Profile of Bacterial Communities in Copper Mine Tailings Revealed through High-Throughput Sequencing
Abstract
1. Introduction
2. Materials and Methods
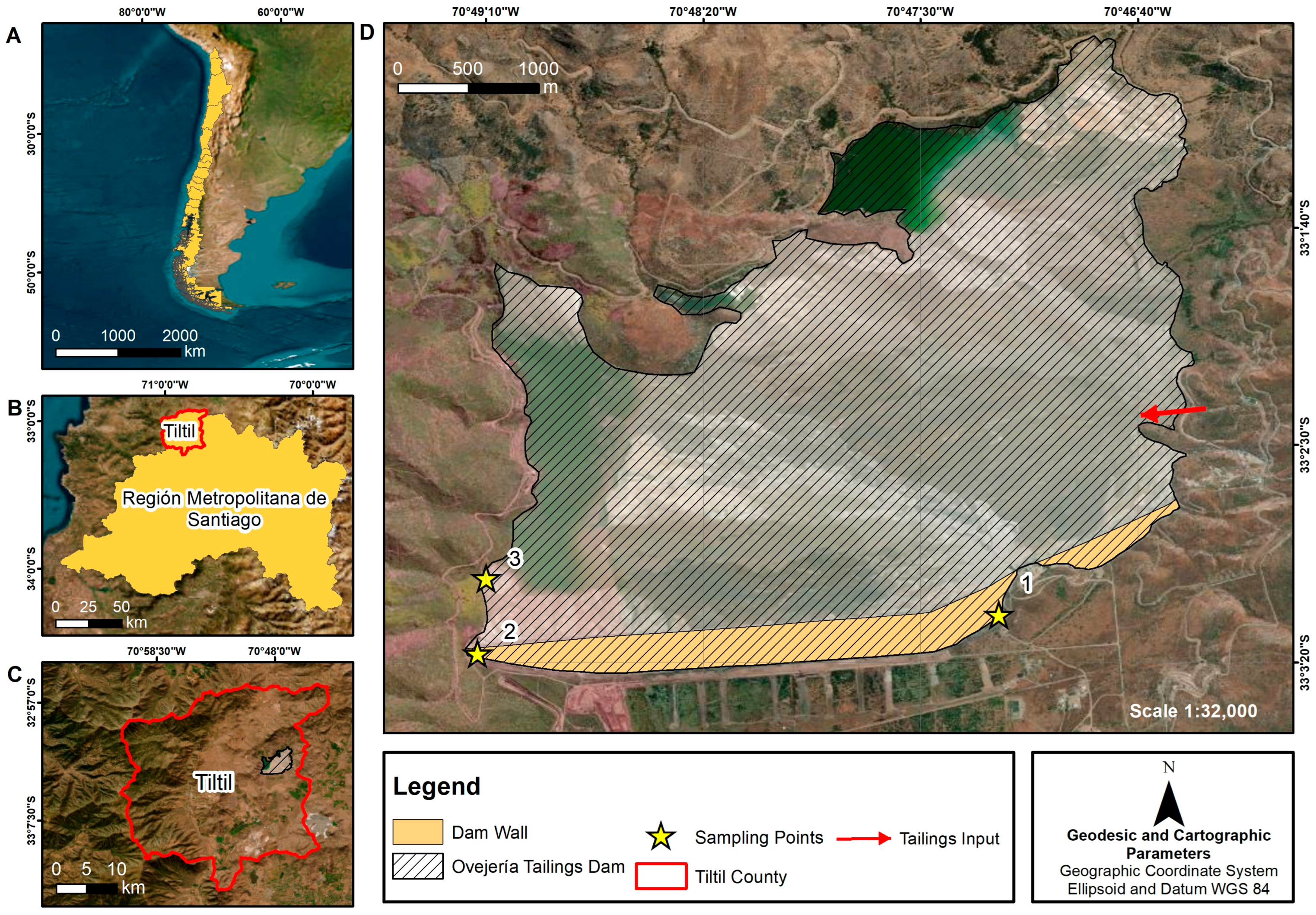
2.1. Study Area
2.2. Tailing Sampling
2.3. Physicochemical Parameters of the Mine Tailings
2.4. DNA Extraction and Sequencing
2.5. Bioinformatic Analysis
3. Results
3.1. Chemical Characterization
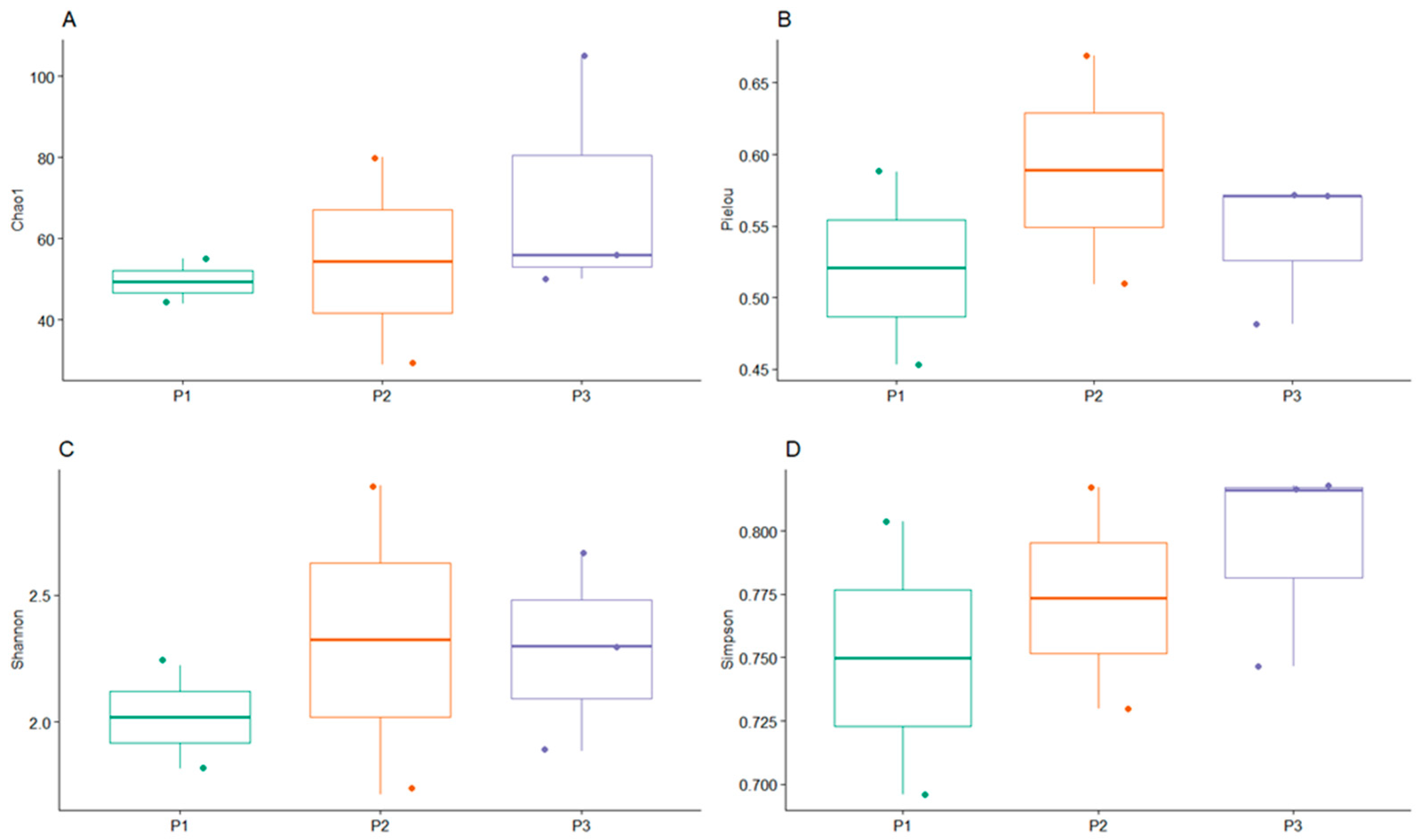
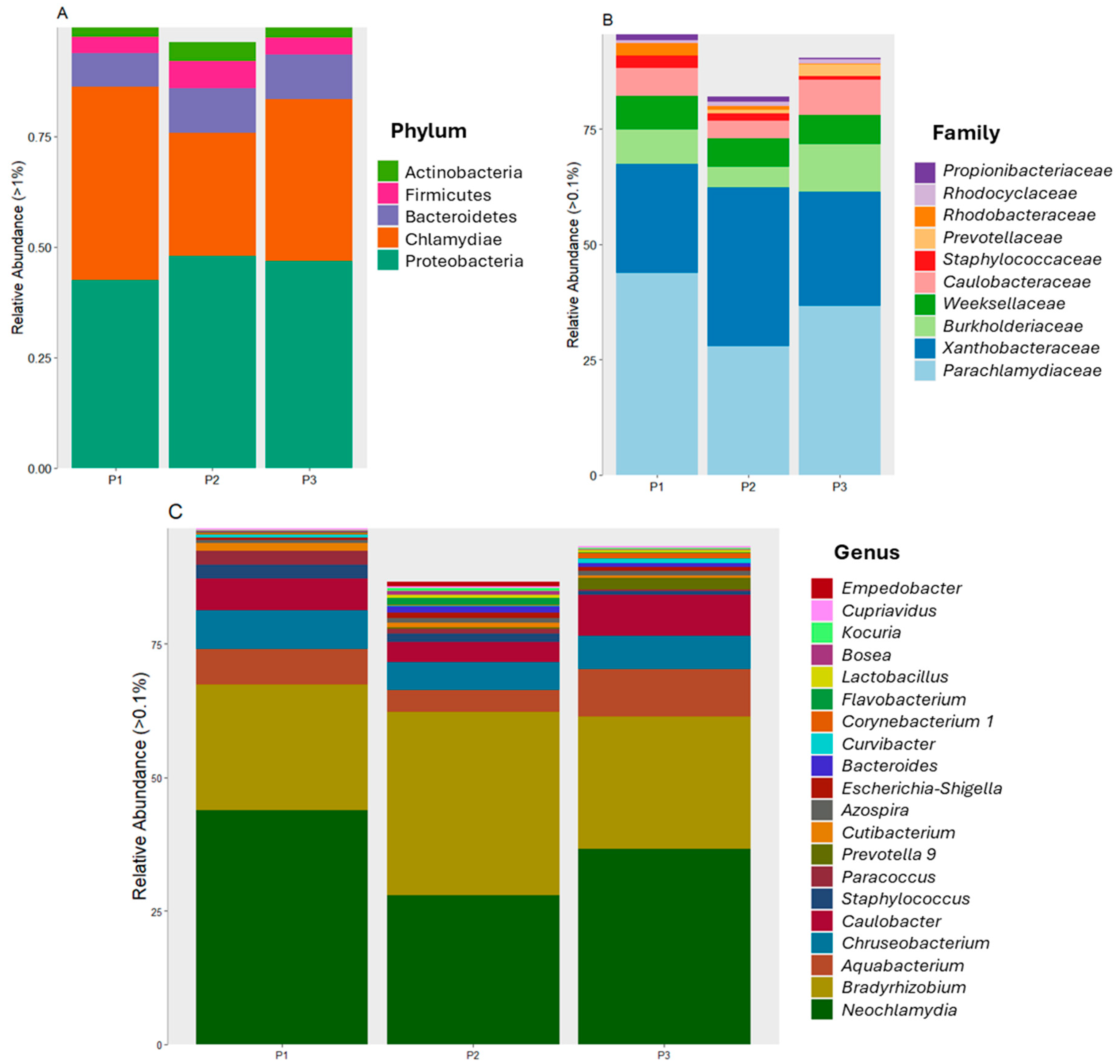
3.2. Bacterial Community Diversity and Distribution
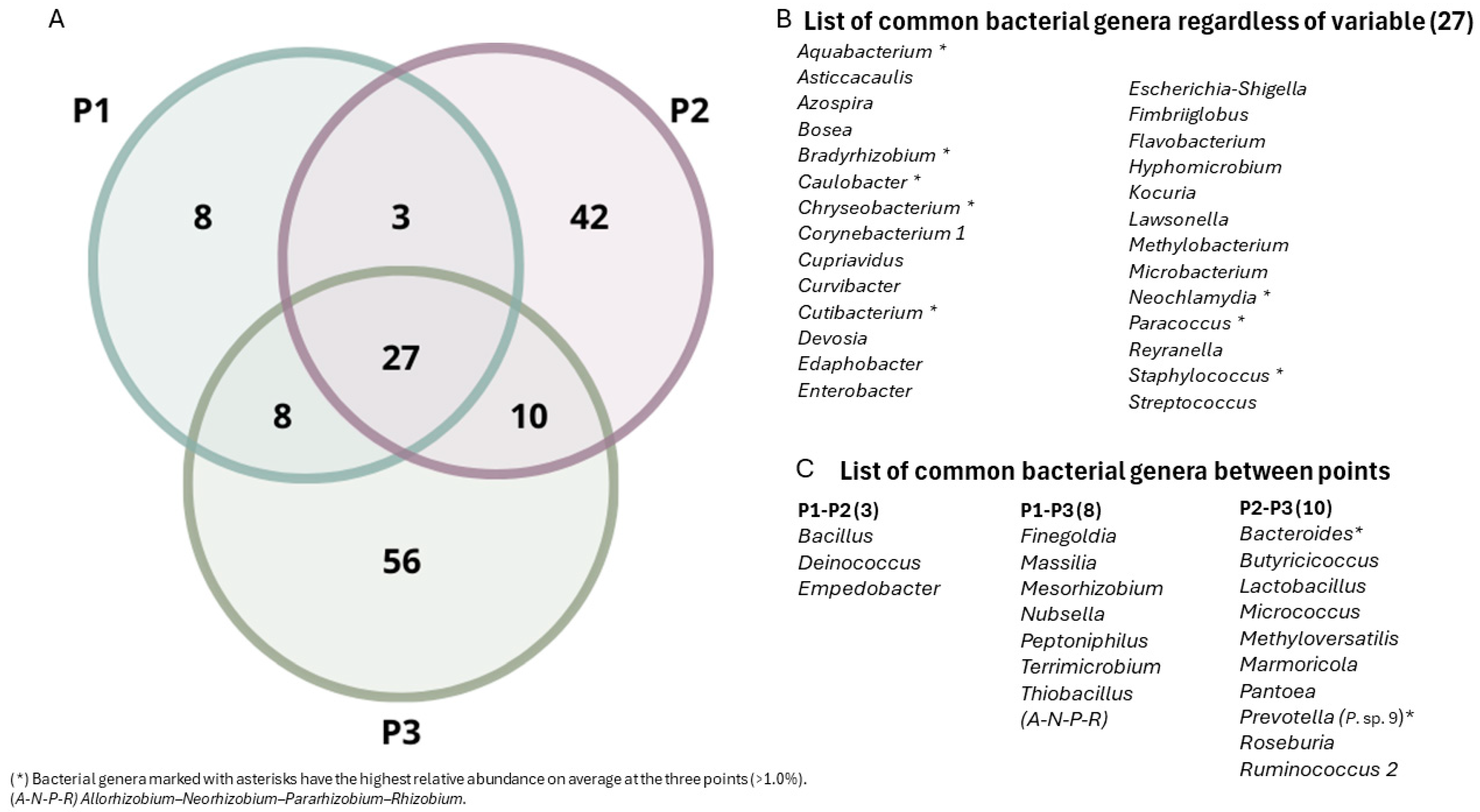
3.3. Biodiversity Analysis of Bacteria Associated with Mining Tailings across Three Zones
4. Discussion
4.1. Chemical Parameters of the Tailing Samples
4.2. Insights into the Variability and Spatial Distribution of Bacterial Communities
4.3. Possible Physical Locations Where Bacterial Communities Can Be Found
4.4. Venn Diagram and Associated Genera
4.5. Conservation, Biodiversity, and Remediation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailing dams: Characteristics, failures, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef]
- Sibanda, T.; Selvarajan, R.; Msagati, T.; Venkatachalam, S.; Meddows-Taylor, S. Defunct gold mine tailings are natural reservoir for unique bacterial communities revealed by high-throughput sequencing analysis. Sci. Total. Environ. 2019, 650, 2199–2209. [Google Scholar] [CrossRef]
- Oberle, B.; Brereton, D.; Mihaylova, A. Towards Zero Harm: A Compendium of Papers for Global Tailings Review, 1st ed.; Global Tailing Review: St. Gallen, Switzerland, 2020; p. 135. [Google Scholar]
- Dold, B. Submarine tailings disposal (STD): A review. Minerals 2014, 4, 642–666. [Google Scholar] [CrossRef]
- Neaman, A.; Ginocchio, R.; Yáñez, C. Restoration and Conservation Actions: Chilean Studies on Phytoremediation of Metal-Polluted, Acidic Soils. In Ecotoxicology in Latin America, 1st ed.; Araújo, C., Shinn, C., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 551–561. [Google Scholar]
- Tapia, Y.; García, A.; Acuña, E.; Salazar, O.; Casanova, M.; Najera, F.; Kremer, C.; Castillo, B.; Joven, A.; Diaz, O.; et al. Monitoring of Chemical Species in Soils, Waters and Plants Near the Active Copper Mine Tailing Dam Ovejeria (Central Chile). Water Air Soil Pollut. 2024, 235, 176. [Google Scholar] [CrossRef]
- Godfrid, J.; Poo, P.; Palmisano, T.; Fuentes, C. Pasivos Ambientales Mineros en Chile: Insumos y Propuestas para una Gestión Sostenible, 1st ed.; Universidad Autónoma de Chile: Santiago, Chile, 2024; p. 66. ISBN 978-956-417-023-7. [Google Scholar]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: A review. J. Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef] [PubMed]
- Keshri, J.; Mankazana, B.B.J.; Momba, M.N.B. Profile of bacterial communities in South African mine water samples using Illumina next-generation sequencing platform. Appl. Microbiol. Biotechnol. 2014, 99, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Kyrpides, N.C.; Hugenholtz, P.; Eisen, J.A.; Woyke, T.; Göker, M.; Parker, C.T.; Amann, R.; Beck, B.J.; Chain, P.S.; Chun, J.; et al. Genomic encyclopedia of bacteria and archaea: Sequencing a myriad of type strains. PLoS Biol. 2014, 12, e1001920. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hajyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Aghababai Beni, A. Comprehensive analysis of heavy metal soil contamination in mining Environments: Impacts, monitoring techniques, and remediation strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Li, Q.; Lesseur, C.; Srirangam, P.; Kaur, K.; Hermetz, K.; Caudle, W.M.; Fiedler, N.; Panuwet, P.; Prapamontol, T.; Naksen, W.; et al. Association between Prenatal Organophosphate Pesticide Exposure and Placental Gene Networks. Environ. Res. 2023, 224, 115490. [Google Scholar] [CrossRef]
- Navarro, M.C.; Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; Vidal, J.; Tovar, P.J.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Punia, A. Carbon dioxide sequestration by mines: Implications for climate change. Clim. Change 2021, 165, 10. [Google Scholar] [CrossRef]
- Punia, A.; Bharti, R.; Kumar, P. Impact of mine pit lake on metal mobility in groundwater. Environ. Earth Sci. 2021, 80, 245. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Soil Reclamation of Abandoned Mine Land by Revegetation: A Review. Int. J. Soil Sediment. Water 2010, 3, 21. [Google Scholar]
- Qian, L.; Lin, H.; Li, B.; Dong, Y. Physicochemical characteristics and microbial communities of rhizosphere in complex amendment-assisted soilless revegetation of gold mine tailings. Chemosphere 2023, 320, 138052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, K.; Struik, P.; Nadeem, M.; Zhang, T.; Zhao, Y.; Wu, R.; Jin, K.; Li, Y. Alteration of microbial carbon and nitrogen metabolism within the soil metagenome with grazing intensity at semiarid steppe. J. Environ. Manag. 2023, 347, 119078. [Google Scholar] [CrossRef]
- Navarro-Noya, Y.E.; Jan-Roblero, J.; González-Chávez, D.C.; Hernández-Gama, R.; Hernández-Rodríguez, C. Bacterial communities associated with the rhizosphere of pioneer plants (Bahia xylopoda and Viguiera linearis) growing on heavy metals-contaminated soils. Antonie Van Leeuwenhoek 2010, 97, 335–349. [Google Scholar] [CrossRef]
- Dhal, P.K.; Sar, P. Microbial communities in uranium mine tailings and mine water sediment from Jaduguda U mine, India: A culture independent analysis. J. Envion. Sci. Health Part A Tox Hazard Subst Environ Eng 2014, 49, 694–709. [Google Scholar] [CrossRef]
- Callender, K.L.; Roy, S.; Khasa, D.P.; Whyte, L.G.; Greer, C.W. Actinorhizal alder phytostabilization alters microbial community dynamics in gold mine waste rock from northern Quebec: A greenhouse study. PLoS ONE 2016, 11, e0150181. [Google Scholar] [CrossRef]
- Orell, A.; Navarro, C.A.; Arancibia, R.; Mobarec, J.C.; Jerez, C.A. Life in blue: Copper resistance mechanisms of bacteria and Archaea used in industrial biomining of minerals. Biotechnol. Adv. 2010, 28, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Faoro, H.; Alves, A.C.; Souza, E.M.; Rigo, L.U.; Cruz, L.M.; Al-Janabi, S.M.; Monteiro, R.A.; Baura, V.A.; Pedrosa, F.O. Influence of soil characteristics on the diversity of bacteria in the southern Brazilian Atlantic Forest. Appl. Environ. Microbiol. 2010, 76, 4744–4749. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The Unseen Majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- He, Z.; Gentry, T.J.; Schadt, C.W.; Wu, L.; Liebich, J.; Chong, S.C.; Huang, Z.; Wu, W.; Gu, B.; Jardine, P.; et al. GeoChip: A comprehensive microarray for investigating biogeochemical, ecological, and environmental processes. ISME J. 2007, 1, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Handelsmen, J.; Tiedje, J.; Alvarez-Cohen, L.; Ashburner, M.; Cann, I.O.; Delong, E.; Doolittle, W.F.; Fraser-Liggett, C.; Godzik, A.; Gordon, J.; et al. Committee on Metagenomics: Challenges and Functional Applications, 1st ed.; National Academy of Sciences: Washington, DC, USA, 2007; ISBN-13: 978-0-309-10676-4. [Google Scholar]
- Hudson-Edwards, K. Tackling mine wastes. Science 2016, 352, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hua, Z.S.; Chen, L.X.; Kuang, J.L.; Li, S.J.; Shu, W.S.; Huang, L.N. Correlating microbial diversity patterns with geochemistry in an extreme and heterogeneous environment of mine tailings. Appl. Environ. Microbiol. 2014, 80, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Akoto, R.; Anning, A.K.; Belford, E.J.D. Effects of ethylenediaminetetraacetic acid-assisted phytoremediation on soil physicochemical and biological properties. Int. J. Environ. Sci. Technol. 2022, 19, 8995–9010. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Xue, Q.; Chen, Z.; Zhou, Y.; Poon, C. Bacterial-induced mineralization (BIM) for soil solidification and heavy metal stabilization: A critical review. Sci. Total. Environ. 2020, 746, 140967. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Li, Y.; Wang, G.; Wang, Z.; Wen, J. Microbial community and metabolic pathway succession driven by changed nutrient inputs in tailings: Effects of different nutrients on tailing remediation. Sci. Rep. 2017, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, M.; Li, B.; Dong, Y. Mechanisms, application advances and future perspectives of microbial-induced heavy metal precipitation: A review. Int. Biodeterior. Biodegrad. 2023, 178, 105544. [Google Scholar] [CrossRef]
- Li, M.; Yao, J.; Sunahara, G.; Hawari, J.; Duran, R.; Liu, J.; Liu, B.; Cao, Y.; Pang, W.; Li, H.; et al. Novel microbial consortia facilitate metalliferous immobilization in non-ferrous metal(loid)s contaminated smelter soil: Efficiency and mechanisms. Environ. Pollut. 2022, 313, 120042. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, Z.; Li, M.; Shang, J.; Niu, W. Evaluation and empirical research on green mine construction in coal industry based on the AHP-SPA model. Resour. Policy 2023, 82, 103503. [Google Scholar] [CrossRef]
- Narayanan, M.; Devarajan, N.; He, Z.; Kandasamy, S.; Ashokkumar, V.; Raja, R.; Carvalho, I.S. Assessment of microbial diversity and enumeration of metal-tolerant autochthonous bacteria from tailings of magnesite and bauxite mines. Mater. Today Proc. 2020, 33, 4391–4401. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of Acid Mine Waste. In The Environmental Geochemistry of ore Deposits. Part A: Processes, Techniques, and Health Issues. Reviews in Economic Geology; Plumlee, G.S., Logsdon, M.J., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; pp. 133–160. [Google Scholar]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef]
- Gadd, G.M. Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr. Opin. Biotechnol. 2000, 11, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, S.A.; Berumen, J.A.G.; Cabriales, J.J.P. El papel de los microorganismos en la biorremediación de suelos contaminados con metales pesados. Acta Univ. 2015, 25, 40–45. [Google Scholar] [CrossRef][Green Version]
- Valls, M.; de Lorenzo, V. Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol. Rev. 2002, 26, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Abdelouas, A. Uranium Mill Tailings: Geochemistry, Mineralogy, and Environmental Impact. Elements 2006, 2, 335–341. [Google Scholar] [CrossRef]
- Domingues, V.S.; de Souza Monteiro, A.; Lopes, A.D.; Lemos, A.; dos Santos, V. Diversity of Metal-Resistant and Tensoactive-Producing Culturable Heterotrophic Bacteria Isolated from a Copper Mine in Brazilian Amazonia. Sci. Rep. 2020, 10, 6171. [Google Scholar] [CrossRef]
- Tapia, Y.; Casanova, M.; Castillo, B.; Acuña, E.; Covarrubias, J.; Antilén, M.; Masaguer, A. Availability of copper in mine tailings with humic substance addition and uptake by Atriplex halimus. Environ. Monit. Assess. 2019, 191, 651. [Google Scholar] [CrossRef]
- Mining Council. CODELCO. Available online: https://consejominero.cl/comunicaciones/plataforma-de-relaves/codelco/ (accessed on 26 July 2024).
- Tapia, Y.; Bustos, P.; Salazar, O.; Casanova, M.; Castillo, B.; Acuña, E.; Masaguer, A. Phytostabilization of Cu in mining tailings using native plant Carpobrotus aequilaterus and the addition of potassium humates. J. Geochem. Explor. 2017, 183, 102–113. [Google Scholar] [CrossRef]
- Ramos-Tapia, I.; Nuñez, R.; Salinas, C.; Salinas, P.; Soto, J.; Paneque, M. Study of Wetland Soils of the Salar de Atacama with different azonal vegetative formations reveals changes in the microbiota associated with hygrophile plant types on the soil surface. Microbiol. Spectr. 2022, 10, e00533-22. [Google Scholar] [CrossRef]
- Núñez Salazar, R.; Aguirre, C.; Soto, J.; Salinas, P.; Salinas, C.; Prieto, H.; Paneque, M. Physicochemical parameters affecting the distribution and diversity of the water column microbial community in the high-altitude Andean lake systems of La Brava and La Punta. Microorganisms 2020, 8, 1181. [Google Scholar] [CrossRef]
- Sims, J.R.; Haby, V.A. Simplified colorimetric determination of Soil Organic Matter. Soil Sci. 1971, 112, 137–141. [Google Scholar] [CrossRef]
- Chapman, H.D. Cation-Exchange Capacity. In Method of Soil Analysis, 1st ed.; Black, C.A., Ed.; Soil Science Society of America: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar]
- Mulvaney, R.L. Nitrogen—Inorganic Forms. In Methods of Soil Analysis: Part 3: Chemical Methods, 1st ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 1123–1184. ISBN 978-0-8911-8866-7. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis: Part 3: Chemical Methods, 1st ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 1123–1184. ISBN 978-0-8911-8866-7. [Google Scholar]
- Dewis, J.; Freitas, F. Physical and Chemical Methods of Soil and Water Analysis; FAO Soils Bulletin 10: Rome, Italy, 1970; p. 286. [Google Scholar]
- Sing, R.; Bhumbla, D.K.; Keefer, R.F. Recommended Soil Sulphate-S Test. In Recommended Soil Testing Procedures for the Northeastern United States, 3rd ed.; University of Delaware: Newark, DE, USA, 1995; p. 493. [Google Scholar]
- Dold, B.; Fontboté, L. Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J. Geochem. Explor. 2001, 74, 3–55. [Google Scholar] [CrossRef]
- Baker, D.E.; Amacher, M.C. Nickel, Copper, Zinc, and Cadmium. In Methods of Soil Analysis: Part 2: Chemical and Microbiological Properties; Page, A.L., Millers, R.H., Keeney, D.R., Eds.; Soil Science Society of America: Madison, WI, USA, 1982; Volume 9, pp. 323–336. ISBN 978-0-8911-8977-0. [Google Scholar]
- Ascar, L.; Ahumada, I.; Richter, P. Effect of biosolid incorporation on arsenic distribution in Mollisol soils in central Chile. Chemosphere 2008, 70, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.; Peñalosa, J.; Carpena, R.; Esteban, E. Comparison of arsenic resistance in Mediterranean woody shrubs used in restoration activities. Chemosphere 2008, 71, 466–473. [Google Scholar] [CrossRef]
- Guerra, V.; Beule, L.; Lehtsaar, E.; Liao, H.L.; Karlovsky, P. Improved protocol for DNA extraction from subsoils using phosphate lysis buffer. Microorganisms 2020, 8, 532. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Hurt, R.A.; Qiu, X.; Wu, L.; Roh, Y.; Palumbo, A.V.; Tiedje, J.M.; Zhou, J. Simultaneous Recovery of RNA and DNA from Soils and Sediments. Appl. Environ. Microbiol. 2001, 67, 4495–4503. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, D.; Silverstein, K.; Young, N. Genomic characterization of the LEED.PEEDs, a Gene Family Unique to the Medicago Lineage. G3 Genes Genomes Genet. 2014, 4, 2003–2012. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software, version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2: Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Holmes, S. Exact sequence variants should replace operational taxonomic units in marker gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Chao, A. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. Available online: http://www.jstor.org/stable/4615964 (accessed on 30 June 2024).
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Limitada, D.M.C. Capítulo II: Descripción del Proyecto. Estudio de Impacto Ambiental. Ficha del Proyecto: Sistema de Disposición de Relaves a Largo Plazo. Proyecto Embalse Ovejería; SEIA: Santiago, Chile, 1993; Available online: https://seia.sea.gob.cl/archivos/EIA/2013082001/EIA_157_Descripcion_del_proyecto.pdf (accessed on 20 August 2024).
- Lottermoser, B.G. Introduction to Mine Wastes. In Mine Wastes; Springer: Berlin/Heidelberg, Germany, 2010; p. 404. [Google Scholar] [CrossRef]
- Hossner, L.; Shahandeh, H. Rehabilitation of Minerals Processing Residue (Tailings). In Encyclopedia of Soil Science; Lal, R., Ed.; Marcel Dekker: New York, NY, USA, 2005; pp. 1450–1455. [Google Scholar]
- Kelly, R.T. Proceedings of Conference on Reclamation of Contaminated Land; Society of the Chemical Industry: London, UK, 1979; p. 567. [Google Scholar]
- Li, X.; Huang, L.; Bond, P.L.; Lu, Y.; Vink, S. Bacterial diversity in response to direct revegetation in the Pb–Zn–Cu tailings under subtropical and semi-arid conditions. Ecol. Eng. 2014, 68, 233–240. [Google Scholar] [CrossRef]
- Gupta, A.; Dutta, A.; Panigrahi, M.K.; Sar, P. Geomicrobiology of Mine Tailings from Malanjkhand Copper Project, India. Geomicrobiol. J. 2020, 38, 97–114. [Google Scholar] [CrossRef]
- Karelová, E.; Harichová, J.; Stojnev, T.; Pangallo, D.; Ferianc, P. The isolation of heavy-metal-resistant culturable bacteria and resistance determinants from a heavy-metal-contaminated site. Biologia 2011, 66, 18–26. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Emenike, C.U.; Agamuthu, P.; Fauziah, S.H.; Omo-Okoro, P.; Jayanthi, B. Enhanced bioremediation of metal-contaminated soil by consortia of Proteobacteria. Water Air Soil Pollut. 2023, 234, 731. [Google Scholar] [CrossRef]
- Horn, M.; Wagner, M. Evidence for additional genus-level diversity of Chlamydiales in the environment. FEMS Microbiol. Lett. 2001, 204, 71–74. [Google Scholar] [CrossRef][Green Version]
- Dary, M.; Chamber-Pérez, M.A.; Palomares, A.J.; Pajuelo, E. “In Situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef]
- Salmi, A.; Boulila, F. Heavy metals multi-tolerant Bradyrhizobium isolated from mercury mining region in Algeria. J. Envrion. Manag. 2021, 289, 112547. [Google Scholar] [CrossRef] [PubMed]
- VanInsberghe, D.; Maas, K.R.; Cardenas, E.; Strachan, C.R.; Hallam, S.J.; Mohn, W.W. Non-symbiotic Bradyrhizobium ecotypes dominate North American forest soils. ISME J. 2015, 9, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Hu, G.; Hu, C.; Xu, C.; Zhang, Z.; Ning, K. Comparative genomics analysis reveals genetic characteristics and nitrogen fixation profile of Bradyrhizobium. iScience 2024, 27, 108948. [Google Scholar] [CrossRef]
- Jin, C.-Z.; Wu, X.-W.; Zhuo, Y.; Yang, Y.; Li, T.; Jin, F.-J.; Lee, H.-G.; Jin, L. Genomic insights into a free-living, nitrogen-fixing but non-nodulating novel species of Bradyrhizobium sediminis from freshwater sediment: Three isolates with the smallest genome within the genus Bradyrhizobium. Syst. Appl. Microbiol. 2022, 45, 126353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jin, C.Z.; Zhuo, Y.; Li, T.; Jin, F.J.; Lee, H.G.; Jin, L. Genetic diversity into a novel free-living species of Bradyrhizobium from contaminated freshwater sediment. Front. Microbiol. 2023, 14, 1295854. [Google Scholar] [CrossRef]
- Seneviratne, M.; Gunaratne, S.; Bandara, T.; Weerasundara, L.; Rajakaruna, N.; Seneviratne, G.; Vithanage, M. Plant growth promotion by Bradyrhizobium japonicum under heavy metal stress. S. Afr. J. Bot. 2016, 105, 19–24. [Google Scholar] [CrossRef]
- Gerrity, D.; Arnold, M.; Dickenson, E.; Moser, D.; Sackett, J.D.; Wert, E.C. Microbial community characterization of ozone biofiltration systems in drinking water and potable reuse applications. Water Res. 2018, 135, 207–219. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Wang, Z.; Cui, Y.; Zhang, Y.; Dai, H.; Li, D. Distinct bacterial communities and resistance genes enriched by triclocarban-contaminated polyethylene microplastics in antibiotics and heavy metals polluted sewage environment. Sci. Total. Environ. 2022, 839, 156330. [Google Scholar] [CrossRef] [PubMed]
- Kroeksakul, P.; Ngamniyom, A.; Silprasit, K.; Sutthisaksopon, P.; Sriyapai, T.; Phowan, N.; Singhaboot, P. Evaluation of Pesticide and Heavy Metal Contamination on Soil Properties and Microbiota in Thailand’s mountainous region. J. Ecol. Eng. 2023, 24, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, R.; Okeke, B.C.; Pieniz, S.; Bortolon, L.; Lambais, M.R.; Camargo, F.A. Effects of Stimulation of Copper bioleaching on microbial community in vineyard soil and Copper mining waste. Biol. Trace Element Res. 2012, 146, 124–133. [Google Scholar] [CrossRef]
- Stouthamer, A.H. Metabolic regulation, including anaerobic metabolism in Paracoccus denitrificans. J. Bioenerg. Biomembr. 1991, 23, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Anttila, J.; Heinonen, P.; Nenonen, T.; Pino, A.; Iwaï, H.; Kauppi, E.; Soliymani, R.; Baumann, M.; Saksi, J.; Suni, N.; et al. Is coproporphyrin III a copper acquisition compound in Paracoccus denitrificans? Biochim. Et Biophys. Acta (BBA)—Bioenerg. 2011, 1807, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Gan, L.; Lin, J.; Chen, Z. Aerobic denitrification by Paracoccus sp. YF1 in the presence of Cu(II). Sci. Total. Environ. 2018, 658, 80–86. [Google Scholar] [CrossRef]
- Xu, L.; Su, J.; Li, K.; Hu, R.; Yan, H.; Liang, E.; Zhou, Z.; Shi, J. Performance of hydrogel immobilized bioreactors combined with different iron ore wastes for denitrification and removal of copper and lead: Optimization and possible mechanism. Water Res. 2022, 225, 119196. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Q.; Peng, H.; Feng, J.; He, J.; Zhang, Y.; Lin, B.; Wu, N.; Xiang, Y. Exploring the impact of intensity and duration of Cu (II) depression on aniline-degrading biosystem: Performance, sludge activity, and microbial diversity. Bioresour. Technol. 2022, 360, 127548. [Google Scholar] [CrossRef]
- Shi, X.; Duan, Z.; Wang, J.; Zhou, W.; Jiang, M.; Li, T.; Ma, H.; Zhu, X. Simultaneous removal of multiple heavy metals using single-chamber microbial electrolysis cells with biocathode in the microaerobic environment. Chemosphere 2023, 318, 137982. [Google Scholar] [CrossRef]
- Bernardet, J.F.; Hugo, C.; Bruun, B. The Genera: Chryseobacterium and Elizabethkingia. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 638–676. [Google Scholar] [CrossRef]
- Majewska, M.; Wdowiak-Wróbel, S.; Marek-Kozaczuk, M.; Nowak, A.; Tyśkiewicz, R. Cadmium-resistant Chryseobacterium sp. DEMBc1 strain: Characteristics and potential to assist phytoremediation and promote plant growth. Environ. Sci. Pollut. Res. 2022, 29, 83567–83579. [Google Scholar] [CrossRef]
- Glibota, N.; Grande Burgos, M.J.; Gálvez, A.; Ortega, E. Copper tolerance and antibiotic resistance in soil bacteria from olive tree agricultural fields routinely treated with copper compounds. J. Sci. Food Agric. 2019, 99, 4677–4685. [Google Scholar] [CrossRef]
- Yang, E.; Sun, L.; Ding, X.; Sun, D.; Liu, J.; Wang, W. Complete genome sequence of Caulobacter flavus RHGG3T, a type species of the genus Caulobacter with plant growth-promoting traits and heavy metal resistance. 3 Biotech 2019, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Karaturovíc, D.M.; Newell, P.C. A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can. J. Microbiol. 1995, 41, 533–536. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Benyehuda, G.; Coombs, J.; Ward, P.L.; Balkwill, D.; Barkay, T. Metal resistance among aerobic chemoheterotrophic bacteria from the deep terrestrial subsurface. Can. J. Microbiol. 2003, 49, 151–156. [Google Scholar] [CrossRef]
- Inagaki, F.; Takai, K.; Hirayama, H.; Yamato, Y.; Nealson, K.H.; Horikoshi, K. Distribution and phylogenetic diversity of the subsurface microbial community in a Japanese epithermal gold mine. Extremophiles 2003, 7, 307–317. [Google Scholar] [CrossRef]
- North, N.N.; Dollhopf, S.L.; Petrie, L.; Istok, J.D.; Balkwill, D.L.; Kostka, J.E. Changes in bacterial community structure during in situ biostimulation of subsurface sediment contaminated with uranium and nitrate. Appl. Envrion. Microbiol 2004, 70, 4911–4920. [Google Scholar] [CrossRef]
- Maertens, L.; Cherry, P.; Tilquin, F.; Van Houdt, R.; Matroule, J.-Y. Environmental conditions modulate the transcriptomic responses of both Caulobacter crescentus morphotypes to Cu stress. Microorganisms 2021, 9, 1116. [Google Scholar] [CrossRef]
- Salam, N.; Jiao, J.Y.; Zhang, X.T.; Li, W.J. Update on the classification of higher ranks in the phylum Actinobacteria. Int. J. Syst. Evol. Microbiol. 2020, 70, 1331–1355. [Google Scholar] [CrossRef]
- Huang, S.; Chen, C.; Jaffé, P.R. Seasonal distribution of nitrifiers and denitrifiers in urban river sediments affected by agricultural activities. Sci. Total. Environ. 2018, 642, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Tsementzi, D.; Hatt, J.K.; Bivins, A.; Khelurkar, N.; Brown, J.; Tripathi, S.N.; Konstantinidis, K.T. Intensive allochthonous inputs along the Ganges River and their effect on microbial community composition and dynamics. Environ. Microbiol. 2019, 21, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Godoy, R.G.; Marcondes, M.A.; Pessôa, R.; Nascimento, A.; Victor, J.R.; Duarte, A.J.D.S.; Clissa, P.B.; Sanabani, S.S. Bacterial community composition and potential pathogens along the Pinheiros River in the southeast of Brazil. Sci. Rep. 2020, 10, 9331. [Google Scholar] [CrossRef]
- Lenchi, N.; Ahmedi, W.N.E.H.; Llirós, M. Simultaneous removal of crude oil and heavy metals by highly adapted bacterial strain Cutibacterium sp. NL2 isolated from Algerian oilfield. Int. Microbiol. 2024, 27, 615–630. [Google Scholar] [CrossRef]
- Lin, Y.; Lay, J.J.; Shieh, W.K. Diesel degradation in soil catalyzed by the addition of a bioagent. Int. J. Environ. Sci. Technol. 2016, 13, 551–560. [Google Scholar] [CrossRef][Green Version]
- Lawal, O.U.; Fraqueza, M.J.; Worning, P.; Bouchami, O.; Bartels, M.D.; Goncalves, L.; Paixão, P.; Goncalves, E.; Toscano, C.; Empel, J.; et al. Staphylococcus saprophyticus Causing Infections in Humans Is Associated with High Resistance to Heavy Metals. Antimicrob. Agents Chemother. 2021, 65, e0268520. [Google Scholar] [CrossRef]
- Padmavathi, A.R.; Sriyutha Murthy, P.; Das, A.; Nishad, P.A.; Pandian, R.; Rao, T.S. Copper oxide nanoparticles as an effective anti-biofilm agent against a copper tolerant marine bacterium, Staphylococcus lentus. Biofouling 2019, 35, 1007–1025. [Google Scholar] [CrossRef]
- Zapotoczna, M.; Riboldi, G.P.; Moustafa, A.M.; Dickson, E.; Narechania, A.; Morrissey, J.A.; Planet, P.J.; Holden, M.T.G.; Waldron, K.J.; Geoghegan, J.A. Mobile-Genetic-Element-Encoded Hypertolerance to Copper Protects Staphylococcus aureus from Killing by Host Phagocytes. mBio 2018, 9, e00550-18. [Google Scholar] [CrossRef]
- Parades-Aguilar, J.; Calderon, K.; Agustin-Salazar, S.; Cerruti, P.; Ambrogi, V.; Gamez-Meza, N.; Medina-Juarez, L.A. Isolation and identification of metallotolerant bacteria with a potential biotechnological application. Sci. Rep. 2024, 14, 3663. [Google Scholar] [CrossRef]
- Ni, G.; Lappan, R.; Hernández, M.; Santini, T.; Tomkins, A.G.; Greening, C. Functional basis of primary succession: Traits of the pioneer microbes. Environ. Microbiol. 2023, 25, 171–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, J.; Xia, F.; Zhao, H.-B.; Xu, Z.; Liu, G.; Zhong, F.; Zhang, X.; Liu, Y.; Du, G.; et al. Textures and chemical compositions of muscovite and quartz: Implications for granite-hosted high-purity quartz mineralization and exploration in South China. Ore Geol. Rev. 2023, 161, 105635. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk-Żak, K.; Szczesny, P.; Gromadka, R.; Zielenkiewicz, U. Taxonomic and chemical assessment of exceptionally abundant rock mine biofilm. PeerJ 2017, 5, e3635. [Google Scholar] [CrossRef][Green Version]
- Sajjad, W.; Ilahi, N.; Kang, S.; Bahadur, A.; Zada, S.; Iqbal, A. Microbios endolíticos de las rocas, su comunidad, función y estrategias de supervivencia. Int. Biodeterior. Biodegrad. 2022, 169, 105387. [Google Scholar] [CrossRef]
- Thiel, J.; Byrne, J.M.; Kappler, A.; Schink, B.; Pester, M. Pyrite formation from FeS and HS is mediated through microbial redox activity. Proc. Natl. Acad. Sci. USA 2019, 116, 6897–6902. [Google Scholar] [CrossRef]
- Moncur, M.C.; Ptacek, C.J.; Lindsay, M.B.J.; Blowes, D.W.; Jambor, J.L. Long-term mineralogical and geochemical evolution of sulfide mine tailings under a shallow water cover. Appl. Geochem. 2015, 57, 178–193. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Bamford, N.C.; MacPhee, C.E.; Stanley-Wall, N.R. Microbial Primer: An introduction to biofilms—What they are, why they form and their impact on built and natural environments. Microbiology 2023, 169, 001338. [Google Scholar] [CrossRef]
- Ishida, K.; Sekizuka, T.; Hayashida, K.; Matsuo, J.; Takeuchi, F.; Kuroda, M.; Nakamura, S.; Yamazaki, T.; Yoshida, M.; Takahashi, K.; et al. Amoebal endosymbiont Neochlamydia genome sequence illuminates the bacterial role in the defense of the host amoebae against Legionella pneumophila. PLoS ONE 2014, 9, e95166. [Google Scholar] [CrossRef]
- Ortega-Peña, S.; Hernández-Zamora, E. Biopelículas microbianas y su impacto en áreas médicas: Fisiopatología, diagnóstico y tratamiento. Boletín Médico Hosp. Infant. México 2018, 75, 79–88. [Google Scholar] [CrossRef]
- Entcheva-Dimitrov, P.; Spormann, A.M. Dynamics and Control of Biofilms of the Oligotrophic Bacterium Caulobacter crescentus. J. Bacteriol. 2004, 186, 24. [Google Scholar] [CrossRef]
- Rossy, T.; Nadell, C.D.; Persat, A. Cellular advective-diffusion drives the emergence of bacterial surface colonization patterns and heterogeneity. Nat. Commun. 2019, 10, 2471. [Google Scholar] [CrossRef]
- Persat, A.; Stone, H.; Gitai, Z. The curved shape of Caulobacter crescentus enhances surface colonization in flow. Nat. Commun. 2014, 5, 3824. [Google Scholar] [CrossRef]
- Bae, H.-S.; Rash, B.A.; Rainey, F.A.; Nobre, M.F.; Tiago, I.; da Costa, M.S.; Moe, W.M. Description of Azospira restricta sp. nov., a nitrogen-fixing bacterium isolated from groundwater. Int. J. Syst. Evol. Microbiol. 2007, 57, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Peng, D.; Walker, S.L.; Cao, B.; Gao, C.; Huang, Q.; Cai, P. Bacillus subtilis biofilm development in the presence of soil clay minerals and iron oxides. NPJ Biofilms Microbiomes 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Matlakowska, R.; Sklodowska, A. The culturable bacteria isolated from organic-rich black shale potentially useful in biometallurgical procedures. J. Appl. Microbiol. 2009, 107, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar] [CrossRef]
- Silva, N.B.S.; Marques, L.A.; R€oder, D.D.B. Diagnosis of biofilm infections: Current methods used, challenges and perspectives for the future. J. Appl. Microbiol. 2021, 131, 2148–2160. [Google Scholar] [CrossRef]
- Xu, L.; Su, J.; Ali, A.; Chang, Q.; Shi, J.; Yang, Y. Denitrification performance of nitrate–dependent ferrous (Fe²⁺) oxidizing Aquabacterium sp. XL4: Adsorption mechanisms of bio–precipitation of phenol and estradiol. J. Hazard. Mater. 2022, 427, 127918. [Google Scholar] [CrossRef]
- Musso, J.; Suazo, G. Determinación de la curva de retención de agua para relaves multimetálicos de la industria minera de Chile. Obras Y Proy. 2019, 25, 22–29. [Google Scholar] [CrossRef][Green Version]
- Foote, A.; Schutz, K.; Zhao, Z.; DiGianivittorio, P.; Korwin-Mihavics, B.R.; LiPuma, J.J.; Wargo, M.J. Characterizing Biofilm Interactions between Ralstonia insidiosa and Chryseobacterium gleum. Microbiol. Spectr. 2023, 11, e0410522. [Google Scholar] [CrossRef]
- Morinaga, K.; Yoshida, K.; Takahashi, K.; Nomura, N.; Toyofuku, M. Peculiarities of biofilm formation by Paracoccus denitrificans. Appl. Microbiol. Biotechnol. 2020, 104, 2427–2433. [Google Scholar] [CrossRef]
- Deng, Z.-S.; Zhao, L.-F.; Xu, L.; Kong, Z.-Y.; Zhao, P.; Qin, W.; Chang, J.-L.; Wei, G.-H. Paracoccus sphaerophysae sp. nov., a siderophore-producing endophytic bacterium isolated from root nodules of Sphaerophysa salsula. Int. J. Syst. Evol. Microbiol. 2011, 61, 593–598. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, G.; Jiang, X.; Shao, K.; Tang, X.; Gao, G. The Relationships between the Free-Living and Particle-Attached Bacterial Communities in Response to Elevated Eutrophication. Front. Microbiol. 2020, 11, 423. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Littmann, E.R.; Fontana, E.; Moody, T.U.; Kohout, C.E.; Gjonbalaj, M.; Eaton, V.; Seok, R.; Leiner, I.M.; Pamer, E.G. Functional and Genomic Variation between Human-Derived Isolates of Lachnospiraceae Reveals Inter- and Intra-Species Diversity. Cell Host Microbe 2020, 28, 134–146.e4. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Borsanelli, A.C.; Gaetti-Jardim, E.J.; Schweitzer, C.M.; Viora, L.; Busin, V.; Riggio, M.P.; Dutra, I.S. Black-pigmented anaerobic bacteria associated with ovine periodontitis. Vet. Microbiol. 2017, 203, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kinyua, M.N. Identification and classification of the Tetrasphaera genus in enhanced biological phosphorus removal process: A review. Rev. Environ. Sci. Biotechnol. 2020, 19, 699–715. [Google Scholar] [CrossRef]
- Wang, X.; Hu, M.; Xia, Y.; Wen, X.; Ding, K. Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl. Environ. Microbiol. 2012, 78, 7042–7047. [Google Scholar] [CrossRef]
- Luo, Y.; Yuan, H.; Zhao, J.; Qi, Y.; Cao, W.-W.; Liu, J.-M.; Guo, W.; Bao, Z.-H. Multiple factors influence bacterial community diversity and composition in soils with rare earth element and heavy metal co-contamination1. Ecotoxicol. Environ. Saf. 2021, 225, 112749. [Google Scholar] [CrossRef] [PubMed]
- Mahabub, M.S.; Alahi, F.; Al Imran, M. Unlocking the potential of microbes: Biocementation technology for mine tailings restoration—A comprehensive review. Environ. Sci. Pollut. Res. 2023, 30, 91676–91709. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, P.F.M.; Lombardi, A.T.; Nogueira, M.M. Cylindrospermopsis raciborskii exudate–Cu complexes: Impact on copper dynamics and bioavailability in an aquatic food chain. Environ. Sci. Pollut. Res. 2012, 19, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, X.; Huot, H.; Liu, W.; Liu, C.; Guo, M.; Li, Y.; Fei, Y.; Chao, Y.; Wang, S.; et al. Reclamation with organic amendments and plants remodels the diversity and structure of bacterial community in ion-adsorption rare earth element mine tailings. J. Soils Sediments 2020, 20, 3669–3680. [Google Scholar] [CrossRef]
- Pereira, S.G.; Albuquerque, L.; Nobre, M.F.; Tiago, I.; Veríssimo, A.; Pereira, A.; da Costa, M.S. Pullulanibacillus uraniitolerans sp. nov., an acidophilic, U(VI)-resistant species isolated from an acid uranium mill tailing effluent and emended description of the genus Pullulanibacillus. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 1, 158–162. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Vinothkanna, A.; Yin, H.; Liu, X.; Meng, D. Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment—A review. Ecotoxicol. Environ. Saf. 2021, 226, 112863. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yang, M.; Zhang, S.; Zhao, D.; Duan, J.; Wang, W.; Yan, L. Iron and sulfur oxidation pathways of Acidithiobacillus ferrooxidans. World J. Microbiol. Biotechnol. 2019, 35, 60. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of microbial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2022, 106, 6933–6952. [Google Scholar] [CrossRef]
- Álvarez-Márquez, D.E.; Bernal-González, M.; Durán-Domínguez-de-Bazúa, M.D.C. Principales microorganismos sulfato reductores (MSR) de reactores anaerobios alimentados con efluentes ácidos, una revisión bibliográfica. Rev. Colomb. Biotecnología. 2022, 24, 62–76. [Google Scholar] [CrossRef]
- Paredes-Mendoza, M.; Espinosa-Victoria, D. Ácidos orgánicos producidos por rizobacterias que solubilizan fosfato: Una revisión crítica. Terra Latinoam. 2010, 28, 61–70. [Google Scholar]
- Corrales, L.; Arévalo, Z.; Moreno, V. Solubilización de fosfatos: Una función microbiana importante en el desarrollo vegetal. Nova 2014, 12, 68–79. [Google Scholar]
- Albelda-Berenguer, M.; Monachon, M.; Joseph, E. Siderophores: From natural roles to potential applications. Adv. Appl. Microbiol. 2019, 106, 193–225. [Google Scholar] [CrossRef]
- Villarreal-Delgado, M.F.; Villa-Rodríguez, E.D.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L. El género Bacillus como agente de control biológico y sus implicaciones en la bioseguridad agrícola. Rev. Mex. Fitopato. 2018, 36, 95–130. [Google Scholar] [CrossRef]
- Morrissey, J.A.; Cockayne, A.; Hill, P.J.; Williams, P. Molecular cloning and analysis of a putative siderophore ABC transporter from Staphylococcus aureus. Infect. Immun. 2000, 68, 6281–6288. [Google Scholar] [CrossRef] [PubMed]

| Total Metals (mg kg−1) | P1 | P2 | P3 |
| Cd | 0.66 ± 0.04 a | 0.63 ± 0.03 a | 0.65 ± 0.09 a |
| Cr | 13.2 ± 1.62 a | 7.18 ± 0.53 b | 8.09 ± 1.17 b |
| Cu | 2488 ± 258 a | 2986 ± 102 a | 2911 ± 806 a |
| Fe | 35,960 ± 3680 a | 26,583 ± 2508 b | 34,604 ± 2156 a |
| Mn | 185.8 ± 20.3 a | 236.2 ± 15.7 b | 268.6 ± 18.1 b |
| Mo | 145.6 ± 8.90 a | 268.8 ± 84.1 a | 169.03 ± 21.8 a |
| Pb | 9.53 ± 0.73 a | 8.63 ± 1.33 a | 11.62 ± 3.56 a |
| Available Metals (DTPA) (mg kg−1) | P1 | P2 | P3 |
| Cd | 0.032 ± 0.002 a | 0.029 ± 0.003 a | 0.033 ± 0.001 a |
| Cr | 0.193 ± 0.014 a | 0.211 ± 0.012 a | 0.206 ± 0.011 a |
| Cu | 67.1 ± 2.9 a | 69.8 ± 4.1 a | 80.4 ± 4.3 b |
| Fe | 7.34 ± 0.32 a | 6.3 ± 0.82 a | 7.13 ± 0.8 a |
| Mn | 6.69 ± 0.2 a | 1.25 ± 0.08 b | 10.5 ± 1.14 c |
| Mo | nd | 0.55 ± 0.07 | nd |
| Pb | 0.16 ± 0.02 a | 0.25 ± 0.02 ab | 0.40 ± 0.1 b |
| Other Variables | P1 | P2 | P3 |
| pH | 6.70 | 6.85 | 6.20 |
| Electrical conductivity (dS m−1) | 4.08 | 2.22 | 2.11 |
| SO4−(mmol/L) | 16.75 | 2.11 | 3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Venegas, J.; Zamora-Leiva, L.; Univaso, L.; Soto, J.; Tapia, Y.; Paneque, M. Profile of Bacterial Communities in Copper Mine Tailings Revealed through High-Throughput Sequencing. Microorganisms 2024, 12, 1820. https://doi.org/10.3390/microorganisms12091820
Jiménez-Venegas J, Zamora-Leiva L, Univaso L, Soto J, Tapia Y, Paneque M. Profile of Bacterial Communities in Copper Mine Tailings Revealed through High-Throughput Sequencing. Microorganisms. 2024; 12(9):1820. https://doi.org/10.3390/microorganisms12091820
Chicago/Turabian StyleJiménez-Venegas, Joseline, Leonardo Zamora-Leiva, Luciano Univaso, Jorge Soto, Yasna Tapia, and Manuel Paneque. 2024. "Profile of Bacterial Communities in Copper Mine Tailings Revealed through High-Throughput Sequencing" Microorganisms 12, no. 9: 1820. https://doi.org/10.3390/microorganisms12091820
APA StyleJiménez-Venegas, J., Zamora-Leiva, L., Univaso, L., Soto, J., Tapia, Y., & Paneque, M. (2024). Profile of Bacterial Communities in Copper Mine Tailings Revealed through High-Throughput Sequencing. Microorganisms, 12(9), 1820. https://doi.org/10.3390/microorganisms12091820








