.; Deng, Z.; Lin, S.; Liang, R. Identification of an Efficient Phenanthrene-Degrading Pseudarthrobacter Sp. L1SW and Characterization of Its Metabolites and Catabolic Pathway. J. Hazard. Mater. 2024, 465, 133138. [Google Scholar] [CrossRef]
- Huizenga, J.M.; Schindler, J.; Simonich, M.T.; Truong, L.; Garcia-Jaramillo, M.; Tanguay, R.L.; Semprini, L. PAH Bioremediation with Rhodococcus rhodochrous ATCC 21198: Impact of Cell Immobilization and Surfactant Use on PAH Treatment and Post-Remediation Toxicity. J. Hazard. Mater. 2024, 470, 134109. [Google Scholar] [CrossRef]
- Kotoky, R.; Singha, L.P.; Pandey, P. Draft Genome Sequence of Heavy Metal-Resistant Soil Bacterium Serratia marcescens S2I7, Which Has the Ability to Degrade Polyaromatic Hydrocarbons. Genome Announc. 2017, 5, e01338-17. [Google Scholar] [CrossRef]
- Gran-Scheuch, A.; Fuentes, E.; Bravo, D.M.; Jiménez, J.C.; Pérez-Donoso, J.M. Isolation and Characterization of Phenanthrene Degrading Bacteria from Diesel Fuel-Contaminated Antarctic Soils. Front. Microbiol. 2017, 8, 1634. [Google Scholar] [CrossRef]
- Kotoky, R.; Ogawa, N.; Pandey, P. The Structure-Function Relationship of Bacterial Transcriptional Regulators as a Target for Enhanced Biodegradation of Aromatic Hydrocarbons. Microbiol. Res. 2022, 262, 127087. [Google Scholar] [CrossRef]
- Mohanty, S.; Jasmine, J.; Mukherji, S. Practical Considerations and Challenges Involved in Surfactant Enhanced Bioremediation of Oil. BioMed Res. Int. 2013, 2013, 328608. [Google Scholar] [CrossRef] [PubMed]
- French, K.E.; Terry, N. A High-Throughput Fluorescence-Based Assay for Rapid Identification of Petroleum-Degrading Bacteria. Front. Microbiol. 2019, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Roy, A.; Pal, S.; Mohapatra, B.; Kazy, S.K.; Maiti, M.K.; Sar, P. Enrichment and Characterization of Hydrocarbon-Degrading Bacteria from Petroleum Refinery Waste as Potent Bioaugmentation Agent for In Situ Bioremediation. Bioresour. Technol. 2017, 242, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Benedek, T.; Szentgyörgyi, F.; Szabó, I.; Farkas, M.; Duran, R.; Kriszt, B.; Táncsics, A. Aerobic and Oxygen-Limited Naphthalene-Amended Enrichments Induced the Dominance of Pseudomonas Spp. from a Groundwater Bacterial Biofilm. Appl. Microbiol. Biotechnol. 2020, 104, 6023–6043. [Google Scholar] [CrossRef]
- Remizovschi, A.; Carpa, R.; Forray, F.L.; Chiriac, C.; Roba, C.-A.; Beldean-Galea, S.; Andrei, A.-S.; Szekeres, E.; Baricz, A.; Lupan, I.; et al. Mud Volcanoes and the Presence of PAHs. Sci. Rep. 2020, 10, 1253. [Google Scholar] [CrossRef]
- Bento, F.M.; Camargo, F.A.O.; Okeke, B.C.; Frankenberger, W.T. Comparative Bioremediation of Soils Contaminated with Diesel Oil by Natural Attenuation, Biostimulation and Bioaugmentation. Bioresour. Technol. 2005, 96, 1049–1055. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Thompson, I.P.; Van Der Gast, C.J.; Ciric, L.; Singer, A.C. Bioaugmentation for Bioremediation: The Challenge of Strain Selection. Environ. Microbiol. 2005, 7, 909–915. [Google Scholar] [CrossRef]
- Urgun-Demirtas, M.; Stark, B.; Pagilla, K. Use of Genetically Engineered Microorganisms (GEMs) for the Bioremediation of Contaminants. Crit. Rev. Biotechnol. 2006, 26, 145–164. [Google Scholar] [CrossRef]
- Staninska-Pięta, J.; Czarny, J.; Piotrowska-Cyplik, A.; Juzwa, W.; Wolko, Ł.; Nowak, J.; Cyplik, P. Heavy Metals as a Factor Increasing the Functional Genetic Potential of Bacterial Community for Polycyclic Aromatic Hydrocarbon Biodegradation. Molecules 2020, 25, 319. [Google Scholar] [CrossRef]
- Dastgheib, S.M.M.; Amoozegar, M.A.; Khajeh, K.; Shavandi, M.; Ventosa, A. Biodegradation of Polycyclic Aromatic Hydrocarbons by a Halophilic Microbial Consortium. Appl. Microbiol. Biotechnol. 2012, 95, 789–798. [Google Scholar] [CrossRef]
- Gou, Y.; Song, Y.; Yang, S.; Yang, Y.; Cheng, Y.; Li, J.; Zhang, T.; Cheng, Y.; Wang, H. Polycyclic Aromatic Hydrocarbon Removal from Subsurface Soil Mediated by Bacteria and Archaea under Methanogenic Conditions: Performance and Mechanisms. Environ. Pollut. 2022, 313, 120023. [Google Scholar] [CrossRef] [PubMed]
- Khemili-Talbi, S.; Kebbouche-Gana, S.; Akmoussi-Toumi, S.; Angar, Y.; Gana, M.L. Isolation of an Extremely Halophilic Arhaeon Natrialba Sp. C21 Able to Degrade Aromatic Compounds and to Produce Stable Biosurfactant at High Salinity. Extremophiles 2015, 19, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Giwa, O.E.; Ibitoye, F.O. Bioremediation of Heavy Metal in Crude Oil Contaminated Soil Using Isolated Indigenous Microorganism Cultured with E Coli DE3 BL21. Int. J. Eng. Appl. Sci. 2017, 4, 67–70. [Google Scholar]
- Ma, X.-K.; Li, T.-T.; Fam, H.; Charles Peterson, E.; Zhao, W.-W.; Guo, W.; Zhou, B. The Influence of Heavy Metals on the Bioremediation of Polycyclic Aromatic Hydrocarbons in Aquatic System by a Bacterial-Fungal Consortium. Environ. Technol. 2018, 39, 2128–2137. [Google Scholar] [CrossRef]
- Feng, B.; Mao, Z.; Yu, J.; Wang, Y.; Zhang, Z.; Xu, L.; Lu, D. The Remediation of Polycyclic Aromatic Hydrocarbon Contaminated Soil by Immobilized Microorganisms Using Distiller’s Grains. Environ. Sci. Pollut. Res. Int. 2024, 31, 21415–21429. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a Strategy for Cleaning up of Soils Contaminated with Aromatic Compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef]
- Shen, T.; Pi, Y.; Bao, M.; Xu, N.; Li, Y.; Lu, J. Biodegradation of Different Petroleum Hydrocarbons by Free and Immobilized Microbial Consortia. Environ. Sci. Process. Impacts 2015, 17, 2022–2033. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. A New Look on Factors Affecting Microbial Degradation of Petroleum Hydrocarbon Pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
- Kotoky, R.; Pandey, P. Difference in the Rhizosphere Microbiome of Melia Azedarach during Removal of Benzo(a)Pyrene from Cadmium Co-Contaminated Soil. Chemosphere 2020, 258, 127175. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Singh, S.; Patel, A.; Jain, K.; Amin, S.; Madamwar, D. Synergistic Biodegradation of Phenanthrene and Fluoranthene by Mixed Bacterial Cultures. Bioresour. Technol. 2019, 284, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Książek-Trela, P.; Figura, D.; Węzka, D.; Szpyrka, E. Degradation of a Mixture of 13 Polycyclic Aromatic Hydrocarbons by Commercial Effective Microorganisms. Open Life Sci. 2024, 19, 20220831. [Google Scholar] [CrossRef] [PubMed]
- Monteiro1, A.A.; Boaventura, R.A.; Rodrigues, A.E. Phenol Biodegradation by Pseudomonas Putida DSM 548 in a Batch Reactor. Biochem. Eng. J. 2000, 6, 45–49. [Google Scholar] [CrossRef]
- Rayu, S.; Karpouzas, D.G.; Singh, B.K. Emerging Technologies in Bioremediation: Constraints and Opportunities. Biodegradation 2012, 23, 917–926. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ramaswamy, S. Non-Heme Iron-Dependent Dioxygenases: Unravelling Catalytic Mechanisms for Complex Enzymatic Oxidations. Curr. Opin. Chem. Biol. 2008, 12, 134–140. [Google Scholar] [CrossRef]
- Que, L., Jr.; Ho, R.Y.N. Dioxygen Activation by Enzymes with Mononuclear Non-Heme Iron Active Sites. Chem. Rev. 1996, 96, 2607–2624. [Google Scholar] [CrossRef]
- Vaillancourt, F.H.; Bolin, J.T.; Eltis, L.D. The Ins and Outs of Ring-Cleaving Dioxygenases. Crit. Rev. Biochem. Mol. Biol. 2006, 41, 241–267. [Google Scholar] [CrossRef]
- Adams, M.A.; Singh, V.K.; Keller, B.O.; Jia, Z. Structural and Biochemical Characterization of Gentisate 1,2-Dioxygenase from Escherichia Coli O157:H7. Mol. Microbiol. 2006, 61, 1469–1484. [Google Scholar] [CrossRef]
- Miyauchi, K.; Adachi, Y.; Nagata, Y.; Takagi, M. Cloning and Sequencing of a Novel Meta-Cleavage Dioxygenase Gene Whose Product Is Involved in Degradation of γ-Hexachlorocyclohexane in Sphingomonas paucimobilis. J. Bacteriol. 1999, 181, 6712–6719. [Google Scholar] [CrossRef]
- Fetzner, S. Ring-Cleaving Dioxygenases with a Cupin Fold. Appl. Environ. Microbiol. 2012, 78, 2505–2514. [Google Scholar] [CrossRef]
- Chua, C.H.; Feng, Y.; Yeo, C.C.; Khoo, H.E.; Poh, C.L. Identification of Amino Acid Residues Essential for Catalytic Activity of Gentisate 1,2-Dioxygenase from Pseudomonas alcaligenes NCIB 9867. FEMS Microbiol. Lett. 2001, 204, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Harayama, S.; Kok, M.; Neidle, E.L. Functional and Evolutionary Relationships among Diverse Oxygenases. Annu. Rev. Microbiol. 1992, 46, 565–601. [Google Scholar] [CrossRef]
- Chauhan, A.; Chakraborti, A.K.; Jain, R.K. Plasmid-Encoded Degradation of p-Nitrophenol and 4-Nitrocatechol by Arthrobacter Protophormiae. Biochem. Biophys. Res. Commun. 2000, 270, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Kato, S.; Shintani, N.; Kamini, N.R.; Nakajima-Kambe, T. Microbial Degradation of Aliphatic and Aliphatic-Aromatic Co-Polyesters. Appl. Microbiol. Biotechnol. 2014, 98, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, W.; Xu, L.; Zheng, X.; Chu, X.; Tian, J.; Wu, N.; Fan, Y. Identification of the Para-Nitrophenol Catabolic Pathway, and Characterization of Three Enzymes Involved in the Hydroquinone Pathway, in Peudomonas Sp. 1-7. BMC Microbiol. 2012, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.B.; O’Halloran, T.V. Overproduction, Purification, and Characterization of Chlorocatechol Dioxygenase, a Non-Heme Iron Dioxygenase with Broad Substrate Tolerance. Biochemistry 1991, 30, 7349–7358. [Google Scholar] [CrossRef]
- Tawfeeq, H.R.; Al-Jubori, S.S.; Mussa, A.H. Purification and Characterization of Catechol 1,2-Dioxygenase (EC 1.13.11.1; Catechol-Oxygen 1,2-Oxidoreductase; C12O) Using the Local Isolate of Phenol-Degrading Pseudomonas putida. Folia Microbiol. 2024, 69, 579–593. [Google Scholar] [CrossRef]
- Adachi, K.; Iwabuchi, T.; Sano, H.; Harayama, S. Structure of the Ring Cleavage Product of 1-Hydroxy-2-Naphthoate, an Intermediate of the Phenanthrene-Degradative Pathway of Nocardioides sp. Strain KP7. J. Bacteriol. 1999, 181, 757–763. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Wang, M.; Zhu, G.; Liu, D.; Sun, F.; Hao, N.; Li, X.; Rao, Z.; Zhang, X.C. Crystal Structure and Mutagenic Analysis of GDOsp, a Gentisate 1,2-Dioxygenase from Silicibacter pomeroyi. Protein Sci. 2008, 17, 1362–1373. [Google Scholar] [CrossRef]
- Ferraroni, M.; Matera, I.; Steimer, L.; Bürger, S.; Scozzafava, A.; Stolz, A.; Briganti, F. Crystal Structures of Salicylate 1,2-Dioxygenase-Substrates Adducts: A Step towards the Comprehension of the Structural Basis for Substrate Selection in Class III Ring Cleaving Dioxygenases. J. Struct. Biol. 2012, 177, 431–438. [Google Scholar] [CrossRef]
- Li, X.; Guo, M.; Fan, J.; Tang, W.; Wang, D.; Ge, H.; Rong, H.; Teng, M.; Niu, L.; Liu, Q.; et al. Crystal Structure of 3-Hydroxyanthranilic Acid 3,4-Dioxygenase from Saccharomyces cerevisiae: A Special Subgroup of the Type III Extradiol Dioxygenases. Protein Sci. 2006, 15, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, C.; Min, J.; Li, B.; Li, J.; Chen, W.; Kong, Y.; Hu, X. The Engineered Biphenyl Dioxygenases Enhanced the Metabolism of Dibenzofuran. Int. Biodeterior. Biodegrad. 2021, 161, 105228. [Google Scholar] [CrossRef]
- Meier, M.J.; Paterson, E.S.; Lambert, I.B. Use of Substrate-Induced Gene Expression in Metagenomic Analysis of an Aromatic Hydrocarbon-Contaminated Soil. Appl. Env. Microbiol. 2016, 82, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Yang, H.; Li, J.; Peng, T.; Huang, T.; Hu, Z. The Unique Biodegradation Pathway of Benzo[a]Pyrene in Moderately Halophilic Pontibacillus chungwhensis HN14. Chemosphere 2024, 354, 141705. [Google Scholar] [CrossRef]
- Silva, A.S.; Jacques, R.J.S.; Andreazza, R.; Bento, F.M.; Roesch, L.F.W.; Camargo, F.A.O. Properties of Catechol 1,2-Dioxygenase in the Cell Free Extract and Immobilized Extract of Mycobacterium fortuitum. Braz. J. Microbiol. 2013, 44, 291–297. [Google Scholar] [CrossRef]
- Haq, I.; Kalamdhad, A.S.; Malik, A. Bioremediation of Petroleum Refinery Wastewater Using Bacillus subtilis IH-1 and Assessment of Its Toxicity. Arch. Microbiol. 2024, 206, 296. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, T.; Sumetzberger-Hasinger, M.; Braunschmid, V.; Konegger, H.; Heipieper, H.J.; Guebitz, G.M.; Lackner, M.; Ribitsch, D.; Loibner, A.P. Laccase-Mediated Degradation of Petroleum Hydrocarbons in Historically Contaminated Soil. Chemosphere 2024, 348, 140733. [Google Scholar] [CrossRef]
- Burton, S.G. Oxidizing Enzymes as Biocatalysts. Trends Biotechnol. 2003, 21, 543–549. [Google Scholar] [CrossRef]
- Canada, K.A.; Iwashita, S.; Shim, H.; Wood, T.K. Directed Evolution of Toluene Ortho-Monooxygenase for Enhanced 1-Naphthol Synthesis and Chlorinated Ethene Degradation. J. Bacteriol. 2002, 184, 344–349. [Google Scholar] [CrossRef]
- Di Gennaro, P.; Conforti, P.; Lasagni, M.; Bestetti, G.; Bernasconi, S.; Orsini, F.; Sello, G. Dioxygenation of Naphthalene by Pseudomonas Fluorescens N3 Dioxygenase: Optimization of the Process Parameters. Biotechnol. Bioeng. 2006, 93, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.; Tao, Y.; Rui, L.; Wood, T.K. Controlling the Regiospecific Oxidation of Aromatics via Active Site Engineering of Toluene Para-Monooxygenase of Ralstonia pickettii PKO1. J. Biol. Chem. 2005, 280, 506–514. [Google Scholar] [CrossRef]
- Tao, Y.; Bentley, W.E.; Wood, T.K. Phenol and 2-Naphthol Production by Toluene 4-Monooxygenases Using an Aqueous/Dioctyl Phthalate System. Appl. Microbiol. Biotechnol. 2005, 68, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, R.; Hofrichter, M. Enzymatic Hydroxylation of Aromatic Compounds. Cell Mol. Life Sci. 2007, 64, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Vardar, G.; Wood, T.K. Protein Engineering of Toluene-o-Xylene Monooxygenase from Pseudomonas Stutzeri OX1 for Synthesizing 4-Methylresorcinol, Methylhydroquinone, and Pyrogallol. Appl. Environ. Microbiol. 2004, 70, 3253–3262. [Google Scholar] [CrossRef]
- Duetz, W.A.; van Beilen, J.B.; Witholt, B. Using Proteins in Their Natural Environment: Potential and Limitations of Microbial Whole-Cell Hydroxylations in Applied Biocatalysis. Curr. Opin. Biotechnol. 2001, 12, 419–425. [Google Scholar] [CrossRef]
- Li, Z.; van Beilen, J.B.; Duetz, W.A.; Schmid, A.; de Raadt, A.; Griengl, H.; Witholt, B. Oxidative Biotransformations Using Oxygenases. Curr. Opin. Chem. Biol. 2002, 6, 136–144. [Google Scholar] [CrossRef]
- Vilbert, A.C.; Kontur, W.S.; Gille, D.; Noguera, D.R.; Donohue, T.J. Engineering Novosphingobium Aromaticivorans to Produce Cis,Cis-Muconic Acid from Biomass Aromatics. Appl. Environ. Microbiol. 2024, 90, e0166023. [Google Scholar] [CrossRef]
- Xu, M.; He, Z.; Zhang, Q.; Liu, J.; Guo, J.; Sun, G.; Zhou, J. Responses of Aromatic-Degrading Microbial Communities to Elevated Nitrate in Sediments. Environ. Sci. Technol. 2015, 49, 12422–12431. [Google Scholar] [CrossRef]
- Wang, H.; Tang, L.-X.; Ye, Y.-F.; Ma, J.-X.; Li, X.; Si, J.; Cui, B.-K. Laccase Immobilization and Its Degradation of Emerging Pollutants: A Comprehensive Review. J. Environ. Manag. 2024, 359, 120984. [Google Scholar] [CrossRef]
- Kaur, T.; Lakhawat, S.S.; Kumar, V.; Sharma, V.; Neeraj, R.R.K.; Sharma, P.K. Polyaromatic Hydrocarbon Specific Ring Hydroxylating Dioxygenases: Diversity, Structure, Function, and Protein Engineering. Curr. Protein Pept. Sci. 2023, 24, 7–21. [Google Scholar] [CrossRef]
- Ramya, R.K.; Theraka, K.; Ramprasadh, S.V.; Bharathi, S.V.; Srinivasan, S.; Jacob, S.; Kuila, A. Pragmatic Treatment Strategies for Polyaromatic Hydrocarbon Remediation and Anti-Biofouling from Surfaces Using Nano-Enzymes: A Review. Appl. Biochem. Biotechnol. 2023, 195, 5479–5496. [Google Scholar] [CrossRef]
- Medić, A.B.; Karadžić, I.M. Pseudomonas in Environmental Bioremediation of Hydrocarbons and Phenolic Compounds- Key Catabolic Degradation Enzymes and New Analytical Platforms for Comprehensive Investigation. World J. Microbiol. Biotechnol. 2022, 38, 165. [Google Scholar] [CrossRef]
- Nazari, M.T.; Simon, V.; Machado, B.S.; Crestani, L.; Marchezi, G.; Concolato, G.; Ferrari, V.; Colla, L.M.; Piccin, J.S. Rhodococcus: A Promising Genus of Actinomycetes for the Bioremediation of Organic and Inorganic Contaminants. J. Environ. Manag. 2022, 323, 116220. [Google Scholar] [CrossRef]
- Thacharodi, A.; Hassan, S.; Singh, T.; Mandal, R.; Chinnadurai, J.; Khan, H.A.; Hussain, M.A.; Brindhadevi, K.; Pugazhendhi, A. Bioremediation of Polycyclic Aromatic Hydrocarbons: An Updated Microbiological Review. Chemosphere 2023, 328, 138498. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Wan Azelee, N.I.; Jeon, B.-H.; et al. Polyaromatic Hydrocarbons (PAHs) in the Water Environment: A Review on Toxicity, Microbial Biodegradation, Systematic Biological Advancements, and Environmental Fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef]
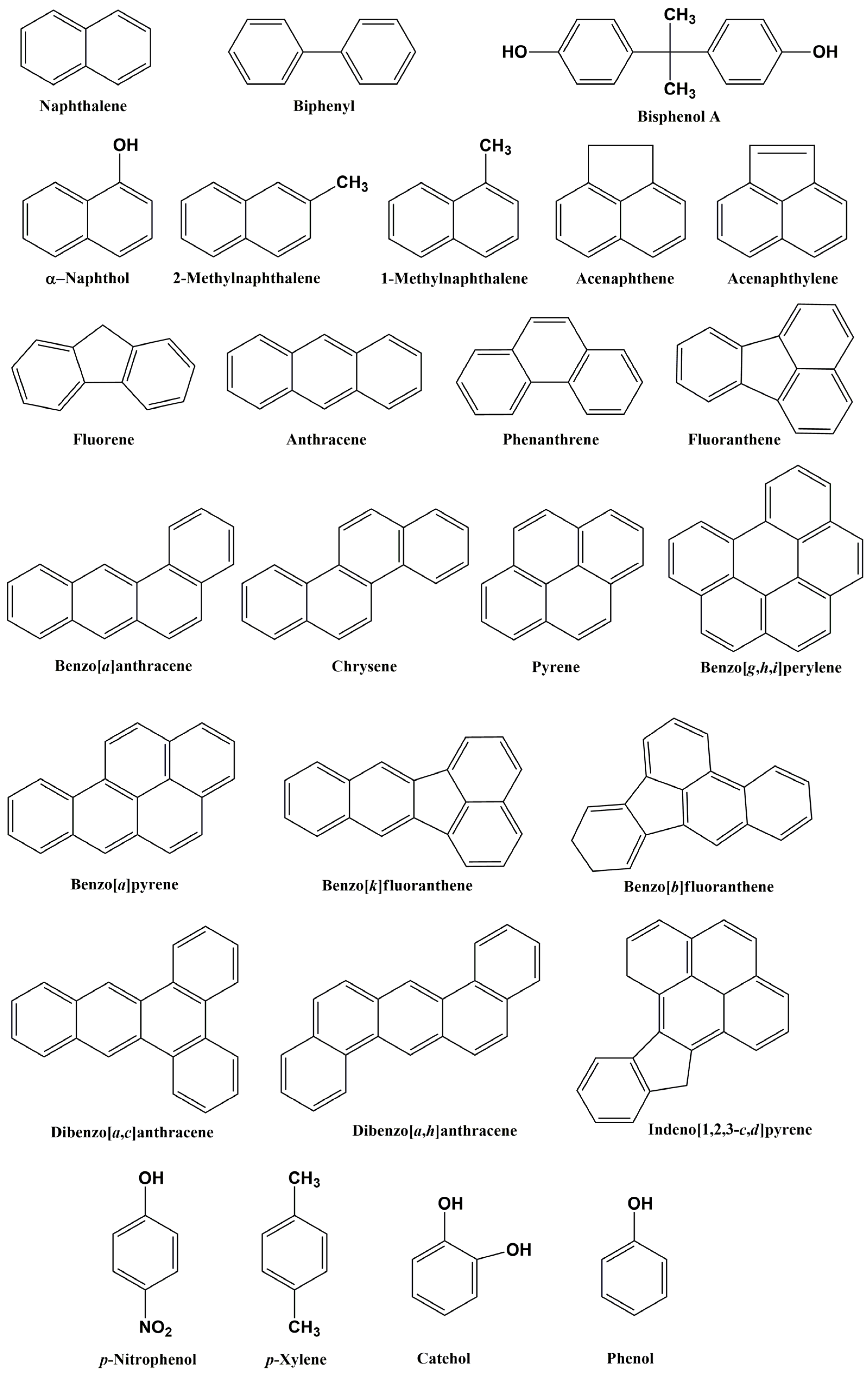

| Bacteria | Sample Source | Registered Degradation of PAH Compound | Specific Features * | References |
|---|---|---|---|---|
| Achromobacter aegrifaciens | Crude-oil-contaminated seawater (Bizerte, Tunisia) | Chrysene | Production of biosurfactant | [91] |
| Acinetobacter baumannii INP1, Pseudomonas taiwanensis PYR1 | PAH-contaminated estuarine wetlands (China) | Pyrene, indeno(1,2,3-cd)pyrene | [92] | |
| Aeribacillus pallidus UCPS2, Bacillus axarquiensis UCPD1, Bacillus siamensis GHP76, Bacillus subtilis subsp. inaquosorum U277 | Unkeshwar hot springs (India) | Anthracene, fluorene, phenanthrene, pyrene | Degradation of a PAH mixture at 50 °C within a consortium | [69] |
| Arthrobacter sp. YC-RL1 | Petroleum-contaminated soil (Xingtai City, Hebei Province, China) | p-Xylene, naphthalene, phenanthrene, biphenyl, p-nitrophenol, and bisphenol A | Degrading a mixture of PAHs | [93] |
| Bacillus sp. ASDC2, Burkholderia sp. ASDC3, Rhodococcus sp. ASDC1 | Polluted soil sediments (Amlakhadi Canal, Ankleshwar, India) | Chrysene | Higher efficiency of degradation in consortia | [94] |
| Bacillus anthracis, B. cereus, B. mojavensis, B. subtilis | Oil-contaminated sludge, soil, and sea water (Borg Al Arab City, Egypt) | Anthracene, α-naphthol, catechol | [95] | |
| Bacillus subtilis SR1 | Petroleum-contaminated rhizosphere soil | Benzo[a]pyrene | Resistant to the presence of several heavy metals | [96] |
| Bacillus velezensis, Microbacterium schleiferi, Pseudomonas aeruginosa, Xanthomonas boreopolis | Crude-oil-contaminated soil | Naphthalene, anthracene, acenaphthylene, fluorene, acenaphthene, phenanthrene, pyrene, benzo[a]pyrene | The ability to degrade PAHs present in crude oil; higher efficiency of degradation in consortia | [66] |
| Bacillus licheniformis MTCC 5514 | Marine samples (India) | Anthracene | Production of biosurfactant | [86] |
| Burkholderia cepacia complex | Sludge samples | Benzo[a]pyrene | Production of biosurfactant | [97] |
| Cobetia marina, Rhodococcus soli, Pseudoalteromonas agarivorans | Sediment samples (Sinduri beach in Taean, Republic of Korea) | Naphthalene, phenanthrene, pyrene | [98] | |
| Cupriavidus sp. MTS-7 | Site of a former gas plant in Australia that has been contaminated for a long time | Benzo[a]pyrene | Ability to degrade PAHs across a wide pH range, especially acidic pH, and in the presence of low concentrations of Cu, Pb, Zn, and Cd | [99] |
| Dietzia psychralcaliphila | Sediments (Deception Island, Antarctica) | Phenanthrene | [9] | |
| Klebsiella michiganensis EF4, K. oxytoca ETN19 | PAH-contaminated farmland soil (Zhenjiang City, Jiangsu, China) | Phenanthrene | Higher efficiency of degradation in consortia | [100] |
| K. pneumoniae AWD5 | Soil from automobile workshop (India) | Pyrene | Stimulate plant growth | [101,102] |
| Microbacterium esteraromaticum, Ochrobactrum anthropi, Pseudomonas aeruginosa, Pseudomonas mendocina, Stenotrophomonas maltophilia | Contaminated soil from oil refinery and a tyre waste dump site (India) | Naphthalene, fluorene, phenanthrene, benzo[b]fluoranthene | The ability to degrade PAHs present in crude oil; higher efficiency of degradation in consortia | [84] |
| Pelagerythrobacter sp. N7 | Saline soil samples (Shanxi Province, China) | Phenanthrene | Resistant to the presence of elevated salt concentrations | [103] |
| Pseudarthrobacter sp. L1SW | Contaminated soil from petroleum refinery (China) | Phenanthrene | Stimulates plant growth; resistant to the presence of heavy metals (Ni, Zn, and Cr) | [104] |
| Pseudomonas aeruginosa | Garden soil | Phenanthrene, anthracene | [83] | |
| Rhodococcus opacus | Petroleum-contaminated soil (Samara, Russia) | Phenol, catechol | [87,88] | |
| Rhodococcus rhodochrous ATCC 21198 | Commercially available from the American Type Culture Collection | Fluorene, phenanthrene, anthracene, pyrene | Degrading a mixture of PAHs | [105] |
| Serratia marcescens S2I7 | Petroleum-contaminated soil (India) | Benzo[a]pyrene | Cadmium sustainability | [106] |
| Sphingobium xenophagum D43FB | Soil samples (South Shetland Islands, Antarctica) | Phenanthrene | Cadmium sustainability | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletgildeeva, A.T.; Kuznetsov, N.A. Bioremediation of Polycyclic Aromatic Hydrocarbons by Means of Bacteria and Bacterial Enzymes. Microorganisms 2024, 12, 1814. https://doi.org/10.3390/microorganisms12091814
Davletgildeeva AT, Kuznetsov NA. Bioremediation of Polycyclic Aromatic Hydrocarbons by Means of Bacteria and Bacterial Enzymes. Microorganisms. 2024; 12(9):1814. https://doi.org/10.3390/microorganisms12091814
Chicago/Turabian StyleDavletgildeeva, Anastasiia T., and Nikita A. Kuznetsov. 2024. "Bioremediation of Polycyclic Aromatic Hydrocarbons by Means of Bacteria and Bacterial Enzymes" Microorganisms 12, no. 9: 1814. https://doi.org/10.3390/microorganisms12091814






