Abstract
Burkholderia (sensu lato) is a diverse group of β-Proteobacteria that exists worldwide in various environments. The SBE clade of this group was thought to be mutualistic with stinkbugs. Riptortus–Burkholderia was suggested as an ideal model system for studying insect–microbe symbiosis. To explore the strain-level diversity of Burkholderia at the individual and population levels of Riptortus stinkbugs (Hemiptera: Alydidae), and to uncover the factors affecting the Burkholderia community, large-scale sampling of two Riptortus species and deep sequencing data (16S amplicon) were used in the present study. Our results showed that: (1) the proportions of facultative symbiotic bacteria Burkholderia were very high, with an average proportion of 87.1% in the samples; (2) only six out of 1373 Burkholderia amplicon sequence variants (ASVs) did not belong to the SBE clade, accounting for only 0.03% of Burkholderia; (3) a relatively small number of Burkholderia ASVs had a large number of sequences, with 22, 54, and 107 ASVs accounting for more than 1.0%, 0.1%, and 0.01% of the total Burkholderia sequences, respectively; (4) multiple Burkholderia ASVs were present in most Riptortus individuals, but there was one dominant or two codominant ASVs, and codominance was more likely to occur when the genetic distance between the two codominant ASVs was small; and (5) the beta diversity of Burkholderia was significantly different between the two host species (PerMANOVA: both Jaccard and Bray–Curtis, p < 0.001) and among localities (PerMANOVA: both Jaccard and Bray–Curtis, p < 0.001). Two-way PerMANOVA also indicated that both the host (Bray–Curtis, p = 0.020; Jaccard, p = 0.001) and geographical location (Bray–Curtis, p = 0.041; Jaccard, p = 0.045) influence Burkholderia communities; furthermore, Mantel tests showed that the Burkholderia communities were significantly correlated with the geographical distance of sample locations (R = 0.056, p = 0.001). Together, our findings demonstrate the fine-scale diversity of Burkholderia symbionts and suggest a region- and host-dependent pattern of Burkholderia in Riptortus stinkbugs.
1. Introduction
Insects lack metabolic pathways for the synthesis of essential amino acids and most vitamins [1,2]. Accordingly, they use two strategies to obtain missing nutrients: directly from food sources or through the synthesis of symbiotic microbial partners [1]. With piercing-sucking mouthparts, hemipteran insects (aphids, cicadas, planthoppers, leafhoppers, stinkbugs, etc.) face the challenge of a nutritionally unbalanced diet, and they cannot obtain enough nutrients (e.g., essential amino acids and B vitamins) from their food. Instead, hemipteran insects usually overcome this nutrient obstacle by harboring specific beneficial microbes in their specialized symbiotic organs, either intracellularly [3,4,5] or extracellularly [6,7,8].
Burkholderia sensu lato is a diverse group of Gram-negative nonfermenting β-Proteobacteria that exist worldwide in various aquatic and terrestrial environments that can be mutualistic or pathogenic to plants, fungi, and animals [7,9,10,11]. Generally, Burkholderia sensu lato is phylogenetically divided into the following three main clades: the pathogenic Burkholderia cepacia complex and Burkholderia pseudomallei group (BCC&P); the nonpathogenic plant-associated beneficial and environmental group (PBE); and the stinkbug-associated beneficial and environmental group (SBE) [7,12,13]. Burkholderia sensu lato has undergone a series of taxonomic revisions since it was proposed in 1992 [14,15,16,17,18,19]. In fact, the meaning of this group is more complex than we once thought. Under the ongoing updated classification framework, Burkholderia sensu lato contains three major genera, as follows: Burkholderia sensu stricto (i.e., the BCC&P clade); Paraburkholderia (i.e., the PBE clade); and Caballeronia (i.e., the SBE clade). In addition, there are several small genera, namely, Robbsia, Mycetohabitans, Trinickia, and Pararobbsia [20,21,22]. In the following work, Burkholderia refers to sensu lato unless otherwise specified.
Riptortus–Burkholderia was suggested as an ideal model system for studying insect–microbe symbiosis [7,12]. The SBE clade of Burkholderia (i.e., Caballeronia in some references) has been shown to be a mutually beneficial bacterium with some stinkbugs (Hemiptera: Heteroptera), mainly in Lygaeoidea and Coreoidea [7,23,24,25,26], while PBE clade (i.e., Paraburkholderia) has been shown in Pyrrhocoroidea [27]. Burkholderia consumes specific nutrients and metabolic wastes in the midgut of stinkbugs; the bacterial symbiont provides the bug with essential nutrients that are lacking in the food of the bug [28].
In these stinkbugs (however, see Itoh et al., 2014) [29], Burkholderia is not transmitted vertically from mother to offspring but is instead acquired from the environmental soil of each generation [30]. As with other infraorder Pentatomomorpha species, specialized “crypts” are found in the posterior midgut region of Riptortus stinkbugs (Coreoidea: Alydidae) that consist of a large number of sac-like structures that facilitate better colonization by symbiotic microbes [31]. The middle part of the midgut is a constricted region filled with a mucus-like matrix that has the ability to prevent nonsymbiotic or pathogenic microbes from entering the posterior midgut during the environmental acquisition of symbiotic bacteria from extremely diverse soil microbiota [13,32,33,34]. The symbiotic bacteria colonize crypts starting from a limited number of infecting bacteria acquired from the environment through feeding and a massive extracellular population is established in the midgut crypts within a few days [33,35]. Under laboratory conditions, nonsymbiotic Burkholderia strains and even Pandoraea could also stably colonize midgut crypts and benefit Riptortus pedestris; however, further co-inoculation experiments showed that native Burkholderia continuously outcompeted nonnative bacteria inside crypts, which may explain the predominance of the native Burkholderia symbiont in R. pedestris [13].
The symbiosis of Riptortus–Burkholderia symbionts at the host population level has been reported in several studies. For example, the cladistic composition of Burkholderia in South Korean populations of R. pedestris was characterized via diagnostic PCR analysis [36,37]. The species diversity of Burkholderia (sensu lato) in laboratory-raised R. pedestris individuals who provided field soil environments was analyzed by cloning sequencing [34]. Although a relaxed specificity at the strain level of Burkholderia in stinkbugs has been suggested [27], the symbiosis of Riptortus–Burkholderia has not been investigated at the bacterial strain level. Large-scale sampling of two Riptortus species (R. pedestris and R. linearis) (Figure 1) and deep sequencing data (16S amplicon) were used in the present study. We aim to answer the following questions: (1) What is the strain-level diversity of Burkholderia at the individual and population levels of the host? (2) Do host insect species and geographical distance influence Burkholderia communities? This study provides further insights into the symbiosis of stinkbugs and Burkholderia in nature.
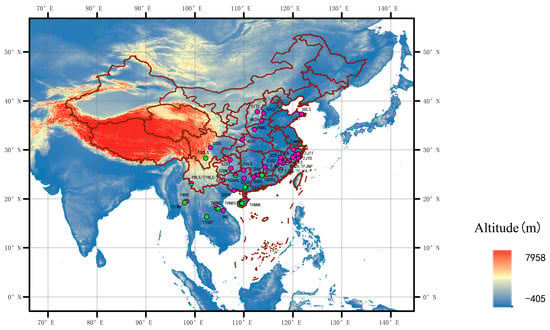
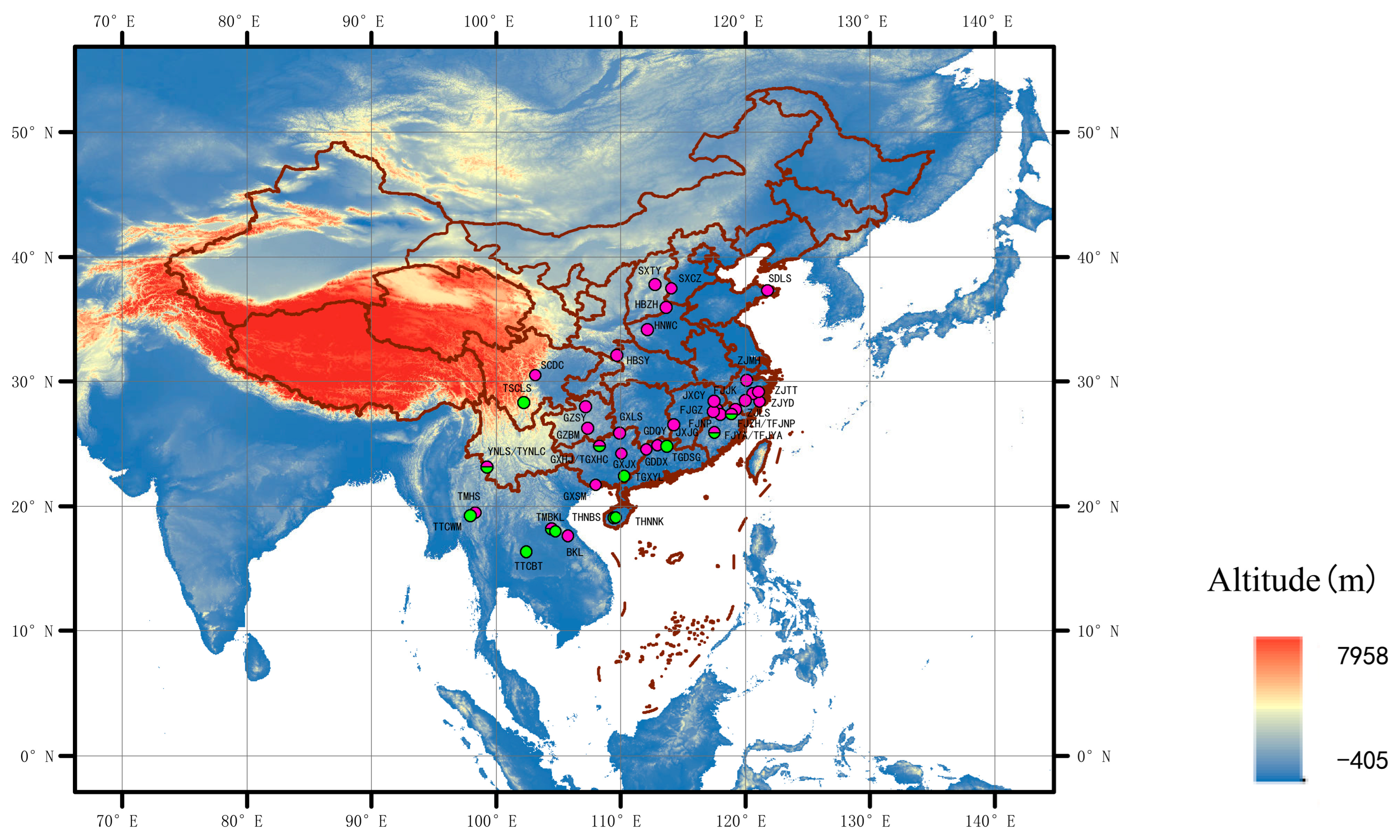
Figure 1.
Collection sites of Riptortus pedestris and R. linearis (the pink dots represent R. pedestris and the green dots represent R. linearis). For details of the abbreviations in the figure, see Supplementary Table S1.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
Adult specimens of Riptortus stinkbugs were collected across China, Thailand, and Laos from 2013 to 2019, preserved in 100% alcohol, and stored at −30 °C until DNA extraction. In the present study, 206 individuals of R. pedestris from 32 sites and 46 individuals of R. linearis from 13 sites (Figure 1; Supplementary Table S1) were used for DNA extraction.
The guts of alcohol-preserved specimens could not be fully detached; the whole abdominal contents were thus dissected. DNA was extracted using a Universal Genomic DNA Kit (CWBIO, Taizhou, China) according to the instructions.
2.2. Sequencing and Sequence Data Processing
The obtained DNA was tested for quality and then used for gene amplification. The V3-V4 hypervariable region of the 16S rRNA was amplified using the universal bacterial primer set 341f (5′-CCTAGGGRBGCASCAG-3′) and 806r (5′-GACTACNNGGGTATCTAAT-3′). The thermocycler settings were as follows: denaturation at 95 °C for 3 min; 30 cycles of denaturation at 95 °C for 30 s; primer annealing at 55 °C for 30 s; extension at 68 °C for 50 s; and a final extension at 68 °C for 10 min. The amplicons were sequenced on the Illumina NovaSeq sequencing platform at Novogene Biotech Co., Ltd, Beijing, China.
The sample data were divided according to the barcode sequence and PCR amplification primer sequence. After the amputation of barcode and primer sequences using FLASH (version 1.2.11, https://ccb.jhu.edu/software/FLASH/) [38], the reads of each sample were connected; the resulting splicing sequence was a raw tag. Fastp software (version 0.23.1) was used to strictly filter the raw tags obtained by splicing to achieve clean tags [39]. The tags acquired after the above processing were denoised by DADA2 [40] in QIIME2 (Version Qiime2-202202) [41] to yield the final amplicon sequence variants (ASVs) and feature list. Species annotation for each representative sequence of ASV was performed using QIIME2 in silva 138.1 (Silva database https://www.arb-silva.de/ for 16S) [42].
2.3. Bacterial Composition Calculation
The total bacterial sequences obtained from 252 Riptortus individuals were calculated. Most of the samples attained high sequencing data outputs. To make more efficient use of the data, the samples with sequencing depths less than 10,000 were excluded from further analysis. The absolute and relative contents of Burkholderia in the remaining samples were statistically analyzed.
2.4. Molecular Phylogenetic Analysis
Since the sequences of Burkholderia sensu lato were annotated as “Burkholderia-Caballeronia-Paraburkholderia” in the Silva 138.1 database, to further clarify the Burkholderia ASVs in Riptortus stinkbugs, maximum likelihood (ML) trees were constructed with 1393 sequences including the 1373 Burkholderia ASVs obtained in this study; 19 representative sequences of the SBE clade (i.e., Caballeronia); the BCC&P clade (i.e., Burkholderia sensu stricto); the PBE clade (i.e., Paraburkholderia); and one representative sequence of Pandoraea as an outgroup [30,43]. The sequences were aligned using MAFFT (version 7.520) according to the default settings [44]. ML analysis was performed using IQ-TREE (version 1.6.12) [45]. The best-fit model for amino acid sequence evolution was selected using Bayesian information criterion scores. Weights (BIC) were automatically selected using the ModelFinder module [45]. In the analysis, 1000 ultrafast bootstrap repeats were used to evaluate branch support [46,47,48]. The resulting tree was visualized and edited in FigTree (version 1.4.4) [49].
2.5. Strain-Level Diversity of Burkholderia
To obtain a more accurate estimate of the strain-level diversity of Burkholderia at the individual and population levels of the hosts, samples with fewer than 5000 Burkholderia reads were excluded when the data were limited to the genus Burkholderia.
The absolute and relative content of each Burkholderia ASV in the total sequences, the number of Burkholderia ASVs in each sample, and the relative content of each Burkholderia ASV in that sample, were calculated. To reflect the differences more directly, simple bar charts, pie charts, pile charts, and Venn diagrams were created. All the statistical analyses and diagrams were performed in R (version 4.3.2) using the R packages “tidyverse”, “readxl”, “scatterpie”, “ggpmisc”, and “VennDiagram”.
To estimate the genetic differentiation among major ASVs, we calculated the pairwise genetic distance of the 159 major ASVs of Burkholderia and calculated the genetic distance between each pair of the first and second highest ASVs in each individual using MEGAX (version 7.0.14) software [50]. To show the relationship between these major ASVs more clearly, the distribution of ASVs was summarized using a statistical parsimony haplotype network inferred in TCS 1.2.1 [51].
2.6. Symbiotic Microbial Community Analysis
To control for differences in sequencing depth between samples, the samples were rarefied to the minimum library size before alpha and beta diversity analyses. Flattening was performed with the R package “vegan”. The observed species richness (Sobs) index was calculated to construct rarefaction curves.
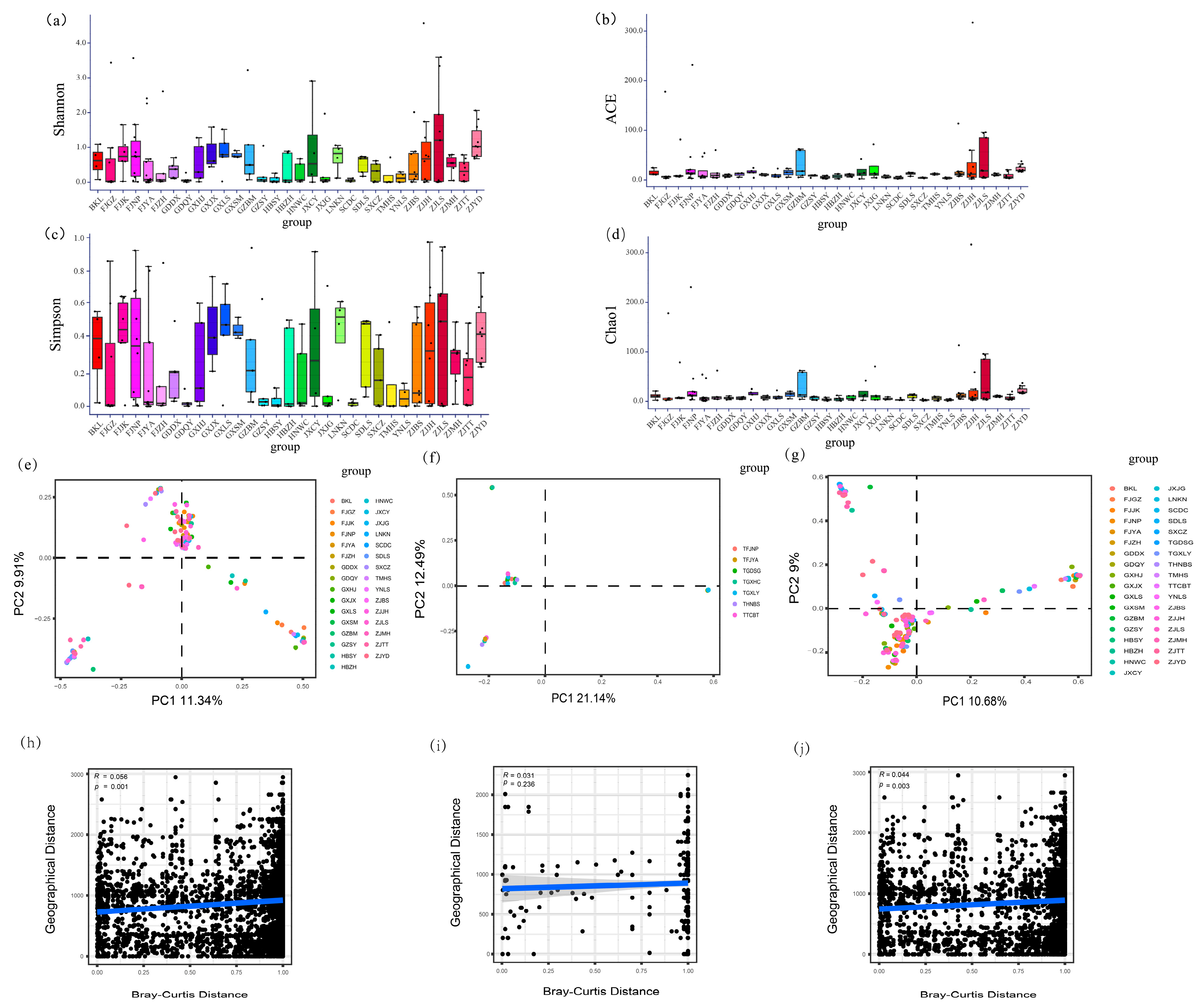
Two non-parametric richness indices, abundance-based coverage estimator (ACE) and bias-corrected Chao1, and two alpha diversity indices, Shannon (emphasizes richness and rare species) and Simpson (emphasizes evenness and dominant species) were determined for each sample. Nonparametric Mann–Whitney (for two groups) or Kruskal–Wallis (for multiple groups) tests were used to assess whether there were differences among locations and between host species for each of these estimators. SPSS (version 26.0.0) software was used for the above statistical analyses [52]. All analyses and charts were carried out in R (version 4.3.2) using the R packages “RcolorBrewer”, “dplyr”, “picante”, “vegan”, “ggprism”, “ggsignif”, “ggpubr”, and “ggplot2” [53,54].
Beta diversity comparisons were performed with the presence/absence metric Jaccard and the relative abundance metric Bray–Curtis. A PCoA map based on the Bray–Curtis index and Jaccard distance algorithm was used to show the beta diversity among the groups. ANOSIM and PerMANOVA were used to analyze the differences between host species and to compare the differences among locations (R. pedestris, R. linearis, and pooled samples were analyzed). All analyses and charts were performed in R (v4.3.2) using the packages “ggprism”, “vegan”, and “ggplot2” [53,54].
R. pedestris and R. linearis from five shared locations were selected to analyze the effects of both host species and geographic location on the structure of Burkholderia communities. In this analysis, two-way PerMANOVA (with 9999 permutations) was conducted using PAST software (version 4) [55], with “host species” and “location” as the main effects.
Mantel tests were used to further test whether there were correlations between Burkholderia communities and the geographic distance of sample sites [56,57]. R. pedestris, R. linearis, and pooled samples were analyzed. The R packages “tidyverse” and “vegan” were used to calculate the sample geographic distance and Bray–Curtis distance, respectively, and to construct a distance matrix. To directly reflect the correlation between the two factors, the R packages “ggExtra”, “ggpubr”, and “ggplot2” were applied to construct scatter plots [53,54].
Finally, the correlations between Burkholderia communities within the two Riptortus species and bioclimatic factors were tested. Nineteen bioclimatic factors with a 2.5 arc-min resolution from 1970 to 2000 on WorldClim 2.1 (https://worldclim.org/) [58] were accessed as a basic environment dataset (accessed on 3 September 2024). The correlation between each bioclimatic factor and each Riptortus species was tested using Mantel tests [56,57]. The analyses were performed in R (v4.3.2) using the packages “rgdal”,“raster”,“tidyverse”, and “patchwork” [53,54].
3. Results
3.1. Bacterial Composition of Riptortus Stinkbugs
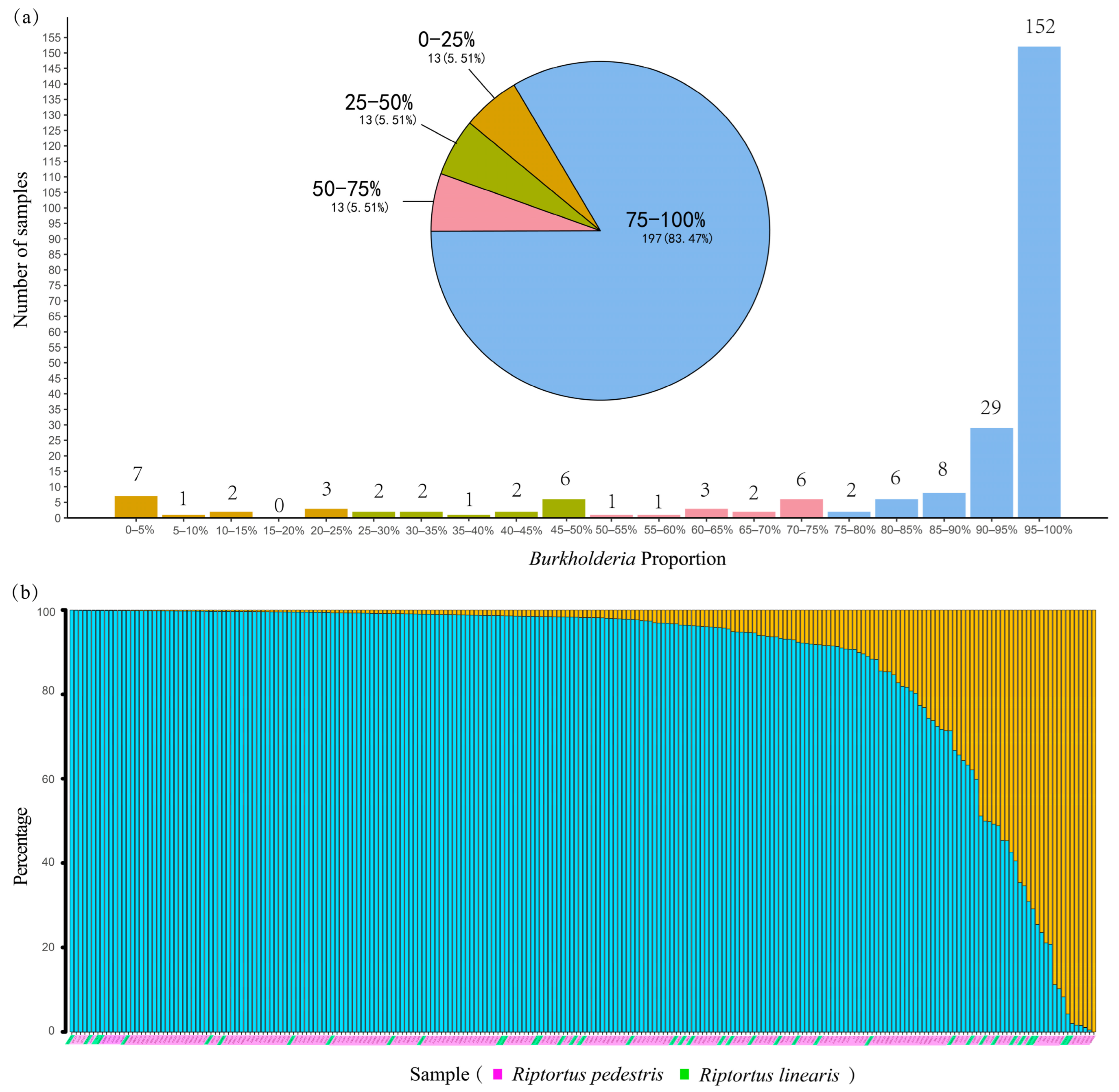
After sequence processing, quality filtering, and the removal of contaminants, a total of 12,012,828 bacterial sequences were obtained from 252 Riptortus individuals, with an average of 47,670 sequences (Supplementary Table S2). Most of the samples had high sequencing data outputs; 19 samples with sequencing depths less than 10,000 sequences were discarded from further analysis. In the remaining 233 samples, with an average of 51,258 sequences, a total of 12,065 bacterial ASVs were obtained. Among them, 1373 ASVs were assigned to Burkholderia-Caballeronia-Paraburkholderia (i.e., Burkholderia sensu lato). Burkholderia reads accounted for a high proportion of the reads in most samples, with an average proportion of 87.1%. In 181 samples (76.7%), Burkholderia accounted for more than 90% of the total bacterial reads. In only 26 samples (11.0%), Burkholderia accounted for less than 50% of the bacterial reads (Figure 2a). Burkholderia was undetected in only one (SXTY3) of the 233 individuals (Figure 2b). In addition to Burkholderia, other genera account for relatively high proportions of Serratia, Enterococcus, Lactococcus, Wolbachia, and Bartonella (Supplementary Table S2).
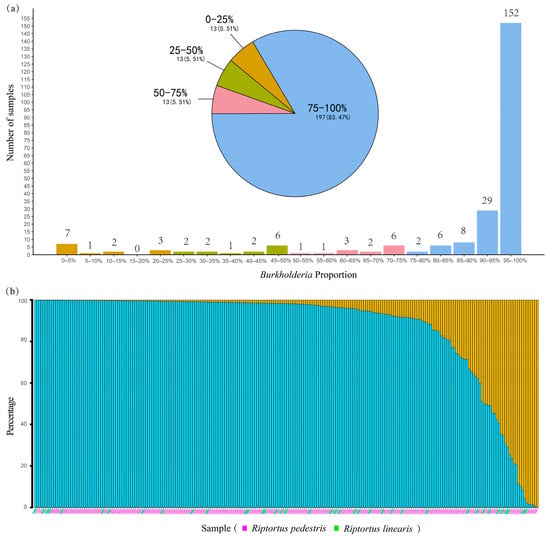
Figure 2.
The proportion of Burkholderia in the total number of bacterial sequences in the Riptortus samples: (a) the bar chart and pie chart show the number of samples with different percentages of Burkholderia bacteria; and (b) bar charts show the percentage of Burkholderia bacteria in the total number of bacterial sequences for each sample (red represents Burkholderia, green represents other bacteria). The 233 samples with sequencing depths greater than 10,000 sequences are shown.
3.2. Phylogenetic Placement of Burkholderia Associated with Riptortus Stinkbugs
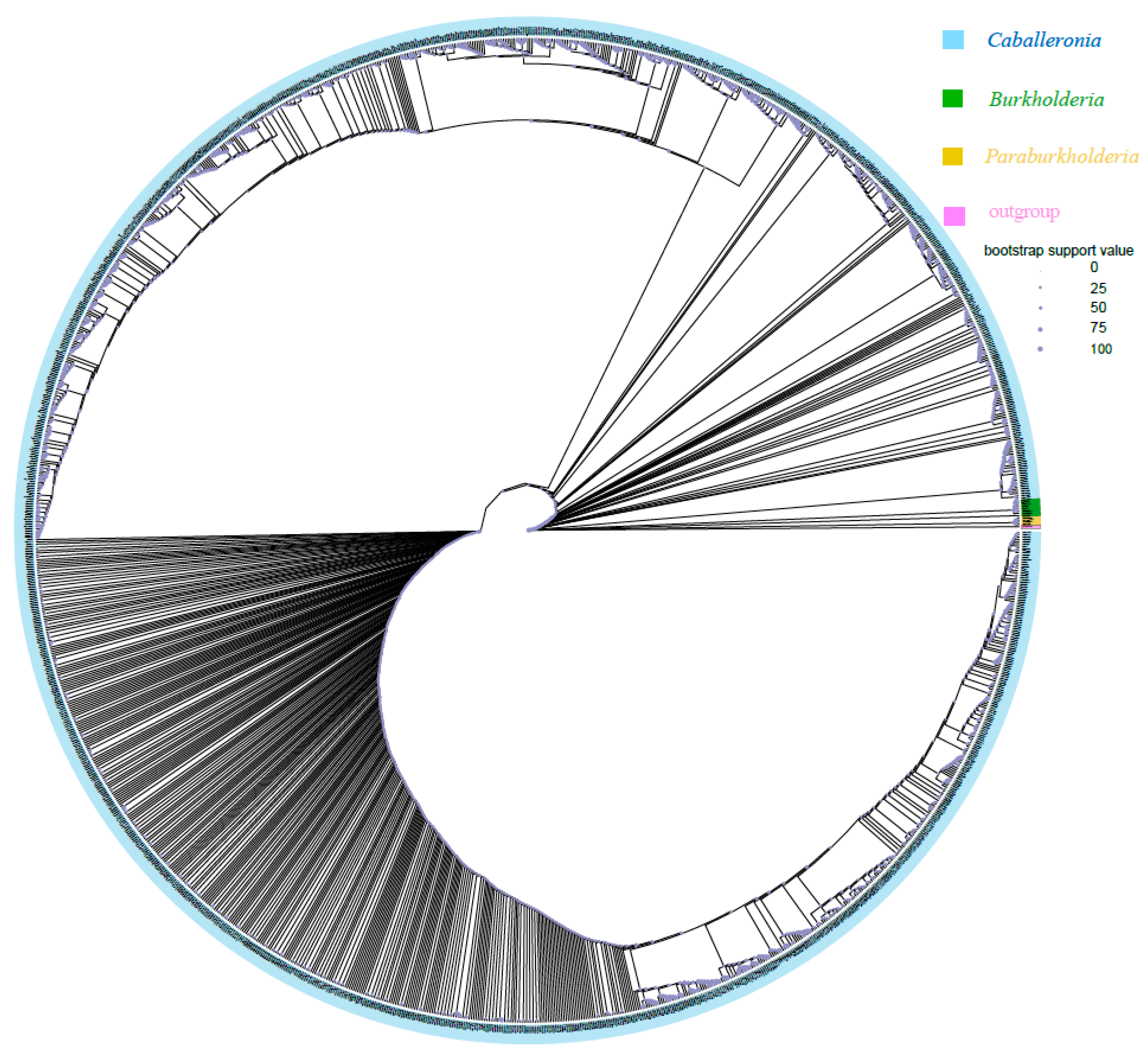
Phylogenetic analyses based on 16S rRNA gene sequences demonstrated that most ASVs obtained in the present study (1367/1373) of Burkholderia sensu lato belonged to the SBE clade (i.e., Caballeronia) (Figure 3). Only one ASV (ASV1099, 159 sequences in total) belonged to the PBE clade (i.e., Paraburkholderia) and was only detected in R. linearis; five ASVs (ASV177, ASV337, ASV8375, ASV12112, and ASV12129; 3212 sequences in total) belonged to the BCC&P clade (i.e., Burkholderia sensu stricto) and were only detected in R. pedestris. The above two clades accounted for only 0.03% of Burkholderia sensu lato (11,107,919 sequences in total). However, subclades of SBE (SBE-α, SBE-β, SBE-γ, Coreoidea clade, etc.) have recently been proposed [59,60] that could not be clearly divided here, probably because the amplicon sequences were too short.
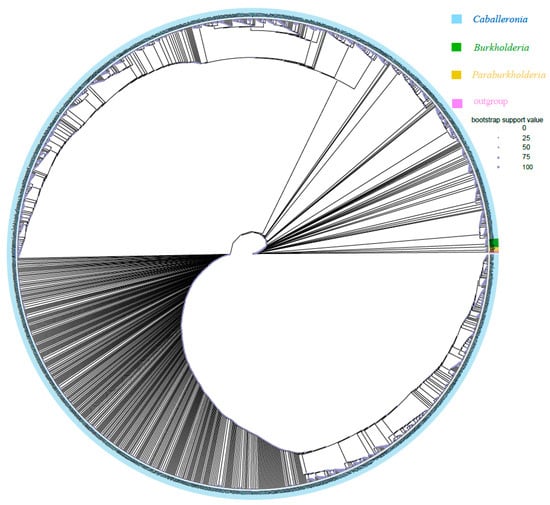
Figure 3.
Maximum likelihood (ML) tree of Burkholderia reconstructed using IQ-TREE based on the V3-V4 hypervariable region of 16S rRNA (430 bp). The tree was constructed with 1393 sequences, including 1373 Burkholderia ASVs from Riptortus pedestris and R. linearis obtained in this study, 19 downloaded Burkholderia sequences from NCBI, and one representative sequence from Pandoraea as an outgroup. Blue, green, yellow, and pink represent the SBE clade (i.e., Caballeronia), BCC&P clade (i.e., Burkholderia sensu stricto), and PBE clade (i.e., Paraburkholderia) and Pandoraea, respectively. The size of the circle indicates the bootstrap support value.
3.3. Strain-Level Diversity of Burkholderia
After excluding 12 samples with fewer than 5000 Burkholderia sequences, 11,048,782 sequences and 1373 ASVs were obtained from the remaining 221 samples (185 from R. pedestris and 36 from R. linearis). Of these 1373 ASVs, 1174 ASVs were detected in only R. pedestris, 81 were detected in only R. linearis, and 118 were shared between R. pedestris and R. linearis (Supplementary Figure S1a). Only relatively few ASVs had a large number of sequences, and the numbers of ASVs that accounted for more than 1.0%, 0.1%, and 0.01% of the total sequences were 22, 54, and 107, respectively. If an ASV exceeding 1.0% of the sequences in any host sample was retained, 160 ASVs could be obtained. Many ASVs are specific and detected in only a small number of individuals. In R. pedestris, 94.8% of the ASVs were present in 1–3 individuals (836, 162, and 115 ASVs were present in one, two, and three samples, respectively). Some other ASVs were detected in a greater number of individuals; for example, ASV1, ASV3, ASV16, ASV6, and ASV12 existed in more than 40 samples (Supplementary Figure S1b). Similarly, in R. linearis, 87.9% of the ASVs were present in one or two individuals (142 and 33 ASVs existed in one and two samples, respectively), while ASV1, ASV16, ASV19, and ASV5 were detected in more than eight samples (Supplementary Figure S1c).
To visually present the relationships between major ASVs, ASVs with a relative content of more than 1% in each sample were used to construct a haplotype network. Under this threshold, 159 ASVs were retained (141 ASVs occurred in R. pedestris, 64 occurred in R. linearis, 45 ASVs were shared, and ASV89, with only 215 bp, was excluded) (Supplementary Table S3). Among the 159 ASVs, the difference among the pairings was between 1 and 29 bases (Supplementary Figure S2; Supplementary Table S3).
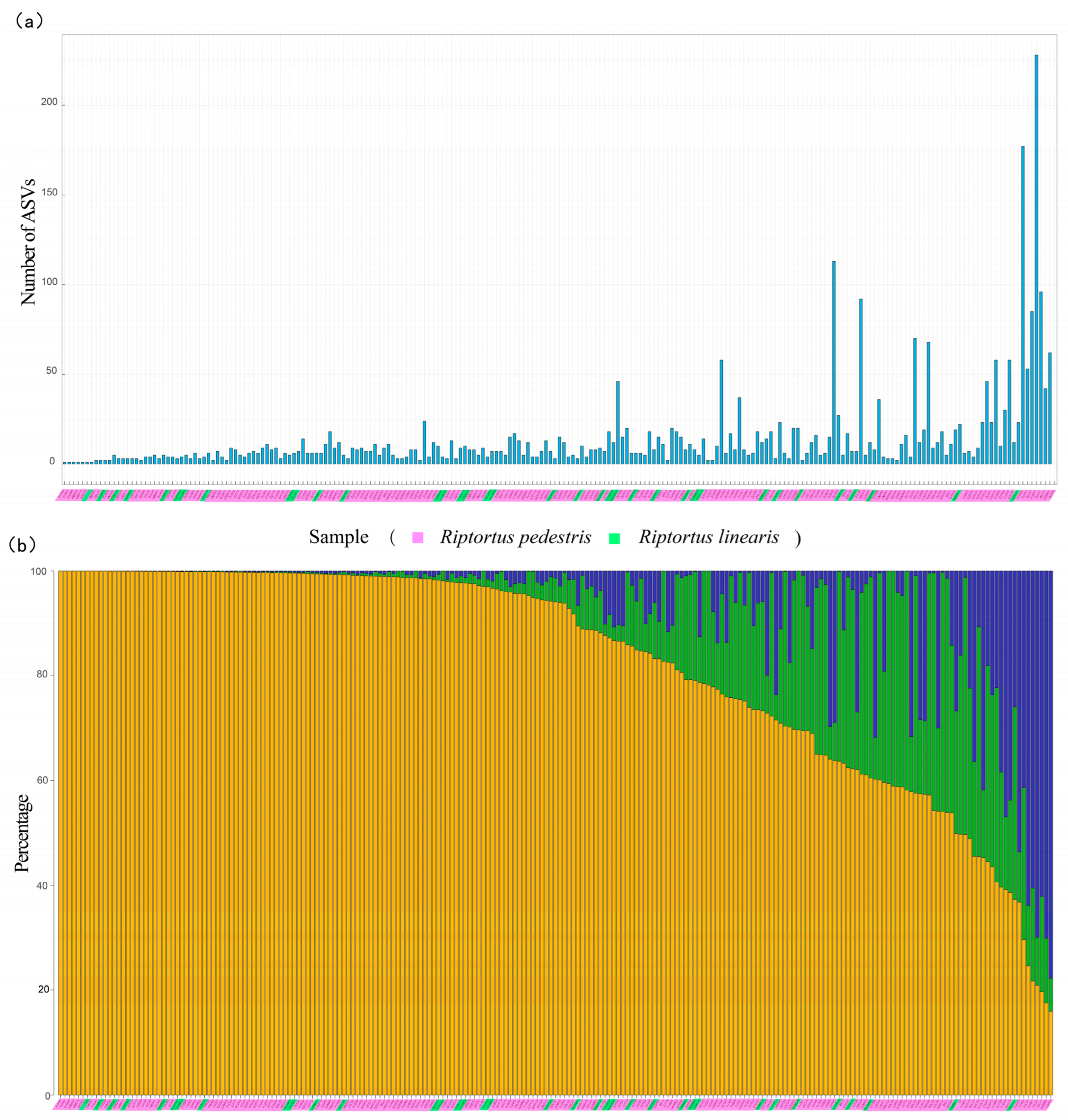
Most of the samples contained fewer than 20 Burkholderia ASVs (87% of the samples from R. pedestris and 89% of those from R. linearis) (Figure 4a). Although multiple Burkholderia ASVs were present in a Riptortus individual in most cases (single ASVs were detected in only six R. pedestris samples and one R. linearis sample), there were one or two dominant ASVs in most samples. In 61.6% of the R. pedestris individuals (114/185), and in 69.4% of the R. linearis individuals (25/36), the first dominant ASV accounted for more than 80% of all the Burkholderia sequences (Figure 4b). Overall, in 62.9% of the Riptortus individuals (139/221), the proportion of the first dominant ASV exceeded 80%. When the first two dominant ASVs were considered, 87.8% of these were Riptortus individuals (194/221) with Burkholderia greater than 80% (Figure 4b).
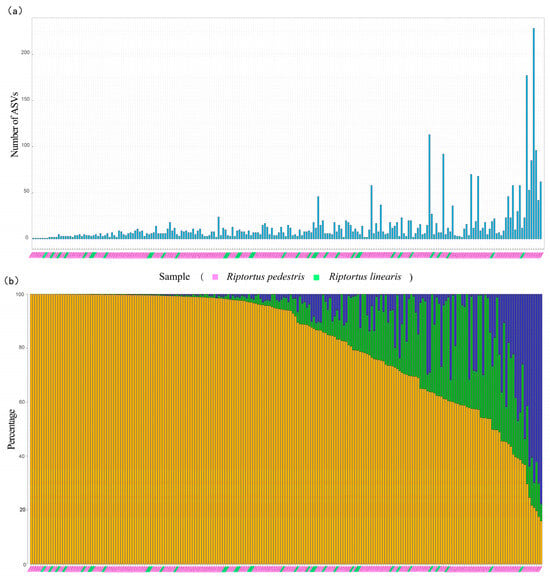
Figure 4.
The number of Burkholderia ASVs and the proportions of the two most dominant Burkholderia ASVs in each sample of Riptortus species: (a) the number of Burkholderia ASVs in each sample of Riptortus species; and (b) the proportion of the two highest Burkholderia ASVs in each sample of Riptortus. The yellow bar represents the relative content of the first most abundant ASV in the sample; the green bar represents the relative content of the second most abundant ASV in the sample; and the blue bar represents the relative content of other ASVs. The pink shade on the horizontal axis represents the samples of R. pedestris, and the green shade represents the samples of R. linearis. The 221 samples (185 R. pedestris and 36 R. linearis) with more than 5000 Burkholderia reads are shown. The individuals are arranged along the horizontal axis according to the proportion of the first most abundant Burkholderia ASV in samples. The individuals of (a,b) (i.e., above and below) correspond one to one.
If the first two dominant ASVs occupied the majority of sequences (e.g., >80%) in the sample and were similar in number, they were called codominant ASVs. If most of the sequences in the sample belonged to one ASV, and the second ASV had significantly fewer sequences, we assumed that the first ASV had a significant advantage in terms of competition for colonization. To explore the competition pattern of symbiotic Burkholderia strains during the colonization process, we focused on samples containing two major ASVs to analyze the genetic differences between them (Supplementary Table S4). The samples with only one ASV or with the sum of the first two dominant ASVs comprising <80% of the entire Burkholderia community were treated as “no obvious codominant ASVs” and excluded from further analysis. There were 1–31 mutations between the first and second dominant ASVs in the 187 Riptortus individuals (Supplementary Table S4). Under different thresholds (the first dominant ASV/the second dominant ASV < 5, 3, 2, and 1.5), the proportion of codominance was significantly greater when there were fewer mutations between ASV pairs. For example, when 5 was used as the threshold, the proportion of codominance was 16.09% when the number of nucleotide mutations between ASV pairs was 11–31; the proportion of codominance was 52% when the number of mutations between ASV pairs was 1–2 (Table 1).

Table 1.
Codominant analysis of the first two dominant ASVs in 187 Riptortus individuals under multiple thresholds. The samples with only one ASV (7 samples) or with the sum of the first two dominant ASVs comprising < 80% of the entire Burkholderia community (27 samples) were treated as “no obvious codominant ASVs” and excluded from the analysis.
3.4. Factors Influencing Burkholderia Communities of Riptortus Stinkbugs
To ensure even sequencing depth across the samples, the dataset was subsampled to a depth of 5000 reads per sample before alpha and beta diversity analysis. All the rarefaction curves reached an asymptote, indicating adequate sequencing depth (Supplementary Figure S3).
There were significant differences in two alpha diversity indicators (Kruskal–Wallis tests, Shannon, p = 0.025; Chao1, p < 0.001) but no significant differences in the other two indicators (Kruskal–Wallis tests, Simpson, p = 0.089; ACE, p = 0.052) among localities of R. pedestris (Figure 5a–d; Supplementary Table S5). However, none of the alpha diversity indicators differed among localities of R. linearis (Kruskal–Wallis tests, p > 0.05 in all cases) (Supplementary Figure S4; Supplementary Table S6). The results also suggested that none of the indicators differed significantly between the two host species (Mann–Whitney tests, p > 0.05 in all cases) (Supplementary Figure S5).
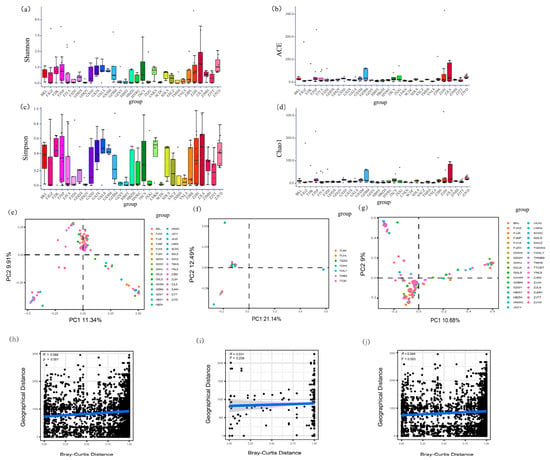
Figure 5.
Factors influencing Burkholderia communities of Riptortus stinkbugs: (a–d) alpha diversity of symbiotic bacterial communities (including Shannon index, Chao1 index, Simpson index, and ACE index; Kruskal–Wallis tests, p = 0.025, p < 0.001, p = 0.089 and p = 0.052, respectively) in R. pedestris; (e–g) principal coordinate analysis (PCoA) of Burkholderia communities in Riptortus stinkbugs, PCoA using the Bray–Curtis distance method and based on data from R. pedestris, R. linearis, and the pooled data of both species, respectively; and (h–j) Mantel test between the Burkholderia community (Bray–Curtis distance) and geographic distance of the sample location using R. pedestris, R. linearis, and the pooled data of both species, respectively.
Both the ANOSIM and PerMANOVA suggested that the beta diversity of Burkholderia was significantly different between the two species (PerMANOVA: both Bray–Curtis and Jaccard, p < 0.001; ANOSIM: Bray–Curtis, p = 0.010, Jaccard, p = 0.014) (Supplementary Figure S6; Supplementary Table S7). The Burkholderia community in R. pedestris was significantly different among localities (both ANOSIM and PerMANOVA: p < 0.001 in all cases) (Figure 5e; Supplementary Figure S7a; Supplementary Table S8). However, there was no significant difference among localities of R. linearis (both ANOSIM and PerMANOVA: p > 0.05 in all cases) (Figure 5f; Supplementary Figure S7b; Supplementary Table S8). A significant difference among localities was suggested when the data of two species were pooled together (both ANOSIM and PerMANOVA: p < 0.001 in all cases) (Figure 5g; Supplementary Figure S7c; Supplementary Table S8). Further two-way PerMANOVA based on samples from two host species with the same localities indicated that localities had a significant effect on the Burkholderia community (Bray–Curtis, F = 1.521, p = 0.020; Jaccard, F = 1.311, p = 0.001) and that host species also had a marginally significant effect on the Burkholderia community (Bray–Curtis, F = 1.842, p = 0.041; Jaccard, F = 1.298, p = 0.045). However, the interaction between host species and locality had no significant effect on the Burkholderia community (Bray–Curtis, F = 0.854, p = 0.768; Jaccard, F = 0.984, p = 0.566) (Table 2).

Table 2.
The role of host species and location in structuring Burkholderia communities in Riptortus stinkbugs. The analyses were carried out using two-way PerMANOVAs and used “host species” and “locality” as the main effects.
Mantel tests revealed that the Burkholderia community of R. pedestris was significantly correlated with the geographical distance of the sample sites (R = 0.044, p = 0.003) (Figure 5h); however, this correlation was not significant for R. linearis (R = 0.031, p = 0.236) (Figure 5i). Mantel tests also showed that the Burkholderia community was significantly correlated with geographical distance when the data for the two Riptortus species were pooled together (R = 0.056, p = 0.001) (Figure 5j). Mantel tests also suggested that there were significant correlations between the Burkholderia community and several bioclimatic factors related to temperature (bio1, bio4, bio8, bio9, bio10 and bio11 for Riptortus pedestris; bio1, bio6, bio9 and bio11 for R. linearis) at the threshold of p = 0.01. However, there was no significant correlation between the Burkholderia community and any bioclimatic factor related to precipitation (Supplementary Table S9).
4. Discussion
We studied the diversity of Burkholderia in the gut of Riptortus stinkbugs from China, Thailand, and Laos. Burkholderia was detected in 232 of 233 individuals (99.6%) based on 16S rRNA gene amplicon sequencing data (Figure 2). The high infection rate of Riptortus was consistent with previous studies; for example, infection rates of Burkholderia were 97.8% in Japanese populations [61] and 80.62–93.1% in South Korean populations [36,37] of R. pedestris estimated by diagnostic PCR.
Burkholderia accounted for a very high proportion of the total bacterial sequences, with an average proportion of 87.1%. In fact, in the present study, DNA samples were extracted from the whole abdomen, which might have resulted in the detection of bacteria from abdomen regions other than the midgut M4 region; thus, the proportion of Burkholderia in the midgut M4 region may have been underestimated. High proportions were also found in two studies using deep sequencing methods; the genus Caballeronia (i.e., the SBE clade of Burkholderia) comprised more than 80% of the entire bacterial community in 66 field-collected individuals of Paradieuches dissimilis (Rhyparochromidae) [60]; and in 15 individuals of Physopelta species (Largidae), Burkholderia occupied more than 94% of the bacterial communities [27].
Phylogenetic analysis indicated that most ASVs of Burkholderia sensu lato belong to the SBE clade (i.e., genus Caballeronia); among the 1373 ASVs, only one belonged to the PBE clade (i.e., Paraburkholderia), and only five belonged to the BCC&P clade (i.e., Burkholderia sensu stricto). However, 70.4% of R. pedestris individuals from South Korea harbored unclassified Burkholderia clades, whereas 22.2% and 7.4% of individuals harbored SBE clades and BCC clades, respectively [36]. In another study based on field-collected samples from South Korea, the BCC clade was the most frequently detected clade (66.06%) among SBE, BCC and PBE; furthermore, 47.06% of R. pedestris individuals were found to harbor an unidentified clade [37]. The discrepancy among studies may be explained by a region-dependent pattern due to the physiological characteristics of different host populations or the differences in soil microorganisms available in different regions. Previous studies showed that different clades of Burkholderia provide various fitness benefits to their stinkbug hosts [13,62]. Even within the same clade (e.g., the SBE clade), not all strains are equivalently beneficial to the stinkbugs [63]. This means that the observed variation in symbiont strains could potentially result in different outcomes for stinkbug performance/fitness. In the present study, a total of 12,065 bacterial ASVs were detected in 233 samples and 1373 Burkholderia ASVs were detected in 221 screened samples (reads of Burkholderia > 5000) of Riptortus species. However, the number of ASVs that accounted for more than 1%, 0.1%, and 0.01% of all Burkholderia sequences was only 22, 54, and 107, respectively. The environment, especially the soil, contains highly diverse bacterial species, including a considerable number of Burkholderia species/strains, even at small spatial scales [34]. As we noted here, the DNA samples were extracted from the contents of the whole abdomen; therefore, a large variety of bacteria may be detected that enter the gut from the environment but do not colonize the M4 region of the midgut. This could explain why the diversity of the bacteria was so high while the high abundance of Burkholderia ASV was limited in this study. In a previous study, 341 bacterial ASVs and 61 Burkholderia ASVs were detected in 352 field-collected samples of Jalysus species [64]. The low number of ASVs in that study may be due to the lower sequencing depth, resulting in the absence of some low-abundance ASVs (6741 sequences per sample in the Ravenscraft study versus 51,258 sequences per sample in our study).
Burkholderia exhibit host specificity and exclusiveness when it is considered at a high taxonomic level; most R. pedestris individuals harbor a single clade or species of Burkholderia according to diagnostic PCR analysis [34,36,37]. Although competition assays have shown that native Burkholderia species routinely outcompete nonnative bacteria [13] and that subclade SBE-β outcompetes SBE-α [59] inside the midgut crypts of stinkbugs, multiple strains/OTUs of Burkholderia species are commonly detected, such as in Physopelta species [27], Jalysus species [64], and Paradieuches dissimilis [60]. In a recent study, bacteria belonging to one or more genera (98.65% similarity as a threshold) were also detected in individuals of R. pedestris by cloning methods [65]. As found in Jalysus [64], in our study, Burkholderia showed a highly promiscuous pattern in Riptortus stinkbugs. Using deep sequencing, more than one ASV (up to 44 ASVs) was detected in most Riptortus samples (214/221) (Figure 4). However, one or two dominant ASVs accounted for a high proportion of the entire Burkholderia community in most Riptortus individuals. In 52.0% of individuals, one dominant ASV accounted for more than 90% of the Burkholderia community, suggesting absolute dominance and an obvious competition pattern. Furthermore, in 78.3% of individuals, the first two dominant ASVs combined accounted for more than 90% of the Burkholderia community (Figure 4b). In the latter case, the first ASV may be clearly dominant over the second ASV in some individuals, also suggesting a competition pattern. In some other individuals, the difference in proportion between the two ASVs was not large, suggesting a codominant pattern (Supplementary Table S4). We found that the two codominant ASVs in a single host individual may be genetically far apart or differ by only one base, which to some extent supports the hypothesis that Burkholderia has relaxed specificity at the strain level in the stinkbug–Burkholderia system [27]. However, our results further suggested that the two closely related ASVs were more likely to be codominant (Table 1). This may be due to intense competition between distant strains or the host’s specific selectivity for Burkholderia. The relative abundances of the ASVs within individual stinkbugs could be determined by several potential drivers. As proposed above, differences in inter-ASV competitive abilities during colonization are the first one. Priority effects are another potential driver, as there is some potential for one ASV to arrive in the midgut M4 region earlier than a second ASV within a narrow time window for colonization [33,35]. The third potential driver of ASV abundances is differences in competitive ability within the midgut M4 region post-colonization. Based on the current data, it is difficult to distinguish the relative importance of these three drivers.
External factors affecting the Burkholderia community have been analyzed in several studies. For example, in the dock bug Coreus marginatus, a region-dependent pattern was suggested between European and Japanese populations [24]. Location was considered the most important factor in shaping the Burkholderia community in Jalysus wickhami and J. spinosus [64]. Additionally, host plant species of Jalysus can also lead to differences [64]. In the western conifer seed bug Leptoglossus occidentalis, symbiotic associations are influenced by the host rather than geography [59]. In R. pedestris, bacterial community structure is affected by season and geography [65]. In the present study, the PerMANOVA suggested that the beta diversity of Burkholderia was significantly different between the two host species and among localities. The two-way PerMANOVA also showed that both the host and geography influence the Burkholderia community; however, geography may play a more important role than host species (Table 2). Furthermore, Mantel tests showed that the Burkholderia community was significantly correlated with geographical distance (Figure 5), indicating a region-dependent pattern. It should be noted that the first two principal coordinates can explain only a small amount (approximately 20%) of the total variation, regardless of whether location or host species are considered (Figure 5e–g). A large number of individual-specific ASVs, but a small number of shared ASVs, may reduce the resolution. For R. linearis, the nonsignificant differences between the PerMANOVA test and the Mantel test may be due to the smaller sample size: R. pedestris had 183 samples, covering 31 sites, while R. linearis had only 28 samples, covering seven sites.
Through pairwise genetic distance calculations and haplotype network diagram analysis, we found that the dominant ASVs in samples from the same location may be the same or different, and that the base differences between unique ASVs may be large or small. Stinkbugs acquire Burkholderia from the environment in each generation during second instars and the microbiome varies greatly even at small spatial scales. Therefore, the association between stinkbugs and Burkholderia strains is very unstable [66], which might explain why samples from the same location were occupied by different strains of Burkholderia. Whether the colonization of symbiotic Burkholderia strains in the host midgut crypts is random, or if there is a density-dependent pattern of Burkholderia strains in competition or a host-specific selectivity for symbiotic strains, requires further study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12091885/s1, Figure S1: Diversity of Burkholderia found in Riptortus pedestris and R. linearis; Figure S2: Maximum parsimony network for V3-V4 hypervariable region of the 16S rRNA from Burkholderia; Figure S3: Rarefaction curves for Burkholderia; Figure S4: Alpha diversity of symbiotic bacterial communities (including Shannon index, Chao1 index, Simpson index, and ACE index) in R. linearis; Figure S5: Comparing of Alpha diversity of symbiotic bacterial communities (including Shannon index, Chao1 index, Simpson index, and ACE index) between Riptortus pedestris and R. linearis; Figure S6: Principal coordinates analysis (PCoA) of Burkholderia communities in Riptortus pedestris and R. linearis; Figure S7: Principal coordinates analysis (PCoA) of Burkholderia communities in Riptortus stinkbugs using the Jaccard distance method; Table S1: Sample collection information of Riptortus pedestris and R. linearis; Table S2: ASV feature table of bacteria; Table S3: Pairwise genetic distance of 159 ASVs of Burkholderia; Table S4: The proportion of the two highest Burkholderia ASVs and genetic distance between them in each sample of Riptortus; Table S5: Alpha diversity indicators of Burkholderia in 183 Riptortus pedestris samples; Table S6: Alpha diversity indicators of Burkholderia in 28 Riptortus linearis samples; Table S7: The role of locations in structuring Burkholderia communities between two Riptortus species; Table S8: The role of locations in structuring Burkholderia communities within Riptortus species; Table S9: The correlations between Burkholderia communities within two Riptortus species and bioclimatic factors.
Author Contributions
Conceptualization, W.-J.B. and H.-J.X.; Methodology, X.-R.H. and Y.W.; Validation, S.-Y.F.; Formal analysis, X.-R.H., S.-Y.F., Y.W., J.-Y.Z., T.-Y.Q. and Y.-F.L.; Investigation, S.-Y.F., J.-Y.Z., T.-Y.Q. and Y.-F.L.; Writing—original draft, X.-R.H.; Writing—review & editing, W.-J.B. and H.-J.X.; Visualization, X.-R.H.; Project administration, H.-J.X.; Funding acquisition, H.-J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 32470442) and the Fundamental Research Funds for the Central Universities (Grant No. 63213120).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Kai Dang, Yu-Jie Duan, Min Li, Jing-Yu Liang, Hua-Xi Liu, Shu-Jing Wang, Qiang Xie and Xiu-Xiu Zhu for their field collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salem, H.; Bauer, E.; Strauss, A.S.; Vogel, H.; Marz, M.; Kaltenpoth, M. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc. R. Soc. B Biol. Sci. 2014, 281, 10. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.A.; Park, H.; Moran, N.A. Genome evolution of the obligate endosymbiont Buchnera aphidicola. Mol. Biol. Evol. 2019, 36, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Ann. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Ann. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Luan, J.B. Insect bacteriocytes: Adaptation, development, and evolution. Ann. Rev. Entomol. 2024, 69, 81–98. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kikuchi, Y.; Nikoh, N.; Shimada, M.; Fukatsu, T. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006, 4, 1841–1851. [Google Scholar] [CrossRef]
- Kaltenpoth, M.; Flórez, L.V. Versatile and dynamic symbioses between insects and Burkholderia bacteria. Ann. Rev. Entomol. 2020, 65, 145–170. [Google Scholar] [CrossRef]
- Moriyama, M.; Fukatsu, T. Host’s demand for essential amino acids is compensated by an extracellular bacterial symbiont in a hemipteran insect model. Front. Physiol. 2022, 13, 14. [Google Scholar] [CrossRef]
- Compant, S.; Nowak, J.; Coenye, T.; Clement, C.; Barka, E.A. Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol. Rev. 2008, 32, 607–626. [Google Scholar] [CrossRef]
- Suárez-Moreno, Z.R.; Caballero-Mellado, J.; Coutinho, B.G.; Mendonça-Previato, L.; James, E.K.; Venturi, V. Common features of environmental and potentially beneficial plant-associated Burkholderia. Microbiol. Ecol. 2012, 63, 249–266. [Google Scholar] [CrossRef]
- Depoorter, E.; Bull, M.J.; Peeters, C.; Coenye, T.; Vandamme, P.; Mahenthiralingam, E. Burkholderia: An update on taxonomy and biotechnological potential as antibiotic producers. Appl. Microbiol. Biotechnol. 2016, 100, 5215–5229. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, K.; Kikuchi, Y. Riptortus pedestris and Burkholderia symbiont: An ideal model system for insect-microbe symbiotic associations. Res. Microbiol. 2017, 168, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Jang, S.; Takeshita, K.; Ohbayashi, T.; Ohnishi, N.; Meng, X.Y.; Mitani, Y.; Kikuchi, Y. Host-symbiont specificity determined by microbe-microbe competition in an insect gut. Proc. Natl. Acad. Sci. USA 2019, 116, 22673–22682. [Google Scholar] [CrossRef] [PubMed]
- Sawana, A.; Adeolu, M.; Gupta, R.S. Molecular signatures and phylogenomic analysis of the genus Burkholderia: Proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov harboring environmental species. Front. Genet. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Dobritsa, A.P.; Samadpour, M. Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov to accommodate twelve species of the genera Burkholderia and Paraburkholderia. Int. J. Syst. Evol. Microbiol. 2016, 66, 2836–2846. [Google Scholar] [CrossRef]
- Beukes, C.W.; Palmer, M.; Manyaka, P.; Chan, W.Y.; Avontuur, J.R.; van Zyl, E.; Huntemann, M.; Clum, A.; Pillay, M.; Palaniappan, K.; et al. Genome data provides high support for generic boundaries in Burkholderia sensu lato. Front. Microbiol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Lopes-Santos, L.; Castro, D.B.A.; Ferreira-Tonin, M.; Corrêa, D.B.A.; Weir, B.S.; Park, D.; Ottoboni, L.M.M.; Neto, J.R.; Destéfano, S.A.L. Reassessment of the taxonomic position of Burkholderia andropogonis and description of Robbsia andropogonis gen. nov., comb. nov. Anton. Leeuw. Int. J. G. 2017, 110, 727–736. [Google Scholar] [CrossRef]
- Estrada-de los Santos, P.; Palmer, M.; Chávez-Ramírez, B.; Beukes, C.; Steenkamp, E.T.; Briscoe, L.; Khan, N.; Maluk, M.; Lafos, M.; Humm, E.; et al. Whole genome analyses suggests that Burkholderia sensu lato contains two additional novel genera (Mycetohabitans gen. nov., and Trinickia gen. nov.): Implications for the evolution of diazotrophy and nodulation in the Burkholderiaceae. Genes 2018, 9, 389. [Google Scholar] [CrossRef]
- Lin, Q.H.; Lv, Y.Y.; Gao, Z.H.; Qiu, L.H. Pararobbsia silviterrae gen. nov., sp. nov., isolated from forest soil and reclassification of Burkholderia alpina as Pararobbsia alpina comb. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 1412–1420. [Google Scholar] [CrossRef]
- Takeshita, K.; Kikuchi, Y. Genomic comparison of insect gut symbionts from divergent Burkholderia subclades. Genes 2020, 11, 744. [Google Scholar] [CrossRef]
- Bach, E.; Sant’Anna, F.H.; Seger, G.D.D.; Passaglia, L.M.P. Pangenome inventory of Burkholderia sensu lato, Burkholderia sensu stricto, and the Burkholderia cepacian complex reveals the uniqueness of Burkholderia catarinensis. Genomics 2022, 114, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Jouan, R.; Lextrait, G.; Lachat, J.; Yokota, A.; Cossard, R.; Naquin, D.; Timchenko, T.; Kikuchi, Y.; Ohbayashi, T.; Mergaert, P. Transposon sequencing reveals the essential gene set and genes enabling gut symbiosis in the insect symbiont Caballeronia insecticola. ISME Commun. 2024, 4, ycad001. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, T.; Itoh, H.; Lachat, J.; Kikuchi, Y.; Mergaert, P. Burkholderia gut symbionts associated with European and Japanese populations of the dock bug Coreus marginatus (Coreoidea: Coreidae). Microbes Environ. 2019, 34, 219–222. [Google Scholar] [CrossRef]
- Sato, Y.; Jang, S.; Takeshita, K.; Itoh, H.; Koike, H.; Tago, K.; Hayatsu, M.; Hori, T.; Kikuchi, Y. Insecticide resistance by a host-symbiont reciprocal detoxification. Nat. Commun. 2021, 12, 6432. [Google Scholar] [CrossRef]
- Shan, H.W.; Wu, W.; Sun, Z.T.; Chen, J.P.; Li, H.J. The gut microbiota of the insect infraorder Pentatomomorpha (Hemiptera: Heteroptera) for the light of ecology and evolution. Microorganisms 2021, 9, 464. [Google Scholar] [CrossRef]
- Takeshita, K.; Matsuura, Y.; Itoh, H.; Navarro, R.; Hori, T.; Sone, T.; Kamagata, Y.; Mergaert, P.; Kikuchi, Y. Burkholderia of plant-beneficial group are symbiotically associated with bordered plant bugs (Heteroptera: Pyrrhocoroidea: Largidae). Microbes Environ. 2015, 30, 321–329. [Google Scholar] [CrossRef]
- Ohbayashi, T.; Futahashi, R.; Terashima, M.; Barrière, Q.; Lamouche, F.; Takeshita, K.; Meng, X.Y.; Mitani, Y.; Sone, T.; Shigenobu, S.; et al. Comparative cytology, physiology and transcriptomics of Burkholderia insecticola in symbiosis with the bean bug Riptortus pedestris and in culture. ISME J. 2019, 13, 1469–1483. [Google Scholar] [CrossRef]
- Itoh, H.; Aita, M.; Nagayama, A.; Meng, X.Y.; Kamagata, Y.; Navarro, R.; Hori, T.; Ohgiya, S.; Kikuchi, Y. Evidence of environmental and vertical transmission of Burkholderia Symbionts in the oriental chinch bug, Cavelerius saccharivorus (Heteroptera: Blissidae). Appl. Environ. Microbiol. 2014, 80, 5974–5983. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 2011, 5, 446–460. [Google Scholar] [CrossRef]
- Hosokawa, T.; Kikuchi, Y.; Nikoh, N.; Meng, X.Y.; Hironaka, M.; Fukatsu, T. Phylogenetic position and peculiar genetic traits of a midgut bacterial symbiont of the stinkbug Parastrachia japonensis. Appl. Environ. Microbiol. 2010, 76, 4130–4135. [Google Scholar] [CrossRef]
- Ohbayashi, T.; Takeshita, K.; Kitagawa, W.; Nikoh, N.; Koga, R.; Meng, X.Y.; Tago, K.; Hori, T.; Hayatsu, M.; Asano, K.; et al. Insect’s intestinal organ for symbiont sorting. Proc. Natl. Acad. Sci. USA 2015, 112, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Ohbayashi, T.; Jang, S.; Mergaert, P. Burkholderia insecticola triggers midgut closure in the bean bug Riptortus pedestris to prevent secondary bacterial infections of midgut crypts. ISME J. 2020, 14, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Gook, D.H.; Jung, M.; Kim, S.; Lee, D.H. Species diversity of environmentally transmitted bacteria colonizing Riptortus pedestris (Hemiptera: Alydidae) and symbiotic effects of the most dominant bacteria. Sci. Rep. 2023, 13, 15166. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Yumoto, I. Efficient colonization of the bean bug Riptortus pedestris by an environmentally transmitted Burkholderia symbiont. Appl. Environ. Microbiol. 2013, 79, 2088–2091. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, D.H. Abundance and diversity of gut-symbiotic bacteria, the genus Burkholderia in overwintering Riptortus pedestris (Hemiptera: Alydidae) populations and soil in South Korea. PLoS ONE 2019, 14, 10. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, M.; Lee, D.H. Characterization of Burkholderia bacteria clade compositions in soil and Riptortus pedestris (Hemiptera: Alydidae) in South Korea. J. Asia-Pac. Entomol. 2022, 25, 8. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Kuechler, S.M.; Matsuura, Y.; Dettner, K.; Kikuchi, Y. Phylogenetically diverse Burkholderia associated with midgut crypts of spurge bugs, Dicranocephalus spp. (Heteroptera: Stenocephalidae). Microbes Environ. 2016, 31, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating Maximum-Likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Anisimova, M.; Gil, M.; Dufayard, J.F.; Dessimoz, C.; Gascuel, O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree (Version 1.4.4). 2018. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 6 July 2022).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 29, 1657–1659. [Google Scholar] [CrossRef]
- Corp, I. IBM SPSS Statistics for Windows, Version 20.0; IBM Corp: New York, NY, USA, 2013; Volume 440, p. 394. [Google Scholar]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2023, 14, 927–930. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. A Stat. Soc. 2011, 174, 245. [Google Scholar] [CrossRef]
- Hammer, Y.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2011, 4, 1–9. [Google Scholar]
- Mantel, N. The detection of disease clustering as a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Ohbayashi, T.; Cossard, R.; Lextrait, G.; Hosokawa, T.; Lesieur, V.; Takeshita, K.; Tago, K.; Mergaert, P.; Kikuchi, Y. Intercontinental diversity of Caballeronia gut symbionts in the conifer pest bug Leptoglossus occidentalis. Microbes Environ. 2022, 37, Me22042. [Google Scholar] [CrossRef]
- Ishigami, K.; Jang, S.; Itoh, H.; Kikuchi, Y. Obligate gut symbiotic association with Caballeronia in the mulberry seed bug Paradieuches dissimilis (Lygaeoidea: Rhyparochromidae). Microbiol. Ecol. 2023, 86, 1307–1318. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Meng, X.Y.; Fukatsu, T. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa Chinensis (Heteroptera: Alydidae). Appl. Environ. Microbiol. 2005, 71, 4035–4043. [Google Scholar] [CrossRef]
- Acevedo, T.S.; Fricker, G.P.; Garcia, J.R.; Alcaide, T.; Berasategui, A.; Stoy, K.S.; Gerardo, N.M. The importance of environmentally acquired bacterial symbionts for the squash bug (Anasa tristis), a significant agricultural pest. Front. Microbiol. 2021, 12, 719112. [Google Scholar] [CrossRef]
- Hunter, M.S.; Umanzor, E.F.; Kelly, S.E.; Whitaker, S.M.; Ravenscraft, A. Development of common leaf-footed bug pests depends on the presence and identity of their environmentally acquired symbionts. Appl. Environ. Microbiol. 2022, 88, e01778-21. [Google Scholar] [CrossRef] [PubMed]
- Ravenscraft, A.; Thairu, M.W.; Hansen, A.K.; Hunter, M.S. Continent-scale sampling reveals fine-scale turnover in a beneficial bug symbiont. Front. Microbiol. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Gook, D.; Jung, M.; Lee, D.H. Seasonal and geographical variations in the community structure of environmentally—Transmitted symbiotic bacteria in Riptortus pedestris (Hemiptera: Alydidae). J. Asia-Pac. Entomol. 2024, 27, 10. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. Insect-microbe mutualism without vertical transmission: A stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 2007, 73, 4308–4316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).