pH Nonlinearly Dominates Soil Bacterial Community Assembly along an Altitudinal Gradient in Oak-Dominant Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. Soil Characteristics Analysis
2.3. DNA Extraction and Sequencing
2.4. Sequence Analysis
2.5. Statistical Analysis
3. Results
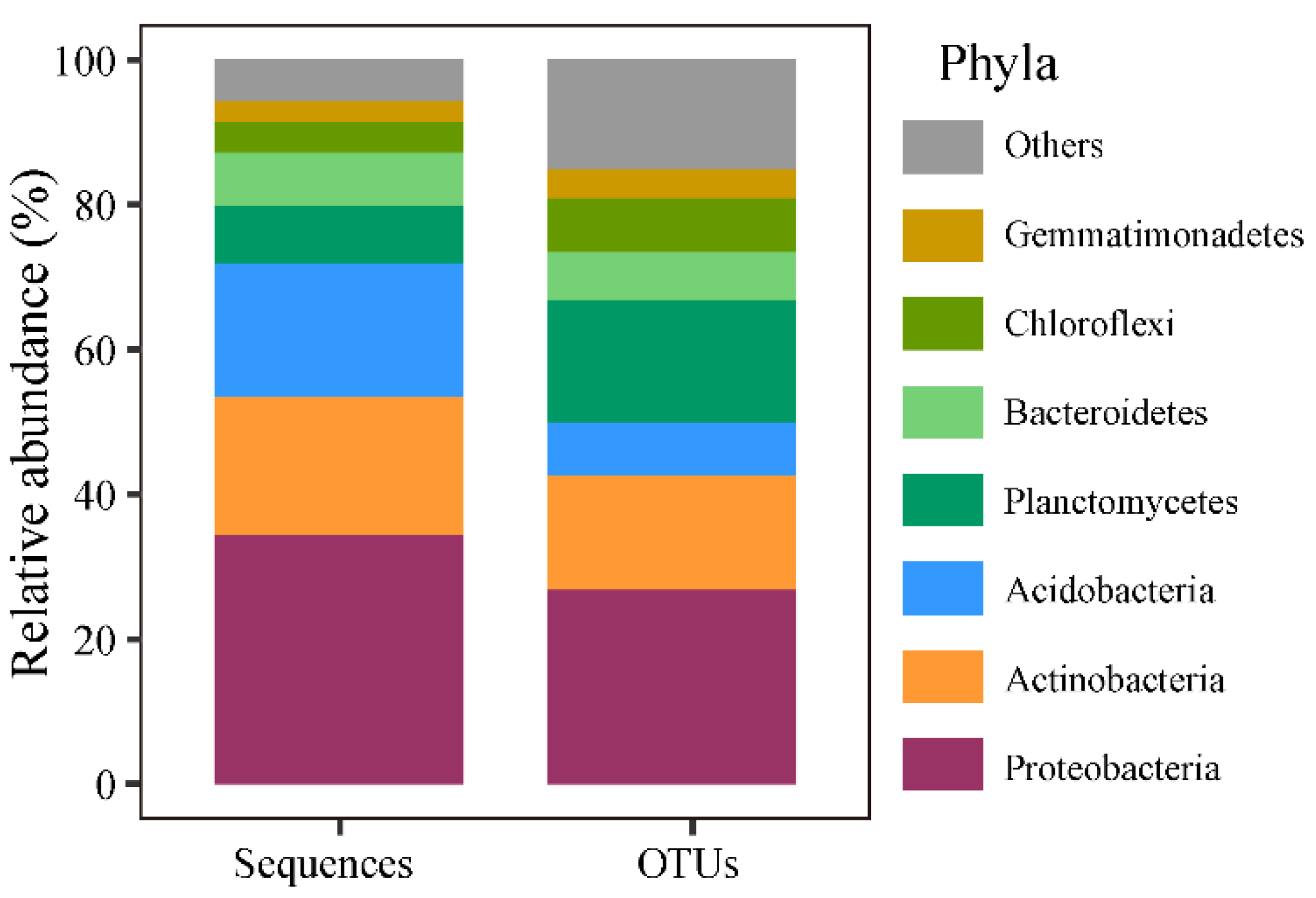
3.1. Characterization of Soil Factors, Plant, and Soil Bacteria
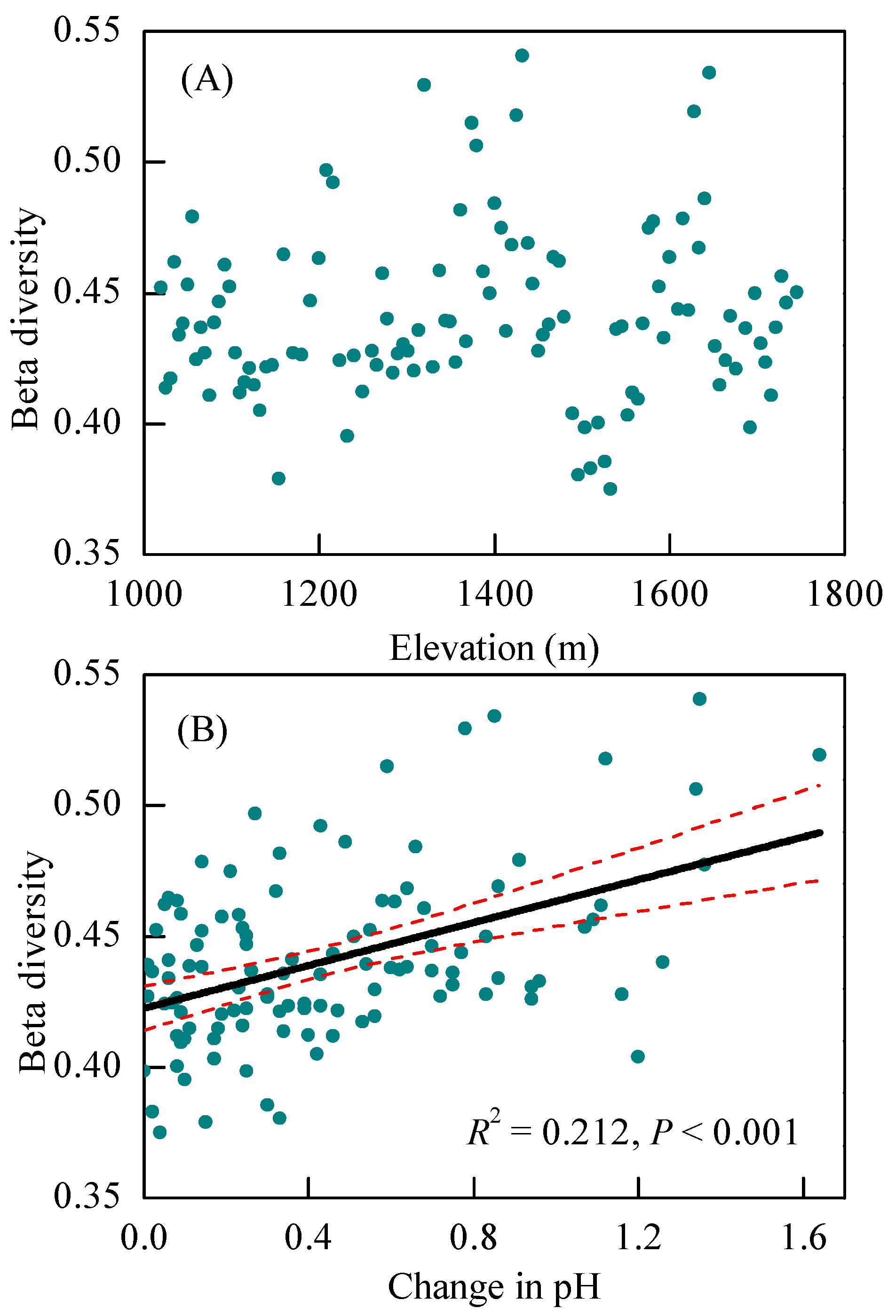
3.2. Changes in Bacterial Diversity Associated with Altitude
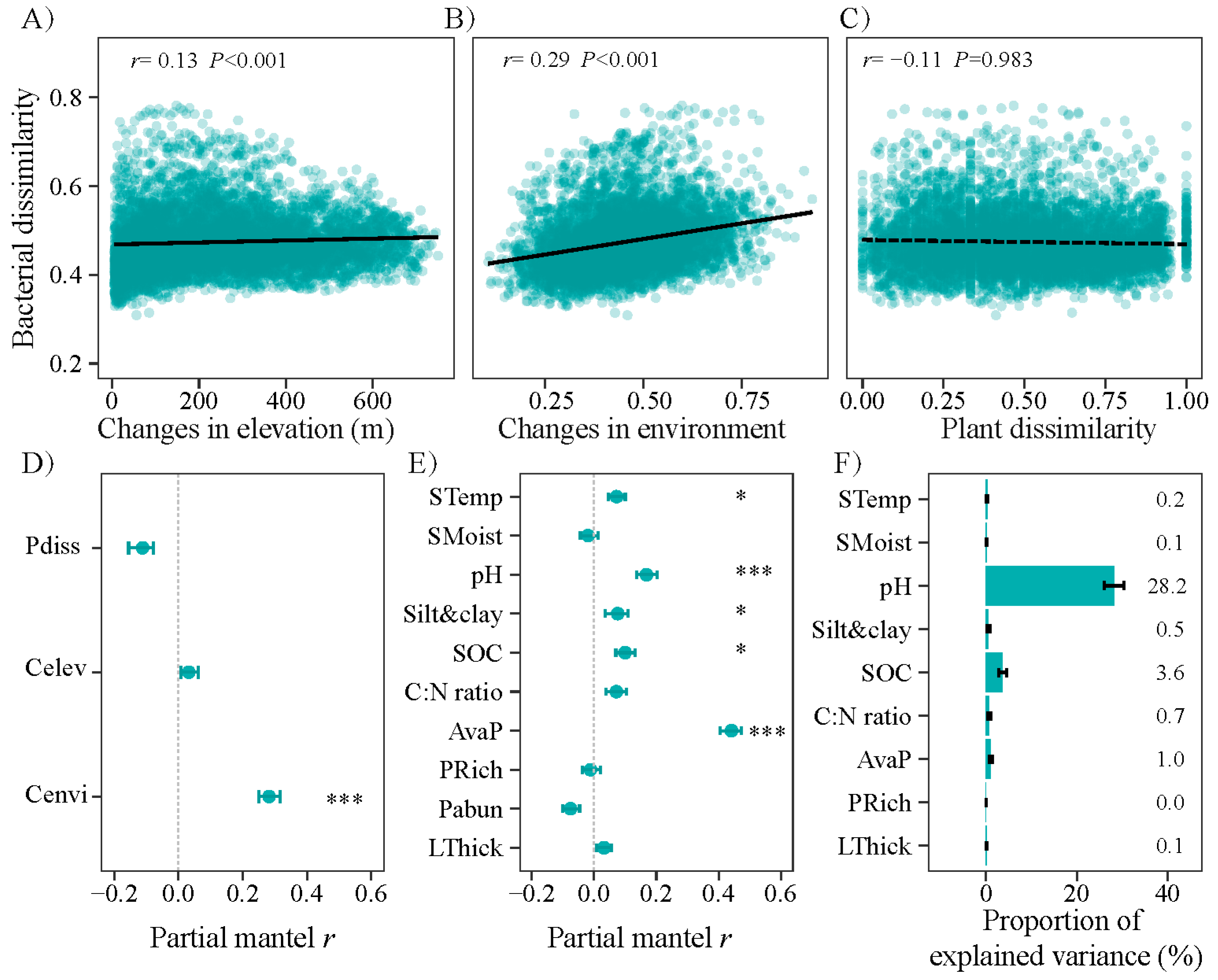
3.3. Changes in Community Composition with Altitude and the Main Impacting Factors
4. Discussion
4.1. Bacterial Diversity and Phylogenetic Structure Decreased with Increasing Altitude
4.2. Bacterial Community Restricted by Soil Condition over Plant Property
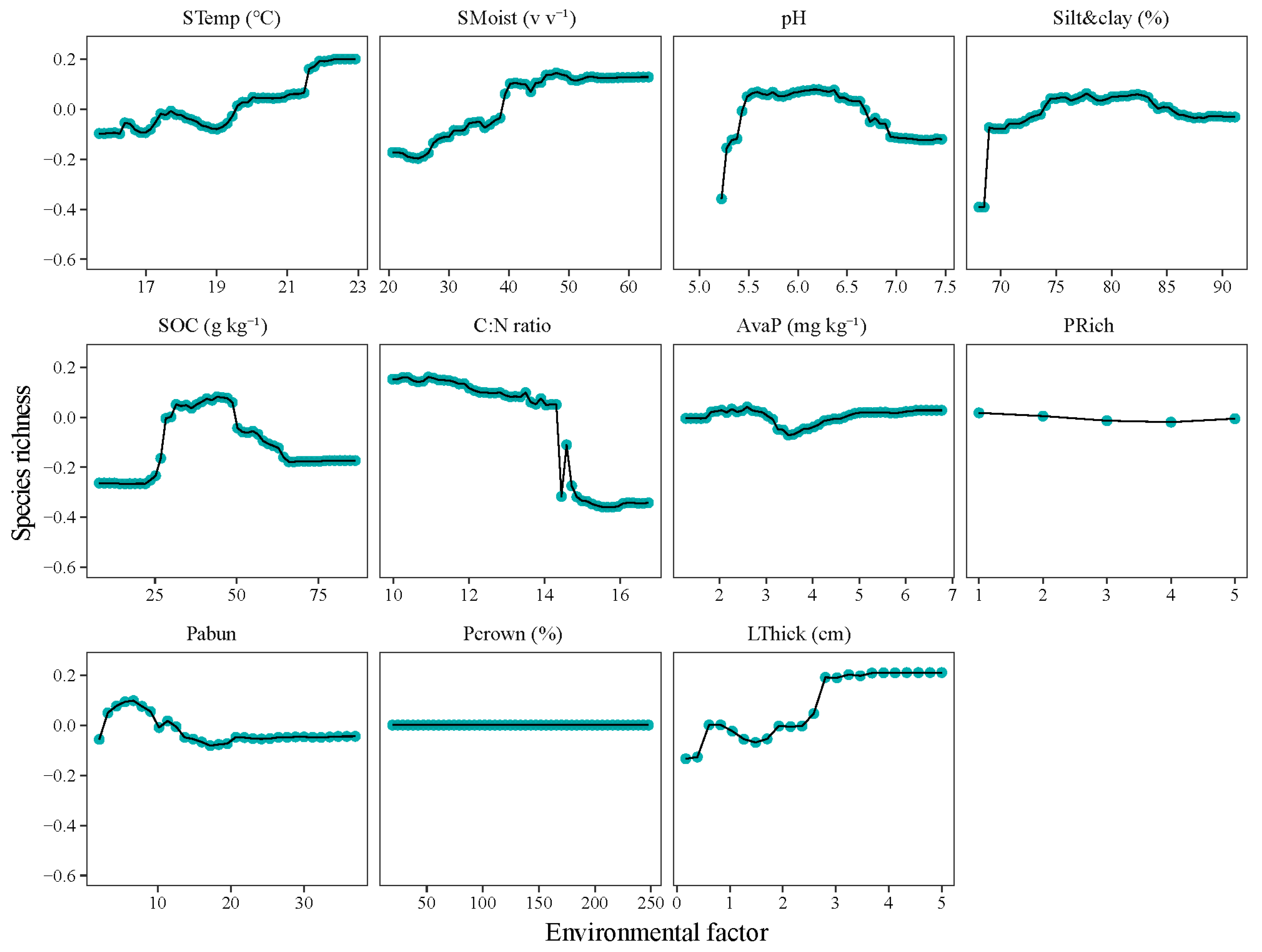
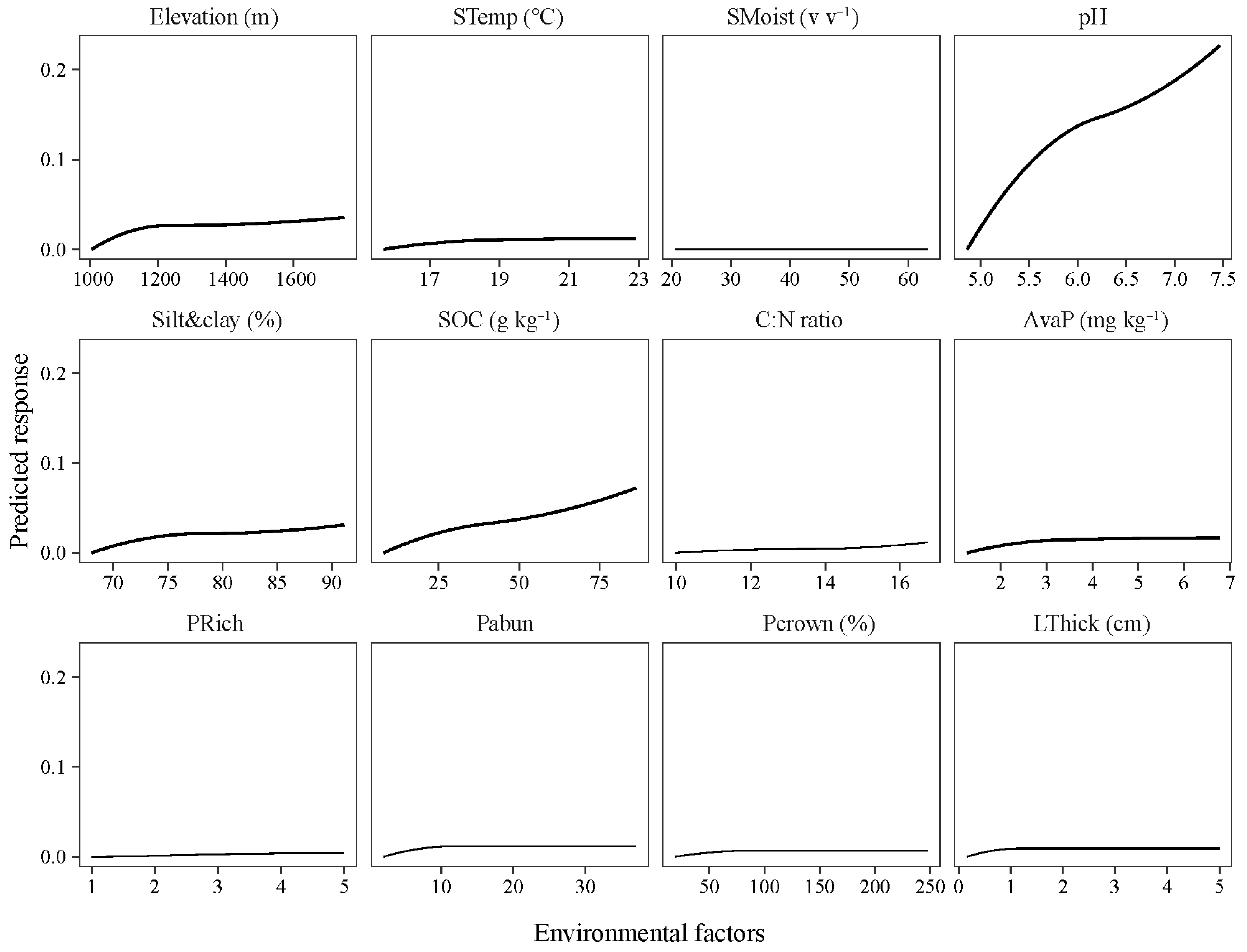
4.3. “Bell-Shaped” Responses of Bacterial Community to Increasing Soil pH and Nutrients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Xu, G.R.; Shen, C.C.; Tang, Y.; Zhang, Y.X.; Ma, K.M. Contrasting elevational diversity patterns for soil bacteria between two ecosystems divided by the treeline. Sci. China Life Sci. 2016, 59, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Zhao, W.Q.; Ren, M.L.; Liu, Y.Q.; Xu, Y.; Wang, J.J. Contrasting elevational patterns and underlying drivers of stream bacteria and fungi at the regional scale on the Tibetan Plateau. FEMS Microbiol. Ecol. 2022, 98, fiac050. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Shi, Y.; Fan, K.K.; He, J.S.; Adams, J.M.; Ge, Y.; Chu, H.Y. Soil pH dominates elevational diversity pattern for bacteria in high elevation alkaline soils on the Tibetan Plateau. FEMS Microbiol. Ecol. 2019, 95, fiz003. [Google Scholar] [CrossRef]
- Ding, Z.Q.; Xu, G.R.; Zhang, Y.X.; Zhang, S.; Ma, K.M. Multi-Scale Altitudinal patterns of soil and litter invertebrate communities in a warm temperate deciduous broadleaf forest. Forests 2023, 14, 2009. [Google Scholar] [CrossRef]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Ma, K.M.; Liu, H.Y.; Tang, Z.Y. A trait-based approach reveals the importance of biotic filter for elevational herb richness pattern. J. Biogeogr. 2018, 45, 2288–2298. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef]
- Zheng, H.F.; Chen, Y.M.; Liu, Y.; Hedenec, P.; Peng, Y.; Xu, Z.F.; Tan, B.; Zhang, L.; Guo, L.; Wang, L.F.; et al. Effects of litter quality diminish and effects of vegetation type develop during litter decomposition of two shrub species in an alpine treeline ecotone. Ecosystems 2021, 24, 197–210. [Google Scholar] [CrossRef]
- Bernard, J.; Wall, C.B.; Costantini, M.S.; Rollins, R.L.; Atkins, M.L.; Cabrera, F.P.; Cetraro, N.D.; Feliciano, C.K.J.; Greene, A.L.; Kitamura, P.K.; et al. Plant part and a steep environmental gradient predict plant microbial composition in a tropical watershed. ISME J. 2021, 15, 999–1009. [Google Scholar] [CrossRef]
- Yang, J.L.; Yu, J.; Wang, J.F.; Li, X.W. Soil microbial community responds to elevation gradient in an arid montane ecosystem in northwest China. Forests 2022, 13, 1360. [Google Scholar] [CrossRef]
- Lin, L.T.; Ruan, Z.Y.; Jing, X.; Wang, Y.G.; Feng, W.T. Soil salinization increases the stability of fungal not bacterial communities in the Taklamakan desert. Soil Ecol. Lett. 2023, 5, 230175. [Google Scholar] [CrossRef]
- Wang, Y.S.; Li, C.N.; Kou, Y.P.; Wang, J.J.; Tu, B.; Li, H.; Li, X.Z.; Wang, C.T.; Yao, M.J. Soil pH is a major driver of soil diazotrophic community assembly in Qinghai-Tibet alpine meadows. Soil Biol. Biochem. 2017, 115, 547–555. [Google Scholar] [CrossRef]
- de Wit, R.; Bouvier, T. ‘Everything is everywhere, but, the environment selects’; what did Baas Becking and Beijerinck really say? Environ. Microbiol. 2006, 8, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.T.; Li, S.Y.; Sun, X.Y.; Zhou, W.Z.; He, L.B.; Zhao, G.Y.; Chen, Z.; Bai, X.T.; Zhang, J.S. The Impact and Determinants of Mountainous Topographical Factors on Soil Microbial Community Characteristics. Microorganisms 2023, 11, 2878. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Soininen, J.; Zhang, Y.; Wang, B.; Yang, X.; Shen, J. Patterns of elevational beta diversity in micro- and macroorganisms. Glob. Ecol. Biogeogr. 2012, 21, 743–750. [Google Scholar] [CrossRef]
- Zhang, Q.; Goberna, M.; Liu, Y.G.; Cui, M.; Yang, H.S.; Sun, Q.X.; Insam, H.; Zhou, J.X. Competition and habitat filtering jointly explain phylogenetic structure of soil bacterial communities across elevational gradients. Environ. Microbiol. 2018, 20, 2386–2396. [Google Scholar] [CrossRef]
- Merino-Martin, L.; Griffiths, R.I.; Gweon, H.S.; Furget-Bretagnon, C.; Oliver, A.; Mao, Z.; Le Bissonnais, Y.; Stokes, A. Rhizosphere bacteria are more strongly related to plant root traits than fungi in temperate montane forests: Insights from closed and open forest patches along an elevational gradient. Plant Soil 2020, 450, 183–200. [Google Scholar] [CrossRef]
- Zi, H.Y.; Jiang, Y.L.; Cheng, X.M.; Li, W.T.; Huang, X.X. Change of rhizospheric bacterial community of the ancient wild tea along elevational gradients in Ailao mountain, China. Sci. Rep. 2020, 10, 9203. [Google Scholar] [CrossRef]
- Wu, M.H.; Chen, S.Y.; Chen, J.W.; Xue, K.; Chen, S.L.; Wang, X.M.; Chen, T.; Kang, S.C.; Rui, J.P.; Thies, J.E.; et al. Reduced microbial stability in the active layer is associated with carbon loss under alpine permafrost degradation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025321118. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Mavengere, N.R.; Ellis, A.G. The structure of legume-rhizobium interaction networks and their response to tree invasions. Aob Plants 2016, 8, plw038. [Google Scholar] [CrossRef] [PubMed]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954. [CrossRef]
- Cornfield, A.J.N. Ammonia released on treating soils with N sodium hydroxide as a possible means of predicting the nitrogen-supplying power of soils. Nature 1960, 187, 260–261. [Google Scholar] [CrossRef]
- Sun, D.-L.; Jiang, X.; Wu, Q.L.; Zhou, N.-Y.J.A. Intragenomic heterogeneity of 16S rRNA genes causes overestimation of prokaryotic diversity. Appl. Environ. Microbiol. 2013, 79, 5962–5969. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B.J.B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L.J.B. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R.J.B. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Webb, C.O. Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 2000, 156, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.; Kembel, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O.J.B. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Martiny, A.C.; Treseder, K.; Pusch, G. Phylogenetic conservatism of functional traits in microorganisms. ISME J. 2013, 7, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Goslee, S.; Urban, D. The ecodist Package: Dissimilarity-based functions for ecological analysis. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Machac, A.; Janda, M.; Dunn, R.R.; Sanders, N.J. Elevational gradients in phylogenetic structure of ant communities reveal the interplay of biotic and abiotic constraints on diversity. Ecography 2011, 34, 364–371. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, D.Y.; Li, H.B.; Huang, R.; Wang, J.J.; Wu, Q.L.L. A monotonically declining elevational pattern of bacterial diversity in freshwater lake sediments. Environ. Microbiol. 2016, 18, 5175–5186. [Google Scholar] [CrossRef]
- Newton, R.J.; Jones, S.E.; Helmus, M.R.; McMahon, K.D. Phylogenetic ecology of the freshwater Actinobacteria acI lineage. Appl. Env. Microb. 2007, 73, 7169–7176. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C: N: P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass. Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Landesman, W.J.; Nelson, D.M.; Fitzpatrick, M.C. Soil properties and tree species drive beta-diversity of soil bacterial communities. Soil Biol. Biochem. 2014, 76, 201–209. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microb. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Martiny, J.B.H.; Jones, S.E.; Lennon, J.T.; Martiny, A.C. Microbiomes in light of traits: A phylogenetic perspective. Science 2015, 350, aac9323. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Tripathi, B.M.; Moroenyane, I.; Takahashi, K.; Kerfahi, D.; Dong, K.; Adams, J.M. Soil pH rather than elevation determines bacterial phylogenetic community assembly on Mt. Norikura. FEMS Microbiol. Ecol. 2019, 95, fiy216. [Google Scholar] [CrossRef]
- Encinas-Viso, F.; Alonso, D.; Klironomos, J.N.; Etienne, R.S.; Chang, E.R. Plant-mycorrhizal fungus co-occurrence network lacks substantial structure. Oikos 2016, 125, 457–467. [Google Scholar] [CrossRef]
- Lin, L.T.; Liu, Y.J.; Wang, Y.G.; Zhang, C.; Feng, W.T. Bacterial communities rather than fungal communities driven soil functions after desert highway shelterbelts construction. Acta Ecol. Sin. 2024, 44, 3612–3622. [Google Scholar]
- Hiiesalu, I.; Schweichhart, J.; Angel, R.; Davison, J.; Dolezal, J.; Kopecky, M.; Macek, M.; Rehakova, K. Plant-symbiotic fungal diversity tracks variation in vegetation and the abiotic environment along an extended elevational gradient in the Himalayas. FEMS Microbiol. Ecol. 2023, 99, fiad092. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Kim, M.; Singh, D.; Lee-Cruz, L.; Lai-Hoe, A.; Ainuddin, A.N.; Go, R.; Rahim, R.A.; Husni, M.H.A.; Chun, J.; et al. Tropical Soil Bacterial Communities in Malaysia: pH Dominates in the Equatorial Tropics Too. Microb. Ecol. 2012, 64, 474–484. [Google Scholar] [CrossRef]
- Kamble, P.N.; Gaikwad, V.B.; Kuchekar, S.R.; Baath, E. Microbial growth, biomass, community structure and nutrient limitation in high pH and salinity soils from Pravaranagar (India). Eur. J. Soil Biol. 2014, 65, 87–95. [Google Scholar] [CrossRef]
- Mod, H.K.; Buri, A.; Yashiro, E.; Guex, N.; Malard, L.; Pinto-Figueroa, E.; Pagni, M.; Niculita-Hirzel, H.; van der Meer, J.R.; Guisan, A. Predicting spatial patterns of soil bacteria under current and future environmental conditions. ISME J. 2021, 15, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Xiong, J.B.; Liu, Y.Q.; Lin, X.G.; Zhang, H.Y.; Zeng, J.; Hou, J.Z.; Yang, Y.P.; Yao, T.D.; Knight, R.; Chu, H.Y. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ. Microbiol. 2012, 14, 2457–2466. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Li, G.; Yu, H.; Ma, K. pH Nonlinearly Dominates Soil Bacterial Community Assembly along an Altitudinal Gradient in Oak-Dominant Forests. Microorganisms 2024, 12, 1877. https://doi.org/10.3390/microorganisms12091877
Lin L, Li G, Yu H, Ma K. pH Nonlinearly Dominates Soil Bacterial Community Assembly along an Altitudinal Gradient in Oak-Dominant Forests. Microorganisms. 2024; 12(9):1877. https://doi.org/10.3390/microorganisms12091877
Chicago/Turabian StyleLin, Litao, Guixiang Li, Huiyi Yu, and Keming Ma. 2024. "pH Nonlinearly Dominates Soil Bacterial Community Assembly along an Altitudinal Gradient in Oak-Dominant Forests" Microorganisms 12, no. 9: 1877. https://doi.org/10.3390/microorganisms12091877






