In Vitro Antimicrobial Synergistic Activity and the Mechanism of the Combination of Naringenin and Amikacin Against Antibiotic-Resistant Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Strains
2.2. Antimicrobial Activity
2.2.1. Determination of MIC and MBC
2.2.2. Antibiotic Synergism Tests
2.2.3. Time-Kill Curve Assays
2.3. The Antibacterial Mechanism
2.3.1. SEM
2.3.2. Cell Wall Permeability
2.3.3. Inner Membrane (IM) Permeability
Detection of K+ and Protein Leakage
IM Permeability of E. coli Was Determined Using CLSM
2.4. Statistical Analysis
3. Results
3.1. Antibacterial Activity of NG
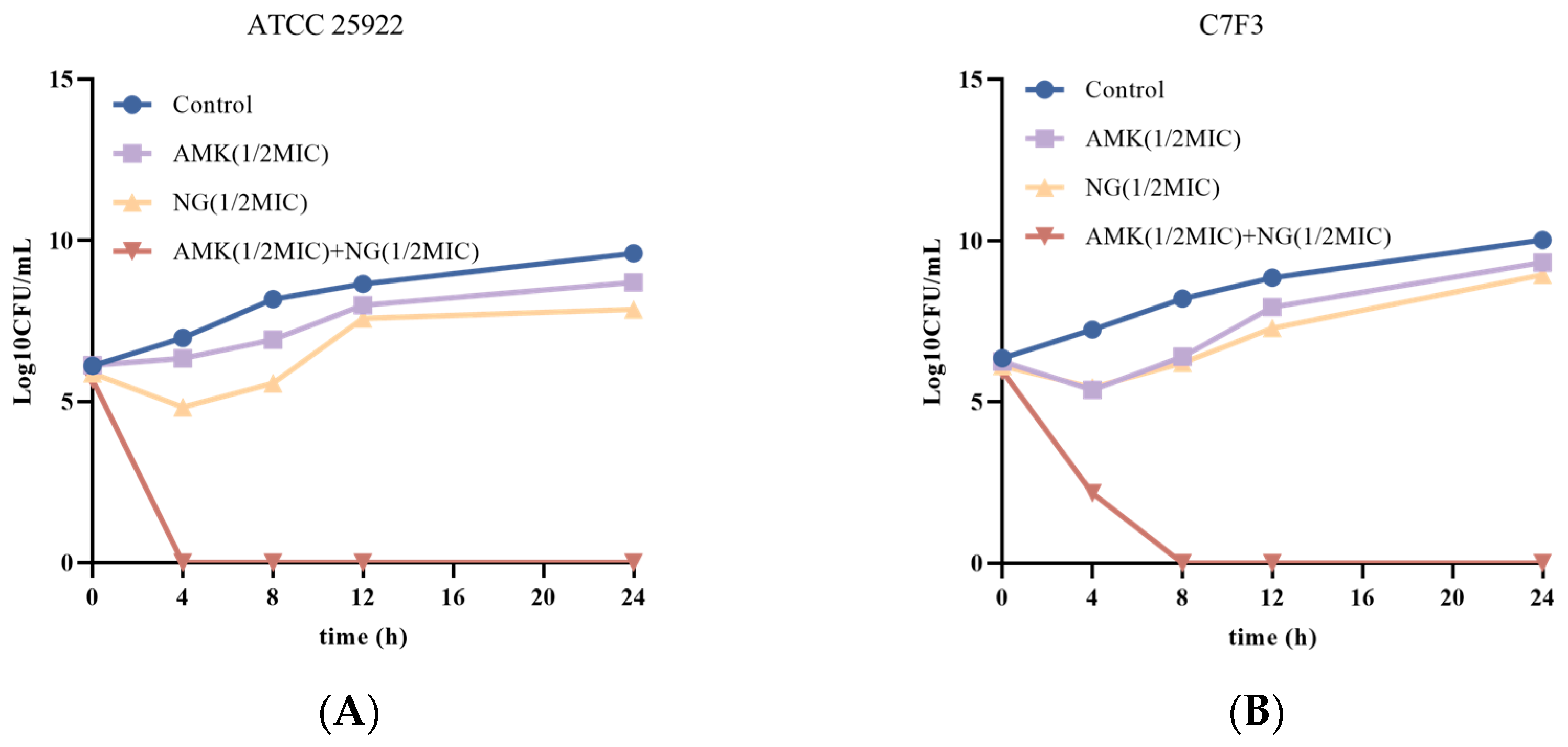
3.2. The Combination of NG and AMK Shows Potent Bactericidal Activity Against E. coli
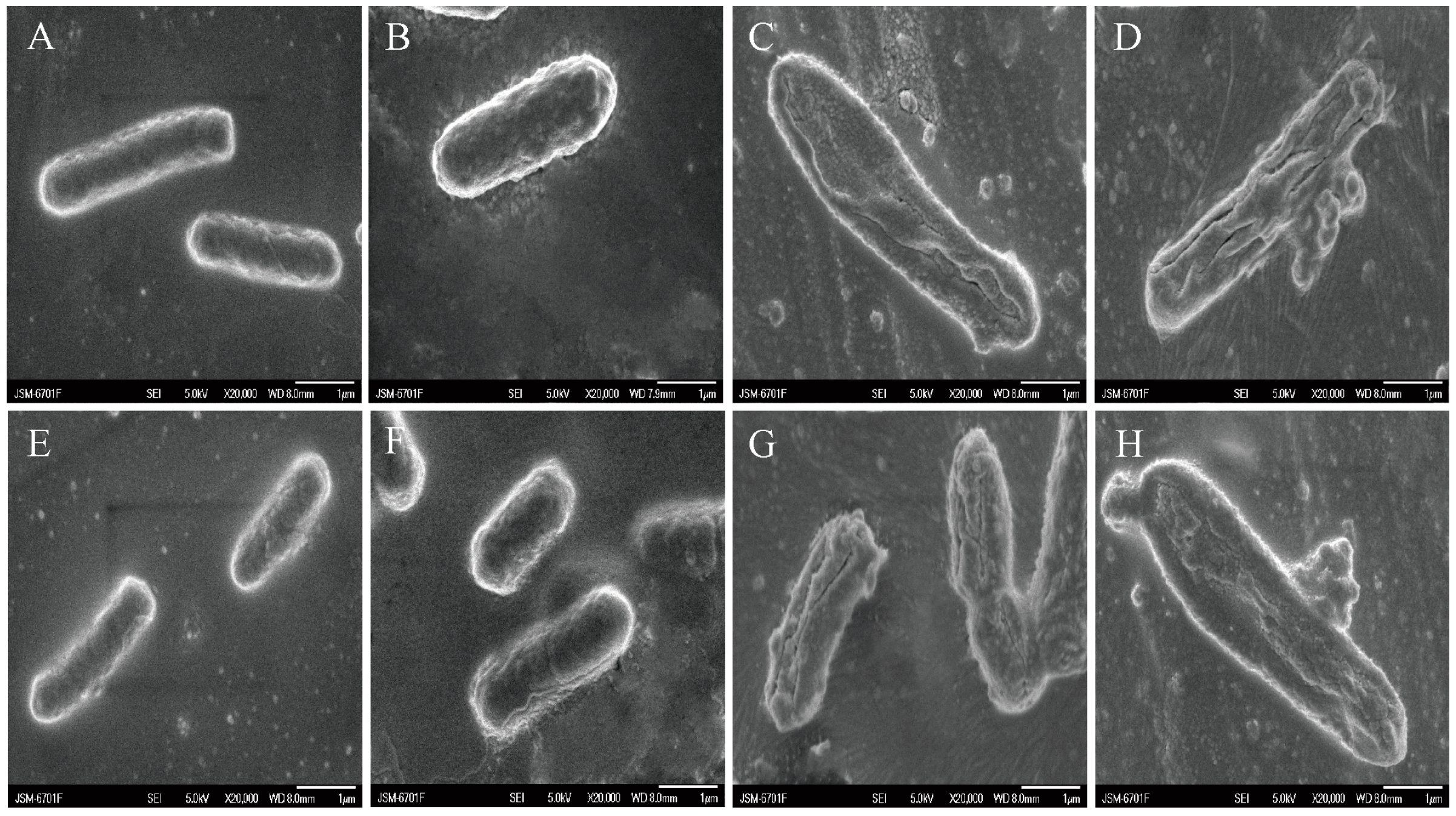
3.3. The Combination of NG and AMK Causes Changes in the Morphology of E. coli
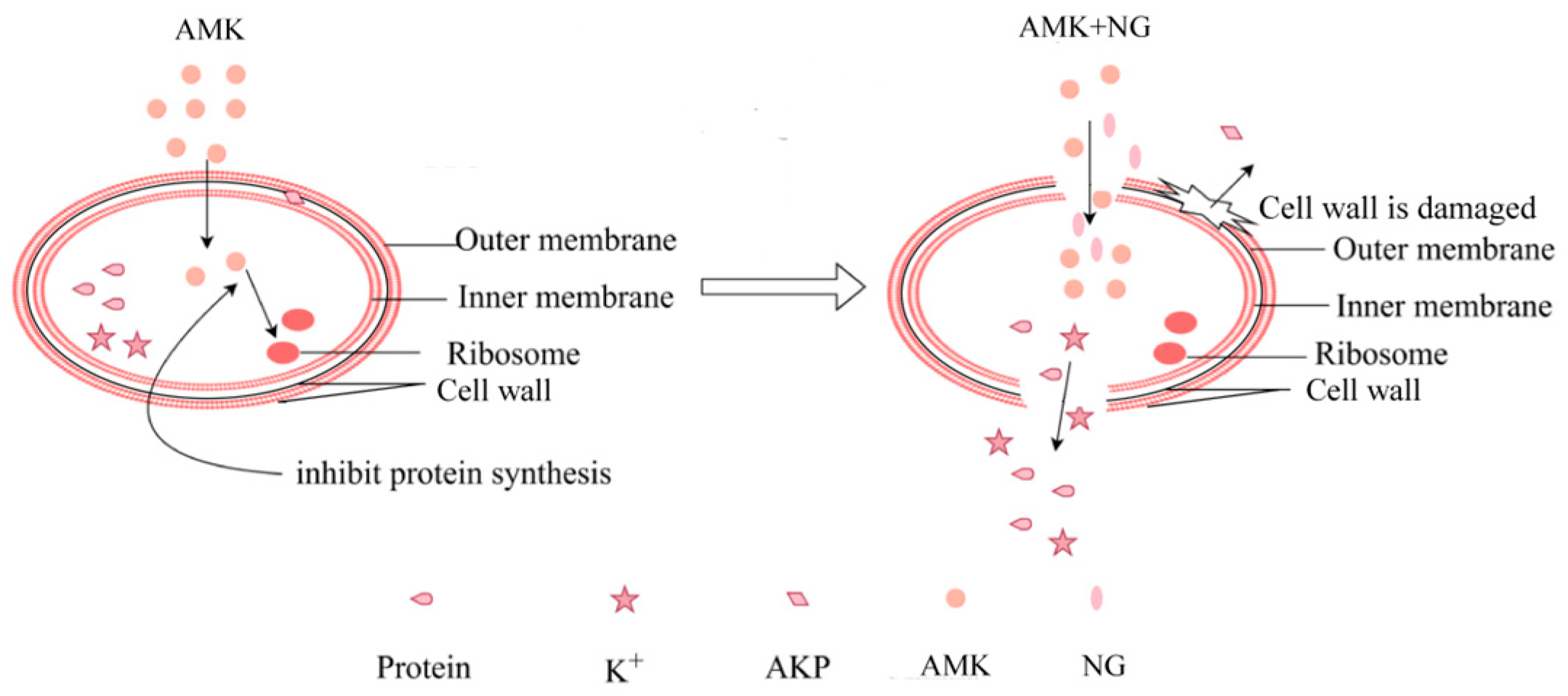
3.4. The Combination of NG and AMK Damages the Cell Wall
3.5. The Combination of NG and AMK Damages the Inner Membrane (IM)
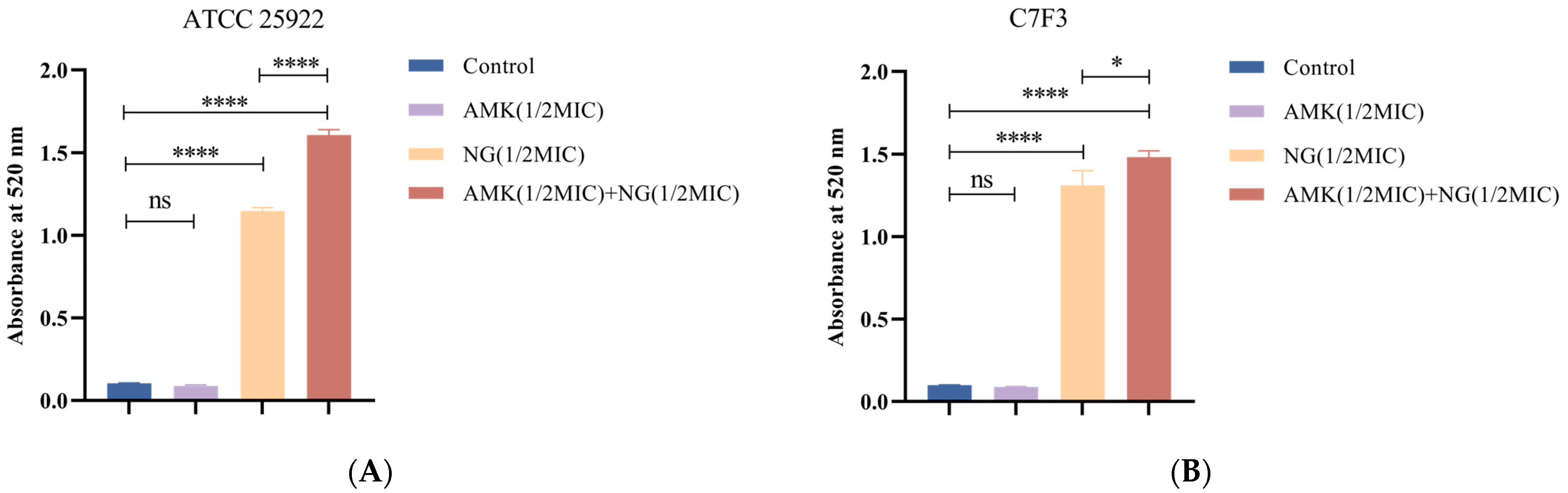
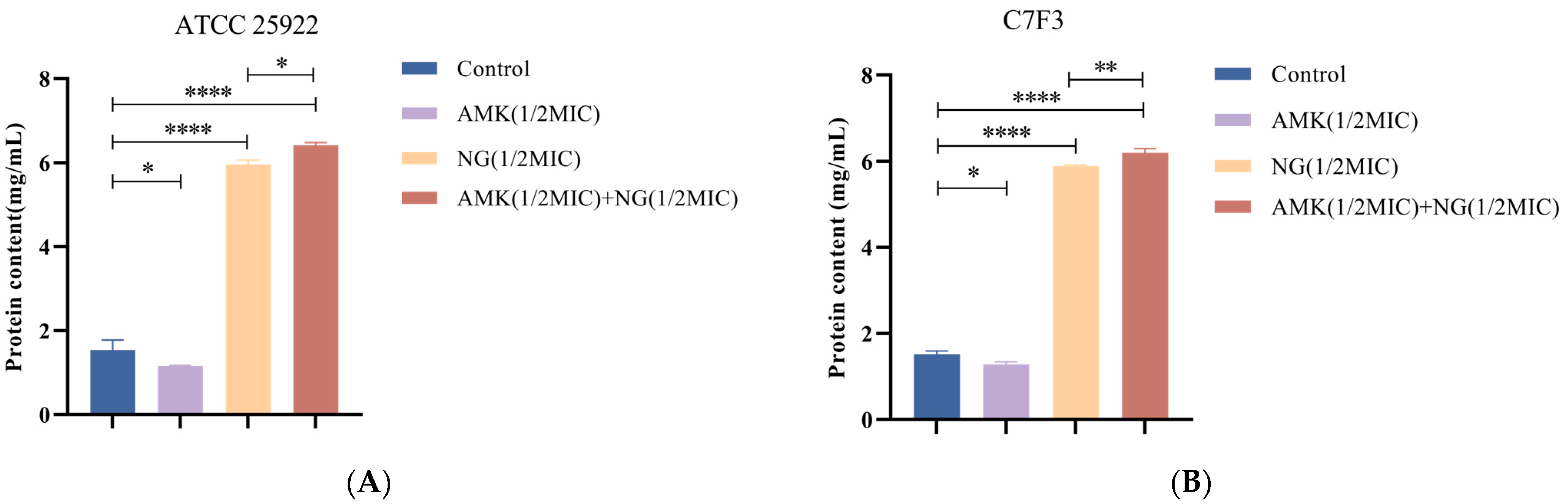
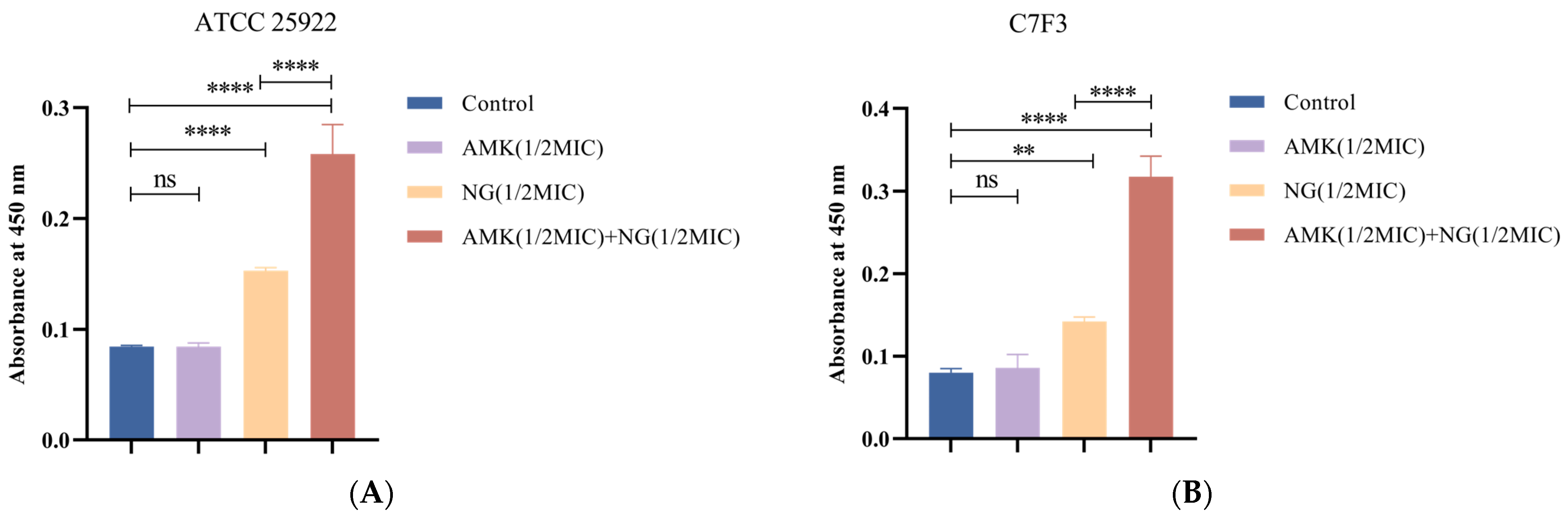
3.5.1. Detection of Protein Leakage
3.5.2. Detection of K+ Leakage
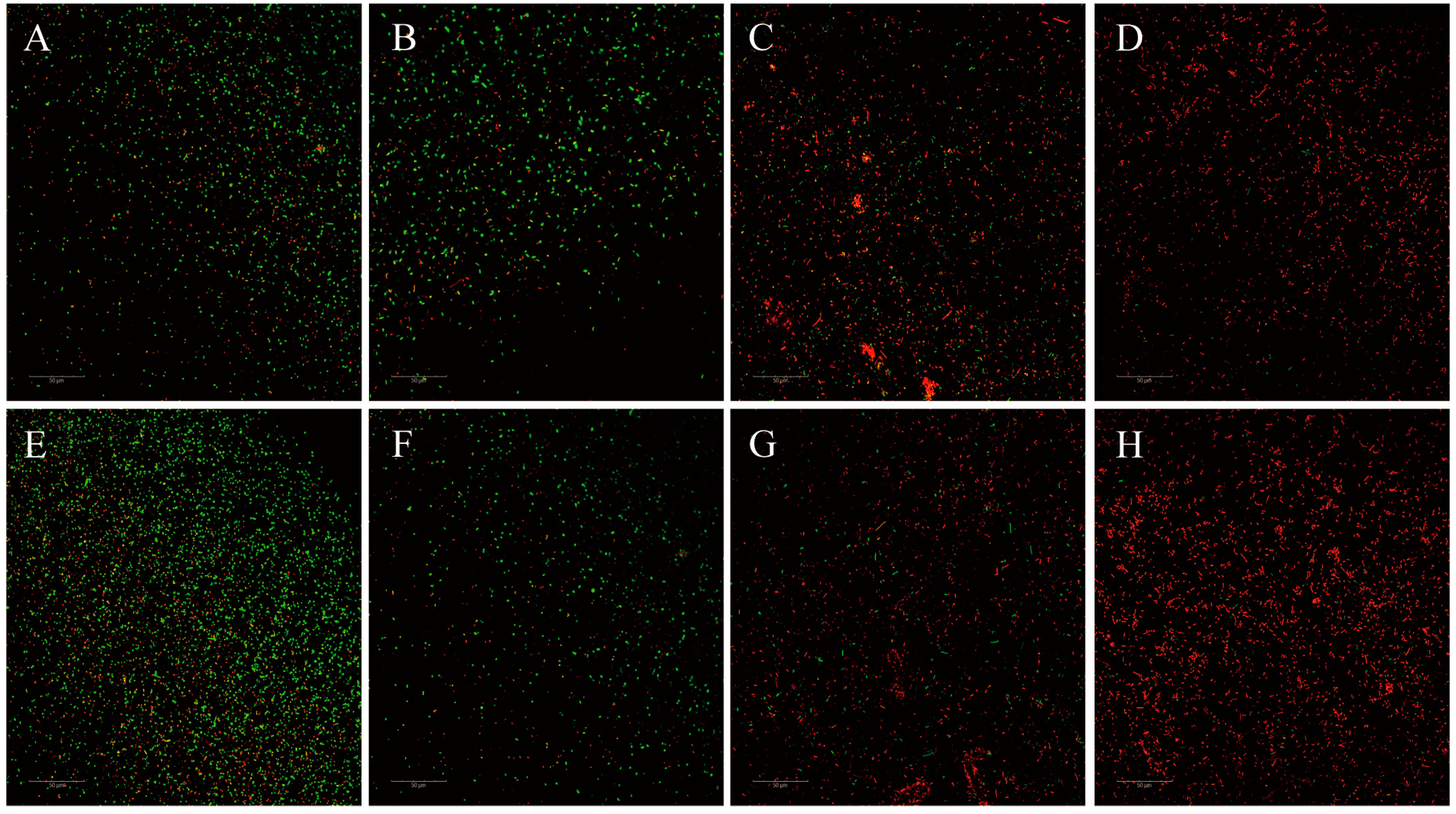
3.5.3. CLSM Examinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mourenza, Á.; Gil, J.A.; Mateos, L.M.; Letek, M. Novel Methods to Identify Oxidative Stress-Producing Antibiotics. Methods Mol. Biol. 2021, 2296, 249–261. [Google Scholar]
- Guo, H.; Tong, Y.; Cheng, J.; Abbas, Z.; Li, Z.; Wang, J.; Zhou, Y.; Si, D.; Zhang, R. Biofilm and Small Colony Variants—An Update on Staphylococcus aureus Strategies toward Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1241. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Wang, W.; Zhang, J. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Vet. Med. Sci. 2022, 8, 1780–1786. [Google Scholar] [CrossRef]
- Zhu, J.; Lv, J.; Zhu, Z.; Wang, T.; Xie, X.; Zhang, H.; Chen, L.; Du, H. Identification of TMexCD-TOprJ-producing carbapenem-resistant Gram-negative bacteria from hospital sewage. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2023, 70, 100989. [Google Scholar] [CrossRef]
- Kanj, S.S.; Bassetti, M.; Kiratisin, P.; Rodrigues, C.; Villegas, M.V.; Yu, Y.; van Duin, D. Clinical data from studies involving novel antibiotics to treat multidrug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents 2022, 60, 106633. [Google Scholar] [CrossRef]
- Udaondo, Z.; Matilla, M.A. Mining for novel antibiotics in the age of antimicrobial resistance. Microb. Biotechnol. 2020, 13, 1702–1704. [Google Scholar] [CrossRef]
- Balabekyan, T.R.; Karapetyan, K.J.; Khachatryan, T.V.; Khachatryan, G.E.; Tatikyan, S.S. Antimicrobial activity of preparations after combined cultivation of lactic acid bacteria and yeast strains. J. Anim. Physiol. Anim. Nutr. 2018, 102, 933–938. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Dossou, S.S.K.; Xu, F.; Cui, X.; Sheng, C.; Zhou, R.; You, J.; Tozo, K.; Wang, L. Comparative metabolomics analysis of different sesame (Sesamum indicum L.) tissues reveals a tissue-specific accumulation of metabolites. BMC Plant Biol. 2021, 21, 352. [Google Scholar] [CrossRef]
- Zafar, A.; Wasti, Y.; Majid, M.; Muntaqua, D.; Bungau, S.G.; Haq, I.U. Artemisia brevifolia Wall. Ex DC Enhances Cefixime Susceptibility by Reforming Antimicrobial Resistance. Antibiotics 2023, 12, 1553. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhao, L.; Hao, Z. Mechanism of Synergy Between Tetracycline and Quercetin Against Antibiotic Resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, M.; Guo, W.; Yao, Z.; Du, X.; Chen, L.; Sun, Y.; Shi, S.; Cao, J.; Zhou, T. The Antibacterial Activity of Kaempferol Combined with Colistin against Colistin-Resistant Gram-Negative Bacteria. Microbiol. Spectr. 2022, 10, e0226522. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Li, C.; Dai, X.; Song, T.; Wang, X.; et al. Naringenin: A flavanone with anti-inflammatory and anti-infective properties. Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef]
- Pan, Z.; He, Q.; Zeng, J.; Li, S.; Li, M.; Chen, B.; Yang, J.; Xiao, J.; Zeng, C.; Luo, H.; et al. Naringenin protects against iron overload-induced osteoarthritis by suppressing oxidative stress. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 105, 154330. [Google Scholar] [CrossRef]
- Sheng, Q.; Hou, X.; Wang, Y.; Wang, N.; Deng, X.; Wen, Z.; Li, D.; Li, L.; Zhou, Y.; Wang, J. Naringenin Microsphere as a Novel Adjuvant Reverses Colistin Resistance via Various Strategies against Multidrug-Resistant Klebsiella pneumoniae Infection. J. Agric. Food Chem. 2022, 70, 16201–16217. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.F.; Carey, R.B.; Carey, R.F.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Paitan, Y. Current Trends in Antimicrobial Resistance of Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 181–211. [Google Scholar]
- Erdal, B.; Yıkmış, S.; Demirok, N.T.; Bozgeyik, E.; Levent, O. Effects of Non-Thermal Treatment on Gilaburu Vinegar (Viburnum opulus L.): Polyphenols, Amino Acid, Antimicrobial, and Anticancer Properties. Biology 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tian, S.; Zhang, T.; Zhang, W.; Lu, Q.; Hu, Q.; Shao, H.; Guo, Y.; Luo, Q. Antibacterial activity mechanism of coptisine against Pasteurella multocida. Front. Cell. Infect. Microbiol. 2023, 13, 1207855. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Geng, D.; Tao, J.; Wang, J.; Liu, S.; Wang, Q.; Xu, F.; Xiao, S.; Wang, R. Synergistic Antibacterial Effect and Mechanism of Allicin and an Enterobacter cloacae Bacteriophage. Microbiol. Spectr. 2023, 11, e0315522. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Qian, Y.; Guo, J.; Mao, M.; An, D. A novel approach for water disinfection by enhanced photoanode oxidation using in-situ generated hydrogen peroxide. J. Clean. Prod. 2023, 416, 138001. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Y.; Yu, Y.; Yin, L.; Zhang, Y. Use of Acetic Acid to Partially Replace Lactic Acid for Decontamination against Escherichia coli O157, H7 in Fresh Produce and Mechanism of Action. Foods 2021, 10, 2406. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef]
- Hermosilla, E.; Díaz, M.; Vera, J.; Seabra, A.B.; Tortella, G.; Parada, J.; Rubilar, O. Molecular Weight Identification of Compounds Involved in the Fungal Synthesis of AgNPs: Effect on Antimicrobial and Photocatalytic Activity. Antibiotics 2022, 11, 622. [Google Scholar] [CrossRef]
- Hussein, M.; Han, M.L.; Zhu, Y.; Zhou, Q.; Lin, Y.-W.; Hancock, R.E.W.; Hoyer, D.; Creek, D.J.; Li, J.; Velkov, T. Metabolomics Study of the Synergistic Killing of Polymyxin B in Combination with Amikacin against Polymyxin-Susceptible and -Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 64, 10–1128. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef]
- Li, Q.; Yu, S.; Han, J.; Wu, J.; You, L.; Shi, X.; Wang, S. Synergistic antibacterial activity and mechanism of action of nisin/carvacrol combination against Staphylococcus aureus and their application in the infecting pasteurized milk. Food Chem. 2022, 380, 132009. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Duan, S.; Hu, Y.; Liang, J.; Tang, Y. Antibacterial activity and synergistic antibiotic mechanism of trialdehyde phloroglucinol against methicillin-resistant Staphylococcus aureus. Phytother. Res. PTR 2023, 37, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; He, K.; Li, T.; Cui, S.; Tang, M.; Kang, S.; Ma, W.; Song, L. Mechanism and antibacterial activity of vine tea extract and dihydromyricetin against Staphylococcus aureus. Sci. Rep. 2020, 10, 21416. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.N.; Wang, F.; Yuan, Y.T.; Liu, J.; Liu, Y.-Z.; Yi, X. Antibacterial Activity and Mode of Action of Dihydromyricetin from Ampelopsis grossedentata Leaves against Food-Borne Bacteria. Molecules 2019, 24, 2831. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, P.; Wang, P.; Ma, C.; Kang, W. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chu, Z.; Ruan, Z.; Wang, X.; Dai, T.; Hu, X. Changes of Intracellular Porphyrin, Reactive Oxygen Species, and Fatty Acids Profiles During Inactivation of Methicillin-Resistant Staphylococcus aureus by Antimicrobial Blue Light. Front. Physiol. 2018, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Moo, C.L.; Osman, M.A.; Yang, S.K.; Yap, W.S.; Ismail, S.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Sci. Rep. 2021, 11, 20824. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Huang, Y.; Chen, S.; Xu, R.; Xu, L.; Qiu, J.; Shi, F.; Liu, S.; Zha, Q.; Ouyang, D.; et al. Dextran sodium sulfate potentiates NLRP3 inflammasome activation by modulating the KCa3.1 potassium channel in a mouse model of colitis. Cell. Mol. Immunol. 2022, 19, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, X.; Wu, H.; Wang, H.; Bian, H.; Zhu, Y.; Xu, W.; Liu, F.; Wang, D.; Fu, L. Antibacterial activity and action mode of chlorogenic acid against Salmonella enteritidis, a foodborne pathogen in chilled fresh chicken. World J. Microbiol. Biotechnol. 2020, 36, 24. [Google Scholar] [CrossRef]
| ATCC 25922 | C7F3 * | C3F1 | C5F3 | B3E2 | B5E1 | A3E2 * | A3F1 | |
|---|---|---|---|---|---|---|---|---|
| Tetracycline | 0.5 (S) | 1024 (R) | 512 (R) | 512 (R) | 512 (R) | 1024 (R) | 512 (R) | 256 (R) |
| Ciprofloxacin | 0.0039 (S) | 4 (R) | 0.125 (S) | 0.5 (I) | 0.25 (S) | 0.03125 (S) | 8 (R) | 0.0625 (S) |
| Meropenem | 0.03125 (S) | 0.0078 (S) | 0.015625 (S) | 0.0625 (S) | 0.03125 (S) | 0.015625 (S) | 16 (R) | 0.0078 (S) |
| Tigecycline | 0.03125 (S) | 16 (R) | 8 (R) | 16 (R) | 16 (R) | 2 (S) | 2 (S) | 4 (I) |
| Polymyxin | 0.5 (I) | 0.5 (I) | 0.25 (I) | 1 (I) | 0.25 (I) | 0.25 (I) | 1 (I) | 0.125 (I) |
| Ceftriaxone | 0.125 (S) | 512 (R) | 512 (R) | 256 (R) | 512 (R) | 0.125 (S) | 256 (R) | 128 (R) |
| Amikacin | 2 (S) | 64 (R) | 4 (S) | 2 (S) | 2 (S) | 4 (S) | 32 (I) | 1 (S) |
| Kanamycin | 1 (S) | 256 (R) | 8 (S) | 4 (S) | 4 (S) | 1024 (R) | 256 (R) | 2 (S) |
| MIC | MBC | |
|---|---|---|
| ATCC 25922 | 1 | 2 |
| C7F3 | 2 | 4 |
| C3F1 | 2 | 8 |
| C5F3 | 8 | 16 |
| B3E2 | 2 | 4 |
| B5E1 | 8 | 16 |
| A3E2 | 2 | 4 |
| A3F1 | 4 | 8 |
| ATCC 25922 | C7F3 | C3F1 | C5F3 | B3E2 | B5E1 | A3E2 | A3F1 | |
|---|---|---|---|---|---|---|---|---|
| Tetracycline | 0.5625 | 1 | 1 | 0.5 | 0.625 | 1 | 1 | 1 |
| Ciprofloxacin | 1 | 1 | 0.75 | 1 | 1 | 0.625 | 0.53125 | 1 |
| Meropenem | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 |
| Tigecycline | 1 | 0.5625 | 0.625 | 1 | 1 | 0.75 | 0.75 | 1 |
| Polymyxin | 0.5 | 1 | 1 | 1 | 0.75 | 0.5625 | 0.75 | 1 |
| Ceftriaxone | 1 | 0.75 | 1 | 0.5 | 1 | 1 | 1 | 0.625 |
| Amikacin | 0.3125 | 0.1875 | 0.375 | 0.75 | 0.5 | 0.75 | 0.625 | 0.375 |
| Kanamycin | 0.75 | 1 | 0.75 | 1 | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, L.; Cao, M.; Chen, X.; Bai, Y.; Wang, W.; Wei, X.; Shi, Y.; Zhang, Y.; Ma, T.; Zhu, Z.; et al. In Vitro Antimicrobial Synergistic Activity and the Mechanism of the Combination of Naringenin and Amikacin Against Antibiotic-Resistant Escherichia coli. Microorganisms 2024, 12, 1871. https://doi.org/10.3390/microorganisms12091871
Yi L, Cao M, Chen X, Bai Y, Wang W, Wei X, Shi Y, Zhang Y, Ma T, Zhu Z, et al. In Vitro Antimicrobial Synergistic Activity and the Mechanism of the Combination of Naringenin and Amikacin Against Antibiotic-Resistant Escherichia coli. Microorganisms. 2024; 12(9):1871. https://doi.org/10.3390/microorganisms12091871
Chicago/Turabian StyleYi, Lankun, Mingze Cao, Xu Chen, Yubin Bai, Weiwei Wang, Xiaojuan Wei, Yuxiang Shi, Yongying Zhang, Tenghe Ma, Zhen Zhu, and et al. 2024. "In Vitro Antimicrobial Synergistic Activity and the Mechanism of the Combination of Naringenin and Amikacin Against Antibiotic-Resistant Escherichia coli" Microorganisms 12, no. 9: 1871. https://doi.org/10.3390/microorganisms12091871




