Streptococcus suis Induces Macrophage M1 Polarization and Pyroptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Animal Experiment
2.3. Cell and Culture
2.4. LDH Release Assay
2.5. Transmission Electron Microscopy
2.6. Confocal Microscopy
2.7. RT-PCR
2.8. Live-Cell Imaging
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. S. suis Promotes M1 Macrophage Activation in Atrophied Thymus of Infected Mice
3.2. S. suis Primes Pyroptosis in Atrophied Thymus of Infected Mice
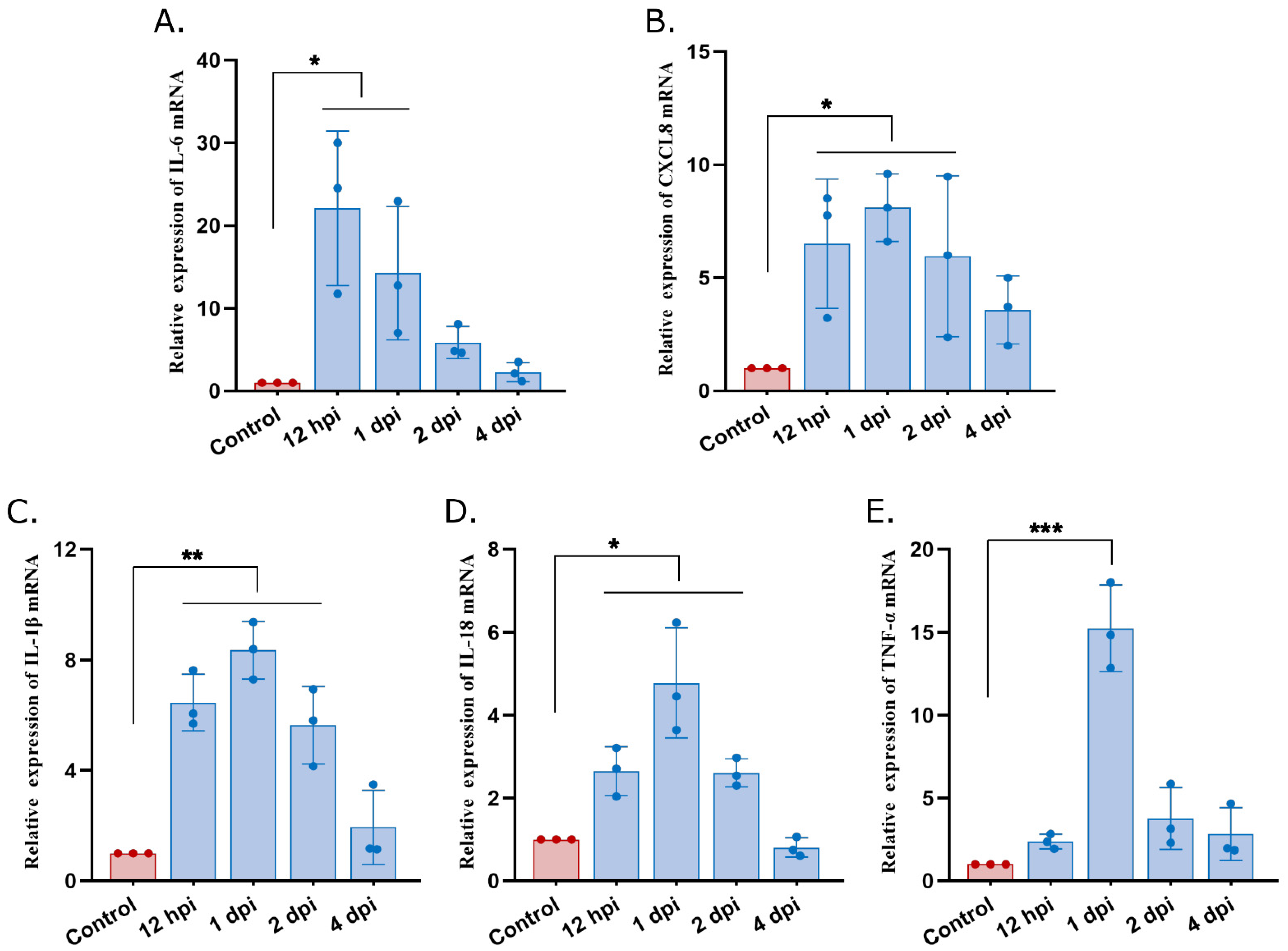
3.3. High Expression of Proinflammatory Mediators in Atrophied Thymus of S. suis-Infected Mice
3.4. S. suis-Induced LDH Positively Correlates with the Infection Time In Vitro
3.5. S. suis Induces Pyroptosis Both in iPAMs and Primary PAMs In Vitro
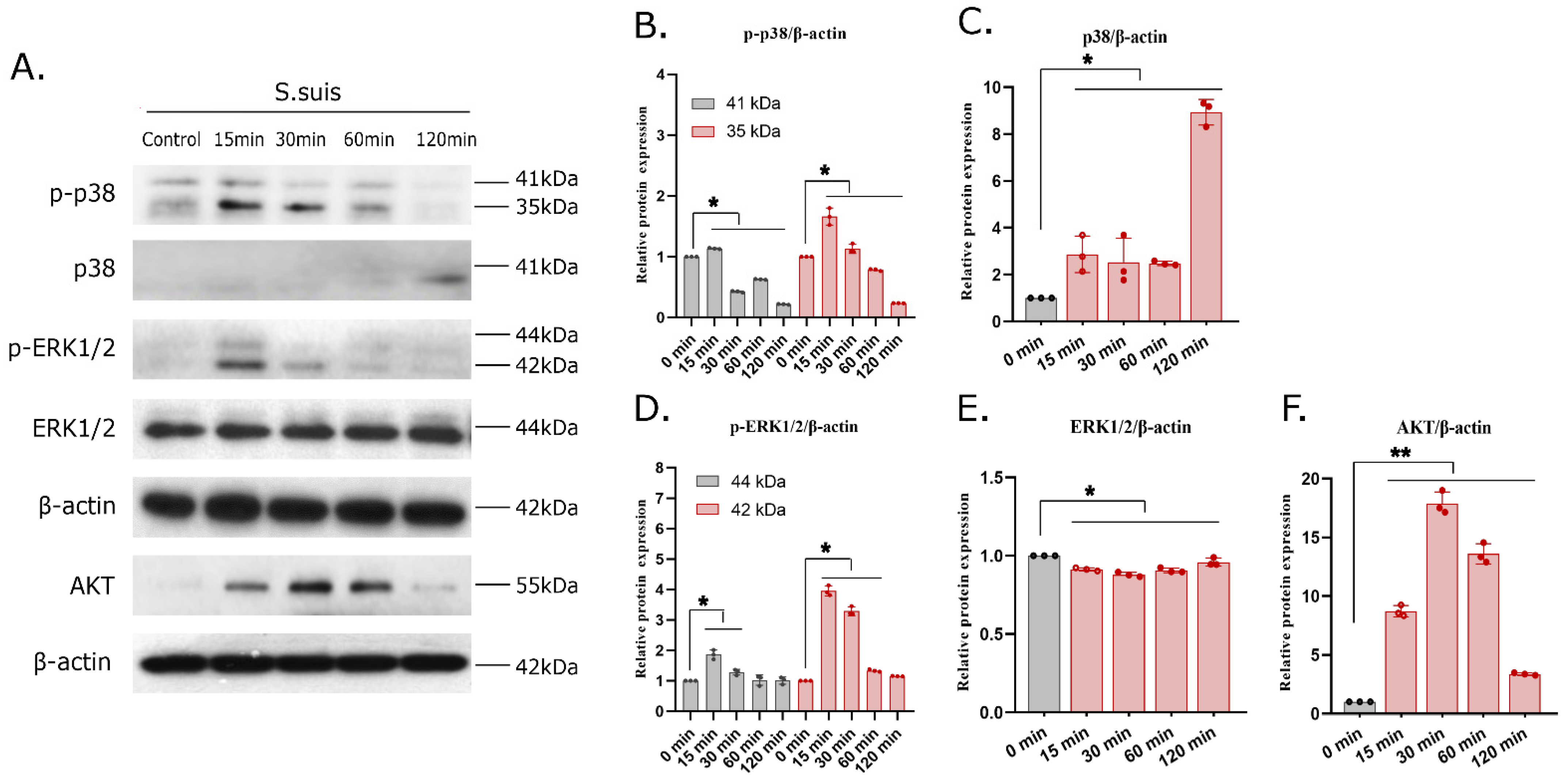
3.6. S. suis Infection Actives the MAPK and AKT Signaling Pathways in PAMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Wang, M.; Hao, H.; Yang, R.; Xie, J.; Su, J.; Lin, M.; Cui, Y.; Jiang, Y. Genomic epidemiological investigation of a Streptococcus suis outbreak in Guangxi, China, 2016. Infect. Genet. Evol. 2019, 68, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, M.J.; Ornelas, M.A.S.; Amarie, R.E.; Manzanilla, E.G.; Martínez-Subiela, S.; Tecles, F.; Tvarijonaviciute, A.; Escribano, D.; González-Bulnes, A.; Cerón, J.J.; et al. Changes in salivary biomarkers of stress, inflammation, redox status, and muscle damage due to Streptococcus suis infection in pigs. BMC Vet. Res. 2023, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Grenier, D. Understanding the virulence of Streptococcus suis: A veterinary, medical, and economic challenge. Med. Mal. Infect. 2018, 48, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, S.; Okura, M.; Takamatsu, D.; Corsaut, L.; Gottschalk, M. Development of a mismatch amplification mutation assay to correctly serotype isolates of Streptococcus suis serotypes 1, 2, 1/2, and 14. J. Vet. Diagn. Investig. 2020, 32, 490–494. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Nghia, H.D.; Hoa, N.T.; Linh, L.D.; Campbell, J.; Diep, T.S.; Chau, N.V.; Mai, N.T.; Hien, T.T.; Spratt, B.; Farrar, J.; et al. Human case of Streptococcus suis serotype 16 infection. Emerg. Infect. Dis. 2008, 14, 155–157. [Google Scholar] [CrossRef]
- Kerdsin, A.; Akeda, Y.; Takeuchi, D.; Dejsirilert, S.; Gottschalk, M.; Oishi, K. Genotypic diversity of Streptococcus suis strains isolated from humans in Thailand. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 917–925. [Google Scholar] [CrossRef]
- Lun, Z.R.; Wang, Q.P.; Chen, X.G.; Li, A.X.; Zhu, X.Q. Streptococcus suis: An emerging zoonotic pathogen. The Lancet. Infect. Dis. 2007, 7, 201–209. [Google Scholar] [CrossRef]
- Wang, S.; Lyu, C.; Duan, G.; Meng, F.; Yang, Y.; Yu, Y.; He, X.; Wang, Z.; Gottschalk, M.; Li, G.; et al. Streptococcus suis Serotype 2 Infection Causes Host Immunomodulation through Induction of Thymic Atrophy. Infect. Immun. 2020, 88, e00950-19. [Google Scholar] [CrossRef]
- Gottschalk, M.; Segura, M.; Xu, J. Streptococcus suis infections in humans: The Chinese experience and the situation in North America. Anim. Health Res. Rev. 2007, 8, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Navegantes, K.C.; de Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, J.L. Macrophages: Their Untold Story in T Cell Activation and Function. Int. Rev. Cell Mol. Biol. 2019, 342, 73–93. [Google Scholar] [PubMed]
- Hulsmans, M.; Sager, H.B.; Roh, J.D.; Valero-Muñoz, M.; Houstis, N.E.; Iwamoto, Y.; Sun, Y.; Wilson, R.M.; Wojtkiewicz, G.; Tricot, B.; et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 2018, 215, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Rigamonti, E.; Zordan, P.; Sciorati, C.; Rovere-Querini, P.; Brunelli, S. Macrophage plasticity in skeletal muscle repair. BioMed Res. Int. 2014, 2014, 560629. [Google Scholar] [CrossRef]
- Place, D.E.; Lee, S.; Kanneganti, T.D. PANoptosis in microbial infection. Curr. Opin. Microbiol. 2021, 59, 42–49. [Google Scholar] [CrossRef]
- Jiang, W.; Deng, Z.; Dai, X.; Zhao, W. PANoptosis: A New Insight Into Oral Infectious Diseases. Front. Immunol. 2021, 12, 789610. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, L. Key Components of Inflammasome and Pyroptosis Pathways Are Deficient in Canines and Felines, Possibly Affecting Their Response to SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 592622. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Tang, Y.D.; Qin, L.; Chen, T.; Wang, S.; Bai, Y.; Cai, X.; Wang, S. Interaction between Porcine Alveolar Macrophage-Tang Cells and Streptococcus suis Strains of Different Virulence: Phagocytosis and Apoptosis. Microorganisms 2023, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Done, S.H.; Paton, D.J. Porcine reproductive and respiratory syndrome: Clinical disease, pathology and immunosuppression. Vet. Rec. 1995, 136, 32–35. [Google Scholar] [CrossRef]
- Imori, P.F.; Passaglia, J.; Souza, R.A.; Rocha, L.B.; Falcao, J.P. Virulence-related genes, adhesion and invasion of some Yersinia enterocolitica-like strains suggests its pathogenic potential. Microb. Pathog. 2017, 104, 72–77. [Google Scholar] [CrossRef]
- Li, H.; Yang, D.H.; Zhang, Y.; Zheng, F.; Gao, F.; Sun, J.; Shi, G. Geniposide suppresses NLRP3 inflammasome-mediated pyroptosis via the AMPK signaling pathway to mitigate myocardial ischemia/reperfusion injury. Chin. Med. 2022, 17, 73. [Google Scholar] [CrossRef]
- Savino, W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2006, 2, e62. [Google Scholar] [CrossRef]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Segura, M.; Gottschalk, M. Streptococcus suis interactions with the murine macrophage cell line J774: Adhesion and cytotoxicity. Infect. Immun. 2002, 70, 4312–4322. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Ou, Q.; Wu, B.; Li, S.; Wu, M.; Qiu, J.; Cen, N.; Hu, K.; Che, Y.; Ma, Y.; et al. TcpC Inhibits M1 but Promotes M2 Macrophage Polarization via Regulation of the MAPK/NF-κB and Akt/STAT6 Pathways in Urinary Tract Infection. Cells 2022, 11, 2674. [Google Scholar] [CrossRef]
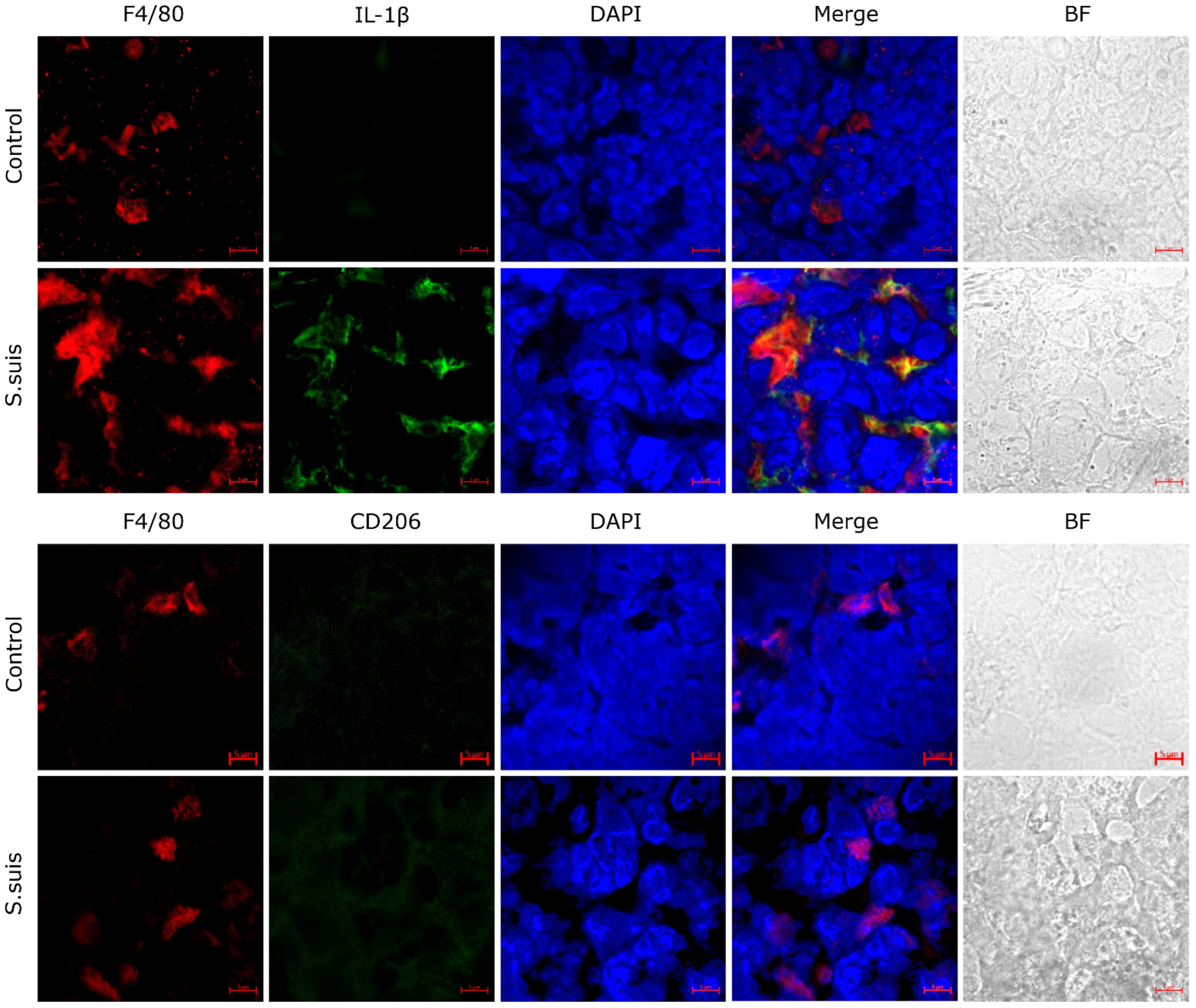



| Cell Surface | Antibody | Dilution, Temperature, Time | Fluorescent Secondary Antibody Temperature, Time |
|---|---|---|---|
| M1 Macrophage | IL-1β | 1:200, overnight, 4 °C | Alexa Fluor 488-conjugated goat anti-mouse IgG, RT, 1 h |
| M2 Macrophage | CD206 | 1:100, overnight, 4 °C | Alexa Fluor 488-conjugated goat anti-mouse IgG, RT, 1 h |
| Macrophage | F4/80 | 1:50, overnight, 4 °C | Alexa Fluor 647-conjugated rabbit anti-mouse F4/80, RT, 1 h |
| Pyroptosis | GSDMD-N | 1:50, overnight, 4 °C | Alexa Fluor 568-conjugated goat anti-rabbit antibody, RT, 1 h |
| Nuclei | DAPI | RT, 20 min |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| IL-6 | TGTGCAATGGCAATTCTGAT | GGTACTCCAGAAGACCAGAGGA |
| IL-8 | GGTCTTCCTGCTTGAATGGCTTGA | GCGGTGTCCTGATTATCGTCCTC |
| IL-18 | GCCATGTCAGAAGACTCTTGCGTC | GTACAGTGAAGTCGGCCAAAGTTGTC |
| IL-1β | GGTGTGTGACGTTCCCATTAGAC | AGGGTGGGTGTGCCGTCTT |
| TNF-α | GTCTACTGAACTTCGGGGTGATCG | TGGGCTACAGGCTTGTCACTCG |
| β-actin | GCTTCTTTGCAGCTCCTTCGT | CCTTCTGACCCATTCCCACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, T.; Gao, K.; Yang, Y.-B.; Qi, B.; Wang, C.; An, T.; Cai, X.; Wang, S. Streptococcus suis Induces Macrophage M1 Polarization and Pyroptosis. Microorganisms 2024, 12, 1879. https://doi.org/10.3390/microorganisms12091879
Li S, Chen T, Gao K, Yang Y-B, Qi B, Wang C, An T, Cai X, Wang S. Streptococcus suis Induces Macrophage M1 Polarization and Pyroptosis. Microorganisms. 2024; 12(9):1879. https://doi.org/10.3390/microorganisms12091879
Chicago/Turabian StyleLi, Siqi, Tianfeng Chen, Kexin Gao, Yong-Bo Yang, Baojie Qi, Chunsheng Wang, Tongqing An, Xuehui Cai, and Shujie Wang. 2024. "Streptococcus suis Induces Macrophage M1 Polarization and Pyroptosis" Microorganisms 12, no. 9: 1879. https://doi.org/10.3390/microorganisms12091879








