Effect of the Bioprotective Properties of Lactic Acid Bacteria Strains on Quality and Safety of Feta Cheese Stored under Different Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Microbial Cultures Preparation
2.3. Na-Alginate Edible Film Preparation
2.4. Inoculation of Feta Cheese Samples and Packaging of the Cheeses
2.5. Microbiological Analysis
2.6. pH and aw Measurements
2.7. Sensory Analysis
2.8. FTIR-ATR Spectroscopy
2.9. Image Acquisition and Segmentation
2.10. Data Analysis
2.11. Statistical Analysis
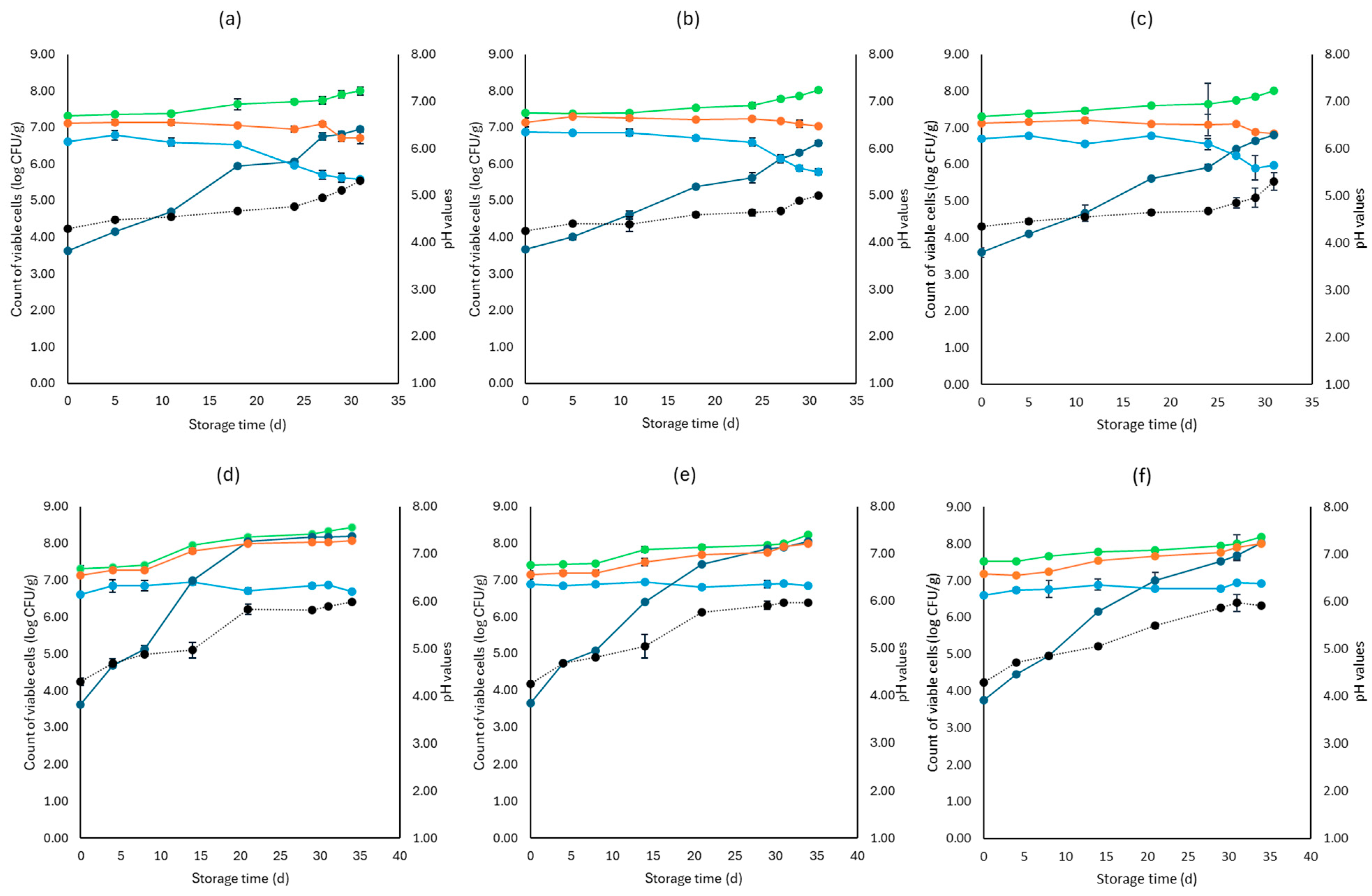
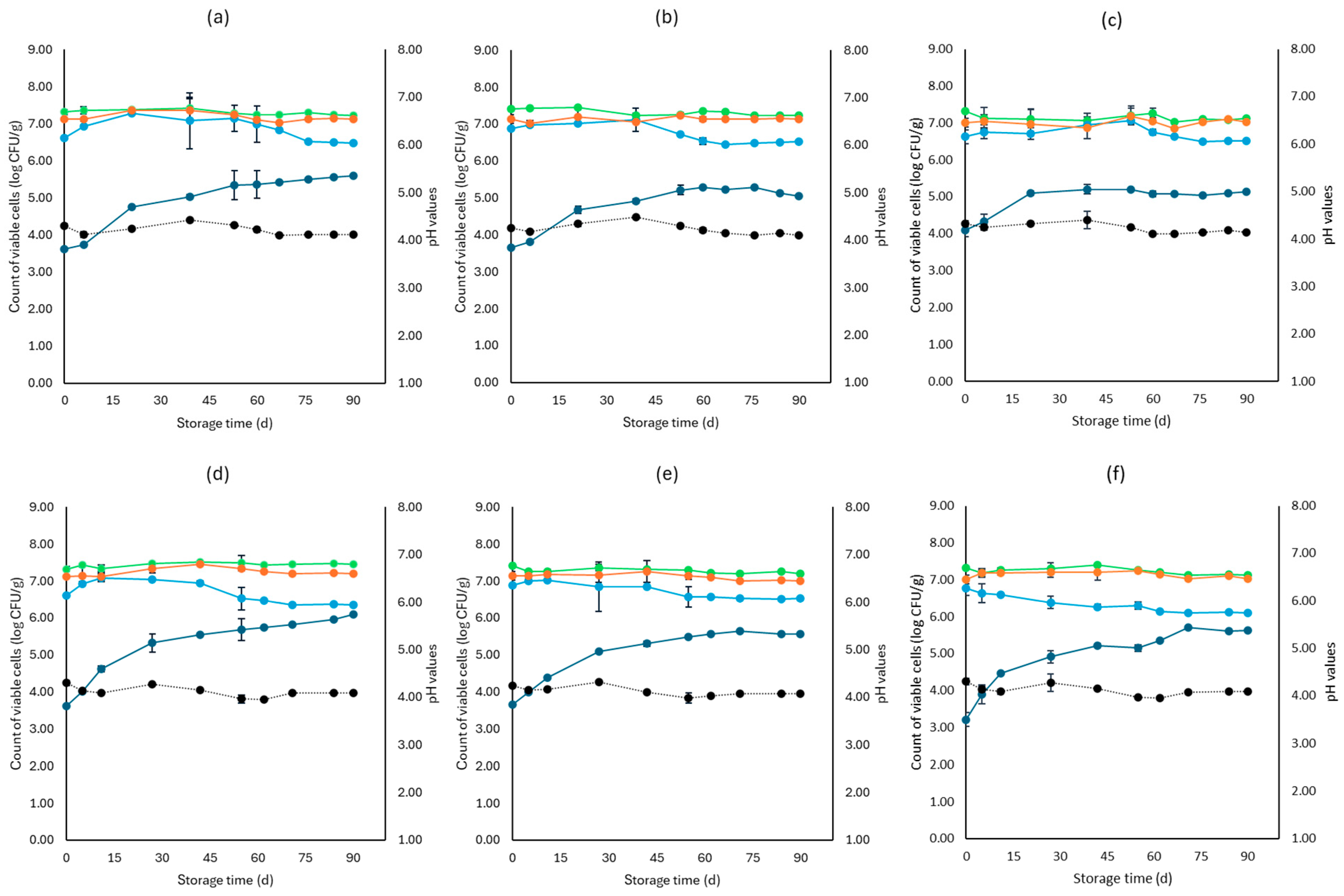
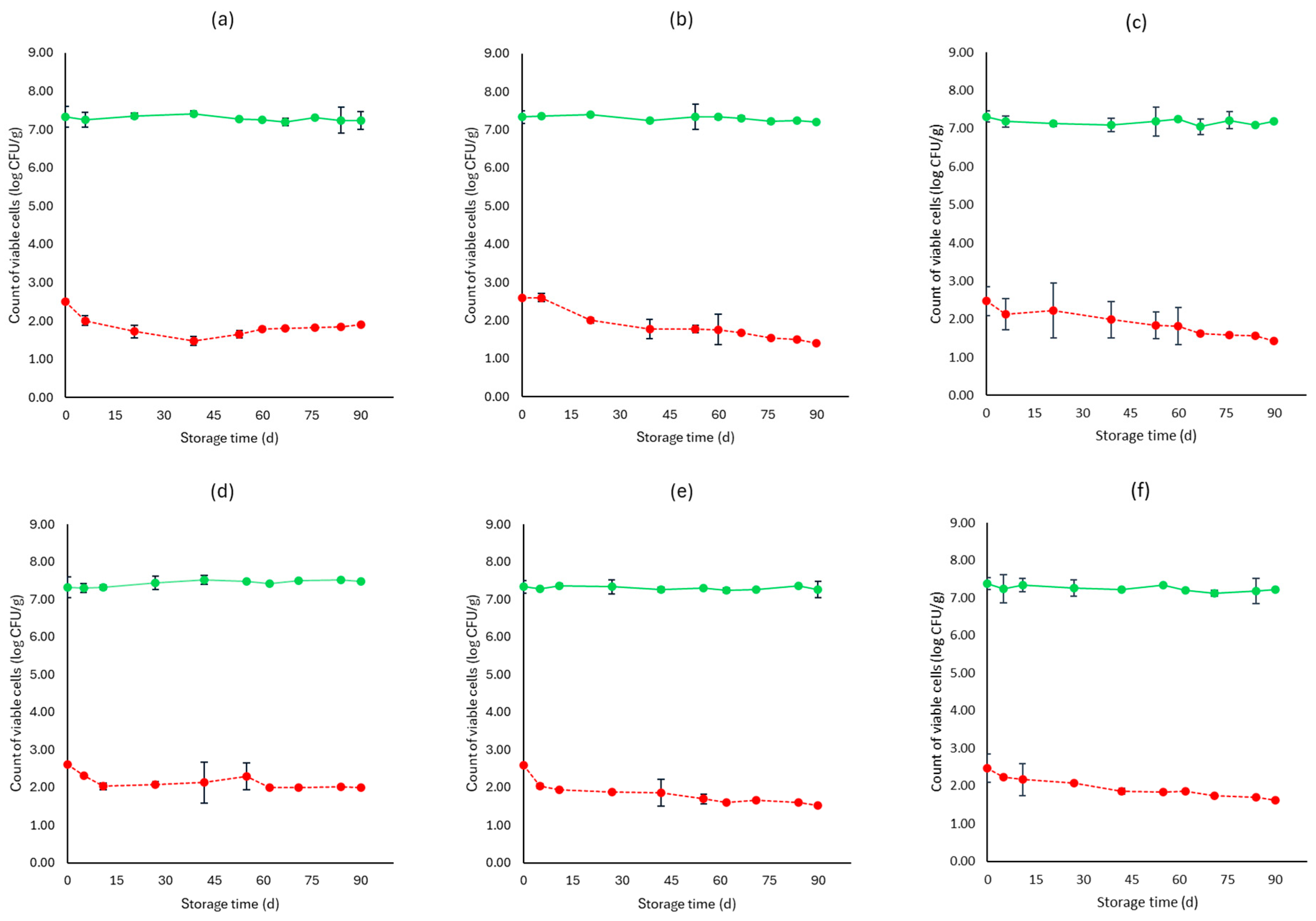
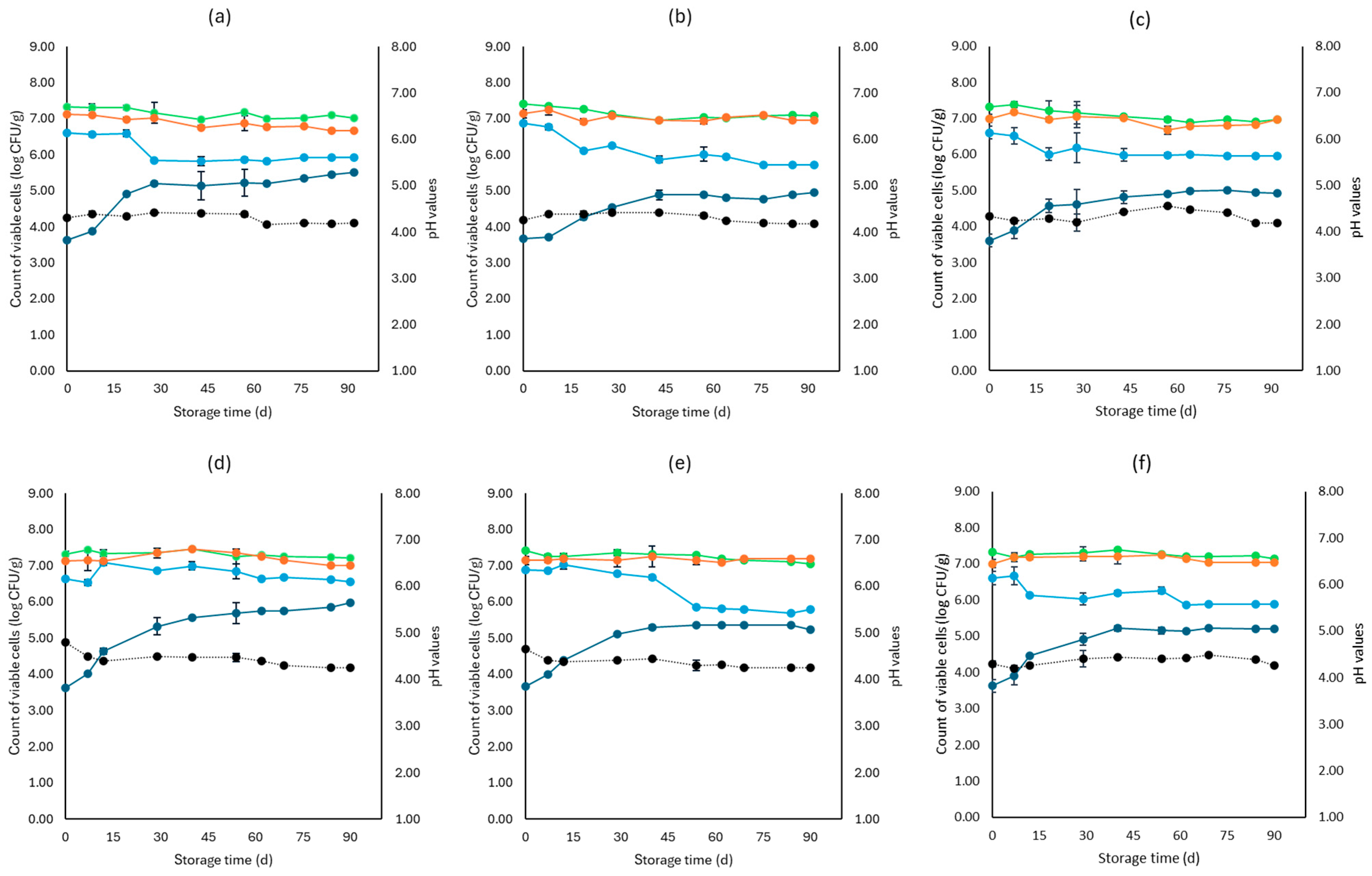
3. Results
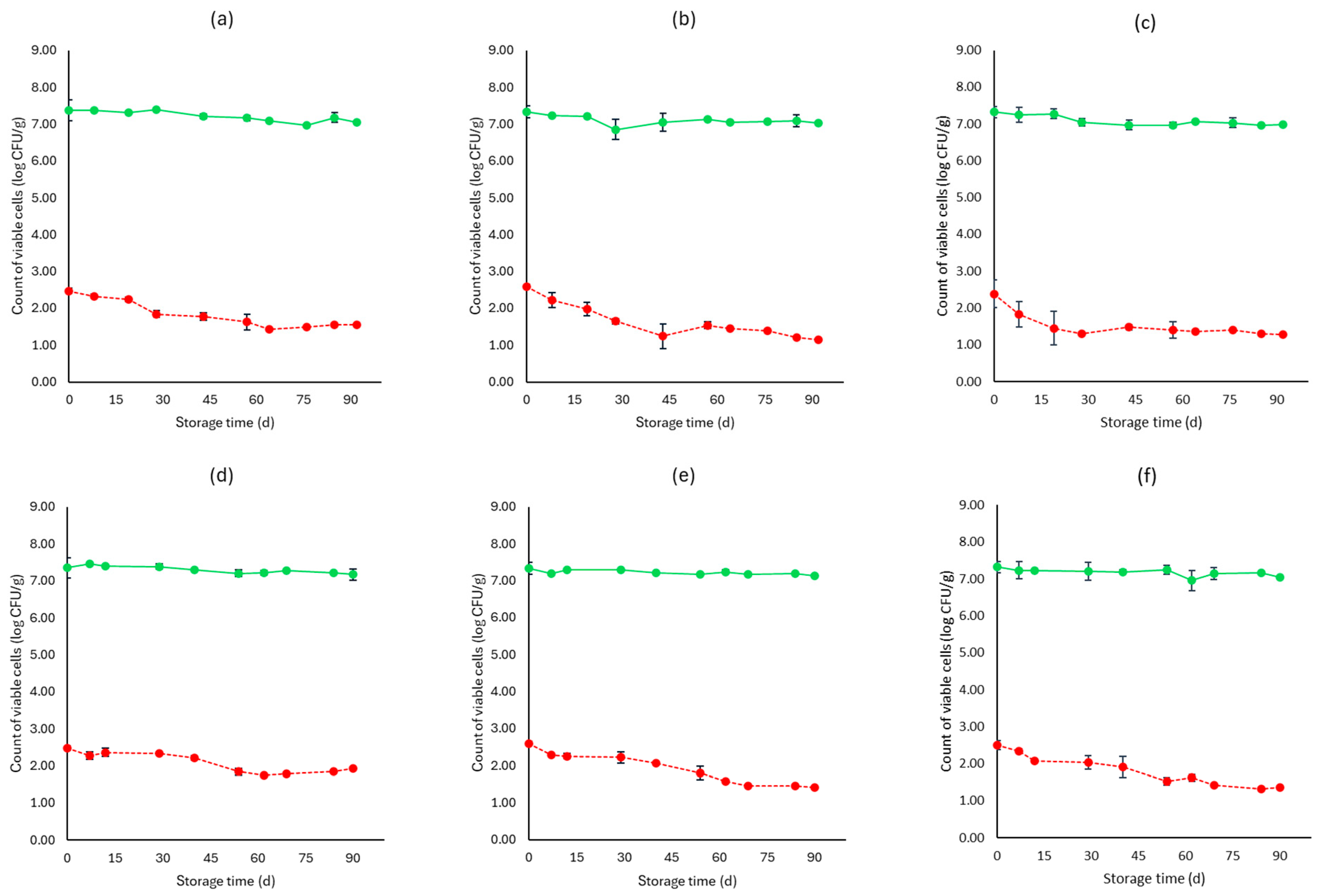
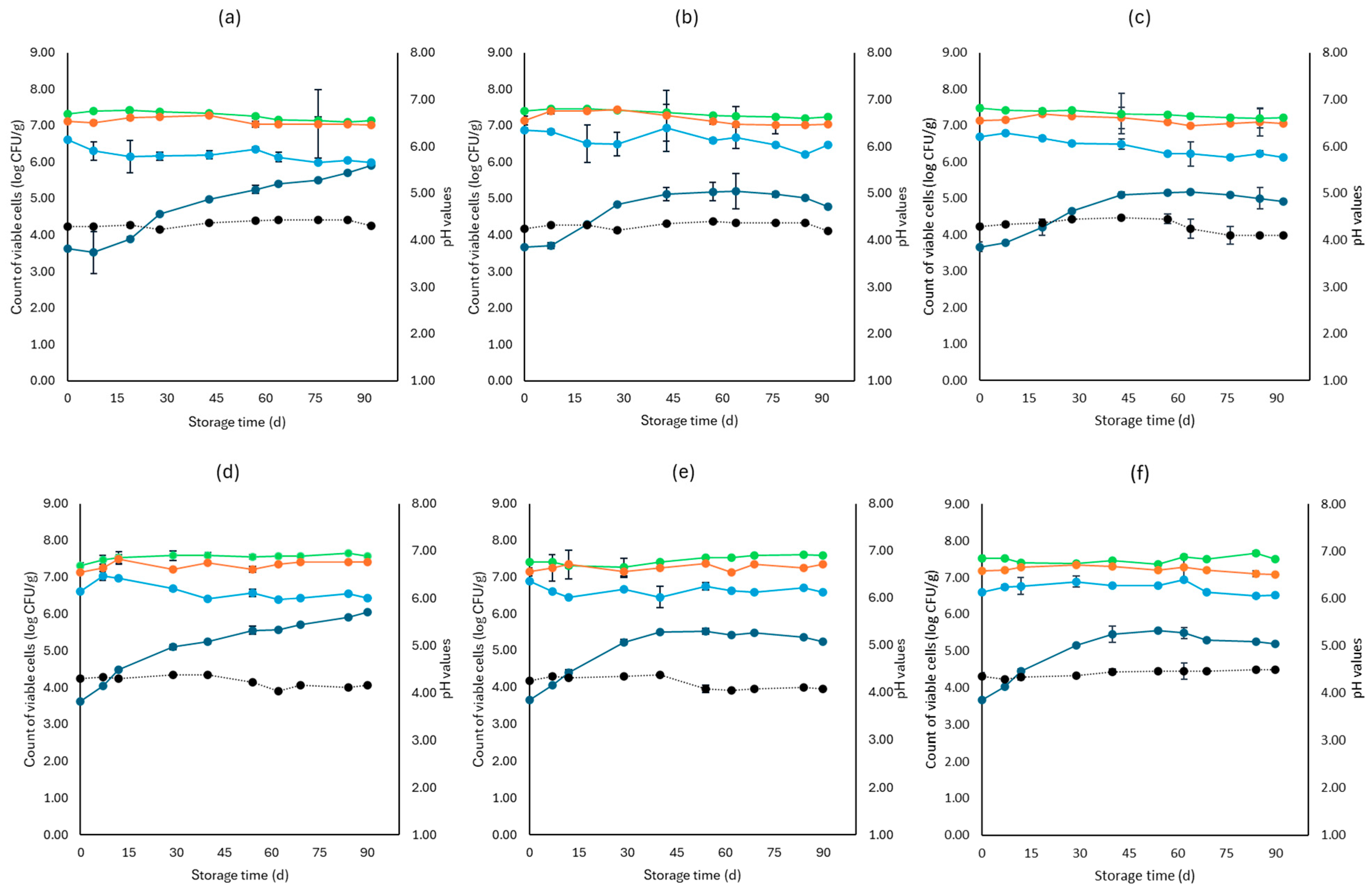
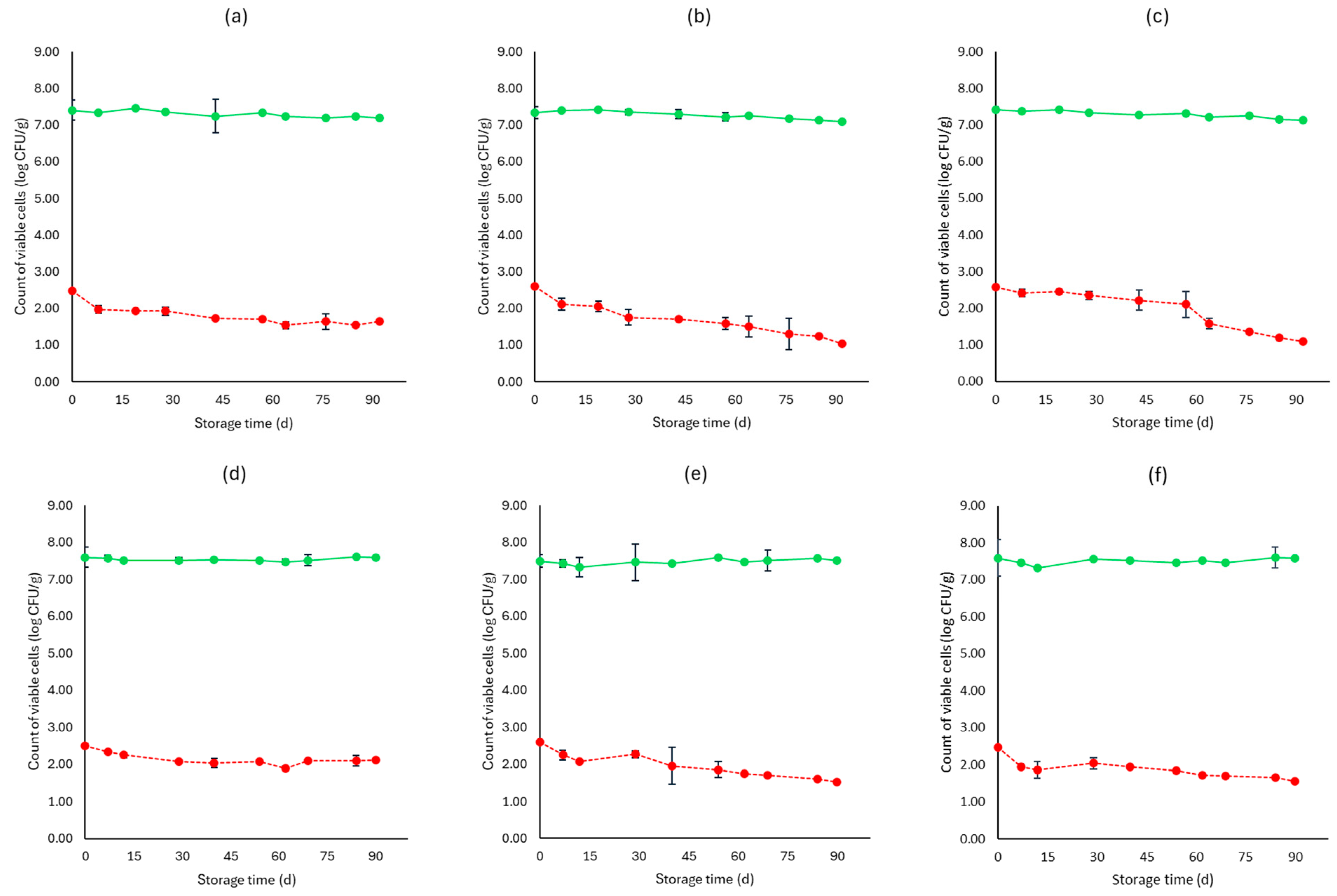
3.1. Microbiological Analyses, pH, and Water Activity (aw)
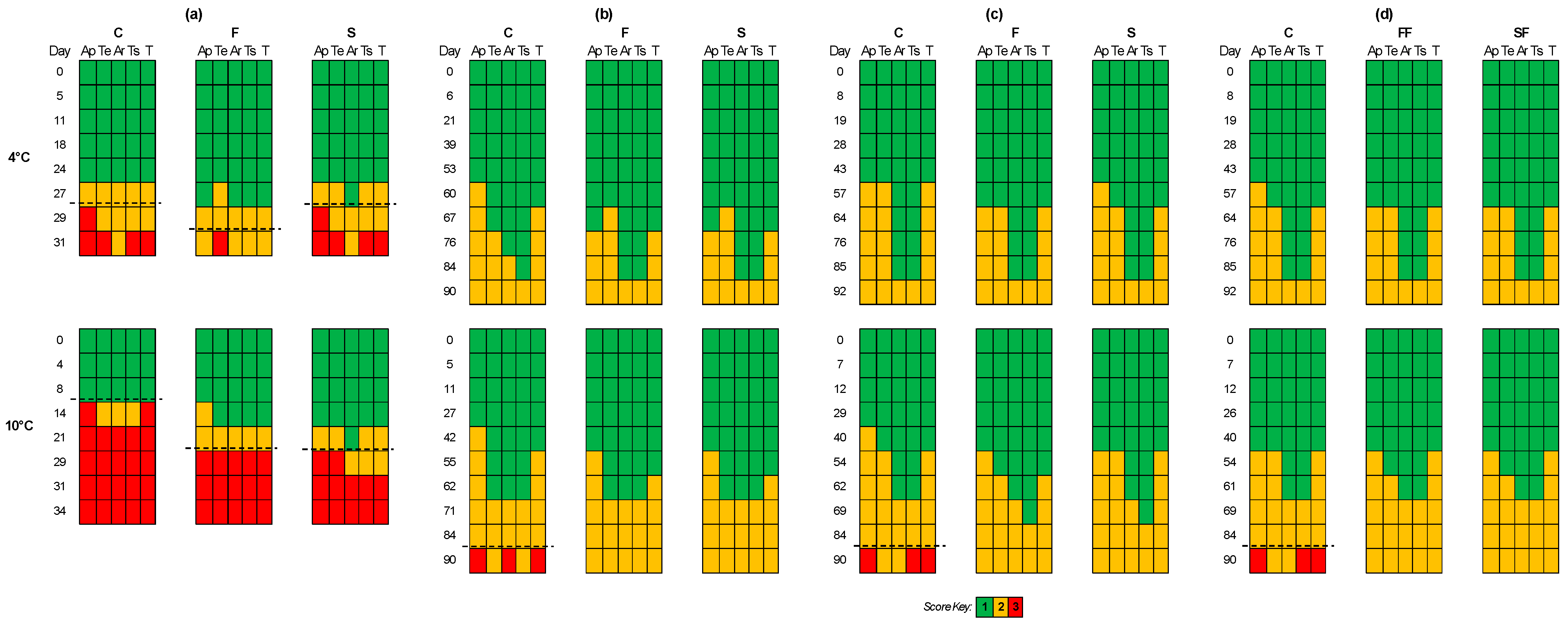
3.2. Sensory Evaluation
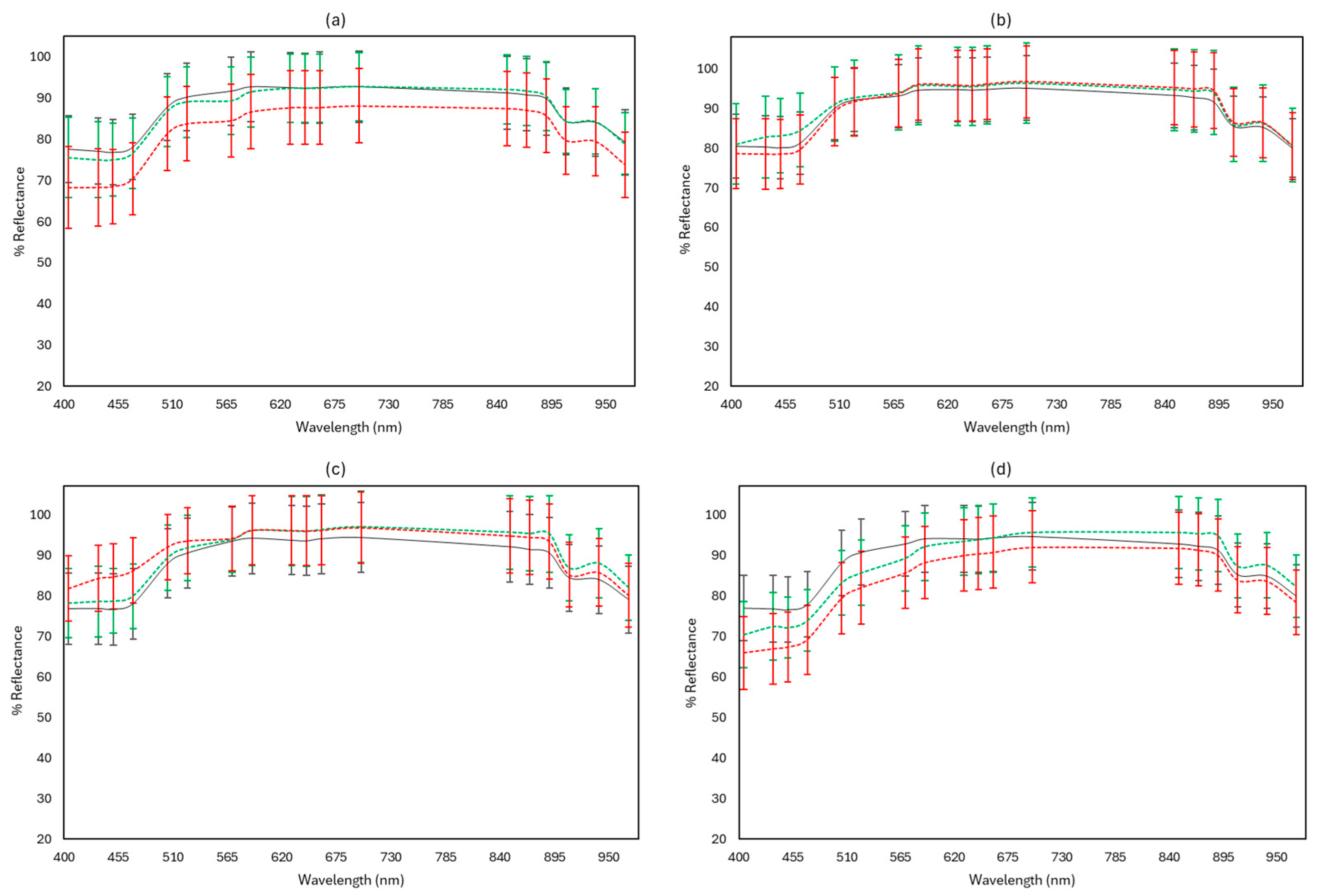
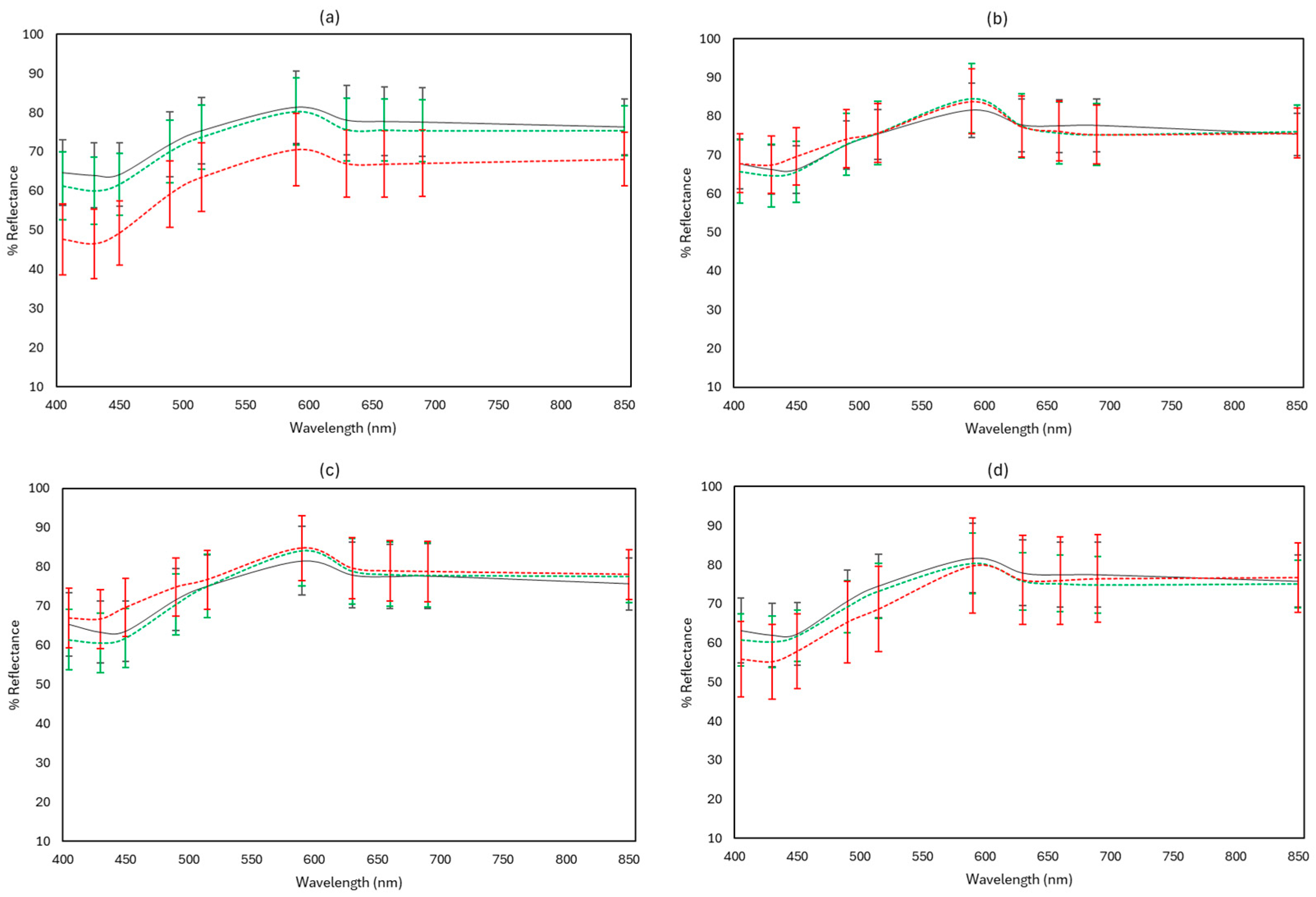
3.3. FTIR and MSI Spectral Data
3.4. Rapid Sensory Class Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Parliament and Council. Regulation (EC) No 1829/2002 Amending the Annex to Regulation (EC) No 1107/96 with Regard to the Name ‘Feta’; European Parliament: Brussels, Belgium, 2002.
- FAO. FAOSTAT; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/faostat/en/ (accessed on 18 December 2022).
- Papadopoulou, O.S.; Argyri, A.A.; Varzakis, E.E.; Tassou, C.C.; Chorianopoulos, N.G. Greek Functional Feta Cheese: Enhancing Quality and Safety Using a Lactobacillus Plantarum Strain with Probiotic Potential. Food Microbiol. 2018, 74, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Anifantakis, E.M.; Moatsou, G. Feta and Other Balkan Cheeses. In Brined Cheeses; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 43–76. [Google Scholar]
- Bintsis, T.; Papademas, P. Microbiological Quality of White-Brined Cheeses: A Review. Int. J. Dairy Technol. 2002, 55, 113–120. [Google Scholar] [CrossRef]
- Manolopoulou, E.; Sarantinopoulos, P.; Zoidou, E.; Aktypis, A.; Moschopoulou, E.; Kandarakis, I.G.; Anifantakis, E.M. Evolution of Microbial Populations during Traditional Feta Cheese Manufacture and Ripening. Int. J. Food Microbiol. 2003, 82, 153–161. [Google Scholar] [CrossRef]
- Pappas, C.P.; Kondyli, E.; Voutsinas, L.P.; Mallatou, H. Effects of Salting Method and Storage Time on Composition and Quality of Feta Cheese. Int. J. Dairy Technol. 1996, 49, 113–118. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Anastasiou, R.; Georgalaki, M.; Bounenni, R.; Paximadaki, A.; Charmpi, C.; Alexandraki, V.; Kazou, M.; Tsakalidou, E. Comparison of the Microbiome of Artisanal Homemade and Industrial Feta Cheese through Amplicon Sequencing and Shotgun Metagenomics. Microorganisms 2022, 10, 1073. [Google Scholar] [CrossRef]
- Tzanetakis, N.; Litopoulou-Tzanetaki, E. Changes in Numbers and Kinds of Lactic Acid Bacteria in Feta and Teleme, Two Greek Cheeses from Ewes’ Milk. J. Dairy Sci. 1992, 75, 1389–1393. [Google Scholar] [CrossRef]
- Rantsiou, K.; Urso, R.; Dolci, P.; Comi, G.; Cocolin, L. Microflora of Feta Cheese from Four Greek Manufacturers. Int. J. Food Microbiol. 2008, 126, 36–42. [Google Scholar] [CrossRef]
- Nacef, M.; Lelièvre-Desmas, M.; Drider, D.; Flahaut, C.; Chollet, S. Artisanal and Industrial Maroilles Cheeses: Are They Different? Comparison Using Sensory, Physico-Chemical and Microbiological Approaches. Int. Dairy J. 2019, 89, 42–52. [Google Scholar] [CrossRef]
- Tzora, A.; Nelli, A.; Voidarou, C.; Fthenakis, G.; Rozos, G.; Theodorides, G.; Bonos, E.; Skoufos, I. Microbiota “Fingerprint” of Greek Feta Cheese through Ripening. Appl. Sci. 2021, 11, 5631. [Google Scholar] [CrossRef]
- Turbes, G.; Linscott, T.D.; Tomasino, E.; Waite-Cusic, J.; Lim, J.; Meunier-Goddik, L. Evidence of Terroir in Milk Sourcing and Its Influence on Cheddar Cheese. J. Dairy Sci. 2016, 99, 5093–5103. [Google Scholar] [CrossRef]
- Fadda, M.E.; Cosentino, S.; Deplano, M.; Palmas, F. Yeast Populations in Sardinian Feta Cheese. Int. J. Food Microbiol. 2001, 69, 153–156. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Gonzales-Barron, U.; Pilão Cadavez, V.A.; Sant’Ana, A.S.; Schaffner, D.W. Quantitative Risk Assessment of Listeria Monocytogenes in Traditional Minas Cheeses: The Cases of Artisanal Semi-Hard and Fresh Soft Cheeses. Food Control 2018, 92, 370–379. [Google Scholar] [CrossRef]
- Cadavez, V.A.P.; Campagnollo, F.B.; Silva, R.A.; Duffner, C.M.; Schaffner, D.W.; Sant’Ana, A.S.; Gonzales-Barron, U. A Comparison of Dynamic Tertiary and Competition Models for Describing the Fate of Listeria Monocytogenes in Minas Fresh Cheese during Refrigerated Storage. Food Microbiol. 2019, 79, 48–60. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Gkerekou, M.A.; Vitzilaiou, E.S.; Skandamis, P.N. Assessing the Capacity of Growth, Survival, and Acid Adaptive Response of Listeria Monocytogenes during Storage of Various Cheeses and Subsequent Simulated Gastric Digestion. Int. J. Food Microbiol. 2017, 246, 50–63. [Google Scholar] [CrossRef]
- RASFF. The Rapid Alert System for Food and Feed. 2022. Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/582129 (accessed on 6 September 2024).
- Leroy, F.; De Vuyst, L. Lactic Acid Bacteria as Functional Starter Cultures for the Food Fermentation Industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Morales-Camacho, J.I.; Mani-López, E.; López-Malo, A. Assessment of Antifungal Activity of Aqueous Extracts and Protein Fractions from Sourdough Fermented by Lactiplantibacillus Plantarum. Future Foods 2024, 9, 100314. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; López-Malo, A.; Mani-López, E. Antimicrobial Activity and Applications of Fermentates from Lactic Acid Bacteria—A Review. Sustain. Food Technol. 2024, 2, 292–306. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; Palou, E.; López-Malo, A. Sourdoughs as Natural Enhancers of Bread Quality and Shelf Life: A Review. Fermentation 2023, 10, 7. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics Produced by Lactic Acid Bacteria: The next Frontier in Food Safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The Impacts of Antimicrobial and Antifungal Activity of Cell-Free Supernatants from Lactic Acid Bacteria in Vitro and Foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Dal Bello, B.; Cocolin, L.; Zeppa, G.; Field, D.; Cotter, P.D.; Hill, C. Technological Characterization of Bacteriocin Producing Lactococcus Lactis Strains Employed to Control Listeria Monocytogenes in Cottage Cheese. Int. J. Food Microbiol. 2012, 153, 58–65. [Google Scholar] [CrossRef]
- Settanni, L.; Franciosi, E.; Cavazza, A.; Cocconcelli, P.S.; Poznanski, E. Extension of Tosèla Cheese Shelf-Life Using Non-Starter Lactic Acid Bacteria. Food Microbiol. 2011, 28, 883–890. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of Lactic Acid Bacteria: Extending the Family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Rhoades, J.; Kargiotou, C.; Katsanidis, E.; Koutsoumanis, K.P. Use of Marination for Controlling Salmonella Enterica and Listeria Monocytogenes in Raw Beef. Food Microbiol. 2013, 36, 248–253. [Google Scholar] [CrossRef]
- Alvarado, C.; McKee, S. Marination to Improve Functional Properties and Safety of Poultry Meat. J. Appl. Poult. Res. 2007, 16, 113–120. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Shi, G.; Chang, J.; Liu, Z.; Zeng, M. Cooperation of Lactic Acid Bacteria Regulated by the AI-2/LuxS System Involve in the Biopreservation of Refrigerated Shrimp. Food Res. Int. 2019, 120, 679–687. [Google Scholar] [CrossRef]
- Lee, K.J.; Park, H.W.; Choi, E.J.; Chun, H.H. Effects of CFSs Produced by Lactic Acid Bacteria in Combination with Grape Seed Extract on the Microbial Quality of Ready-To-Eat Baby Leaf Vegetables. Cogent Food Agric. 2016, 2. [Google Scholar] [CrossRef]
- Doukaki, A.; Papadopoulou, O.S.; Tzavara, C.; Mantzara, A.-M.; Michopoulou, K.; Tassou, C.; Skandamis, P.; Nychas, G.-J.; Chorianopoulos, N. Monitoring the Bioprotective Potential of Lactiplantibacillus Pentosus Culture on Pathogen Survival in and the Shelf-Life of Fresh Ready-To-Eat Salads Stored under Modified Atmosphere Packaging. Pathogens 2024, 13, 557. [Google Scholar] [CrossRef]
- Vera-Santander, V.E.; Hernández-Figueroa, R.H.; Arrioja-Bretón, D.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Utilization of Whey for Eco-Friendly Bio-Preservation of Mexican-Style Fresh Cheeses: Antimicrobial Activity of Lactobacillus Casei 21/1 Cell-Free Supernatants (CFS). Int. J. Environ. Res. Public Health 2024, 21, 560. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Panagou, E.Z.; Mohareb, F. Novel Approaches for Food Safety Management and Communication. Curr. Opin. Food Sci. 2016, 12, 13–20. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food Authentication: Techniques, Trends & Emerging Approaches. TrAC Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Hassoun, A.; Guðjónsdóttir, M.; Prieto, M.A.; Garcia-Oliveira, P.; Simal-Gandara, J.; Marini, F.; Di Donato, F.; D’Archivio, A.A.; Biancolillo, A. Application of Novel Techniques for Monitoring Quality Changes in Meat and Fish Products during Traditional Processing Processes: Reconciling Novelty and Tradition. Processes 2020, 8, 988. [Google Scholar] [CrossRef]
- G Pavli, F.; A Argyri, A.; Papadopoulou, O.S.; Nychas, G.-J.; Chorianopoulos, N.G.; Tassou, C.C. Probiotic Potential of Lactic Acid Bacteria from Traditional Fermented Dairy and Meat Products: Assessment by in Vitro Tests and Molecular Characterization. J. Probiotics Health 2016, 4, 10-4172. [Google Scholar] [CrossRef]
- Stergiou, O.S.; Tegopoulos, K.; Kiousi, D.E.; Tsifintaris, M.; Papageorgiou, A.C.; Tassou, C.C.; Chorianopoulos, N.; Kolovos, P.; Galanis, A. Whole-Genome Sequencing, Phylogenetic and Genomic Analysis of Lactiplantibacillus pentosus L33, a Potential Probiotic Strain Isolated from Fermented Sausages. Front. Microbiol. 2021, 12, 746659. [Google Scholar] [CrossRef]
- Tegopoulos, K.; Stergiou, O.S.; Kiousi, D.E.; Tsifintaris, M.; Koletsou, E.; Papageorgiou, A.C.; Argyri, A.A.; Chorianopoulos, N.; Galanis, A.; Kolovos, P. Genomic and Phylogenetic Analysis of Lactiplantibacillus plantarum L125, and Evaluation of Its Anti-Proliferative and Cytotoxic Activity in Cancer Cells. Biomedicines 2021, 9, 1718. [Google Scholar] [CrossRef]
- Pavli, F.; Kovaiou, I.; Apostolakopoulou, G.; Kapetanakou, A.; Skandamis, P.; Nychas, G.-J.; Tassou, C.; Chorianopoulos, N. Alginate-Based Edible Films Delivering Probiotic Bacteria to Sliced Ham Pretreated with High Pressure Processing. Int. J. Mol. Sci. 2017, 18, 1867. [Google Scholar] [CrossRef]
- Kamarinou, C.S.; Papadopoulou, O.S.; Doulgeraki, A.I.; Tassou, C.C.; Galanis, A.; Chorianopoulos, N.; Argyri, A.A. Application of Multi-Functional Lactic Acid Bacteria Strains in a Pilot Scale Feta Cheese Production. Front. Microbiol. 2023, 14, 1254598. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Papadopoulou, O.; Carstensen, J.M.; Nychas, G.-J.E. Potential of Multispectral Imaging Technology for Rapid and Non-Destructive Determination of the Microbiological Quality of Beef Filets during Aerobic Storage. Int. J. Food Microbiol. 2014, 174, 1–11. [Google Scholar] [CrossRef]
- Papadopoulou, O.S.; Argyri, A.A.; Kounani, V.; Tassou, C.C.; Chorianopoulos, N. Use of Fourier Transform Infrared Spectroscopy for Monitoring the Shelf Life and Safety of Yogurts Supplemented with a Lactobacillus Plantarum Strain with Probiotic Potential. Front. Microbiol. 2021, 12, 678356. [Google Scholar] [CrossRef]
- Sokolova, M.; Lapalme, G. A Systematic Analysis of Performance Measures for Classification Tasks. Inf. Process. Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: Paris, France, 2023; Available online: https://www.xlstat.com (accessed on 23 August 2024).
- Ellis, D.I.; Broadhurst, D.; Goodacre, R. Rapid and Quantitative Detection of the Microbial Spoilage of Beef by Fourier Transform Infrared Spectroscopy and Machine Learning. Anal. Chim. Acta 2004, 514, 193–201. [Google Scholar] [CrossRef]
- Chen, M.; Irudayaraj, J.; McMahon, D.J. Examination of Full Fat and Reduced Fat Cheddar Cheese during Ripening by Fourier Transform Infrared Spectroscopy. J. Dairy Sci. 1998, 81, 2791–2797. [Google Scholar] [CrossRef]
- Wu, Z.; Bertram, H.C.; Kohler, A.; Böcker, U.; Ofstad, R.; Andersen, H.J. Influence of Aging and Salting on Protein Secondary Structures and Water Distribution in Uncooked and Cooked Pork. A Combined FT-IR Microspectroscopy and 1H NMR Relaxometry Study. J. Agric. Food Chem. 2006, 54, 8589–8597. [Google Scholar] [CrossRef]
- Pappas, C.S.; Tarantilis, P.A.; Moschopoulou, E.; Moatsou, G.; Kandarakis, I.; Polissiou, M.G. Polissiou Identification and Differentiation of Goat and Sheep Milk Based on Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) Using Cluster Analysis. Food Chem. 2008, 106, 1271–1277. [Google Scholar] [CrossRef]
- Nicolaou, N.; Xu, Y.; Goodacre, R. Fourier Transform Infrared Spectroscopy and Multivariate Analysis for the Detection and Quantification of Different Milk Species. J. Dairy Sci. 2010, 93, 5651–5660. [Google Scholar] [CrossRef]
- Aernouts, B.; Polshin, E.; Saeys, W.; Lammertyn, J. Mid-Infrared Spectrometry of Milk for Dairy Metabolomics: A Comparison of Two Sampling Techniques and Effect of Homogenization. Anal. Chim. Acta 2011, 705, 88–97. [Google Scholar] [CrossRef]
- Ammor, M.S.; Argyri, A.; Nychas, G.-J.E. Rapid Monitoring of the Spoilage of Minced Beef Stored under Conventionally and Active Packaging Conditions Using Fourier Transform Infrared Spectroscopy in Tandem with Chemometrics. Meat Sci. 2009, 81, 507–514. [Google Scholar] [CrossRef]
- Izco, J.M.; Tormo, M.; Jiménez-Flores, R. Rapid Simultaneous Determination of Organic Acids, Free Amino Acids, and Lactose in Cheese by Capillary Electrophoresis. J. Dairy Sci. 2002, 85, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Skeie, S.; Lindberg, C.; Narvhus, J. Development of Amino Acids and Organic Acids in Norvegia, Influence of Milk Treatment and Adjunct Lactobacillus. Int. Dairy J. 2001, 11, 399–411. [Google Scholar] [CrossRef]
- Manolaki, P.; Katsiari, M.C.; Alichanidis, E. Effect of a Commercial Adjunct Culture on Organic Acid Contents of Low-Fat Feta-Type Cheese. Food Chem. 2006, 98, 658–663. [Google Scholar] [CrossRef]
- Jo, Y.; Benoist, D.M.; Ameerally, A.; Drake, M.A. Sensory and Chemical Properties of Gouda Cheese. J. Dairy Sci. 2018, 101, 1967–1989. [Google Scholar] [CrossRef]
- Pisano, M.B.; Rosa, A.; Putzu, D.; Cesare Marincola, F.; Mossa, V.; Viale, S.; Fadda, M.E.; Cosentino, S. Influence of Autochthonous Putative Probiotic Cultures on Microbiota, Lipid Components and Metabolome of Caciotta Cheese. Front. Microbiol. 2020, 11, 583745. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Rathnakumar, K.; Awasti, N.; Elfaruk, M.S.; Hammam, A.R.A. Influence of Probiotic Adjunct Cultures on the Characteristics of Low-Fat Feta Cheese. Food Sci. Nutr. 2021, 9, 1512–1520. [Google Scholar] [CrossRef]
- Litopoulou-Tzanetaki, E.; Tzanetakis, N. Microbiological Characteristics of Greek Traditional Cheeses. Small Rumin. Res. 2011, 101, 17–32. [Google Scholar] [CrossRef]
- Makarijoski, B. Microbiological Quality of Macedonian White Brined Cheese. J. Agric. Plant Sci. 2023, 21, 69–74. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Du, Z.; Li, B.; Liu, Y.; Gao, Y.; Zhang, Y.; Zhang, K.; Wang, Q.; Lu, S.; et al. Impact of NSLAB on Kazakh Cheese Flavor. Food Res. Int. 2021, 144, 110315. [Google Scholar] [CrossRef]
- Tsanasidou, C.; Asimakoula, S.; Sameli, N.; Fanitsios, C.; Vandera, E.; Bosnea, L.; Koukkou, A.I.; Samelis, J. Safety Evaluation, Biogenic Amine Formation, and Enzymatic Activity Profiles of Autochthonous Enterocin-Producing Greek Cheese Isolates of the Enterococcus Faecium/Durans Group. Microorganisms 2021, 9, 777. [Google Scholar] [CrossRef]
- Geronikou, A.; Larsen, N.; Lillevang, S.K.; Jespersen, L. Occurrence and Identification of Yeasts in Production of White-Brined Cheese. Microorganisms 2022, 10, 1079. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.-T.; Arias-Roth, E.; Jakob, E. Cheese Yeasts. Yeast 2019, 36, 129–141. [Google Scholar] [CrossRef]
- Kousta, M.; Mataragas, M.; Skandamis, P.; Drosinos, E.H. Prevalence and Sources of Cheese Contamination with Pathogens at Farm and Processing Levels. Food Control 2010, 21, 805–815. [Google Scholar] [CrossRef]
- Silva, H.L.A.; Balthazar, C.F.; Silva, R.; Vieira, A.H.; Costa, R.G.B.; Esmerino, E.A.; Freitas, M.Q.; Cruz, A.G. Sodium Reduction and Flavor Enhancer Addition in Probiotic Prato Cheese: Contributions of Quantitative Descriptive Analysis and Temporal Dominance of Sensations for Sensory Profiling. J. Dairy Sci. 2018, 101, 8837–8846. [Google Scholar] [CrossRef] [PubMed]
- Prezzi, L.E.; Lee, S.H.I.; Nunes, V.M.R.; Corassin, C.H.; Pimentel, T.C.; Rocha, R.S.; Ramos, G.L.P.A.; Guimarães, J.T.; Balthazar, C.F.; Duarte, M.C.K.H.; et al. Effect of Lactobacillus Rhamnosus on Growth of Listeria Monocytogenes and Staphylococcus Aureus in a Probiotic Minas Frescal Cheese. Food Microbiol. 2020, 92, 103557. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Silvetti, T.; Battelli, G.; Brasca, M. Can Lactic Acid Bacteria Be an Efficient Tool for Controlling Listeria Monocytogenes Contamination on Cheese Surface? The Case of Gorgonzola Cheese. Food Control 2019, 96, 499–507. [Google Scholar] [CrossRef]
- Psoni, L.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Characteristics of Batzos Cheese Made from Raw, Pasteurized And/or Pasteurized Standardized Goat Milk and a Native Culture. Food Control 2006, 17, 533–539. [Google Scholar] [CrossRef]
- Viana de Souza, J.; Silva Dias, F. Protective, Technological, and Functional Properties of Select Autochthonous Lactic Acid Bacteria from Goat Dairy Products. Curr. Opin. Food Sci. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Bettera, L.; Levante, A.; Lazzi, C.; Bottari, B.; Gatti, M. Lactic Acid Bacteria in Cow Raw Milk for Cheese Production: Which and How Many? Front. Microbiol. 2023, 13, 1092224. [Google Scholar] [CrossRef]
- Gaglio, R.; Todaro, M.; Settanni, L. Improvement of Raw Milk Cheese Hygiene through the Selection of Starter and Non-Starter Lactic Acid Bacteria: The Successful Case of PDO Pecorino Siciliano Cheese. Int. J. Environ. Res. Public Health 2021, 18, 1834. [Google Scholar] [CrossRef]
- Escobar-Zepeda, A.; Sanchez-Flores, A.; Quirasco Baruch, M. Metagenomic Analysis of a Mexican Ripened Cheese Reveals a Unique Complex Microbiota. Food Microbiol. 2016, 57, 116–127. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Microbiology of Cheese Ripening. In Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; pp. 333–390. [Google Scholar]
- González, L.; Zarate, V. Influence of an Autochthonous Starter Culture and a Commercial Starter on the Characteristics of Tenerife Pasteurised Goats’ Milk Cheese. Int. J. Dairy Technol. 2012, 65, 542–547. [Google Scholar] [CrossRef]
- Bancalari, E.; Montanari, C.; Levante, A.; Alinovi, M.; Neviani, E.; Gardini, F.; Gatti, M. Lactobacillus Paracasei 4341 as Adjunct Culture to Enhance Flavor in Short Ripened Caciotta-Type Cheese. Food Res. Int. 2020, 135, 109284. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Sidira, M.; Koutinas, A.A.; Kourkoutas, Y. Free and Immobilized Lactobacillus Casei ATCC 393 on Whey Protein as Starter Cultures for Probiotic Feta-Type Cheese Production. J. Dairy Sci. 2014, 97, 4675–4685. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Chondrou, P.; Galanis, A.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.; Plessas, S. Application of a Novel Potential Probiotic Lactobacillus Paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese. Microorganisms 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, S.; Pavlou, E.; Pasentsis, K.; Rhoades, J.; Likotrafiti, E.; Argiriou, A. Microbial Profiles of Greek PDO Cheeses Assessed with Amplicon Metabarcoding. Food Microbiol. 2021, 99, 103836. [Google Scholar] [CrossRef] [PubMed]
- Kamarinou, C.S.; Papadopoulou, O.S.; Doulgeraki, A.I.; Tassou, C.C.; Galanis, A.; Chorianopoulos, N.G.; Argyri, A.A. Mapping the Key Technological and Functional Characteristics of Indigenous Lactic Acid Bacteria Isolated from Greek Traditional Dairy Products. Microorganisms 2022, 10, 246. [Google Scholar] [CrossRef]
- Gupta, M.; Bajaj, B.K. Functional Characterization of Potential Probiotic Lactic Acid Bacteria Isolated from Kalarei and Development of Probiotic Fermented Oat Flour. Probiotics Antimicrob. Proteins 2017, 10, 654–661. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Gonçalves dos Santos, M.T.P.; Galván, A.I.; Merchán, A.V.; González, E.; Córdoba, M.d.G.; Benito, M.J. Screening of Autochthonous Lactic Acid Bacteria Strains from Artisanal Soft Cheese: Probiotic Characteristics and Prebiotic Metabolism. LWT 2019, 114, 108388. [Google Scholar] [CrossRef]
- Rhoades, J.; Anastasiou, I.; Michailidou, S.; Koinidis, A.; Doulgerakis, C.; Alexa, E.A.; Alvarez-Ordóñez, A.; Argiriou, A.; Likotrafiti, E. Microbiological Analysis of Greek Protected Designation of Origin Cheeses and Characterisation of the Isolated Lactic Acid Bacteria. Int. Dairy J. 2021, 123, 105183. [Google Scholar] [CrossRef]
- Lappa, I.K.; Natsia, A.; Alimpoumpa, D.; Stylianopoulou, E.; Prapa, I.; Tegopoulos, K.; Pavlatou, C.; Skavdis, G.; Papadaki, A.; Kopsahelis, K. Novel Probiotic Candidates in Artisanal Feta-Type Kefalonian Cheese: Unveiling a Still-Undisclosed Biodiversity. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef]
- Kraggerud, H.; Næs, T.; Abrahamsen, R.K. Prediction of Sensory Quality of Cheese during Ripening from Chemical and Spectroscopy Measurements. Int. Dairy J. 2014, 34, 6–18. [Google Scholar] [CrossRef]
- Lanciotti, R.; Vannini, L.; Lopez, C.C.; Gobbetti, M.; Guerzoni, M.E. Evaluation of the Ability of Yarrowia lipolytica to Impart Strain—Dependent Characteristics to Cheese When Used as a Ripening Adjunct. Int. J. Dairy Technol. 2005, 58, 89–99. [Google Scholar] [CrossRef]
- Subramanian, A.; Alvarez, V.B.; Harper, W.J.; Rodriguez-Saona, L.E. Monitoring Amino Acids, Organic Acids, and Ripening Changes in Cheddar Cheese Using Fourier-Transform Infrared Spectroscopy. Int. Dairy J. 2011, 21, 434–440. [Google Scholar] [CrossRef]


| Benchtop-MSI | Portable-MSI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| From/To | Fresh | Semi-Fresh | Spoiled | Precision (%) | Sensitivity (%) | Fresh | Semi-Fresh | Spoiled | Precision (%) | Sensitivity (%) |
| Fresh | 46 | 14 | 0 | 75.41 | 76.67 | 28 | 6 | 0 | 82.35 | 82.35 |
| Semi-fresh | 15 | 30 | 0 | 54.55 | 66.67 | 5 | 10 | 0 | 29.41 | 66.67 |
| Spoiled | 0 | 11 | 31 | 100 | 73.81 | 1 | 18 | 8 | 100 | 29.63 |
| Accuracy (%) | 72.79 | 60.53 | ||||||||
| FTIR | ||||||||||
| Fresh | 43 | 22 | 0 | 93.49 | 66.15 | |||||
| Semi-fresh | 2 | 12 | 0 | 28.57 | 85.71 | |||||
| Spoiled | 1 | 8 | 7 | 100 | 43.75 | |||||
| Accuracy (%) | 65.26 | |||||||||
| Benchtop-MSI | Portable-MSI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| From/To | Fresh | Semi-Fresh | Spoiled | Precision (%) | Sensitivity (%) | Fresh | Semi-Fresh | Spoiled | Precision (%) | Sensitivity (%) |
| Fresh | 105 | 4 | 0 | 89.74 | 96.33 | 88 | 7 | 0 | 87.13 | 92.63 |
| Semi-fresh | 12 | 21 | 0 | 84.00 | 63.64 | 13 | 21 | 0 | 63.64 | 61.76 |
| Spoiled | 0 | 0 | 5 | 100 | 100 | 0 | 5 | 13 | 100 | 72.22 |
| Accuracy (%) | 89.12 | 82.99 | ||||||||
| FTIR | ||||||||||
| Fresh | 59 | 19 | 0 | 93.65 | 75.64 | |||||
| Semi-fresh | 4 | 20 | 0 | 48.78 | 83.33 | |||||
| Spoiled | 0 | 3 | 0 | 100 | 33.33 | |||||
| Accuracy (%) | 76.19 | |||||||||
| Benchtop-MSI | Portable-MSI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| From/To | Fresh | Semi-Fresh | Spoiled | Precision (%) | Sensitivity (%) | Fresh | Semi-Fresh | Spoiled | Precision (%) | Sensitivity (%) |
| Fresh | 105 | 9 | 0 | 98.13 | 92.11 | 75 | 11 | 0 | 78.13 | 82.21 |
| Semi-fresh | 2 | 4 | 0 | 11.76 | 66.67 | 21 | 12 | 0 | 37.50 | 36.36 |
| Spoiled | 0 | 21 | 7 | 100 | 25.00 | 0 | 9 | 18 | 100 | 66.67 |
| Accuracy (%) | 78.38 | 71.92 | ||||||||
| FTIR | ||||||||||
| Fresh | 51 | 10 | 0 | 94.44 | 83.61 | |||||
| Semi-fresh | 3 | 34 | 0 | 72.34 | 91.89 | |||||
| Spoiled | 0 | 3 | 1 | 100 | 25.0 | |||||
| Accuracy (%) | 84.31 | |||||||||
| Benchtop-MSI | Portable-MSI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| From/To | Fresh | Semi-Fresh | Spoiled | Precision (%) | Sensitivity (%) | Fresh | Semi-Fresh | Spoiled | Precision (%) | Sensitivity (%) |
| Fresh | 55 | 9 | 0 | 77.46 | 85.94 | 43 | 10 | 0 | 64.18 | 81.13 |
| Semi-fresh | 16 | 51 | 0 | 72.86 | 76.12 | 24 | 53 | 0 | 77.94 | 68.83 |
| Spoiled | 0 | 10 | 5 | 100 | 33.33 | 0 | 5 | 11 | 100 | 68.75 |
| Accuracy (%) | 76.03 | 73.29 | ||||||||
| FTIR | ||||||||||
| Fresh | 46 | 25 | 0 | 79.31 | 64.79 | |||||
| Semi-fresh | 12 | 45 | 0 | 59.21 | 78.95 | |||||
| Spoiled | 0 | 6 | 7 | 100 | 53.85 | |||||
| Accuracy (%) | 69.50 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doukaki, A.; Papadopoulou, O.S.; Baraki, A.; Siapka, M.; Ntalakas, I.; Tzoumkas, I.; Papadimitriou, K.; Tassou, C.; Skandamis, P.; Nychas, G.-J.; et al. Effect of the Bioprotective Properties of Lactic Acid Bacteria Strains on Quality and Safety of Feta Cheese Stored under Different Conditions. Microorganisms 2024, 12, 1870. https://doi.org/10.3390/microorganisms12091870
Doukaki A, Papadopoulou OS, Baraki A, Siapka M, Ntalakas I, Tzoumkas I, Papadimitriou K, Tassou C, Skandamis P, Nychas G-J, et al. Effect of the Bioprotective Properties of Lactic Acid Bacteria Strains on Quality and Safety of Feta Cheese Stored under Different Conditions. Microorganisms. 2024; 12(9):1870. https://doi.org/10.3390/microorganisms12091870
Chicago/Turabian StyleDoukaki, Angeliki, Olga S. Papadopoulou, Antonia Baraki, Marina Siapka, Ioannis Ntalakas, Ioannis Tzoumkas, Konstantinos Papadimitriou, Chrysoula Tassou, Panagiotis Skandamis, George-John Nychas, and et al. 2024. "Effect of the Bioprotective Properties of Lactic Acid Bacteria Strains on Quality and Safety of Feta Cheese Stored under Different Conditions" Microorganisms 12, no. 9: 1870. https://doi.org/10.3390/microorganisms12091870
APA StyleDoukaki, A., Papadopoulou, O. S., Baraki, A., Siapka, M., Ntalakas, I., Tzoumkas, I., Papadimitriou, K., Tassou, C., Skandamis, P., Nychas, G.-J., & Chorianopoulos, N. (2024). Effect of the Bioprotective Properties of Lactic Acid Bacteria Strains on Quality and Safety of Feta Cheese Stored under Different Conditions. Microorganisms, 12(9), 1870. https://doi.org/10.3390/microorganisms12091870










