Dual-Emission Fluorescence Resonance Energy Transfer (FRET) PCR Discriminates Salmonella Pullorum and Gallinarum
Abstract
1. Introduction
2. Material and Methods
2.1. Reference Bacterial Strains
2.2. Genomic DNA Extraction
2.3. Differential FRET-PCR Targeting PegB Gene
Primers and Probes
2.4. Testing of PegB-Targeting FRET-PCR with Reference Strains and Clinical Isolates
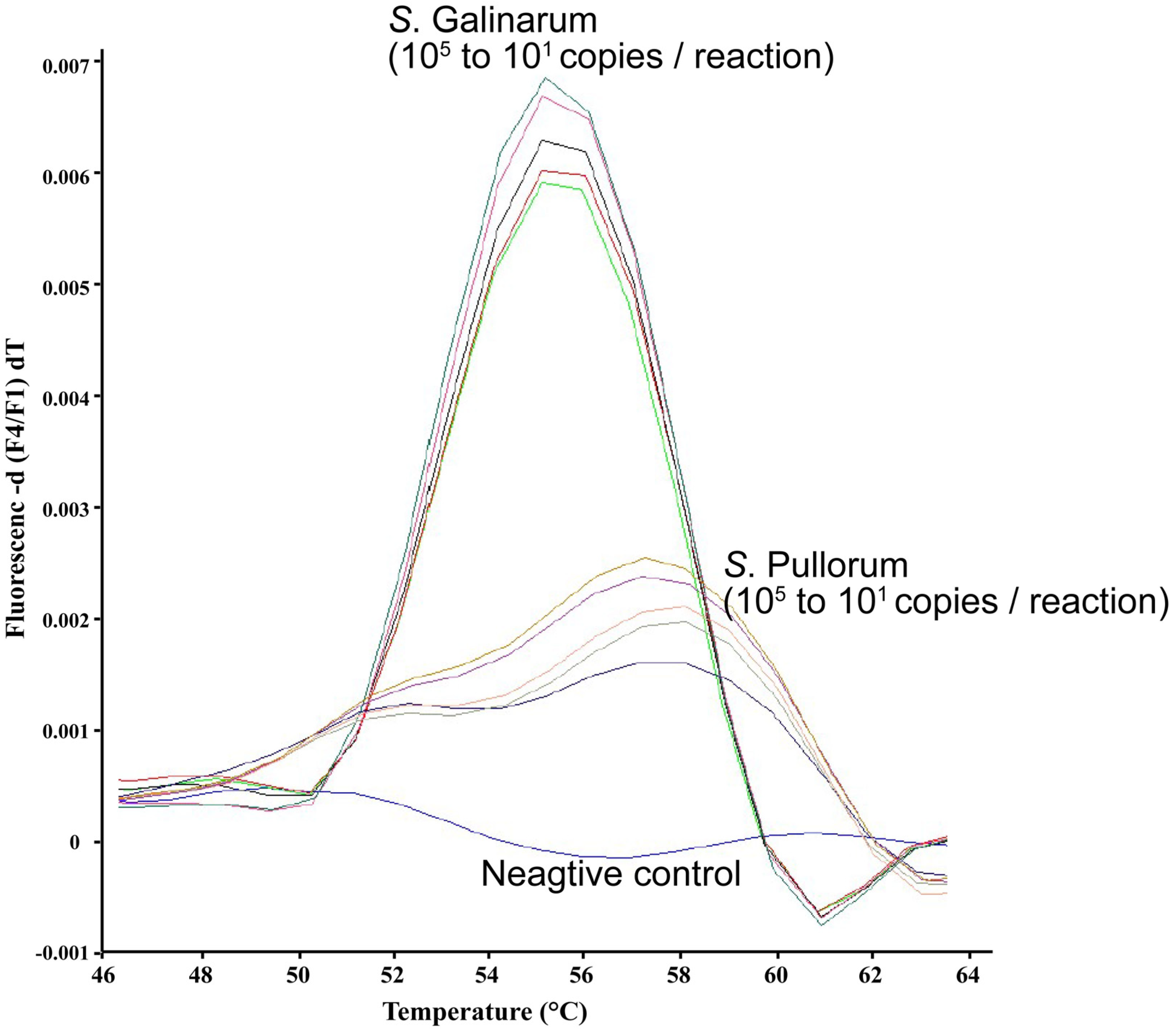
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ao, T.T.; Feasey, N.A.; Gordon, M.A.; Keddy, K.H.; Angulo, F.J.; Crump, J.A. Global burden of invasive nontyphoidal Salmonella disease. 2010. Emerg. Infect. Dis. 2015, 21, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Dopfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.A.; Jones, M.A.; Smith, A.L.; Wigley, P. The long view: Salmonella-the last forty years. Avian Pathol. 2012, 41, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.A.; Freitas Neto, O.C. Pullorum disease and fowl typhoid–New thoughts on old diseases: A review. Avian Pathol. 2011, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Johnston, R.N.; Liu, G.R.; Liu, S.L. Genomic comparison between Salmonella Gallinarum and Pullorum: Differential pseudogene formation under common host restriction. PLoS ONE 2013, 8, e59427. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Rankin, S.C.; Blanchet, R.T.; Nulton, J.D.; Edwards, R.A.; Schifferli, D.M. Diversification of the Salmonella fimbriae: A model of macro- and microevolution. PLoS ONE 2012, 7, e38596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, L.; Kelly, P.; Freeman, M.; Jaegerson, K.; Gong, J.; Xu, B.; Pan, Z.; Xu, C.; Wang, C. Detection of Salmonella spp. using a generic and differential FRET-PCR. PLoS ONE 2013, 16, e76053. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, D.; Vaglenov, A.; Kaltenboeck, B. One-step real-time duplex reverse transcription PCRs simultaneously quantify analyte and housekeeping gene mRNAs. Biotechniques 2004, 36, 508–519. [Google Scholar] [CrossRef] [PubMed]
- DeGraves, F.J.; Gao, D.; Kaltenboeck, B. High-sensitivity quantitative PCR platform. Biotechniques 2003, 34, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S49–S52. [Google Scholar]
- Gong, J.; Zhang, J.; Xu, M.; Zhu, C.; Yu, Y.; Liu, X.; Kelly, P.; Xu, B.; Wang, C. Prevalence and fimbrial genotype distribution of poultry Salmonella isolates in China (2006 to 2012). Appl. Environ. Microbiol. 2014, 80, 687–693. [Google Scholar] [CrossRef]
- Shah, D.H.; Park, J.; Cho, M.; Kim, M.; Chae, J. Allele-specific PCR method based on rfbS sequence for distinguishing Salmonella gallinarum from Salmonella pullorum: Serotype-specific rfbS sequence polymorphism. J. Microbiol. Methods 2005, 60, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kwon, Y.; Jung, B.; Kim, A.; Lee, K.; An, B.; Song, E.; Kwon, J.; Chung, G. Differential identification of Salmonella enterica subsp. enterica serovar Gallinarum biovars Gallinarum and Pullorum based on polymorphic regions of glgC and speC genes. Vet. Microbiol. 2011, 147, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Zhu, H.; Tao, C.; Zhang, B.; Yao, L.; Zhang, Y.; Afayibo, D.J.A.; Li, T.; Tian, M.; Qi, J.; et al. Rapid detection and differentiating of the predominant Salmonella serovars in chicken farm by taqman multiplex real-time PCR assay. Front. Cell Infect. Microbiol. 2021, 11, 759965. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.F.A.; Freitas Neto, O.C.; Lopes, P.D.; Almeida, A.M.; Barrow, P.A.; Berchieri, A., Jr. Polymerase chain reaction assay based on ratA gene allows differentiation between Salmonella enterica subsp. enterica serovar Gallinarum biovars Gallinarum and Pullorum. J. Vet. Diagn. Investig. 2013, 25, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Park, K.Y.; Yoo, H.S.; Park, J.Y.; Park, Y.H.; Kim, S.J. Differentiation of Salmonella enterica serotype gallinarum biotype pullorum from biotype gallinarum by analysis of phase 1 flagellin C gene (fliC). J. Microbiol. Methods 2000, 40, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kisiela, D.; Kuczkowski, M.; Kiczak, L.; Wieliczko, A.; Ugorski, M. Differentiation of Salmonella Gallinarum biovar Gallinarum from Salmonella Gallinarum biovar Pullorum by PCR-RFLP of the fimH gene. J. Vet. Med. B 2005, 52, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Hardick, A.; Hardick, J.; Wood, B.J.; Gaydos, C. Comparison between the Gen-Probe transcription-mediated amplification Trichomonas vaginalis research assay and real-time PCR for Trichomonas vaginalis detection using a Roche LightCycler instrument with female self-obtained vaginal swab samples and male urine samples. J. Clin. Microbiol. 2006, 44, 4197–4199. [Google Scholar] [CrossRef] [PubMed]
- Kaltenboeck, B.; Wang, C. Advances in real-time PCR: Application to clinical laboratory diagnostics. Adv. Clin. Chem. 2005, 40, 219–259. [Google Scholar] [CrossRef] [PubMed]
| Farm | Region | No. of Samples | PegB FRET-PCR Identification | Bacteriological Identification | ||
|---|---|---|---|---|---|---|
| S. Pullorum | S. Gallinarum | S. Pullorum | S. Gallinarum | |||
| A | Jiangdu | 307 | 21 | 21 | ||
| B | Gaoyou | 91 | 4 | 4 | ||
| C | Baoying | 59 | 6 | 6 | ||
| D | Peixian | 58 | 2 | 2 | ||
| E | Yizheng | 57 | 1 | 1 | ||
| F | Wujin | 45 | 4 | 4 | ||
| G | Lishui | 41 | ||||
| H | Haian | 32 | ||||
| I | Peixian | 22 | ||||
| J | Jiangdu | 16 | ||||
| K | Peixian | 11 | 1 | 1 | ||
| Total | 739 | 37 | 2 | 37 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Iduu, N.V.; Zhang, D.; Chenoweth, K.; Wei, L.; Yang, Y.; Dou, X.; Wang, C. Dual-Emission Fluorescence Resonance Energy Transfer (FRET) PCR Discriminates Salmonella Pullorum and Gallinarum. Microorganisms 2024, 12, 1815. https://doi.org/10.3390/microorganisms12091815
Gong J, Iduu NV, Zhang D, Chenoweth K, Wei L, Yang Y, Dou X, Wang C. Dual-Emission Fluorescence Resonance Energy Transfer (FRET) PCR Discriminates Salmonella Pullorum and Gallinarum. Microorganisms. 2024; 12(9):1815. https://doi.org/10.3390/microorganisms12091815
Chicago/Turabian StyleGong, Jiansen, Nneka Vivian Iduu, Di Zhang, Kelly Chenoweth, Lanjing Wei, Yi Yang, Xinhong Dou, and Chengming Wang. 2024. "Dual-Emission Fluorescence Resonance Energy Transfer (FRET) PCR Discriminates Salmonella Pullorum and Gallinarum" Microorganisms 12, no. 9: 1815. https://doi.org/10.3390/microorganisms12091815
APA StyleGong, J., Iduu, N. V., Zhang, D., Chenoweth, K., Wei, L., Yang, Y., Dou, X., & Wang, C. (2024). Dual-Emission Fluorescence Resonance Energy Transfer (FRET) PCR Discriminates Salmonella Pullorum and Gallinarum. Microorganisms, 12(9), 1815. https://doi.org/10.3390/microorganisms12091815








