Decoding the Gut Microbiome in Companion Animals: Impacts and Innovations
Abstract
1. Introduction
2. Techniques Used for the Detection of Gut Microbiome Abundance
3. Factors Influencing the Gut Microbiome
3.1. Age and Breed
3.2. Gender
3.3. Physical Activity
3.4. Antibiotics
3.5. Diet
3.6. Probiotics
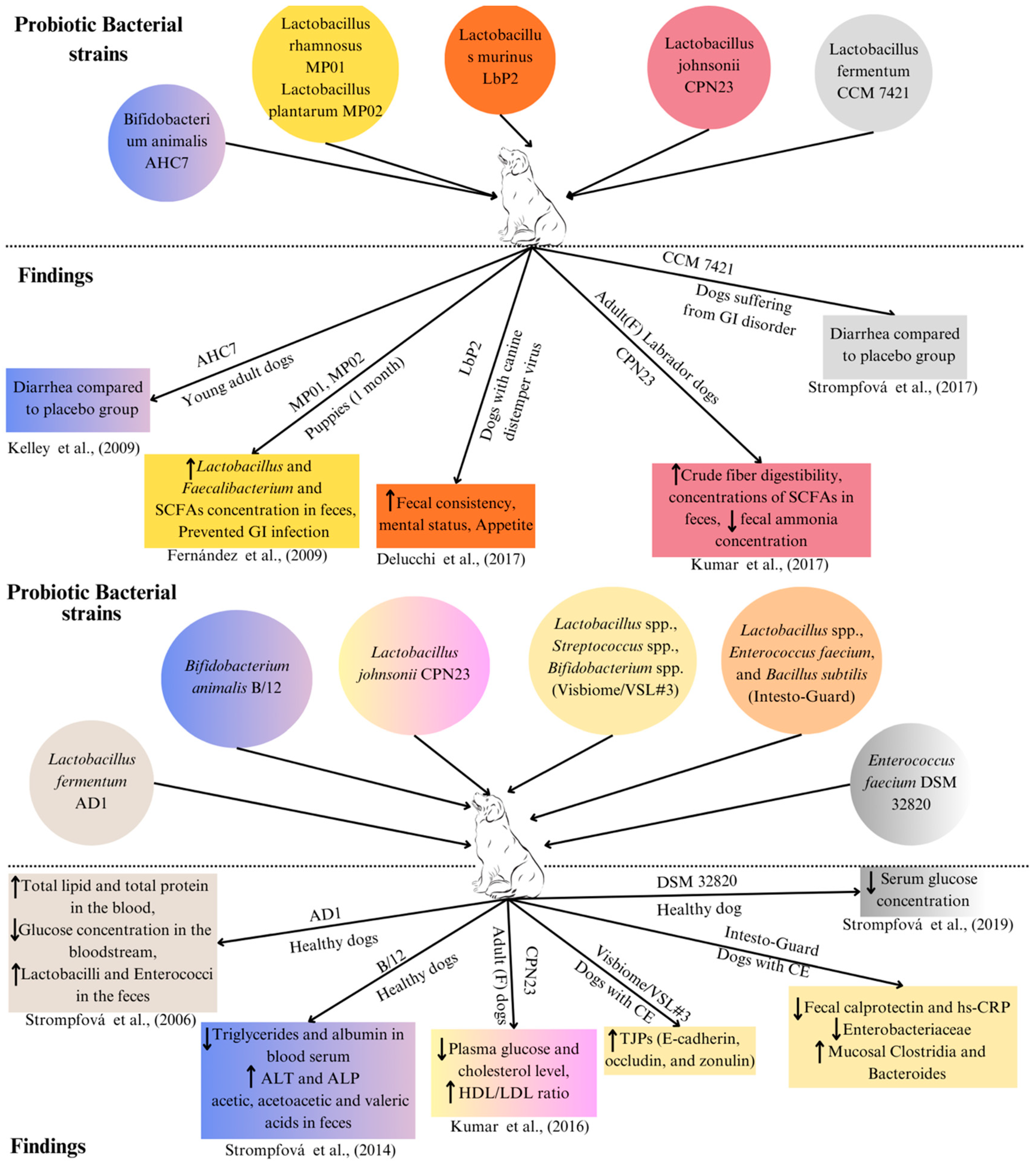
| Bacterial Strains | Amount | Source | Age/Conditions | Assessment | Findings Obtained | Reference |
|---|---|---|---|---|---|---|
| Bifidobacterium animalis AHC7 | 2 × 1010 CFU/day | Canine | Young adult dogs having acute diarrhoea | Managing acute diarrhoea |
| [111,130] |
| Lactobacillus rhamnosus MP01, Lactobacillus plantarum MP02 | 109 CFU/day | Canine | Puppies (1 month) | Infection prevention in puppies |
| [131] |
| Lactobacillus murinus LbP2 | 5 × 109 CFU/day | Canine | Dogs with canine distemper virus (CDV)-associated diarrhoea | Mental and faecal status |
| [132] |
| Lactobacillus johnsonii CPN23 | 2.3 × 108 CFU/day | Canine | Female Labrador dogs (Adult) | Nutrient digestibility and faecal fermentative metabolites |
| [133] |
| Lactobacillus fermentum CCM 7421 | 107–109 CFU/day | Canine | Dogs (having gastrointestinal disorder) | Composition of the faecal microbiome and blood samples |
| [134] |
| Lactobacillus fermentum AD1 | 3 mL of 109 CFU/mL | Canine | Control healthy dogs | Composition of the faecal microbiome and blood samples |
| [135] |
| Bifidobacterium animalis B/12 | 1 mL of 1.04 × 109 CFU/mL | Canine | Control healthy dogs | Composition of the faecal microbiome and Blood samples |
| [136] |
| Lactobacillus johnsonii CPN23 | 108 CFU/mL (0.1 mL/kg BW) | Canine | Female dogs (adult) | Assessment of blood sample profile |
| [141] |
| Enterococcus faecium DSM 32820 | 109 CFU/day | Canine | Control healthy dogs | Blood sample profile |
| [137] |
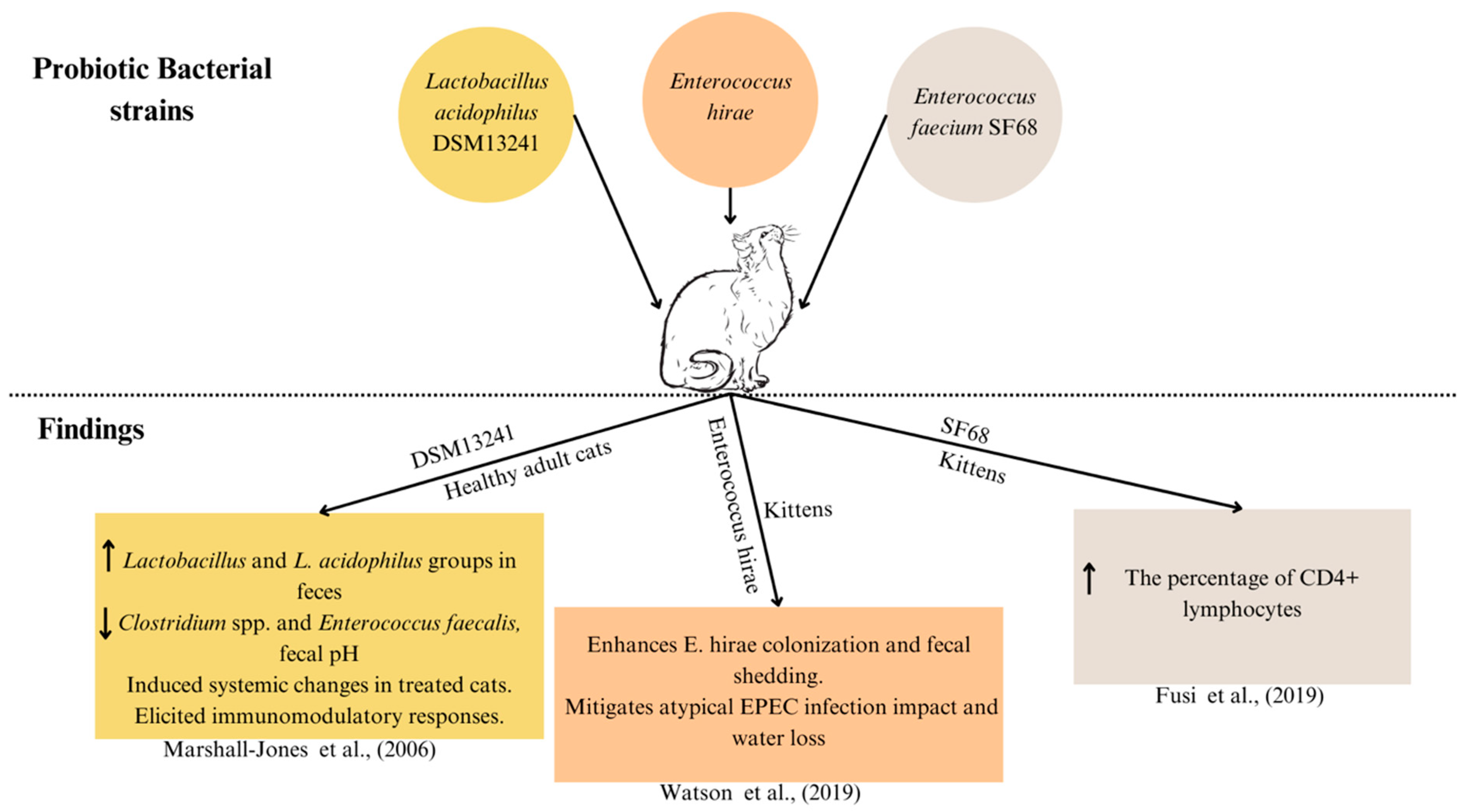
| Lactobacillus acidophilus DSM 13241 | 2 × 108 CFU/day | Feline | Healthy adult cats | Improving intestinal health in cats |
| [139] |
| Enterococcus hirae | 2.85–4.28 × 108 CFU/day | Feline | Kittens | Preventing atypical Enteropathogenic E. coli (EPEC) in kittens |
| [94,138] |
| Enterococcus faecium SF68 | 5 × 109 CFU/day | Feline | Kittens | Enterococcus faecium strain SF68 supplementation on immune function |
| [140] |
| Bacillus subtilis HH2 | 5 × 109 CFU/day | Canine | Beagles with orally administered Enterotoxigenic Escherichia coli (ETEC) | Intestinal barrier integrity, faecal microbiota, and non-specific immunity |
| [142,143] |
| Lactobacillus plantarum DSM 24730, Lactobacillus paracasei DSM 24733, Lactobacillus delbrueckii subsp. bulgaricus DSM 24734, Lactobacillus acidophilus DSM 24735, Streptococcus thermophilus DSM 24731, Bifidobacterium breve DSM 24732, Bifidobacterium longum DSM 24736, and Bifidobacterium infantis DSM 24737 | S. thermopilus 40.55%, Bifidobacteria 12.5%, Lactobacilli 13%, and other excipients 39.05% (112–225 × 109 CFU/10 kg) | Canine | Dogs with CE | Disease activity and mucosal microbiota changes and tight junction protein (TJP) expression |
| [54] |
| Bifidobacterium bifidum, Enterococcus faecium and thermophilus, and Lactobacillus acidophilus, bulgaricus, casei, and lantarum | 5 × 109 CFU/day | Feline | Healthy cats | Faecal microbiome and faecal metabolomics |
| [97] |
| Lactobacillus acidophilus DSM 32241, Lactobacillus helveticus DSM 32242, Lactobacillus paracasei DSM 32243, Lactobacillus plantarum DSM 32244, and Lactobacillus brevis DSM 27961, Streptococcus thermophilus DSM 32245, Bifidobacterium lactis DSM 32246, Bifidobacterium lactis DSM 32247 | 400 billion cfu of lyophilised bacteria/day | Canine | Healthy dogs | Concentration of faecal immunoglobulin IgA, plasma IgG, and faecal microbiota composition |
| [144] |
| Lactobacillus acidophilus, Lactobacillus casei, Enterococcus faecium, and Bacillus subtilis | 1 billion CFU/mL per 2.2 kg of body weight orally twice daily | Canine | Dogs with confirmed CE | Clinical signs, mucosal microbiota, and inflammatory indices |
| [125] |
3.7. Faecal Microbiota Transplantation (FMT)
4. Gut Microbiome and Diseases
4.1. Neurological Disorders/Disease
4.2. Cardiac Health
4.3. Chronic Inflammatory Enteropathies in Dogs and Cats
4.4. Obesity
5. Future Prospects and Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Driscoll, C.A.; Macdonald, D.W.; O’Brien, S.J. From Wild Animals to Domestic Pets, an Evolutionary View of Domestication. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. S1), 9971–9978. [Google Scholar] [CrossRef] [PubMed]
- Aydin, N.; Krueger, J.I.; Fischer, J.; Hahn, D.; Kastenmüller, A.; Frey, D.; Fischer, P. “Man’s Best Friend:” How the Presence of a Dog Reduces Mental Distress after Social Exclusion. J. Exp. Soc. Psychol. 2012, 48, 446–449. [Google Scholar] [CrossRef]
- Serpell, J.A. Factors Influencing Human Attitudes to Animals and Their Welfare. Anim. Welf. 1994, 13, S145–S151. [Google Scholar] [CrossRef]
- Harris Interactive: Harris Polls > Pets Aren’t Just Animals; They Are Members of the Family. Available online: https://www.harrisinteractives.com/NewsRoom/HarrisPolls/tabid/447/ctl/ReadCustomDefault/mid/1508/ArticleId/1076/Default.html (accessed on 11 August 2024).
- Su, Q.; Liu, Q. Factors Affecting Gut Microbiome in Daily Diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An Ecological and Evolutionary Perspective on Human–Microbe Mutualism and Disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the Gut Microbiota in Host Appetite Control: Bacterial Growth to Animal Feeding Behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide Therapeutics: Current Status and Future Directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Jergens, A.E.; Guard, B.C.; Redfern, A.; Rossi, G.; Mochel, J.P.; Pilla, R.; Chandra, L.; Seo, Y.-J.; Steiner, J.M.; Lidbury, J.; et al. Microbiota-Related Changes in Unconjugated Fecal Bile Acids Are Associated with Naturally Occurring, Insulin-Dependent Diabetes Mellitus in Dogs. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Kieler, I.N.; Osto, M.; Hugentobler, L.; Puetz, L.; Gilbert, M.T.P.; Hansen, T.; Pedersen, O.; Reusch, C.E.; Zini, E.; Lutz, T.A.; et al. Diabetic Cats Have Decreased Gut Microbial Diversity and a Lack of Butyrate Producing Bacteria. Sci. Rep. 2019, 9, 4822. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The Gut Microbiota in Human Energy Homeostasis and Obesity. Trends Endocrinol. Metab. 2015, 26, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Savoj, J.; Nakata, M.B.; Vaziri, N.D. Altered Microbiome in Chronic Kidney Disease: Systemic Effects of Gut-Derived Uremic Toxins. Clin. Sci. 2018, 132, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Jackson, M.I.; Jewell, D.E.; Ephraim, E. Chronic Kidney Disease in Cats Alters Response of the Plasma Metabolome and Fecal Microbiome to Dietary Fiber. PLoS ONE 2020, 15, e0235480. [Google Scholar] [CrossRef]
- Penders, J.; Stobberingh, E.E.; van den Brandt, P.A.; Thijs, C. The Role of the Intestinal Microbiota in the Development of Atopic Disorders. Allergy 2007, 62, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Kalenyak, K.; Isaiah, A.; Heilmann, R.M.; Suchodolski, J.S.; Burgener, I.A. Comparison of the Intestinal Mucosal Microbiota in Dogs Diagnosed with Idiopathic Inflammatory Bowel Disease and Dogs with Food-Responsive Diarrhea before and after Treatment. FEMS Microbiol. Ecol. 2018, 94, fix173. [Google Scholar] [CrossRef]
- Wang, S.; Martins, R.; Sullivan, M.C.; Friedman, E.S.; Misic, A.M.; El-Fahmawi, A.; De Martinis, E.C.P.; O’Brien, K.; Chen, Y.; Bradley, C.; et al. Diet-Induced Remission in Chronic Enteropathy Is Associated with Altered Microbial Community Structure and Synthesis of Secondary Bile Acids. Microbiome 2019, 7, 126. [Google Scholar] [CrossRef]
- Silverman, G.J.; Azzouz, D.F.; Alekseyenko, A.V. Systemic Lupus Erythematosus and Dysbiosis in the Microbiome: Cause or Effect or Both? Curr. Opin. Immunol. 2019, 61, 80–85. [Google Scholar] [CrossRef]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the Fecal Microbiota and Serum Metabolite Profiles in Dogs with Idiopathic Inflammatory Bowel Disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762.e14. [Google Scholar] [CrossRef]
- Cintio, M.; Scarsella, E.; Sgorlon, S.; Sandri, M.; Stefanon, B. Gut Microbiome of Healthy and Arthritic Dogs. Vet. Sci. 2020, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Stavroulaki, E.M.; Suchodolski, J.S.; Xenoulis, P.G. Effects of Antimicrobials on the Gastrointestinal Microbiota of Dogs and Cats. Vet. J. 2023, 291, 105929. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Influence of Probiotic Supplementation on Health Status of the Dogs: A Review. Appl. Sci. 2021, 11, 11384. [Google Scholar] [CrossRef]
- Conway, J.; A Duggal, N. Ageing of the Gut Microbiome: Potential Influences on Immune Senescence and Inflammageing. Ageing Res. Rev. 2021, 68, 101323. [Google Scholar] [CrossRef]
- Hernandez, J.; Rhimi, S.; Kriaa, A.; Mariaule, V.; Boudaya, H.; Drut, A.; Jablaoui, A.; Mkaouar, H.; Saidi, A.; Biourge, V.; et al. Domestic Environment and Gut Microbiota: Lessons from Pet Dogs. Microorganisms 2022, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Calalang, J.; Cheung, H.; Lichimo, K.; So, B. Identifying Breed, Dietary, and Reproductive Factors Affecting the Gut Microbiome of Dogs with Inflammatory Bowel Disease. Undergrad. J. Exp. Microbiol. Immunol. (UJEMI) 2021, 26. [Google Scholar]
- Ritchie, L.E.; Steiner, J.M.; Suchodolski, J.S. Assessment of Microbial Diversity along the Feline Intestinal Tract Using 16S RRNA Gene Analysis. FEMS Microbiol. Ecol. 2008, 66, 590–598. [Google Scholar] [CrossRef]
- Allaway, D.; Haydock, R.; Lonsdale, Z.N.; Deusch, O.D.; O’Flynn, C.; Hughes, K.R. Rapid Reconstitution of the Fecal Microbiome after Extended Diet-Induced Changes Indicates a Stable Gut Microbiome in Healthy Adult Dogs. Appl. Environ. Microbiol. 2020, 86, e00562-20. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Camacho, J.; Steiner, J.M. Analysis of Bacterial Diversity in the Canine Duodenum, Jejunum, Ileum, and Colon by Comparative 16S RRNA Gene Analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and Human Inflammatory Bowel Disease Rely on Overlapping yet Distinct Dysbiosis Networks. Nat. Microbiol. 2016, 1, 16177. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium Nucleatum Infection Is Prevalent in Human Colorectal Carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E.N.; Maclean, P.; Thomas, D.G.; Cave, N.J.; Young, W. Key Bacterial Families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) Are Related to the Digestion of Protein and Energy in Dogs. PeerJ 2017, 2017, e3019. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.W.; Lee, J.S.; Jang, D.S.; Shim, S.H. Inhibitory Effects of Compounds Isolated from Roots of Cynanchum Wilfordii on Oxidation and Glycation of Human Low-Density Lipoprotein (LDL). J. Funct. Foods 2019, 59, 281–290. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative Stress, Hormones, and Effects of Natural Antioxidants on Intestinal Inflammation in Inflammatory Bowel Disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef] [PubMed]
- Patani, A.; Balram, D.; Yadav, V.K.; Lian, K.-Y.; Patel, A.; Sahoo, D.K. Harnessing the Power of Nutritional Antioxidants against Adrenal Hormone Imbalance-Associated Oxidative Stress. Front. Endocrinol. 2023, 14, 1271521. [Google Scholar] [CrossRef]
- Mishra, B.P.; Mishra, J.; Paital, B.; Rath, P.K.; Jena, M.K.; Reddy, B.V.V.; Pati, P.K.; Panda, S.K.; Sahoo, D.K. Properties and Physiological Effects of Dietary Fiber-Enriched Meat Products: A Review. Front. Nutr. 2023, 10, 1275341. [Google Scholar] [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic Production: Harnessing the Power of Microbial Metabolites for Health Applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Heilmann, R.M.; Ackermann, M.; Parker, V.; Rudinsky, A.; Winston, J.A.; Bourgois-Mochel, A.; Iennarella, C.A.; Friedberg, I.; Allenspach, K.; et al. Tu1739 Micrornas as Potential Biomarkers for Diagnosis and Monitoring Chronic Inflammatory Enteropathy in Dogs. Gastroenterology 2024, 166, S-1401. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Borcherding, D.C.; Chandra, L.; Jergens, A.E.; Atherly, T.; Bourgois-Mochel, A.; Ellinwood, N.M.; Snella, E.; Severin, A.J.; Martin, M.; et al. Differential Transcriptomic Profiles Following Stimulation with Lipopolysaccharide in Intestinal Organoids from Dogs with Inflammatory Bowel Disease and Intestinal Mast Cell Tumor. Cancers 2022, 14, 3525. [Google Scholar] [CrossRef]
- Ziese, A.-L.; Suchodolski, J.S. Impact of Changes in Gastrointestinal Microbiota in Canine and Feline Digestive Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 155–169. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Wang, Y.; Li, Y.; Zhang, X.; Zheng, H.; Ma, F.; Ma, C.; Lu, B.; Xie, Z.; et al. Beneficial Changes of Gut Microbiota and Metabolism in Weaned Rats with Lactobacillus Acidophilus NCFM and Bifidobacterium Lactis Bi-07 Supplementation. J. Funct. Foods 2018, 48, 252–265. [Google Scholar] [CrossRef]
- Sauter, S.N.; Allenspach, K.; Gaschen, F.; Gröne, A.; Ontsouka, E.; Blum, J.W. Cytokine Expression in an Ex Vivo Culture System of Duodenal Samples from Dogs with Chronic Enteropathies: Modulation by Probiotic Bacteria. Domest. Anim. Endocrinol. 2005, 29, 605–622. [Google Scholar] [CrossRef]
- Cosío-Carpintero, K.E.; Gutiérrez Olvera, C.; Márquez-Mota, C.C.; Ortega-Cerrilla, M.E.; Sánchez González, M.G.; Gutiérrez, L. High Levels of Dietary Digestible Protein Transiently Promote Beneficial Bacteria in Adult Dog Feces. Vet. México OA 2022, 9. [Google Scholar] [CrossRef]
- Janeczko, S.; Atwater, D.; Bogel, E.; Greiter-Wilke, A.; Gerold, A.; Baumgart, M.; Bender, H.; McDonough, P.L.; McDonough, S.P.; Goldstein, R.E.; et al. The Relationship of Mucosal Bacteria to Duodenal Histopathology, Cytokine MRNA, and Clinical Disease Activity in Cats with Inflammatory Bowel Disease. Vet. Microbiol. 2008, 128, 178–193. [Google Scholar] [CrossRef]
- Ranjbar, M.; Salehi, R.; Haghjooy Javanmard, S.; Rafiee, L.; Faraji, H.; Jafarpor, S.; Ferns, G.A.; Ghayour-Mobarhan, M.; Manian, M.; Nedaeinia, R. The Dysbiosis Signature of Fusobacterium Nucleatum in Colorectal Cancer-Cause or Consequences? A Systematic Review. Cancer Cell Int. 2021, 21, 194. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Analysis of the Gut Microbiome in Dogs and Cats. Vet. Clin. Pathol. 2022, 50 (Suppl. S1), 6–17. [Google Scholar] [CrossRef]
- Tettamanti Boshier, F.A.; Srinivasan, S.; Lopez, A.; Hoffman, N.G.; Proll, S.; Fredricks, D.N.; Schiffer, J.T. Complementing 16S RRNA Gene Amplicon Sequencing with Total Bacterial Load To Infer Absolute Species Concentrations in the Vaginal Microbiome. mSystems 2020, 5, 2. [Google Scholar] [CrossRef]
- Garraway, K.; Johannes, C.M.; Bryan, A.; Peauroi, J.; Rossi, G.; Zhang, M.; Wang, C.; Allenspach, K.; Jergens, A.E. Relationship of the Mucosal Microbiota to Gastrointestinal Inflammation and Small Cell Intestinal Lymphoma in Cats. J. Vet. Intern. Med. 2018, 32, 1692–1702. [Google Scholar] [CrossRef]
- White, R.; Atherly, T.; Guard, B.; Rossi, G.; Wang, C.; Mosher, C.; Webb, C.; Hill, S.; Ackermann, M.; Sciabarra, P.; et al. Randomized, Controlled Trial Evaluating the Effect of Multi-Strain Probiotic on the Mucosal Microbiota in Canine Idiopathic Inflammatory Bowel Disease. Gut Microbes 2017, 8, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Lanerie, D.J.; Dowd, S.E.; Paddock, C.G.; Grützner, N.; Steiner, J.M.; Ivanek, R.; Suchodolski, J.S. Effect of a Multi-Species Synbiotic Formulation on Fecal Bacterial Microbiota of Healthy Cats and Dogs as Evaluated by Pyrosequencing. FEMS Microbiol. Ecol. 2011, 78, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Hand, D.; Wallis, C.; Colyer, A.; Penn, C.W. Pyrosequencing the Canine Faecal Microbiota: Breadth and Depth of Biodiversity. PLoS ONE 2013, 8, e53115. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Dowd, S.E.; Westermarck, E.; Steiner, J.M.; Wolcott, R.D.; Spillmann, T.; Harmoinen, J.A. The Effect of the Macrolide Antibiotic Tylosin on Microbial Diversity in the Canine Small Intestine as Demonstrated by Massive Parallel 16S RRNA Gene Sequencing. BMC Microbiol. 2009, 9, 210. [Google Scholar] [CrossRef]
- Sturgeon, A.; Stull, J.W.; Costa, M.C.; Weese, J.S. Metagenomic Analysis of the Canine Oral Cavity as Revealed by High-Throughput Pyrosequencing of the 16S RRNA Gene. Vet. Microbiol. 2013, 162, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, A.; Pinder, S.L.; Costa, M.C.; Weese, J.S. Characterization of the Oral Microbiota of Healthy Cats Using Next-Generation Sequencing. Vet. J. 2014, 201, 223–229. [Google Scholar] [CrossRef]
- Dorn, E.S.; Tress, B.; Suchodolski, J.S.; Nisar, T.; Ravindran, P.; Weber, K.; Hartmann, K.; Schulz, B.S. Bacterial Microbiome in the Nose of Healthy Cats and in Cats with Nasal Disease. PLoS ONE 2017, 12, e0180299. [Google Scholar] [CrossRef]
- Hutchins, R.G.; Bailey, C.S.; Jacob, M.E.; Harris, T.L.; Wood, M.W.; Saker, K.E.; Vaden, S.L. The Effect of an Oral Probiotic Containing Lactobacillus, Bifidobacterium, and Bacillus Species on the Vaginal Microbiota of Spayed Female Dogs. J. Vet. Intern. Med. 2013, 27, 1368–1371. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Noti, M. The Aging Gut Microbiome and Its Impact on Host Immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef] [PubMed]
- You, I.; Kim, M.J. Comparison of Gut Microbiota of 96 Healthy Dogs by Individual Traits: Breed, Age, and Body Condition Score. Animals 2021, 11, 2432. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.Y.; Laga, W.; Wang, Y.; Ma, H.; Sun, Z.; Zhang, H. Oral Administration of Compound Probiotics Improved Canine Feed Intake, Weight Gain, Immunity and Intestinal Microbiota. Front. Immunol. 2019, 10, 666. [Google Scholar] [CrossRef]
- Deusch, O.; O’Flynn, C.; Colyer, A.; Swanson, K.S.; Allaway, D.; Morris, P. A Longitudinal Study of the Feline Faecal Microbiome Identifies Changes into Early Adulthood Irrespective of Sexual Development. PLoS ONE 2015, 10, e0144881. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Young, W.; Butowski, C.F.; Moon, C.D.; Maclean, P.H.; Rosendale, D.; Cave, N.J.; Thomas, D.G. The Fecal Microbiota in the Domestic Cat (Felis Catus) Is Influenced by Interactions between Age and Diet; A Five Year Longitudinal Study. Front. Microbiol. 2018, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Hooda, S.; Minamoto, Y.; Suchodolski, J.S.; Swanson, K.S. Current State of Knowledge: The Canine Gastrointestinal Microbiome. Anim. Health Res. Rev. 2012, 13, 78–88. [Google Scholar] [CrossRef]
- Li, Z.; Di, D.; Sun, Q.; Yao, X.; Wei, J.; Li, B.; Liu, K.; Shao, D.; Qiu, Y.; Liu, H.; et al. Comparative Analyses of the Gut Microbiota in Growing Ragdoll Cats and Felinae Cats. Animals 2022, 12, 2467. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef]
- Sinha, T.; Vich Vila, A.; Garmaeva, S.; Jankipersadsing, S.A.; Imhann, F.; Collij, V.; Bonder, M.J.; Jiang, X.; Gurry, T.; Alm, E.J.; et al. Analysis of 1135 Gut Metagenomes Identifies Sex-Specific Resistome Profiles. Gut Microbes 2019, 10, 358. [Google Scholar] [CrossRef]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex Differences and Hormonal Effects on Gut Microbiota Composition in Mice. Gut Microbes 2016, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Cox-York, K.A.; Sheflin, A.M.; Foster, M.T.; Gentile, C.L.; Kahl, A.; Koch, L.G.; Britton, S.L.; Weir, T.L. Ovariectomy Results in Differential Shifts in Gut Microbiota in Low versus High Aerobic Capacity Rats. Physiol. Rep. 2015, 3, e12488. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal Microbial Determinants of Fecal and Systemic Estrogens and Estrogen Metabolites: A Cross-Sectional Study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef]
- Scarsella, E.; Stefanon, B.; Cintio, M.; Licastro, D.; Sgorlon, S.; Dal Monego, S.; Sandri, M. Learning Machine Approach Reveals Microbial Signatures of Diet and Sex in Dog. PLoS ONE 2020, 15, e0237874. [Google Scholar] [CrossRef] [PubMed]
- Holzhausen, E.A.; Malecki, K.C.; Sethi, A.K.; Gangnon, R.; Cadmus-Bertram, L.; Deblois, C.L.; Suen, G.; Safdar, N.; Peppard, P.E. Assessing the Relationship between Physical Activity and the Gut Microbiome in a Large, Population-Based Sample of Wisconsin Adults. PLoS ONE 2022, 17, e0276684. [Google Scholar] [CrossRef] [PubMed]
- Kieler, I.N.; Kamal, S.S.; Vitger, A.D.; Nielsen, D.S.; Lauridsen, C.; Bjornvad, C.R. Gut Microbiota Composition May Relate to Weight Loss Rate in Obese Pet Dogs. Vet. Med. Sci. 2017, 3, 252. [Google Scholar] [CrossRef]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the Dog and Human Gut Microbiomes in Gene Content and Response to Diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef]
- Wang, D.; Russel, W.A.; Macdonald, K.M.; De Leon, V.M.; Ay, A.; Belanger, K.D. Analysis of the Gut Microbiome in Sled Dogs Reveals Glucosamine- and Activity-Related Effects on Gut Microbial Composition. Front. Vet. Sci. 2024, 11, 1272711. [Google Scholar] [CrossRef]
- Kushwaha, S.O.; Sahu, S.K.; Yadav, V.K.; Rathod, M.C.; Patel, D.; Sahoo, D.K.; Patel, A. Bacteriophages as a Potential Substitute for Antibiotics: A Comprehensive Review. Cell Biochem. Funct. 2024, 42, e4022. [Google Scholar] [CrossRef]
- Turicea, B.; Sahoo, D.K.; Allbaugh, R.A.; Stinman, C.C.; Kubai, M.A. Novel Treatment of Infectious Keratitis in Canine Corneas Using Ultraviolet C (UV-C) Light. Vet. Ophthalmol. 2024. [Google Scholar] [CrossRef]
- Anthony, W.E.; Burnham, C.A.D.; Dantas, G.; Kwon, J.H. The Gut Microbiome as a Reservoir for Antimicrobial Resistance. J. Infect. Dis. 2021, 223, S209–S213. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of Gut Microbiota of Healthy Adults Following Antibiotic Exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Fishbein, S.R.S.; Mahmud, B.; Dantas, G. Antibiotic Perturbations to the Gut Microbiome. Nat. Rev. Microbiol. 2023, 21, 772–788. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Xu, Z.Z.; Chang, M.W.; Marotz, C.A.; Saghatelian, A.; Knight, R.; Panda, S. Antibiotic-Induced Microbiome Depletion Alters Metabolic Homeostasis by Affecting Gut Signaling and Colonic Metabolism. Nat. Commun. 2018, 9, 2872. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, N.; Yahagi, K.; Hara, T.; Watanabe, Y.; Matsumoto, H.; Mori, H.; Higashi, K.; Tsuji, H.; Matsumoto, S.; Kurokawa, K.; et al. Key Bacterial Taxa and Metabolic Pathways Affecting Gut Short-Chain Fatty Acid Profiles in Early Life. ISME J. 2021, 15, 2574–2590. [Google Scholar] [CrossRef]
- Igarashi, H.; Maeda, S.; Ohno, K.; Horigome, A.; Odamaki, T.; Tsujimoto, H. Effect of Oral Administration of Metronidazole or Prednisolone on Fecal Microbiota in Dogs. PLoS ONE 2014, 9, e107909. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Gaschen, F.P.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; AlShawaqfeh, M.K.; et al. Effects of Metronidazole on the Fecal Microbiome and Metabolome in Healthy Dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-Term Impact of Tylosin on Fecal Microbiota and Fecal Bile Acids of Healthy Dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef]
- Espinosa-Gongora, C.; Jessen, L.R.; Kieler, I.N.; Damborg, P.; Bjørnvad, C.R.; Gudeta, D.D.; Pires Dos Santos, T.; Sablier-Gallis, F.; Sayah-Jeanne, S.; Corbel, T.; et al. Impact of Oral Amoxicillin and Amoxicillin/Clavulanic Acid Treatment on Bacterial Diversity and β-Lactam Resistance in the Canine Faecal Microbiota. J. Antimicrob. Chemother. 2020, 75, 351–361. [Google Scholar] [CrossRef]
- Connelly, S.; Fanelli, B.; Hasan, N.A.; Colwell, R.R.; Kaleko, M. Low Dose Oral Beta-Lactamase Protects the Gut Microbiome from Oral Beta-Lactam-Mediated Damage in Dogs. AIMS Public. Health 2019, 6, 477. [Google Scholar] [CrossRef]
- Grønvold, A.M.R.; L’Abée-Lund, T.M.; Sørum, H.; Skancke, E.; Yannarell, A.C.; MacKie, R.I. Changes in Fecal Microbiota of Healthy Dogs Administered Amoxicillin. FEMS Microbiol. Ecol. 2009, 71, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.E.; Jacob, M.E.; Bruno-Bárcena, J.M.; Amirsultan, S.; Stauffer, S.H.; Píqueras, V.O.; Frias, R.; Gookin, J.L. Influence of the Intestinal Microbiota on Disease Susceptibility in Kittens with Experimentally-Induced Carriage of Atypical Enteropathogenic Escherichia Coli. Vet. Microbiol. 2019, 231, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Stavroulaki, E.M.; Suchodolski, J.S.; Pilla, R.; Fosgate, G.T.; Sung, C.H.; Lidbury, J.A.; Steiner, J.M.; Xenoulis, P.G. Short- and Long-Term Effects of Amoxicillin/Clavulanic Acid or Doxycycline on the Gastrointestinal Microbiome of Growing Cats. PLoS ONE 2021, 16, e0253031. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, J.C.; Stokes, J.E.; Laia, N.L.; Price, J.M.; Suchodolski, J.S. Short and Long-Term Effects of a Synbiotic on Clinical Signs, the Fecal Microbiome, and Metabolomic Profiles in Healthy Research Cats Receiving Clindamycin: A Randomized, Controlled Trial. PeerJ 2018, 2018, e5130. [Google Scholar] [CrossRef]
- Whittemore, J.C.; Stokes, J.E.; Price, J.M.; Suchodolski, J.S. Effects of a Synbiotic on the Fecal Microbiome and Metabolomic Profiles of Healthy Research Cats Administered Clindamycin: A Randomized, Controlled Trial. Gut Microbes 2019, 10, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Torres-Henderson, C.; Summers, S.; Suchodolski, J.; Lappin, M.R. Effect of Enterococcus Faecium Strain SF68 on Gastrointestinal Signs and Fecal Microbiome in Cats Administered Amoxicillin-Clavulanate. Top. Companion Anim. Med. 2017, 32, 104–108. [Google Scholar] [CrossRef]
- Clayton, J.B.; Al-Ghalith, G.A.; Long, H.T.; van Tuan, B.; Cabana, F.; Huang, H.; Vangay, P.; Ward, T.; van Minh, V.; Tam, N.A.; et al. Associations between Nutrition, Gut Microbiome, and Health in A Novel Nonhuman Primate Model. Sci. Rep. 2018, 8, 11159. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Dawin, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef]
- Minamoto, Y.; Minamoto, T.; Isaiah, A.; Sattasathuchana, P.; Buono, A.; Rangachari, V.R.; McNeely, I.H.; Lidbury, J.; Steiner, J.M.; Suchodolski, J.S. Fecal Short-Chain Fatty Acid Concentrations and Dysbiosis in Dogs with Chronic Enteropathy. J. Vet. Intern. Med. 2019, 33, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Prajapati, N.; Prajapati, A.; Singh, S.; Joshi, M.; Prajapati, D.; Patani, A.; Sahoo, D.K.; Patel, A. Postbiotic Emissaries: A Comprehensive Review on the Bioprospecting and Production of Bioactive Compounds by Enterococcus Species. Int. J. Food Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Alexander, C.; Cross, T.W.L.; Devendran, S.; Neumer, F.; Theis, S.; Ridlon, J.M.; Suchodolski, J.S.; De Godoy, M.R.C.; Swanson, K.S. Effects of Prebiotic Inulin-Type Fructans on Blood Metabolite and Hormone Concentrations and Faecal Microbiota and Metabolites in Overweight Dogs. Br. J. Nutr. 2018, 120, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Middelbos, I.S.; Vester Boler, B.M.; Qu, A.; White, B.A.; Swanson, K.S.; Fahey, G.C. Phylogenetic Characterization of Fecal Microbial Communities of Dogs Fed Diets with or without Supplemental Dietary Fiber Using 454 Pyrosequencing. PLoS ONE 2010, 5, e9768. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; Dowd, S.E.; Suchodolski, J.S.; Steiner, J.M.; Duclos, L.; Swanson, K.S. Fecal Microbial Communities of Healthy Adult Dogs Fed Raw Meat-Based Diets with or without Inulin or Yeast Cell Wall Extracts as Assessed by 454 Pyrosequencing. FEMS Microbiol. Ecol. 2013, 84, 532–541. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Rossoni Serao, M.C.; de Godoy, M.R.C.; Swanson, K.S.; Guérin-Deremaux, L.; Lynch, G.L.; Wils, D.; Fahey, G.C.; Dilger, R.N. Potato Fiber as a Dietary Fiber Source in Dog Foods. J. Anim. Sci. 2013, 91, 5344–5352. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Kerr, K.R.; Dilger, R.N.; Fahey, G.C.; Guérin-Deremaux, L.; Lynch, G.L.; Wils, D.; Suchodolski, J.S.; Steer, J.M.; Dowd, S.E.; et al. Modulation of the Faecal Microbiome of Healthy Adult Dogs by Inclusion of Potato Fibre in the Diet. Br. J. Nutr. 2015, 113, 125–133. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Kanakupt, K.; Vester Boler, B.M.; Dunsford, B.R.; Fahey, G.C. Effects of Short-Chain Fructooligosaccharides and Galactooligosaccharides, Individually and in Combination, on Nutrient Digestibility, Fecal Fermentative Metabolite Concentrations, and Large Bowel Microbial Ecology of Healthy Adults Cats. J. Anim. Sci. 2011, 89, 1376–1384. [Google Scholar] [CrossRef]
- Barry Kiely, M.; Boileau, T.; Soon Park, J.; Minikhiem, D.; Kiely, B.; Boileau, T.; Park, J.S. Clinical Benefits of Probiotic Canine-Derived Bifidobacterium Animalis Strain AHC7 in Dogs with Acute Idiopathic Diarrhea. Vet. J. 2009, 10, 121–130. [Google Scholar]
- Garcia-Mazcorro, J.F.; Barcenas-Walls, J.R.; Suchodolski, J.S.; Steiner, J.M. Molecular Assessment of the Fecal Microbiota in Healthy Cats and Dogs before and during Supplementation with Fructo-Oligosaccharides (FOS) and Inulin Using High-Throughput 454-Pyrosequencing. PeerJ 2017, 5, e3184. [Google Scholar] [CrossRef]
- Young, W.; Moon, C.D.; Thomas, D.G.; Cave, N.J.; Bermingham, E.N. Pre- and Post-Weaning Diet Alters the Faecal Metagenome in the Cat with Differences in Vitamin and Carbohydrate Metabolism Gene Abundances. Sci. Rep. 2016, 6, 34668. [Google Scholar] [CrossRef] [PubMed]
- Deb-Choudhury, S.; Bermingham, E.N.; Young, W.; Barnett, M.P.G.; Knowles, S.O.; Harland, D.; Clerens, S.; Dyer, J.M. The Effects of a Wool Hydrolysate on Short-Chain Fatty Acid Production and Fecal Microbial Composition in the Domestic Cat (Felis Catus). Food Funct. 2018, 9, 4107–4121. [Google Scholar] [CrossRef] [PubMed]
- Wernimont, S.; Fritsch, D.; Jackson, M.; Badri, D.; Cochrane, C.-Y.; Gross, K. Specialized Dietary Fibers Alter Microbiome Composition & Promote Fermentative Metabolism in the Lower Gastrointestinal Tract of Healthy Adult Cats (P20-045-19). Curr. Dev. Nutr. 2019, 3, nzz040.P20-045-19. [Google Scholar] [CrossRef]
- Hooda, S.; Vester Boler, B.M.; Kerr, K.R.; Dowd, S.E.; Swanson, K.S. The Gut Microbiome of Kittens Is Affected by Dietary Protein:Carbohydrate Ratio and Associated with Blood Metabolite and Hormone Concentrations. Br. J. Nutr. 2013, 109, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kölle, P. The Fecal Microbiome and Metabolome Differs between Dogs Fed Bones and Raw Food (BARF) Diets and Dogs Fed Commercial Diets. PLoS ONE 2018, 13, e0201279. [Google Scholar] [CrossRef]
- Kim, J.; An, J.-U.; Kim, W.; Lee, S.; Cho, S. Differences in the Gut Microbiota of Dogs (Canis Lupus Familiaris) Fed a Natural Diet or a Commercial Feed Revealed by the Illumina MiSeq Platform. Gut Pathog. 2017, 9, 68. [Google Scholar] [CrossRef]
- Herstad, K.M.V.; Gajardo, K.; Bakke, A.M.; Moe, L.; Ludvigsen, J.; Rudi, K.; Rud, I.; Sekelja, M.; Skancke, E. A Diet Change from Dry Food to Beef Induces Reversible Changes on the Faecal Microbiota in Healthy, Adult Client-Owned Dogs. BMC Vet. Res. 2017, 13, 147. [Google Scholar] [CrossRef]
- Vester, B.M.; Dalsing, B.L.; Middelbos, I.S.; Apanavicius, C.J.; Lubbs, D.C.; Swanson, K.S. Faecal Microbial Populations of Growing Kittens Fed High- or Moderate-Protein Diets. Arch. Anim. Nutr. 2009, 63, 254–265. [Google Scholar] [CrossRef]
- Deusch, O.; O’Flynn, C.; Colyer, A.; Morris, P.; Allaway, D.; Jones, P.G.; Swanson, K.S. Deep Illumina-Based Shotgun Sequencing Reveals Dietary Effects on the Structure and Function of the Fecal Microbiome of Growing Kittens. PLoS ONE 2014, 9, e101021. [Google Scholar] [CrossRef]
- Kerr, K.R.; Dowd, S.E.; Swanson, K.S. Faecal Microbiota of Domestic Cats Fed Raw Whole Chicks v. an Extruded Chicken-Based Diet. J. Nutr. Sci. 2014, 3, e22. [Google Scholar] [CrossRef]
- Butowski, C.F.; Thomas, D.G.; Young, W.; Cave, N.J.; McKenzie, C.M.; Rosendale, D.I.; Bermingham, E.N. Addition of Plant Dietary Fibre to a Raw Red Meat High Protein, High Fat Diet, Alters the Faecal Bacteriome and Organic Acid Profiles of the Domestic Cat (Felis Catus). PLoS ONE 2019, 14, e0216072. [Google Scholar] [CrossRef]
- Food: Guidelines for the Evaluation of Probiotics in Food—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Guidelines+for+the+Evaluation+of+Probiotics+in+Food&author=Food+and+Agriculture+Organization+%28FAO%29&publication_year=2002&inst=10763123895732025241 (accessed on 1 August 2024).
- Sahoo, D.K.; Allenspach, K.; Mochel, J.P.; Parker, V.; Rudinsky, A.J.; Winston, J.A.; Bourgois-Mochel, A.; Ackermann, M.; Heilmann, R.M.; Köller, G.; et al. Synbiotic-IgY Therapy Modulates the Mucosal Microbiome and Inflammatory Indices in Dogs with Chronic Inflammatory Enteropathy: A Randomized, Double-Blind, Placebo-Controlled Study. Vet. Sci. 2022, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Urdaneta, V.; Casadesús, J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Chon, H.; Ku, W.; Eom, S.; Seok, M.; Kim, S.; Lee, J.; Kim, D.; Lee, S.; Koo, H.; et al. Bile Acids: Major Regulator of the Gut Microbiome. Microorganisms 2022, 10, 1792. [Google Scholar] [CrossRef]
- Tian, Y.; Gui, W.; Koo, I.; Smith, P.B.; Allman, E.L.; Nichols, R.G.; Rimal, B.; Cai, J.; Liu, Q.; Patterson, A.D. The Microbiome Modulating Activity of Bile Acids. Gut Microbes 2020, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Saxelin, M.; Tynkkynen, S.; Mattila-Sandholm, T.; de Vos, W.M. Probiotic and Other Functional Microbes: From Markets to Mechanisms. Curr. Opin. Biotechnol. 2005, 16, 204–211. [Google Scholar] [CrossRef]
- Schmitz, S.; Suchodolski, J. Understanding the Canine Intestinal Microbiota and Its Modification by Pro-, Pre- and Synbiotics—What Is the Evidence? Vet. Med. Sci. 2016, 2, 71–94. [Google Scholar] [CrossRef]
- Fernández, L.; Martínez, R.; Pérez, M.; Arroyo, R.; Rodríguez, J.M. Characterization of Lactobacillus Rhamnosus MP01 and Lactobacillus Plantarum MP02 and Assessment of Their Potential for the Prevention of Gastrointestinal Infections in an Experimental Canine Model. Front. Microbiol. 2019, 10, 1117. [Google Scholar] [CrossRef]
- Delucchi, L.; Fraga, M.; Zunino, P. Effect of the Probiotic Lactobacillus Murinus LbP2 on Clinical Parameters of Dogs with Distemper-Associated Diarrhea. Can. J. Vet. Res. 2017, 81, 118. [Google Scholar]
- Kumar, S.; Pattanaik, A.K.; Sharma, S.; Jadhav, S.E.; Dutta, N.; Kumar, A. Probiotic Potential of a Lactobacillus Bacterium of Canine Faecal-Origin and Its Impact on Select Gut Health Indices and Immune Response of Dogs. Probiotics Antimicrob. Proteins 2017, 9, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Strompfova, V.; Kubasova, I.; Laukova, A. Health Benefits Observed after Probiotic Lactobacillus Fermentum CCM 7421 Application in Dogs. Appl. Microbiol. Biotechnol. 2017, 101, 6309–6320. [Google Scholar] [CrossRef]
- Strompfová, V.; Marciňáková, M.; Simonová, M.; Bogovič-Matijašić, B.; Lauková, A. Application of Potential Probiotic Lactobacillus Fermentum AD1 Strain in Healthy Dogs. Anaerobe 2006, 12, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Strompfová, V.; Pogány Simonová, M.; Gancarčíková, S.; Mudroňová, D.; Farbáková, J.; Mad’ari, A.; Lauková, A. Effect of Bifidobacterium Animalis B/12 Administration in Healthy Dogs. Anaerobe 2014, 28, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Strompfová, V.; Kubašová, I.; Ščerbová, J.; Maďari, A.; Gancarčíková, S.; Mudroňová, D.; Miltko, R.; Belzecki, G.; Lauková, A. Oral Administration of Bacteriocin-Producing and Non-Producing Strains of Enterococcus Faecium in Dogs. Appl. Microbiol. Biotechnol. 2019, 103, 4953–4965. [Google Scholar] [CrossRef] [PubMed]
- Gookin, J.L.; Strong, S.J.; Bruno-Bárcena, J.M.; Stauffer, S.H.; Williams, S.; Wassack, E.; Azcarate-Peril, M.A.; Estrada, M.; Seguin, A.; Balzer, J.; et al. Randomized Placebo-Controlled Trial of Feline-Origin Enterococcus Hirae Probiotic Effects on Preventative Health and Fecal Microbiota Composition of Fostered Shelter Kittens. Front. Vet. Sci. 2022, 9, 923792. [Google Scholar] [CrossRef]
- Marshall-Jones, Z.V.; Baillon, M.-L.A.; Croft, J.M.; Butterwick, R.F. Effects of Lactobacillus Acidophilus DSM13241 as a Probiotic in Healthy Adult Cats. Am. J. Vet. Res. 2006, 67, 1005–1012. [Google Scholar] [CrossRef]
- Fusi, E.; Rizzi, R.; Polli, M.; Cannas, S.; Giardini, A.; Bruni, N.; Marelli, S.P. Effects of Lactobacillus Acidophilus D2/CSL (CECT 4529) Supplementation on Healthy Cat Performance. Vet Rec Open 2019, 6, e000368. [Google Scholar] [CrossRef]
- Kumar, A.; Vandana; Singh, M.; Singh, P.P.; Singh, S.K.; Singh, P.K.; Pandey, K.D. Isolation of Plant Growth Promoting Rhizobacteria and Their Impact on Growth and Curcumin Content in Curcuma longa L. Biocatal. Agric. Biotechnol. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Lee, D.; Goh, T.W.; Kang, M.G.; Choi, H.J.; Yeo, S.Y.; Yang, J.; Huh, C.S.; Kim, Y.Y.; Kim, Y. Perspectives and Advances in Probiotics and the Gut Microbiome in Companion Animals. J. Anim. Sci. Technol. 2022, 64, 197–217. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Zhou, Z.; Li, C.; Luo, R.; Liu, H.; Fu, H.; Zhong, Z.; Shen, L.; Cao, S.; et al. Protective Effects of Bacillus Subtilis HH2 against Oral Enterotoxigenic Escherichia Coli in Beagles. Vet. Sci. 2023, 10, 432. [Google Scholar] [CrossRef]
- Rossi, G.; Pengo, G.; Galosi, L.; Berardi, S.; Tambella, A.M.; Attili, A.R.; Gavazza, A.; Cerquetella, M.; Jergens, A.E.; Guard, B.C.; et al. Effects of the Probiotic Mixture Slab51® (SivoMixx®) as Food Supplement in Healthy Dogs: Evaluation of Fecal Microbiota, Clinical Parameters and Immune Function. Front. Vet. Sci. 2020, 7, 567174. [Google Scholar] [CrossRef]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [PubMed]
- Tuniyazi, M.; Hu, X.; Fu, Y.; Zhang, N. Canine Fecal Microbiota Transplantation: Current Application and Possible Mechanisms. Vet. Sci. 2022, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Chaitman, J.; Ziese, A.L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs with Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 527840. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.Q.; Gomes, L.A.; Santos, I.S.; Alfieri, A.F.; Weese, J.S.; Costa, M.C. Fecal Microbiota Transplantation in Puppies with Canine Parvovirus Infection. J. Vet. Intern. Med. 2018, 32, 707–711. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Improvement in Clinical Symptoms and Fecal Microbiome After Fecal Microbiota Transplantation in a Dog with Inflammatory Bowel Disease. Vet. Med. Res. Rep. 2019, 10, 197–201. [Google Scholar] [CrossRef]
- Ambrosini, Y.M.; Borcherding, D.; Kanthasamy, A.; Kim, H.J.; Willette, A.A.; Jergens, A.; Allenspach, K.; Mochel, J.P. The Gut-Brain Axis in Neurodegenerative Diseases and Relevance of the Canine Model: A Review. Front. Aging Neurosci. 2019, 11, 130. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Akyuz, E.; Polat, A.K.; Eroglu, E.; Kullu, I.; Angelopoulou, E.; Paudel, Y.N. Revisiting the Role of Neurotransmitters in Epilepsy: An Updated Review. Life Sci. 2021, 265, 118826. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The Role of Short-Chain Fatty Acids in Intestinal Barrier Function, Inflammation, Oxidative Stress, and Colonic Carcinogenesis. Pharmacol. Res. 2021, 165. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Yamashita, R.; Ochi, D.; Watanuki, Y.; Moore, C.; et al. Transport and Release of Chemicals from Plastics to the Environment and to Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan Oligosaccharide Attenuates Cognitive and Behavioral Disorders in the 5xFAD Alzheimer’s Disease Mouse Model via Regulating the Gut Microbiota-Brain Axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, E.H.; Kang, S.S.; Liu, X.; Alam, A.; Ye, K. Gut Dysbiosis Contributes to Amyloid Pathology, Associated with C/EBPβ/AEP Signaling Activation in Alzheimer’s Disease Mouse Model. Sci. Adv. 2020, 6, eaba0466. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized Crossover Trial of a Modified Ketogenic Diet in Alzheimer’s Disease. Alzheimers Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, C.; Wu, J.; Liu, P.; Deng, X.; Zhang, Y.; Peng, B.; Zhu, Y. Ketogenic Diet Ameliorates Cognitive Impairment and Neuroinflammation in a Mouse Model of Alzheimer’s Disease. CNS Neurosci. Ther. 2022, 28, 580. [Google Scholar] [CrossRef]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic Diet Enhances Neurovascular Function with Altered Gut Microbiome in Young Healthy Mice. Sci. Rep. 2018, 8, 6670. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. eBioMedicine 2019, 47, 529. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Afghan, M.; Tarmahi, V.; Behtari, M.; Rahimi Khamaneh, S.; Raeisi, S. Ketogenic Diet: Overview, Types, and Possible Anti-Seizure Mechanisms. Nutr. Neurosci. 2021, 24, 307–316. [Google Scholar] [CrossRef]
- Imdad, K.; Abualait, T.; Kanwal, A.; AlGhannam, Z.T.; Bashir, S.; Farrukh, A.; Khattak, S.H.; Albaradie, R.; Bashir, S. The Metabolic Role of Ketogenic Diets in Treating Epilepsy. Nutrients 2022, 14, 5074. [Google Scholar] [CrossRef]
- Allenspach, K.; Borcherding, D.C.; Iennarella-Servantez, C.A.; Mosichuk, A.P.; Atherly, T.; Sahoo, D.K.; Kathrani, A.; Suchodolski, J.S.; Bourgois-Mochel, A.; Serao, M.R.; et al. Ketogenic Diets in Healthy Dogs Induce Gut and Serum Metabolome Changes Suggestive of Anti-tumourigenic Effects: A Model for Human Ketotherapy Trials. Clin. Transl. Med. 2022, 12, e1047. [Google Scholar] [CrossRef] [PubMed]
- Yudkoff, M.; Daikhin, Y.; Nissim, I.; Lazarow, A.; Nissim, I. Ketogenic Diet, Amino Acid Metabolism, and Seizure Control. J. Neurosci. Res. 2001, 66, 931–940. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Mendonca, A.; Dell, C.; Likhodii, S.S.; Musa, K.; Iracleous, C.; Cunnane, S.C.; Burnham, W.M.I. The MCT Ketogenic Diet: Effects on Animal Seizure Models. Exp. Neurol. 2000, 161, 696–703. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728. [Google Scholar] [CrossRef]
- Hernández-Soberano, C.; Ruíz-Herrera, L.F.; Valencia-Cantero, E. Endophytic Bacteria Arthrobacter Agilis UMCV2 and Bacillus Methylotrophicus M4-96 Stimulate Achene Germination, in Vitro Growth, and Greenhouse Yield of Strawberry (Fragaria × Ananassa). Sci. Hortic. 2020, 261, 109005. [Google Scholar] [CrossRef]
- Handl, S.; German, A.J.; Holden, S.L.; Dowd, S.E.; Steiner, J.M.; Heilmann, R.M.; Grant, R.W.; Swanson, K.S.; Suchodolski, J.S. Faecal Microbiota in Lean and Obese Dogs. FEMS Microbiol. Ecol. 2013, 84, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.D.; Barker, A.K.; Alcott, C.J.; Levine, J.M.; Meren, I.; Wengert, J.; Jergens, A.E.; Suchodolski, J.S. The Association of Specific Constituents of the Fecal Microbiota with Immune-Mediated Brain Disease in Dogs. PLoS ONE 2017, 12, e0170589. [Google Scholar] [CrossRef]
- Mondo, E.; Barone, M.; Soverini, M.; D’Amico, F.; Cocchi, M.; Petrulli, C.; Mattioli, M.; Marliani, G.; Candela, M.; Accorsi, P.A. Gut Microbiome Structure and Adrenocortical Activity in Dogs with Aggressive and Phobic Behavioral Disorders. Heliyon 2020, 6, e03311. [Google Scholar] [CrossRef]
- Muñana, K.R.; Nettifee, J.A.; Griffith, E.H.; Early, P.J.; Yoder, N.C. Evaluation of a Collar-mounted Accelerometer for Detecting Seizure Activity in Dogs. J. Vet. Intern. Med. 2020, 34, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Kubinyi, E.; Bel Rhali, S.; Sándor, S.; Szabó, A.; Felföldi, T. Gut Microbiome Composition Is Associated with Age and Memory Performance in Pet Dogs. Animals 2020, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Sakon, H.; Nagai, F.; Morotomi, M.; Tanaka, R. Sutterella parvirubra sp. nov. and Megamonas funiformis sp. nov., Isolated from Human Faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 970–975. [Google Scholar] [CrossRef]
- Oyama, M.A.; Rush, J.E.; O’Sullivan, M.L.; Williams, R.M.; Rozanski, E.A.; Petrie, J.P.; Sleeper, M.M.; Brown, D.C. Perceptions and Priorities of Owners of Dogs with Heart Disease Regarding Quality versus Quantity of Life for Their Pets. J. Am. Vet. Med. Assoc. 2008, 233, 104–108. [Google Scholar] [CrossRef]
- Seo, J.; Matthewman, L.; Xia, D.; Wilshaw, J.; Chang, Y.M.; Connolly, D.J. The Gut Microbiome in Dogs with Congestive Heart Failure: A Pilot Study. Sci. Rep. 2020, 10, 13777. [Google Scholar] [CrossRef]
- Li, Q.; Larouche-Lebel, É.; Loughran, K.A.; Huh, T.P.; Suchodolski, J.S.; Oyama, M.A. Gut Dysbiosis and Its Associations with Gut Microbiota-Derived Metabolites in Dogs with Myxomatous Mitral Valve Disease. mSystems 2021, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Qian, L. Research on Gut Microbiota-Derived Secondary Bile Acids in Cancer. Integr. Cancer Ther. 2022, 21, 15347354221114100. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Fietz, S.A.; Kalusa, M.; Jergens, A.E.; Sahoo, D.K.; Stewart, T.; Heilmann, R.M. Ultrastructural Changes in Chronic Inflammatory Enteropathies—A Comparison between Dogs and Humans. Front. Cell Dev. Biol. 2024, 12, 1379714. [Google Scholar] [CrossRef]
- Jia, J.; Frantz, N.; Khoo, C.; Gibson, G.R.; Rastall, R.A.; McCartney, A.L. Investigation of the faecal microbiota associated with canine chronic diarrhoea. FEMS Microbiol. Ecol. 2010, 71, 304–312. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Markel, M.E.; Garcia-Mazcorro, J.F.; Unterer, S.; Heilmann, R.M.; Dowd, S.E.; Kachroo, P.; Ivanov, I.; Minamoto, Y.; Dillman, E.M.; et al. The Fecal Microbiome in Dogs with Acute Diarrhea and Idiopathic Inflammatory Bowel Disease. PLoS ONE 2012, 7, e51907. [Google Scholar] [CrossRef]
- Oli, M.W.; Otoo, H.N.; Crowley, P.J.; Heim, K.P.; Nascimento, M.M.; Ramsook, C.B.; Lipke, P.N.; Brady, L.J. Functional Amyloid Formation by Streptococcus Mutans. Microbiology 2012, 158, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Núñez Sellés, A.J.; Vélez Castro, H.T.; Agüero-Agüero, J.; González-González, J.; Naddeo, F.; De Simone, F.; Rastrelli, L. Isolation and Quantitative Analysis of Phenolic Antioxidants, Free Sugars, and Polyols from Mango (Mangifera indica L.) Stem Bark Aqueous Decoction Used in Cuba as a Nutritional Supplement. J. Agric. Food Chem. 2002, 50, 762–766. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Wong, D.; Patani, A.; Paital, B.; Yadav, V.K.; Patel, A.; Jergens, A.E. Exploring the Role of Antioxidants in Sepsis-Associated Oxidative Stress: A Comprehensive Review. Front. Cell. Infect. Microbiol. 2024, 14, 1348713. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.P.; Díaz-Regañón, D.; Gabriel, V.; Livania, V.; Liu, D.; Ahmed, B.H.; Lincoln, A.; Wickham, H.; Ralston, A.; Merodio, M.M.; et al. Changes of Enterocyte Morphology and Enterocyte: Goblet Cell Ratios in Dogs with Protein-Losing and Non-Protein-Losing Chronic Enteropathies. Vet. Sci. 2023, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.W.; Dogan, B.; Rishniw, M.; Goldstein, R.E.; Klaessig, S.; McDonough, P.L.; German, A.J.; Yates, R.M.; Russell, D.G.; Johnson, S.E.; et al. Adherent and Invasive Escherichia Coli Is Associated with Granulomatous Colitis in Boxer Dogs. Infect. Immun. 2006, 74, 4778–4792. [Google Scholar] [CrossRef] [PubMed]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota Alterations in Acute and Chronic Gastrointestinal Inflammation of Cats and Dogs. World J. Gastroenterol. 2014, 20, 16489. [Google Scholar] [CrossRef]
- Cassmann, E.; White, R.; Atherly, T.; Wang, C.; Sun, Y.; Khoda, S.; Mosher, C.; Ackermann, M.; Jergens, A. Alterations of the Ileal and Colonic Mucosal Microbiota in Canine Chronic Enteropathies. PLoS ONE 2016, 11, e0147321. [Google Scholar] [CrossRef]
- Larsen, J.M.; Musavian, H.S.; Butt, T.M.; Ingvorsen, C.; Thysen, A.H.; Brix, S. Chronic Obstructive Pulmonary Disease and Asthma-Associated Proteobacteria, but Not Commensal Prevotella Spp., Promote Toll-like Receptor 2-Independent Lung Inflammation and Pathology. Immunology 2015, 144, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Altomare, A.; Putignani, L.; Del Chierico, F.; Cocca, S.; Angeletti, S.; Ciccozzi, M.; Tripiciano, C.; Dalla Piccola, B.; Cicala, M.; Guarino, M.P.L. Gut Mucosal-Associated Microbiota Better Discloses Inflammatory Bowel Disease Differential Patterns than Faecal Microbiota. Dig. Liver Dis. 2019, 51, 648–656. [Google Scholar] [CrossRef]
- Li, Q.; Lauber, C.L.; Czarnecki-Maulden, G.; Pan, Y.; Hannah, S.S. Effects of the Dietary Protein and Carbohydrate Ratio on Gut Microbiomes in Dogs of Different Body Conditions. mBio 2017, 8, e01703-16. [Google Scholar] [CrossRef]
- Chun, J.L.; Ji, S.Y.; Lee, S.D.; Lee, Y.K.; Kim, B.; Kim, K.H. Difference of Gut Microbiota Composition Based on the Body Condition in Dogs. J. Anim. Sci. Technol. 2020, 62, 239. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.E.; Kim, H.B.; Isaacson, R.E.; Seo, K.W.; Song, K.H. Association of Obesity with Serum Leptin, Adiponectin, and Serotonin and Gut Microflora in Beagle Dogs. J. Vet. Intern. Med. 2015, 29, 43. [Google Scholar] [CrossRef] [PubMed]
- Moinard, A.; Payen, C.; Ouguerram, K.; André, A.; Hernandez, J.; Drut, A.; Biourge, V.C.; Suchodolski, J.S.; Flanagan, J.; Nguyen, P.; et al. Effects of High-Fat Diet at Two Energetic Levels on Fecal Microbiota, Colonic Barrier, and Metabolic Parameters in Dogs. Front. Vet. Sci. 2020, 7, 566282. [Google Scholar] [CrossRef] [PubMed]
- Forster, G.M.; Stockman, J.; Noyes, N.; Heuberger, A.L.; Broeckling, C.D.; Bantle, C.M.; Ryan, E.P. A Comparative Study of Serum Biochemistry, Metabolome and Microbiome Parameters of Clinically Healthy, Normal Weight, Overweight, and Obese Companion Dogs. Top. Companion Anim. Med. 2018, 33, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and Probiotics in Canine and Feline Welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Osto, M.; Lutz, T.A. Translational Value of Animal Models of Obesity—Focus on Dogs and Cats. Eur. J. Pharmacol. 2015, 759, 240–252. [Google Scholar] [CrossRef]
- Tal, M.; Weese, J.S.; Gomez, D.E.; Hesta, M.; Steiner, J.M.; Verbrugghe, A. Bacterial Fecal Microbiota Is Only Minimally Affected by a Standardized Weight Loss Plan in Obese Cats. BMC Vet. Res. 2020, 16, 112. [Google Scholar] [CrossRef]
- Blake, A.B.; Suchodolski, J.S. Importance of Gut Microbiota for the Health and Disease of Dogs and Cats. Anim. Front. 2016, 6, 37–42. [Google Scholar] [CrossRef]
- Apper, E.; Privet, L.; Taminiau, B.; Le Bourgot, C.; Svilar, L.; Martin, J.-C.; Diez, M. Relationships between Gut Microbiota, Metabolome, Body Weight, and Glucose Homeostasis of Obese Dogs Fed with Diets Differing in Prebiotic and Protein Content. Microorganisms 2020, 8, 513. [Google Scholar] [CrossRef]
- Alessandri, G.; Milani, C.; Mancabelli, L.; Longhi, G.; Anzalone, R.; Lugli, G.A.; Duranti, S.; Turroni, F.; Ossiprandi, M.C.; van Sinderen, D.; et al. Deciphering the Bifidobacterial Populations within the Canine and Feline Gut Microbiota. Appl. Environ. Microbiol. 2020, 86, e02875-19. [Google Scholar] [CrossRef]

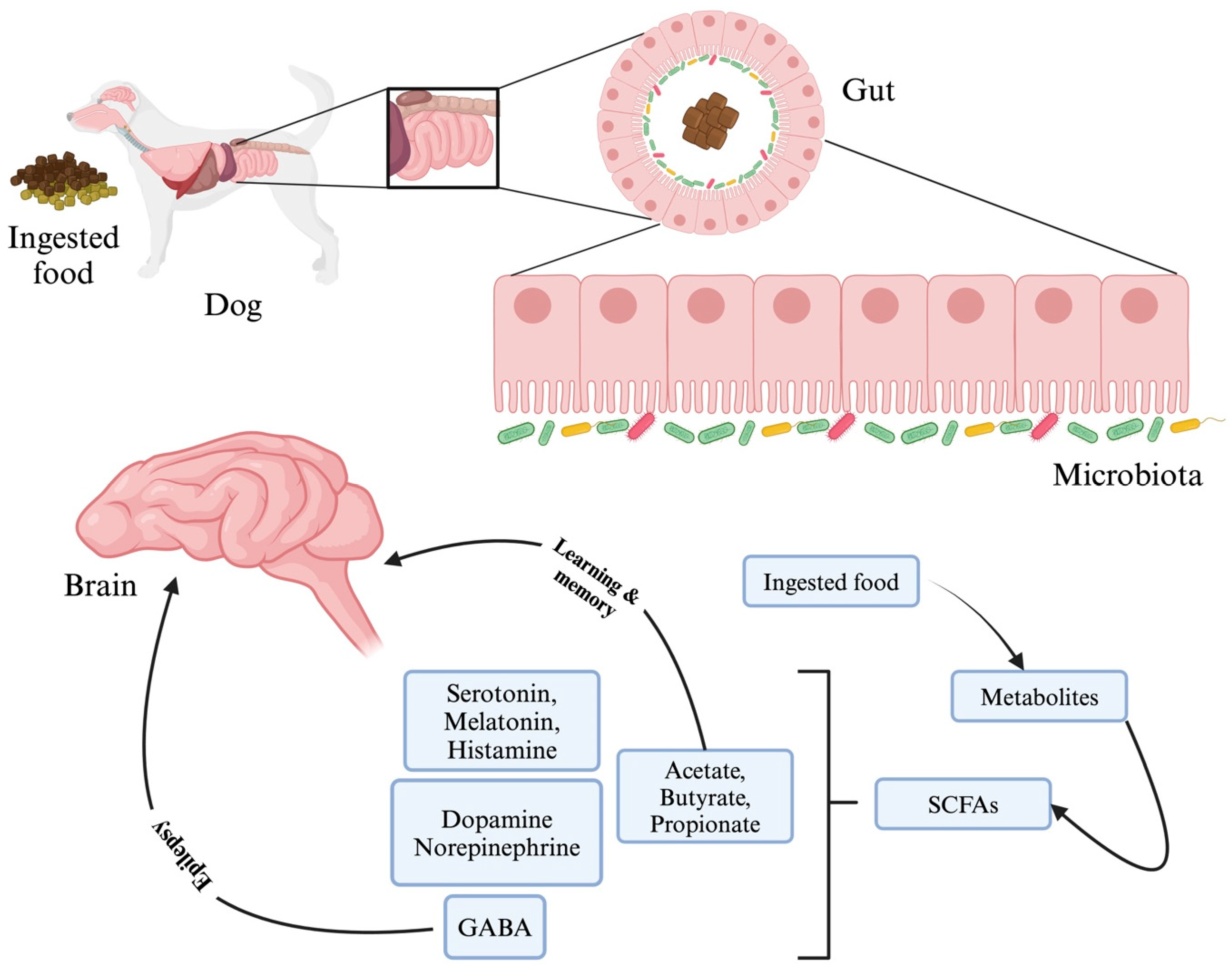
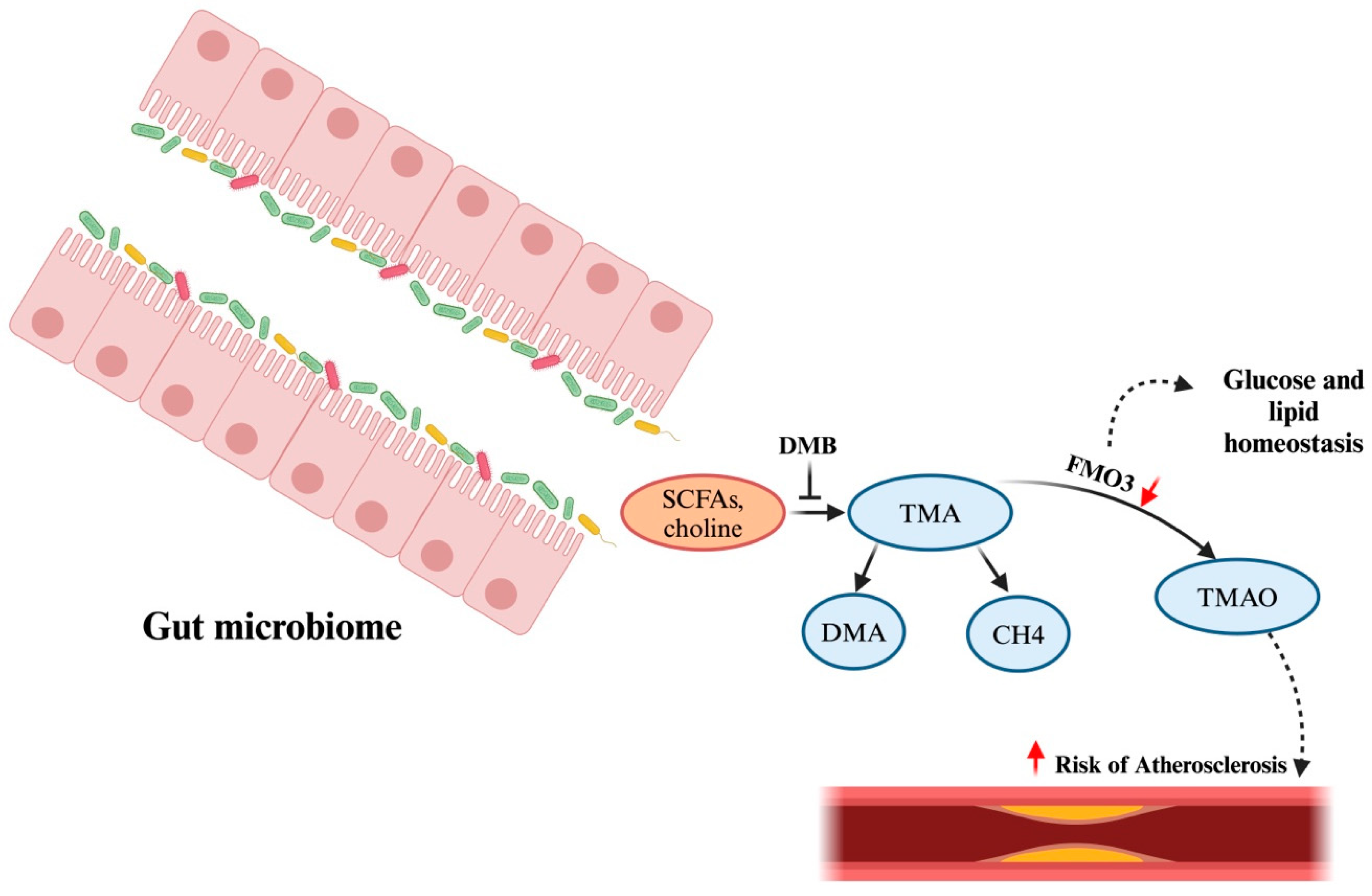
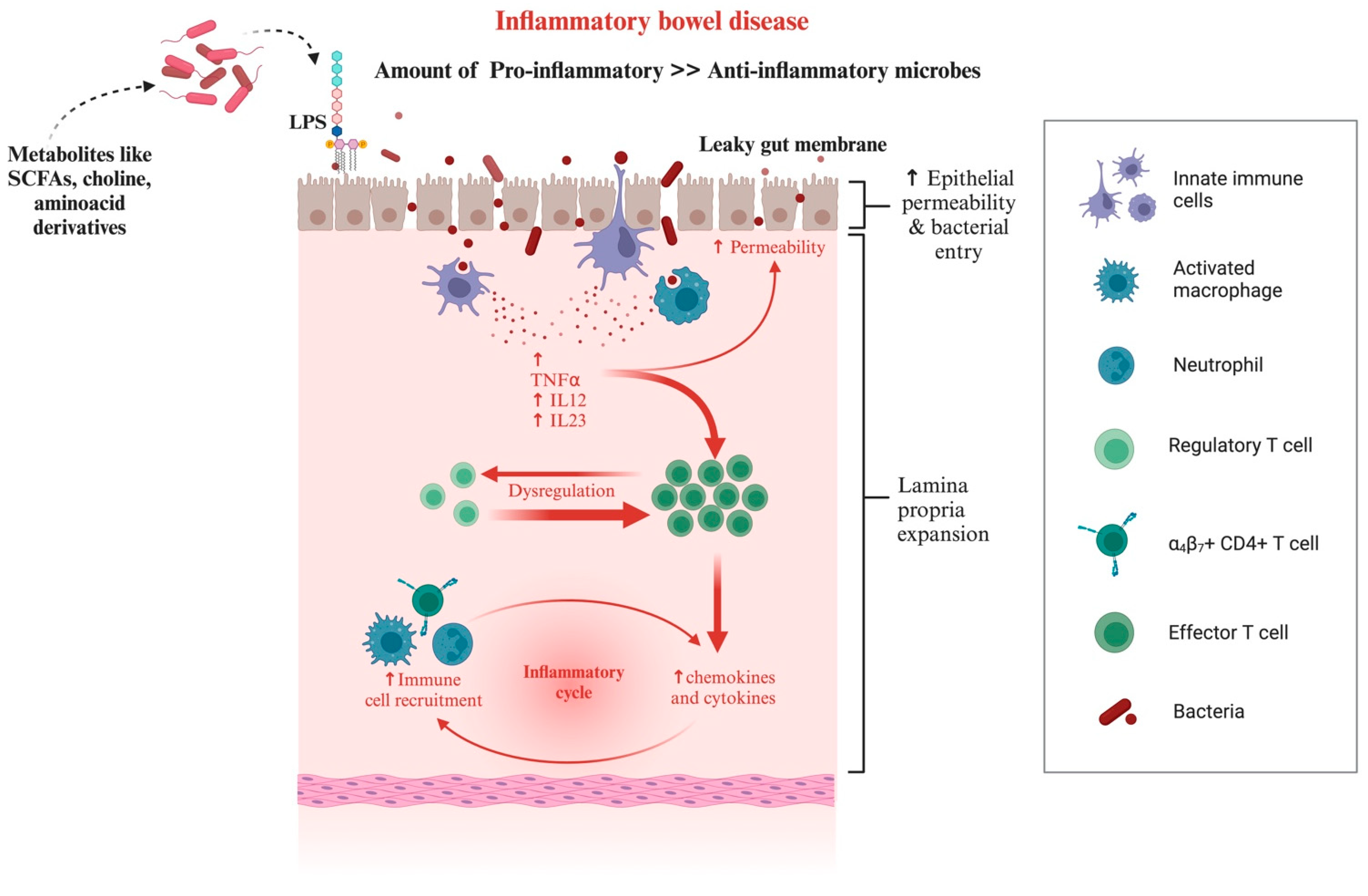
| Phylum | Class | Family | Genus/Species |
|---|---|---|---|
| Proteobacteria | Betaproteobacteria | Alcaligenaceae | Sutterella |
| Gammaproteobacteria | Enterobacteriaceae | E. coli | |
| Fusobacteria | Fusobacteriia | Fusobacteriaceae | Fusobacterium |
| Firmicutes | Bacilli | Turicibacteraceae | Turicibacter |
| Veillonellaceae | Megamonas | ||
| Lactobacillaceae | Lactobacillus | ||
| Streptococcaceae | Streptococcus | ||
| Clostridia | Clostridiaceae | Clostridium | |
| Ruminococcaceae | Faecalibacterium prausnitzii | ||
| Lachnospiraceae | Blautia | ||
| Peptostreptococcaceae | Peptostreptococcus | ||
| Bacteroidetes | Bacteroidetes | Prevotellaceae | Prevotella |
| Bacteroidaceae | Bacteroides | ||
| Actinobacteria | Coriobacteriia | Coriobacteriaceae | Collinsella |
| Disease/Disorder | Microbiota Involved | Author |
|---|---|---|
| Secretory diarrhea | Clostridium hiranonis | [45] |
| Carbohydrate fermentation | Bifidobacterium, Lactobacillus, and Faecalibacterium | [36] |
| Prevents leaky gut syndrome | Clostridiales | [36] |
| Protects against excessive inflammation | Parabacteroides | [46] |
| Mitigates CE | Lactobacillus acidophilus strains and Lactobacillus johnsonii strain | [47] |
| Intestinal disease (in cats) | Bifidobacteria and Bacteroides (decrease), Desulfovibrio (increase) | [48,49] |
| Small cell intestinal lymphoma | Fusobacterium sp. (increase) | [50] |
| Impact on Dogs | ||||
|---|---|---|---|---|
| Diet Type | Technique | Results | Alterations in Abundance | References |
| Inulin-type fructans | 16S rRNA seq. | Firmicutes, Erysipelotrichaceae, and Turicibacteraceae | Increase | [104] |
| Beet pulp | 16S rRNA seq. | Erysipelotrichi and Fusobacteria | Decrease | [105] |
| Firmicutes and Clostridia | Increase | |||
| Yeast cell wall | 16S rRNA seq. | Bifidobacterium | Increase | [106] |
| Inulin | 16S rRNA seq. | Enterobacteriaceae | Decrease | [106] |
| Megamonas and Lactobacillus | Increase | |||
| Potato fibre | 16S rRNA seq. | Faecalibacterium, Lachnospira, faecal acetate, propionate and butyrate | Increase | [107,108] |
| Prevotella and Fusobacterium | Decrease | |||
| Soybean husk | qPCR | Clostridium cluster XI | Decrease | [109] |
| Total Lactobacilli, Faecalibacterium, Bacteroides-Prevotella-Porphyromonas, and Clostridium cluster XIVa | Increase | |||
| Impact on Cats | ||||
| FOS | qPCR | Bifidobacterium | Increase | [110] |
| 16S rRNA seq. | Actinobacteria | Increase | [111] | |
| GOS | qPCR | Bifidobacterium | Increase | [110] |
| Cellulose | 16S rRNA seq. | No changes | — | [111] |
| FOS and GOS | qPCR | Bifidobacterium, total SCFAs, butyrate, and valerate | Increase | [110] |
| FOS and inulin | 16S rRNA seq. | Veillonaceae | Increase | [112] |
| Gammaproteobacteria | Decrease | |||
| Inulin | 16S rRNA seq. | Bifidobacterium | Increase | [113] |
| Faecalibacterium and Fusobacterium | Decrease | |||
| Pectin | 16S rRNA seq. | Firmicutes | Increase | [111] |
| Wool hydrolysate | 16S rRNA seq. | No changes | — | [114] |
| Mixed insoluble fibres | 16S rRNA seq. | Blautia, Bacteroides, Turicibacter, acetic and propionic acids | Increase | [115] |
| Isobutyric, 2-methylbutyric, and isovaleric acids | Decrease | |||
| Inulin and cellulose | 16S rRNA seq. | Prevotella, Bifidobacterium, Lactobacillus, Megamonas, and unclassified Lachnospiraceae | Increase | [116] |
| Clostridium, Fusobacterium, and Eubacterium | Decrease | |||
| Impact on Dogs | |||||
| Diet Type | Results | Feed Duration | Number of Individuals | References | |
| Bones and raw foods (BARF) | ↓ Bifidobacterium and Faecalibacterium; ↑ Fusobacteria, Escherichia coli, Streptococcus, and Clostridium | 4 weeks to 9 years | 27 | [117] | |
| Red meat | ↓ Faecalibacterium, Peptostreptococcus, Bacteroides, and Prevotella ↑ Fusobacterium, Lactobacillus, and Clostridium | 9 weeks | 7 | [35] | |
| Raw diet | ↑ Richness, evenness, Clostridium perfringens, Clostridium hiranonis, Dorea, and Fusobacterium varium | At least 1 year | 6 | [118] | |
| Kibble with boiled beef | ↓ Faecalibacterium prausnitzii ↑ Clostridium hiranonis, Dorea, Slackia, and unidentified Clostridiaceae | 1 week per combination | 11 | [119] | |
| Impact on Cats | |||||
| Diet Type | Results | Other Specifications | Feed Duration | Number of Individuals | References |
| High-protein low-carbohydrate dry food | ↓ Lactobacillus, Bifidobacterium, and Escherichia coli | Kitten, weaning diet | 8 weeks | 7 | [120] |
| ↓ Actinobacteria, Bifidobacterium, Dialister, Acidaminococcus, Megasphera, and Mitsuokella ↑ Fusobacteria, Clostridium, Faecalibacterium, Ruminococcus, Blautia, and Eubacterium | Kitten, weaning diet | 8 weeks | 7 | [116] | |
| ↑ Species diversity; affected 194 metabolic pathways, including amino acid synthesis and metabolism | Kitten, weaning diet | 8 weeks | 6 | [121] | |
| Raw 1 to 3-day-old chicks | ↑ Peptococcus, Pseudobutyrivibrio, and unidentified Lachnospiraceae | Adult | 1.3 weeks | 5 | [122] |
| Raw | ↑ Clostridium, Fusobacterium, Eubacterium, and molar ratio of butyrate | Adult | 3 weeks | 12 | [123] |
| Raw plus plant fibre | ↓ Clostridium, Fusobacterium, and Eubacterium | Adult | 3 weeks | 12 | [123] |
| ↑ Prevotella | |||||
| Canned | ↓ Firmicutes, Bacteroides, Lactobacillus, and Streptococcus | Kitten, weaning diet | 9 weeks | 10 | [67] |
| ↑ Fusobacterium, Clostridium, unidentified Peptostreptococcaceae and Prevotellaceae | |||||
| ↓ Lactobacillus, Megasphera, and Olsenella | Adult | 5 weeks | 16 | [67] | |
| ↑ Species richness, Fusobacteria, Proteobacteria, Clostridium, Blautia, Bacteroides, and unidentified Peptostreptococcaceae | |||||
| ↓ Lactobacillus, Bifidobacterium, and Collinsella | Kitten, weaning diet | 9 weeks | 10 | [113] | |
| ↑ Bacteroides, Clostridium, Fusobacterium, genes involved in vitamin biosynthesis, metabolism, and transport | Kitten, weaning diet | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, H.; Trivedi, M.; Gurjar, T.; Sahoo, D.K.; Jergens, A.E.; Yadav, V.K.; Patel, A.; Pandya, P. Decoding the Gut Microbiome in Companion Animals: Impacts and Innovations. Microorganisms 2024, 12, 1831. https://doi.org/10.3390/microorganisms12091831
Shah H, Trivedi M, Gurjar T, Sahoo DK, Jergens AE, Yadav VK, Patel A, Pandya P. Decoding the Gut Microbiome in Companion Animals: Impacts and Innovations. Microorganisms. 2024; 12(9):1831. https://doi.org/10.3390/microorganisms12091831
Chicago/Turabian StyleShah, Harsh, Mithil Trivedi, Tejas Gurjar, Dipak Kumar Sahoo, Albert E. Jergens, Virendra Kumar Yadav, Ashish Patel, and Parth Pandya. 2024. "Decoding the Gut Microbiome in Companion Animals: Impacts and Innovations" Microorganisms 12, no. 9: 1831. https://doi.org/10.3390/microorganisms12091831
APA StyleShah, H., Trivedi, M., Gurjar, T., Sahoo, D. K., Jergens, A. E., Yadav, V. K., Patel, A., & Pandya, P. (2024). Decoding the Gut Microbiome in Companion Animals: Impacts and Innovations. Microorganisms, 12(9), 1831. https://doi.org/10.3390/microorganisms12091831









