Effects of 5-Aminolevulinic Acid Supplementation on Gas Production, Fermentation Characteristics, and Bacterial Community Profiles In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and In Vitro Procedure
2.2. Data Collection, Sampling, and Analysis
2.3. Bacterial Amplicon Sequencing and Data Processing
2.4. Calculations and Statistical Analysis
3. Results
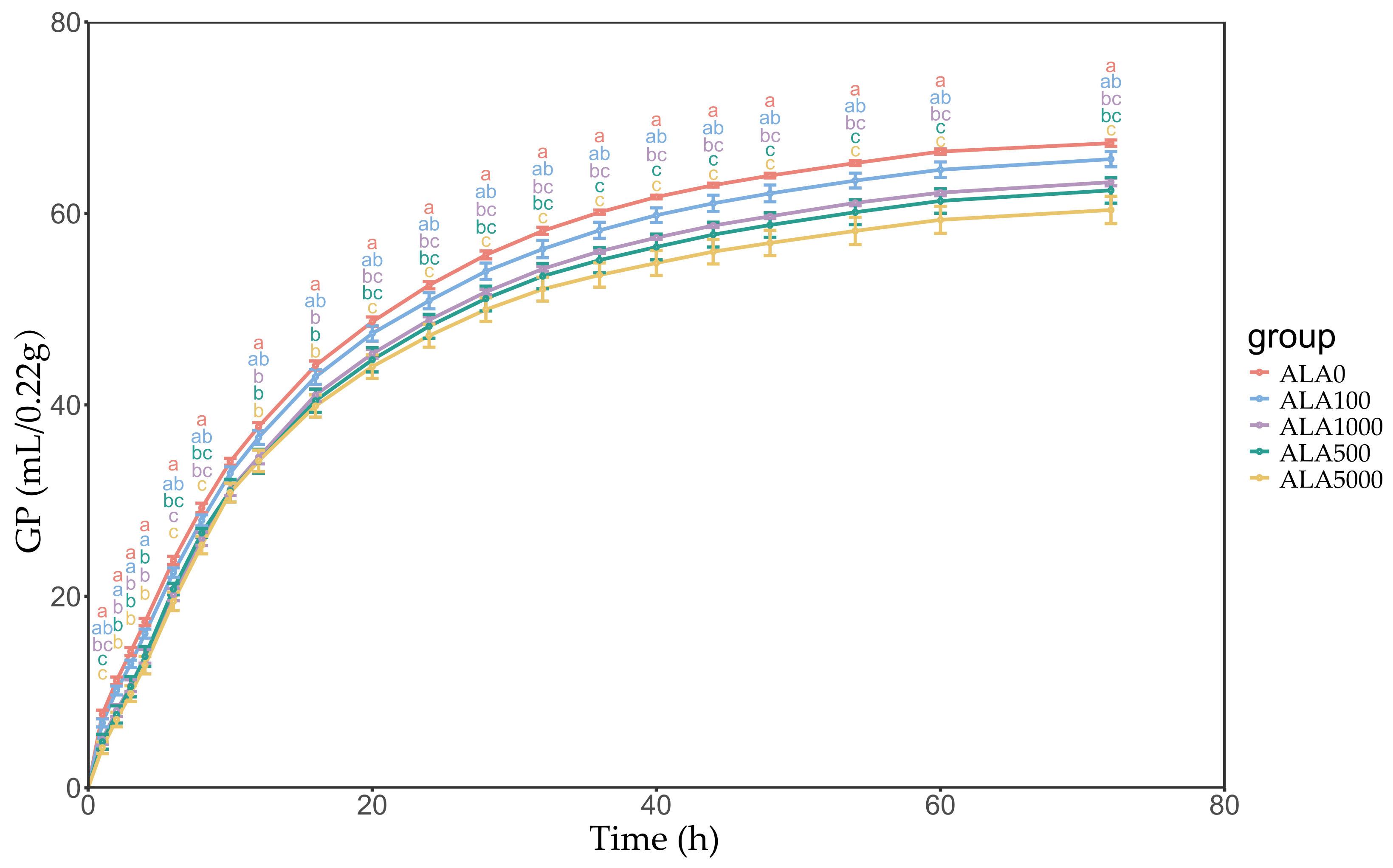
3.1. In Vitro Gas Production Kinetics and Dry Matter Digestibility
3.2. In Vitro Fermentation Parameters
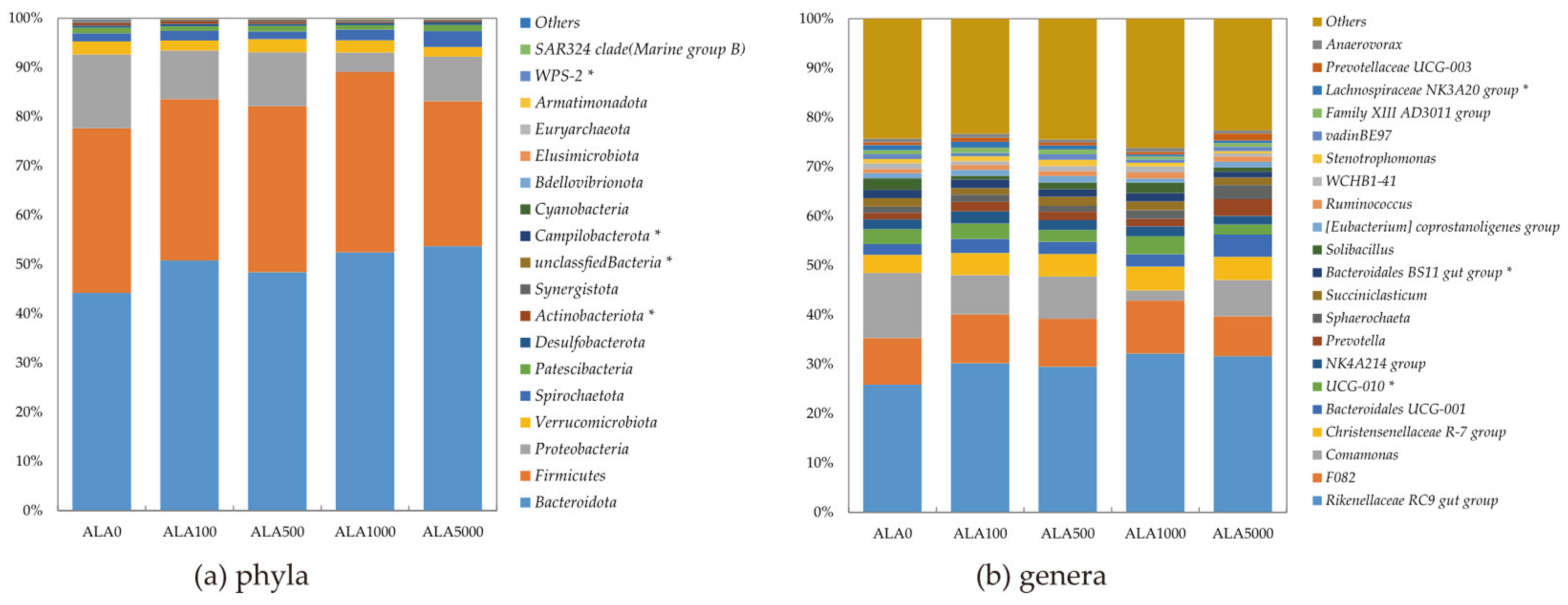
3.3. Sequencing Overview and Bacterial Diversity
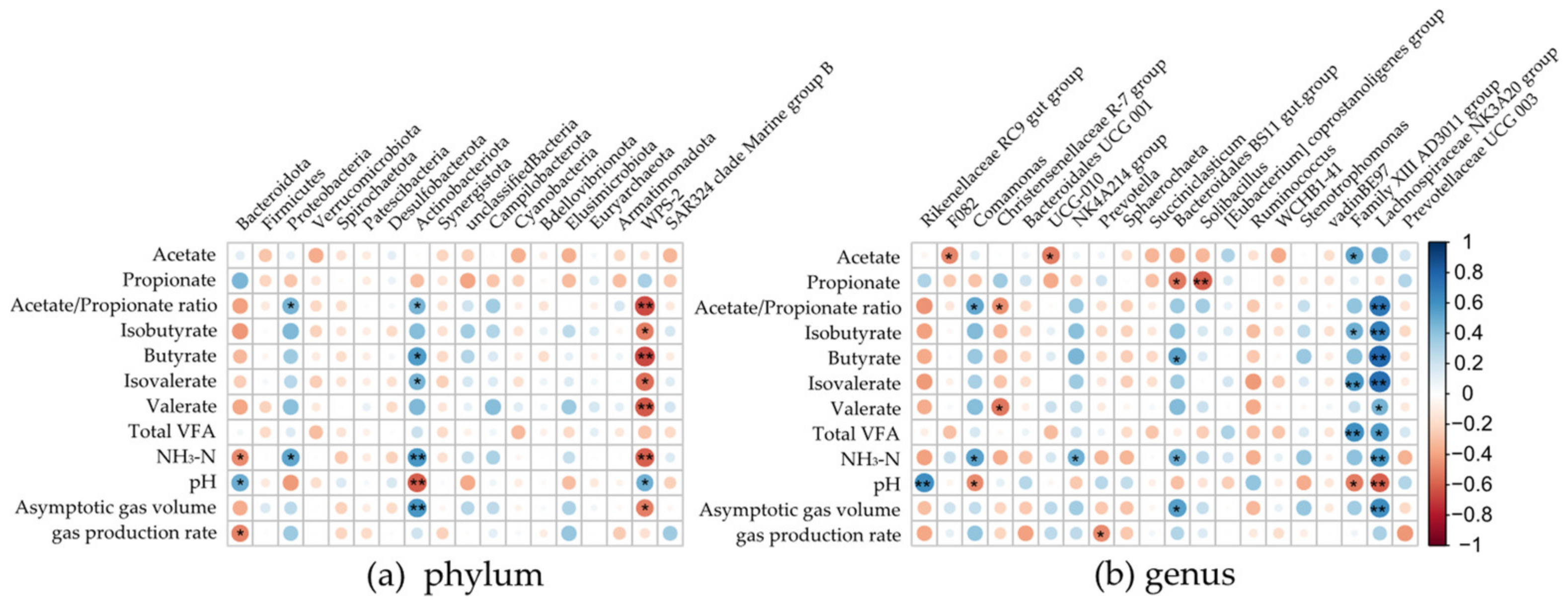
3.4. Correlation of Bacterial Taxa with In Vitro Gas Production and Fermentation Parameters
3.5. Metabolic Pathways Predicted from Bacterial Communities in In Vitro Rumen Cultures with 5-ALA Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suresh, G.; Das, R.K.; Kaur, B.S.; Rouissi, T.; Avalos, R.A.; Chorfi, Y.; Godbout, S. Alternatives to antibiotics in poultry feed: Molecular perspectives. Crit. Rev. Microbiol. 2018, 44, 318–335. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. Molecular nutrition: Interaction of nutrients, gene regulations and performances. Anim. Sci. J. 2016, 87, 857–862. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Zhu, F.; Li, Z.; Lu, N.; Li, Y.; Xu, Q.; Chen, N. Metabolic engineering of an auto-regulated Corynebacterium glutamicum chassis for biosynthesis of 5-aminolevulinic acid. Bioresour. Technol. 2020, 318, 124064. [Google Scholar] [CrossRef]
- Bryant, D.A.; Hunter, C.N.; Warren, M.J. Biosynthesis of the modified tetrapyrroles—The pigments of life. J. Biol. Chem. 2020, 295, 6888–6925. [Google Scholar] [CrossRef] [PubMed]
- Kornitzer, D.; Roy, U. Pathways of heme utilization in fungi. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118817. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.E.; MacMillan, L.; Stevens, J.R.; Brosnan, J.T. Division of labour: How does folate metabolism partition between one-carbon metabolism and amino acid oxidation? Biochem. J. 2015, 472, 135–146. [Google Scholar] [CrossRef]
- Shima, S. The Biological Methane-Forming Reaction: Mechanism Confirmed Through Spectroscopic Characterization of a Key Intermediate. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 13648–13649. [Google Scholar] [CrossRef]
- Sato, K.; Matsushita, K.; Takahashi, K.; Aoki, M.; Fuziwara, J.; Miyanari, S.; Kamada, T. Dietary supplementation with 5-aminolevulinic acid modulates growth performance and inflammatory responses in broiler chickens. Poult. Sci. 2012, 91, 1582–1589. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Shirai, M.; Takeya, H.; Sugimura, S.; Miyanari, S.; Taniguchi, S.; Sato, K. Effects of 5-aminolevulinic acid supplementation on milk production, iron status, and immune response of dairy cows. J. Dairy Sci. 2019, 102, 11009–11015. [Google Scholar] [CrossRef]
- Wang, J.P.; Jung, J.H.; Kim, I.H. Effects of dietary supplementation with delta-aminolevulinic acid on growth performance, hematological status, and immune responses of weanling pigs. Livest. Sci. 2011, 140, 131–135. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kim, I.H.; Cho, J.H.; Min, B.J.; Yoo, J.S.; Wang, Q. Effect of δ-aminolevulinic acid on growth performance, nutrient digestibility, blood parameters and the immune response of weanling pigs challenged with Escherichia coli lipopolysaccharide. Livest. Sci. 2008, 114, 108–116. [Google Scholar] [CrossRef]
- Chang, M.; Li, M.; Li, M.; Xie, Y.; Li, Y.; Yang, W.; Gao, Z. Changes of gut microbiota in pregnant sows induced by 5-Aminolevulinic acid. Res. Vet. Sci. 2021, 136, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Park, J.W.; Kim, I.H. δ-Aminolevulinic acid, and lactulose supplements in weaned piglets diet: Effects on performance, fecal microbiota, and in-vitro noxious gas emissions. Livest. Sci. 2016, 183, 84–91. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Yawei, Z.; Boya, T.; Yuehong, W.; Qiang, L.; Yuanqing, Z. Simultaneous Determination of 8 Short-Chain Organic Acids Concentration in Gastrointestinal Content by High Performance Liquid Chromatography with a Ligand-Exchange Column. Chin. J. Anim. Nutr. 2022, 34, 7411–7420. [Google Scholar] [CrossRef][Green Version]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In vitro Media1. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, X.; Zhang, Y.; Zhang, Y.; Zhang, Y. Amplicon Sequencing Analysis is Based on the QIIME2 Platform Evaluates the Difference in Anaerobic Fungal Structure between Ruminal and Rectal Content of Beef Cattle. Chin. J. Anim. Nutr. 2022, 34, 5107–5115. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Kamra, D.N.; Saha, S.; Bhatt, N.; Chaudhary, L.C.; Agarwal, N. Effect of Diet on Enzyme Profile, Biochemical Changes and In sacco Degradability of Feeds in the Rumen of Buffalo. Asian Austral. J. Anim. 2003, 16, 374–379. [Google Scholar] [CrossRef]
- Blümmel, M.; Steingaβ, H.; Becker, K. The relationship betweenin vitro gas production, in vitro microbial biomass yield and15 N incorporation and its implications for the prediction of voluntary feed intake of roughages. Brit. J. Nutr. 1997, 77, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Ferret, A.; Devant, M. Effects of pH and pH fluctuations on microbial fermentation and nutrient flow from a dual-flow continuous culture system. J. Dairy Sci. 2002, 85, 574–579. [Google Scholar] [CrossRef]
- Abuelfatah, K.; Zuki, A.B.; Goh, Y.M.; Sazili, A.Q.; Abubakr, A. Effects of feeding whole linseed on ruminal fatty acid composition and microbial population in goats. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2016, 2, 323–328. [Google Scholar] [CrossRef]
- Bergman, E.N.; Reid, R.S.; Murray, M.G.; Brockway, J.M.; Whitelaw, F.G. Interconversions and production of volatile fatty acids in the sheep rumen. Biochem. J. 1965, 97, 53–58. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Huan, L.; Xianghui, Z.; Lanjiao, X.; Luhua, W.; Zuodong, C.; Mingren, Z.E.Q. Effects of Nicitinic Acid, Fructooligosaccharides and Rare Earth Citrate Combinations on in vitro Rumen Fermentation Characteristics and Bacterial Community of Beef Cattle. Chin. J. Anim. Nutr. 2018, 30, 1996–2005. [Google Scholar] [CrossRef]
- Jing, W.; Shou-peng, F.; Ju-xiong, L. Research Progress on the Roles of β-hydroxybutyric Acid Played in Central Nervous System and Mechanism. China Anim. Husb. Vet. Med. 2012, 39, 106–110. [Google Scholar] [CrossRef]
- Solden, L.M.; Hoyt, D.W.; Collins, W.B.; Plank, J.E.; Daly, R.A.; Hildebrand, E.; Beavers, T.J.; Wolfe, R.; Nicora, C.D.; Purvine, S.O.; et al. New roles in hemicellulosic sugar fermentation for the uncultivated Bacteroidetes family BS11. ISME J. 2017, 11, 691–703. [Google Scholar] [CrossRef]
- Kaminsky, R.A.; Reid, P.M.; Altermann, E.; Kenters, N.; Kelly, W.J.; Noel, S.J.; Attwood, G.T.; Janssen, P.H. Rumen Lachnospiraceae isolate NK3A20 exhibits metabolic flexibility in response to substrate and coculture with a methanogen. Appl. Environ. Microb. 2023, 89, e00634-23. [Google Scholar] [CrossRef] [PubMed]
- Shengnan, L.; Chuxin, K.; He, H.; Jiaqi, G.; Lina, Z.; Bailiang, L.; Guicheng, H. Butyrate-producing bacteria in the intestinal tract and the physiological function of their metabolite butyrate: A review. Microbiol. China 2021, 48, 948–959. [Google Scholar]
- Chen, J.; Chen, Z.; Wang, Z.; Zheng, A.; Chang, W.; Cai, H.; Liu, G. Dietary 5-aminolevulinic acid supplementation improves growth performance, nutrient utilisation, iron status and antioxidant capacity of broilers. Ital. J. Anim. Sci. 2022, 21, 445–454. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K.; Raatikainen, K.; Holder, V.; Moran, C.A. Conversion of Branched-Chain Amino Acids to Corresponding Isoacids—An in vitro Tool for Estimating Ruminal Protein Degradability. Front. Vet. Sci. 2019, 6, 311. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic Alterations in Yak Rumen Bacteria Community and Metabolome Characteristics in Response to Feed Type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef]
- Hess, M.; Sczyrba, A.; Egan, R.; Kim, T.W.; Chokhawala, H.; Schroth, G.; Luo, S.; Clark, D.S.; Chen, F.; Zhang, T.; et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 2011, 331, 463–467. [Google Scholar] [CrossRef]
- Hook, S.E.; Steele, M.A.; Northwood, K.S.; Dijkstra, J.; France, J.; Wright, A.D.; McBride, B.W. Impact of subacute ruminal acidosis (SARA) adaptation and recovery on the density and diversity of bacteria in the rumen of dairy cows. Fems Microbiol. Ecol. 2011, 78, 275–284. [Google Scholar] [CrossRef]
- Su, X.L.; Tian, Q.; Zhang, J.; Yuan, X.Z.; Shi, X.S.; Guo, R.B.; Qiu, Y.L. Acetobacteroides hydrogenigenes gen. nov., sp. nov., an anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Micr. 2014, 64, 2986–2991. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, L.; Zhang, Y. Research progress on the genus Cellulomonas. Acta Microbiol. Sin. 2019, 59, 1–10. [Google Scholar]
- Fukuda, S.; Toh, H.; Taylor, T.D.; Ohno, H.; Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 2012, 3, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Woo, P.C.Y.; Woo, G.K.S.; Fung, A.M.Y.; Wong, M.K.M.; Chan, K.; Tam, D.M.W.; Yuen, K. Eggerthella hongkongensis sp. nov. and Eggerthella sinensis sp. nov., two novel Eggerthella species, account for half of the cases of Eggerthella bacteremia. Diagn. Micr. Infec. Dis. 2004, 49, 255–263. [Google Scholar] [CrossRef]
- Cavalieri, S.J.; Knoop, F.C. Corynebacterium Infections. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–5. [Google Scholar]
- Kraatz, M.; Wallace, R.J.; Svensson, L. Olsenella umbonata sp. nov., a microaerotolerant anaerobic lactic acid bacterium from the sheep rumen and pig jejunum, and emended descriptions of Olsenella, Olsenella uli and Olsenella profusa. Int. J. Syst. Evol. Micr. 2011, 61, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Mendling, W.; Palmeira-de-Oliveira, A.; Biber, S.; Prasauskas, V. An update on the role of Atopobium vaginae in bacterial vaginosis: What to consider when choosing a treatment? A mini review. Arch. Gynecol. Obstet. 2019, 300, 1–6. [Google Scholar] [CrossRef]
- Sheremet, A.; Jones, G.M.; Jarett, J.; Bowers, R.M.; Bedard, I.; Culham, C.; Eloe Fadrosh, E.A.; Ivanova, N.; Malmstrom, R.R.; Grasby, S.E.; et al. and genomic analyses of candidate phylum WPS-2 bacteria in an unvegetated soil. Environ. Microbiol. 2020, 22, 3143–3157. [Google Scholar] [CrossRef]



| Items | Groups 1 | SEM 2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ALA0 | ALA100 | ALA500 | ALA1000 | ALA5000 | Group | Linear | Quadric | ||
| b 3, mL | 66.10 a | 64.46 ab | 62.70 bc | 61.54 bc | 59.58 c | 0.669 | <0.01 | <0.01 | <0.01 |
| c 4, mL/h | 0.071 | 0.069 | 0.065 | 0.066 | 0.067 | 0.001 | 0.32 | 0.58 | 0.16 |
| DMD, % | 72.23 c | 72.77 bc | 73.80 bc | 75.59 a | 74.39 ab | 0.353 | <0.01 | 0.10 | <0.01 |
| Items | Groups 1 | SEM 2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ALA0 | ALA100 | ALA500 | ALA1000 | ALA5000 | Group | Linear | Quadric | ||
| pH | 6.69 b | 6.70 b | 6.72 b | 6.89 a | 6.82 a | 0.021 | <0.01 | 0.04 | <0.01 |
| Acetate, mmol/L | 50.05 | 50.32 | 50.35 | 48.72 | 50.64 | 0.280 | 0.29 | 0.45 | 0.18 |
| Propionate, mmol/L | 15.28 | 15.58 | 15.71 | 15.74 | 16.06 | 0.106 | 0.17 | 0.03 | 0.55 |
| Acetate/Propionate ratio | 3.28 a | 3.23 ab | 3.20 ab | 3.09 c | 3.15 bc | 0.018 | <0.01 | 0.07 | <0.01 |
| Butyrate, mmol/L | 7.12 a | 6.94 a | 6.48 b | 5.95 c | 5.92 c | 0.129 | <0.01 | <0.01 | <0.01 |
| Isobutyrate, mmol/L | 0.88 a | 0.88 a | 0.86 a | 0.79 b | 0.76 b | 0.013 | <0.01 | <0.01 | <0.01 |
| Valerate, mmol/L | 1.13 a | 1.13 a | 1.06 ab | 1.02 b | 1.02 b | 0.015 | 0.01 | 0.02 | <0.01 |
| Isovalerate, mmol/L | 1.78 a | 1.76 a | 1.72 ab | 1.61b | 1.62 b | 0.023 | 0.02 | 0.02 | <0.01 |
| Total VFA 3, mmol/L | 76.24 | 76.61 | 76.18 | 73.84 | 76.02 | 0.405 | 0.27 | 0.88 | 0.13 |
| NH3-N, mg/dL | 22.20 a | 21.67 ab | 20.48 b | 18.08 c | 16.67 c | 0.570 | <0.01 | <0.01 | <0.01 |
| Items | Groups 1 | SEM 2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ALA0 | ALA100 | ALA500 | ALA1000 | ALA5000 | Group | Linear | Quadric | ||
| Observed features | 1261 | 1269 | 1357 | 1238 | 1246 | 26.44 | 0.67 | 0.60 | 0.87 |
| Shannon index | 8.47 | 8.76 | 8.76 | 8.91 | 8.57 | 0.083 | 0.50 | 0.64 | 0.27 |
| Pielou evenness | 0.82 | 0.85 | 0.84 | 0.87 | 0.83 | 0.007 | 0.25 | 0.72 | 0.16 |
| Faith PD | 102.85 | 104.12 | 107.60 | 100.51 | 97.17 | 1.654 | 0.36 | 0.10 | 0.27 |
| Simpson index | 0.98 | 0.99 | 0.99 | 0.99 | 0.98 | 0.002 | 0.36 | 0.96 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Guo, Z.; Zhang, N.; Wang, J.; Xu, J.; Zhang, W.; Liu, Q.; Wang, C.; Zhang, Y.; Zhang, Y. Effects of 5-Aminolevulinic Acid Supplementation on Gas Production, Fermentation Characteristics, and Bacterial Community Profiles In Vitro. Microorganisms 2024, 12, 1867. https://doi.org/10.3390/microorganisms12091867
Hao Z, Guo Z, Zhang N, Wang J, Xu J, Zhang W, Liu Q, Wang C, Zhang Y, Zhang Y. Effects of 5-Aminolevulinic Acid Supplementation on Gas Production, Fermentation Characteristics, and Bacterial Community Profiles In Vitro. Microorganisms. 2024; 12(9):1867. https://doi.org/10.3390/microorganisms12091867
Chicago/Turabian StyleHao, Zhenkai, Zhuangzhuang Guo, Ning Zhang, Jing Wang, Jiabao Xu, Weiyu Zhang, Qiang Liu, Cong Wang, Yawei Zhang, and Yuanqing Zhang. 2024. "Effects of 5-Aminolevulinic Acid Supplementation on Gas Production, Fermentation Characteristics, and Bacterial Community Profiles In Vitro" Microorganisms 12, no. 9: 1867. https://doi.org/10.3390/microorganisms12091867
APA StyleHao, Z., Guo, Z., Zhang, N., Wang, J., Xu, J., Zhang, W., Liu, Q., Wang, C., Zhang, Y., & Zhang, Y. (2024). Effects of 5-Aminolevulinic Acid Supplementation on Gas Production, Fermentation Characteristics, and Bacterial Community Profiles In Vitro. Microorganisms, 12(9), 1867. https://doi.org/10.3390/microorganisms12091867





