Kupffer Cells and Hepatocytes: A Key Relation in the Context of Canine Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
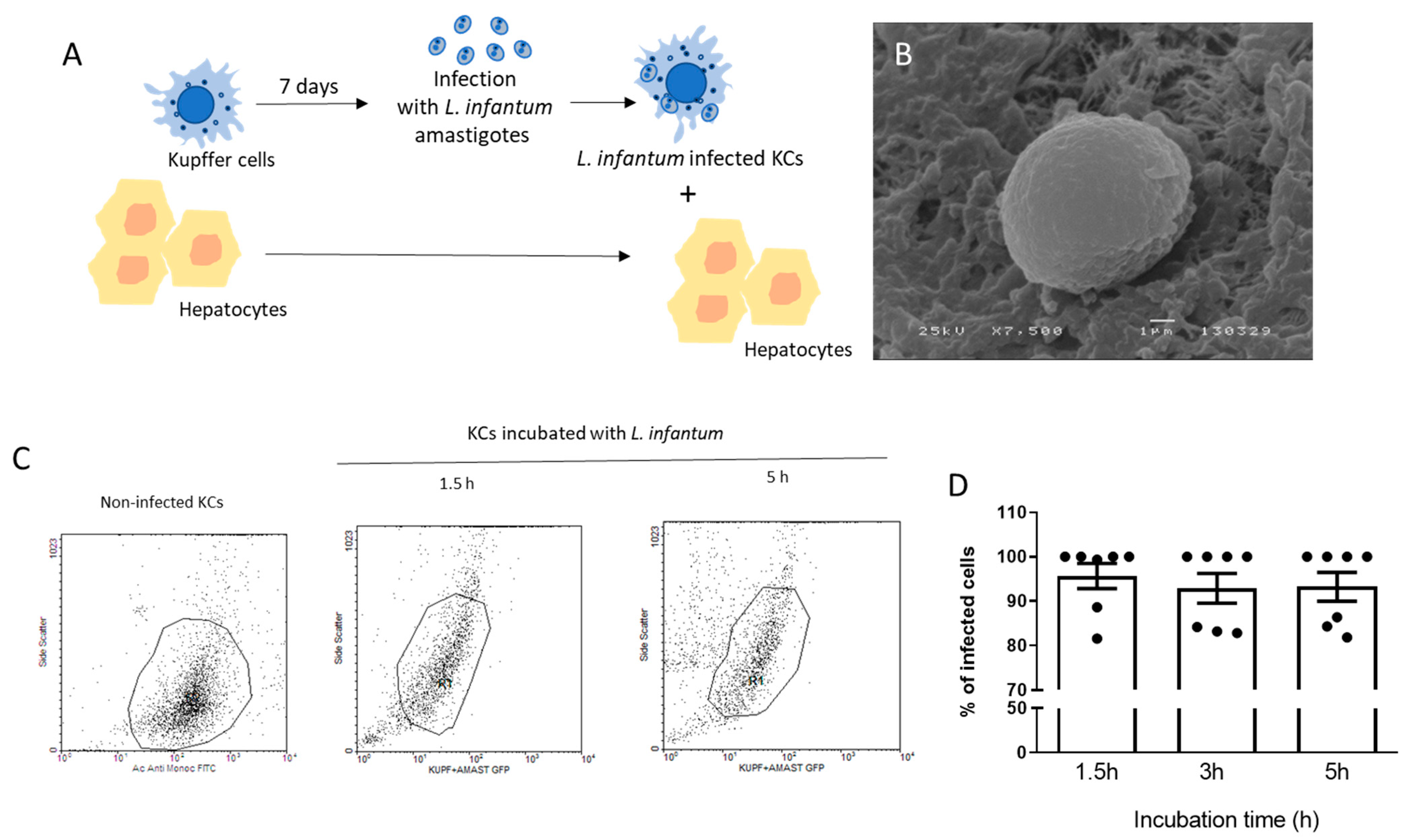
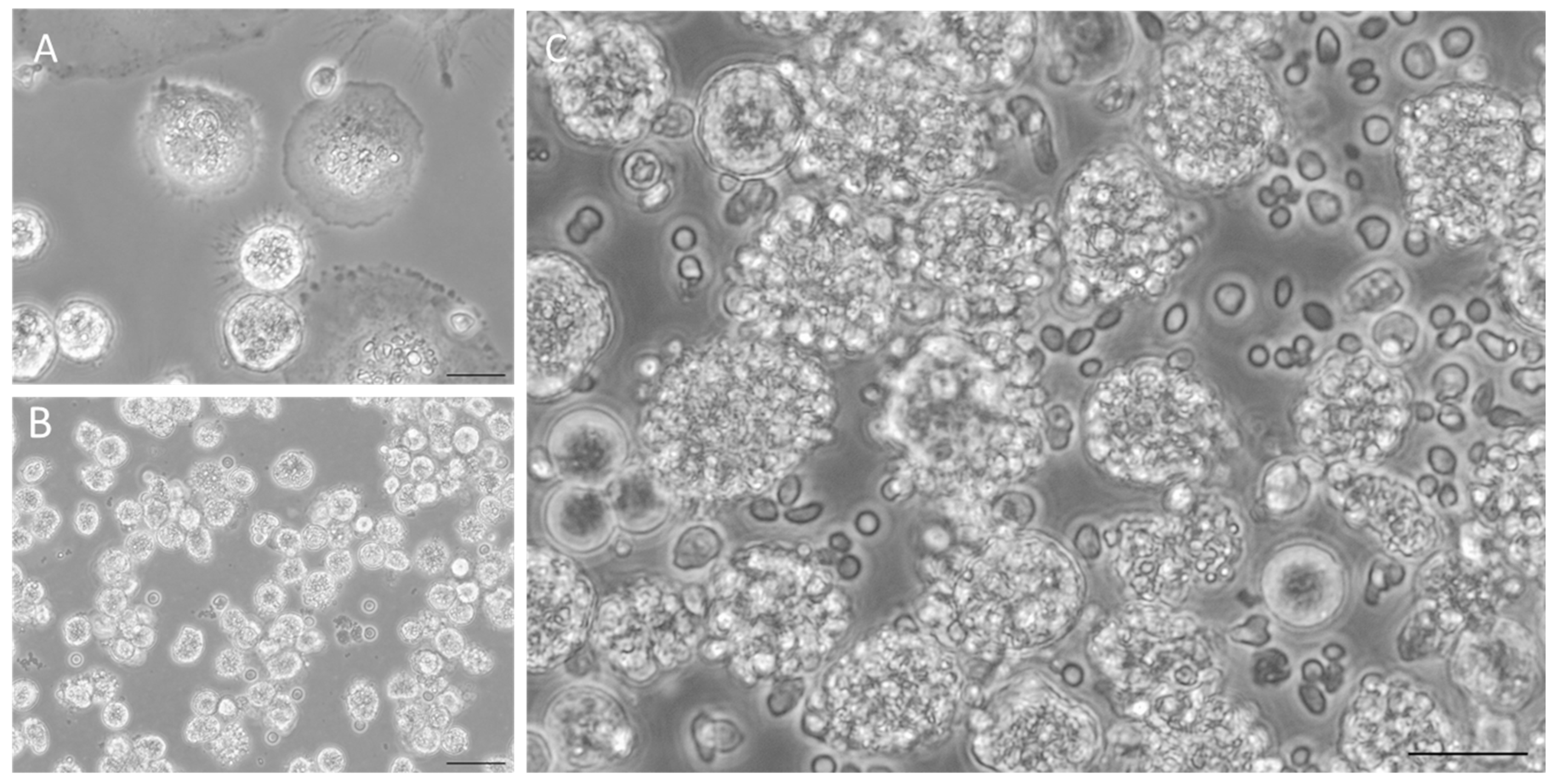
2.1. Isolation of Kupffer Cells and Hepatocytes
2.2. Parasites and Kupffer Cells Infection
2.3. Flow Cytometry
2.4. Scan Electron Microscopy (SEM)
2.5. Co-Culture System
2.6. Real-Time PCR
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results and Discussion
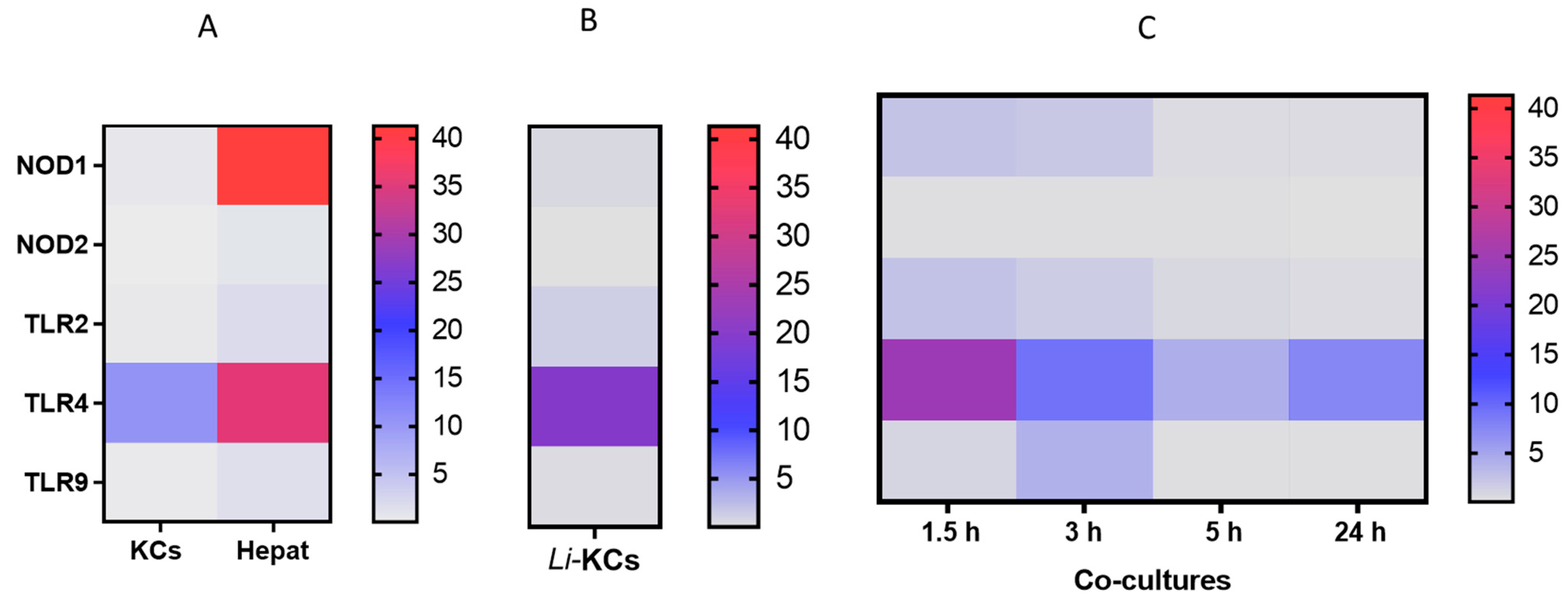
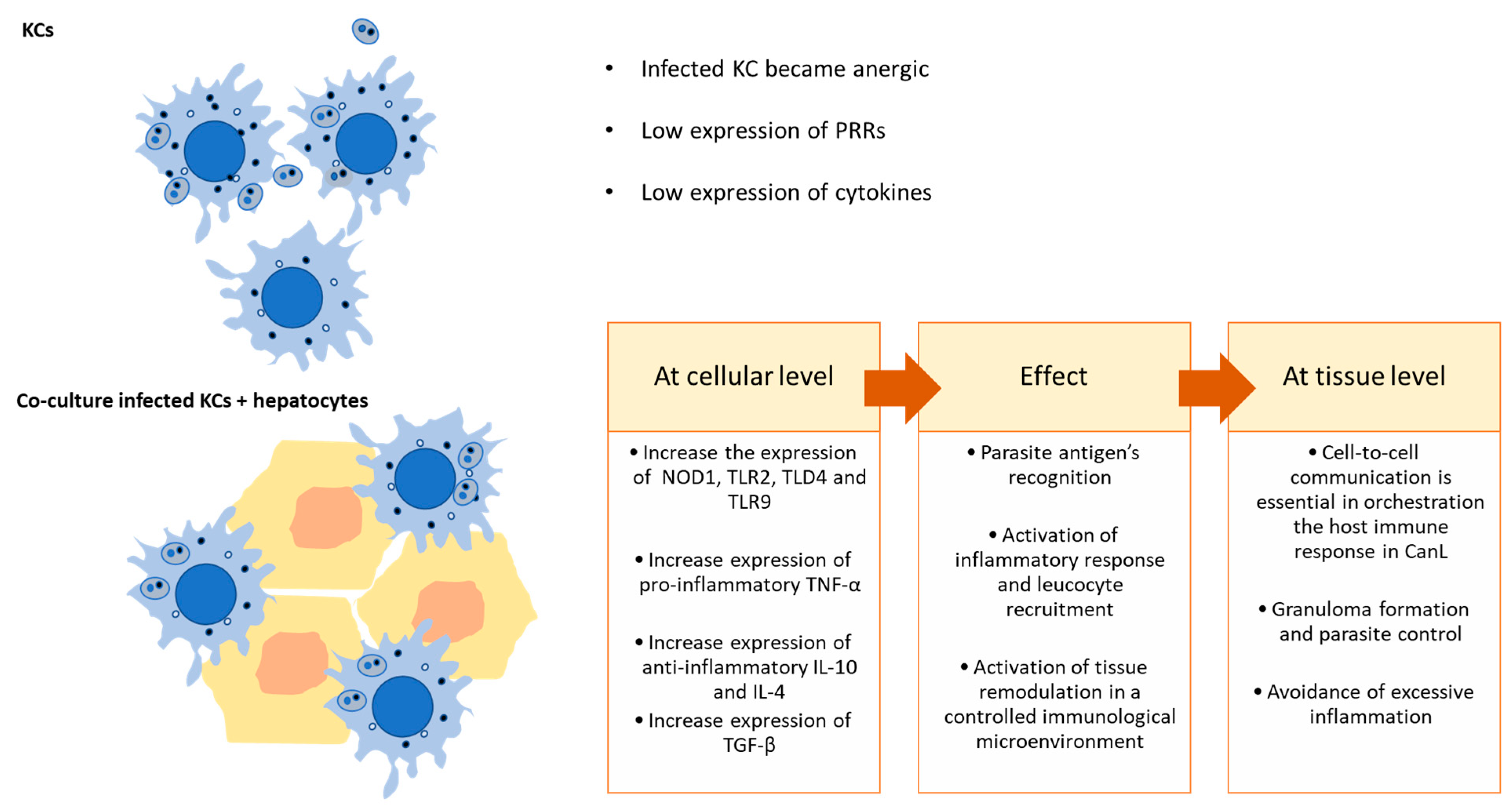
3.1. Co-Cultures Do Not Extensively Activate PRRs
3.2. Co-Cultures Can Generate Key Cytokines and Orchestrate an Immune Response
3.3. The Interaction of L. infantum-Infected KCs with Hepatocytes Is Key in Regulating Local Immune Response
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.; Maia, C.; Courtenay, O.; Llabrés-Brustenga, A.; Lotto Batista, M.; Moirano, G.; van Daalen, K.R.; Semenza, J.C.; Lowe, R. A climatic suitability indicator to support Leishmania infantum surveillance in Europe: A modelling study. Lancet Reg. Health-Eur. 2024, 43, 100971. [Google Scholar] [CrossRef] [PubMed]
- Palatnik-De-Sousa, C.B.; Day, M.J. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasites Vectors 2011, 4, 197. [Google Scholar] [CrossRef] [PubMed]
- TDR|Neglected Tropical Disease (NTD) Research. Visceral Leishmaniasis Research. 2024. Available online: https://tdr.who.int/our-work/research-for-implementation/neglected-tropical-diseases-research/visceral-leishmaniasis-research (accessed on 6 September 2024).
- Vilas-Boas, D.F.; Nakasone, E.K.N.; Gonçalves, A.A.M.; Lair, D.F.; Oliveira, D.S.d.; Pereira, D.F.S.; Silva, G.G.; Conrado, I.d.S.S.; Resende, L.A.; Zaldívar, M.F.; et al. Global Distribution of Canine Visceral Leishmaniasis and the Role of the Dog in the Epidemiology of the Disease. Pathogens 2024, 13, 455. [Google Scholar] [CrossRef]
- Paz, S.; Majeed, A.; Christophides, G.K. Climate change impacts on infectious diseases in the Eastern Mediterranean and the Middle East (EMME)—Risks and recommendations. Clim. Chang. 2021, 169, 40. [Google Scholar] [CrossRef]
- Arikan, A.; Cakir, N. Climate change and future infectious diseases: A growing threat. New Microbes New Infect. 2023, 52, 101088. [Google Scholar] [CrossRef]
- Alexandre-Pires, G.; de Brito, M.T.; Algueró, C.; Martins, C.; Rodrigues, O.R.; da Fonseca, I.P.; Santos-Gomes, G. Canine leishmaniasis. Immunophenotypic profile of leukocytes in different compartments of symptomatic, asymptomatic and treated dogs. Vet. Immunol. Immunopathol. 2010, 137, 275–283. [Google Scholar] [CrossRef]
- Sánchez, M.A.; Diaz, N.L.; Zerpa, O.; Negron, E.; Convit, J.; Tapia, F.J. Organ-specific immunity in canine visceral leishmaniasis: Analysis of symptomatic and asymptomatic dogs naturally infected with Leishmania chagasi. Am. J. Trop. Med. Hyg. 2004, 70, 618–624. [Google Scholar] [CrossRef]
- Gao, B. Basic liver immunology. Cell. Mol. Immunol. 2016, 13, 265–266. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, M.J.; Gao, B. Hepatocytes: A key cell type for innate immunity. Cell. Mol. Immunol. 2016, 13, 301–315. [Google Scholar]
- Gong, J.; Tu, W.; Liu, J.; Tian, D. Hepatocytes: A key role in liver inflammation. Front. Immunol. 2022, 13, 1083780. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Musrati, M.A.; De Baetselier, P.; Movahedi, K.; Van Ginderachter, J.A. Ontogeny, functions and reprogramming of Kupffer cells upon infectious disease. Front. Immunol. 2023, 14, 1238452. [Google Scholar] [CrossRef]
- Rodrigues, A.; Santos-Mateus, D.; Alexandre-Pires, G.; Valério-Bolas, A.; Rafael-Fernandes, M.; Pereira, M.A.; Ligeiro, D.; de Jesus, J.; Alves-Azevedo, R.; Lopes-Ventura, S.; et al. Leishmania infantum exerts immunomodulation in canine Kupffer cells reverted by meglumine antimoniate. Comp. Immunol. Microbiol. Infect. Dis. 2017, 55, 42–52. [Google Scholar] [CrossRef]
- Rodrigues, A.; Alexandre-Pires, G.; Valério-Bolas, A.; Santos-Mateus, D.; Rafael-Fernandes, M.; Pereira, M.A.; Ligeiro, D.; Nunes, T.; Alves-Azevedo, R.; Lopes-Ventura, S.; et al. Dog hepatocytes are key effector cells in the liver innate immune response to Leishmania infantum. Parasitology 2019, 146, 753–764. [Google Scholar] [CrossRef]
- Rodrigues, A.V.; Alexandre-Pires, G.; Valério-Bolas, A.; Santos-Mateus, D.; Rafael-Fernandes, M.; Pereira, M.A.; Ligeiro, D.; Nunes, T.; Alves-Azevedo, R.; Santos, M.; et al. 3D-Hepatocyte Culture Applied to Parasitology: Immune Activation of Canine Hepatic Spheroids Exposed to Leishmania Infantum. Biomedicines 2020, 8, 628. [Google Scholar] [CrossRef]
- Rodrigues, A.V.; Valério-Bolas, A.; Alexandre-Pires, G.; Aires-Pereira, M.; Nunes, T.; Ligeiro, D.; Pereira da Fonseca, I.; Santos-Gomes, G. Zoonotic Visceral Leishmaniasis: New Insights on Innate Immune Response by Blood Macrophages and Liver Kupffer Cells to Leishmania infantum Parasites. Biology 2022, 11, 100. [Google Scholar] [CrossRef]
- Santos-Gomes, G.M.; Abranches, P. Comparative study of infectivity caused by promastigotes of Leishmania infantum MON-1, L. infantum MON-24 and L. donovani MON-18. Folia Parasitol. 1996, 43, 7–12. [Google Scholar]
- Rodrigues, O.R.; Moura, R.A.; Gomes-Pereira, S.; Santos-Gomes, G.M. H-2 complex influences cytokine gene expression in Leishmania infantum-infected macrophages. Cell. Immunol. 2006, 243, 118–126. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, R. Toll-like receptors in acute liver injury and regeneration. Int. Immunopharmacol. 2011, 11, 1433–1441. [Google Scholar] [CrossRef]
- Kesar, V.; Odin, J.A. Toll-like receptors and liver disease. Liver Int. 2014, 34, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Jeong, W.-I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, N.; Kanai, T. Role of Toll-like receptors in immune activation and tolerance in the liver. Front. Immunol. 2014, 5, 221. [Google Scholar] [CrossRef]
- Kiziltas, S. Toll-like receptors in pathophysiology of liver diseases. World J. Hepatol. 2016, 8, 1354–1369. [Google Scholar] [CrossRef]
- Murray, H.W. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Pathol. 2001, 82, 249–267. [Google Scholar] [CrossRef]
- Kaye, P.M.; Beattie, L. Lessons from other diseases: Granulomatous inflammation in leishmaniasis. Semin. Immunopathol. 2016, 38, 249–260. [Google Scholar] [CrossRef]
- Fischer, L.; Lucendo-villarin, B.; Hay, D.C.; O’farrelly, C. Human PSC-Derived Hepatocytes Express Low Levels of Viral Pathogen Recognition Receptors, but Are Capable of Mounting an Effective Innate Immune Response. Int. J. Mol. Sci. 2020, 21, 3831. [Google Scholar] [CrossRef]
- Anand, S.; Wang, P.; Yoshimura, K.; Choi, I.H.; Hilliard, A.; Chen, Y.H.; Wang, C.-R.; Schulick, R.; Flies, A.S.; Flies, D.B.; et al. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J. Clin. Investig. 2006, 116, 1045–1051. [Google Scholar] [CrossRef]
- Osero, B.O.; Aruleba, R.T.; Brombacher, F.; Hurdayal, R. Unravelling the unsolved paradoxes of cytokine families in host resistance and susceptibility to Leishmania infection. Cytokine X 2020, 2, 100043. [Google Scholar] [CrossRef]
- O’Garra, A.; Vieira, P.L.; Vieira, P.; Goldfeld, A.E. IL-10–producing and naturally occurring CD4+ Tregs: Limiting collateral damage. J. Clin. Investig. 2004, 114, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Wang, X.Z. Interleukin-10 and chronic liver disease. World J. Gastroenterol. 2006, 12, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Huang, R.; Chen, Z.; Wang, X.; Chen, F.; Huang, Y. Interleukin-10 gene intervention ameliorates liver fibrosis by enhancing the immune function of natural killer cells in liver tissue. Int. Immunopharmacol. 2024, 127, 111341. [Google Scholar] [CrossRef] [PubMed]
- Poniatowski, L.A.; Wojdasiewicz, P.; Gasik, R.; Szukiewicz, D. Transforming Growth Factor Beta Family: Insight into the Role of Growth Factors in Regulation of Fracture Healing Biology and Potential Clinical Applications. Mediat. Inflamm. 2015, 2015, 137823. [Google Scholar] [CrossRef]
- Lehn, M.; Kandil, O.; Arena, C.; Rein, M.S.; Remold, H.G. Interleukin-4 fails to inhibit interferon-γ-induced activation of human colostral macrophages. Cell. Immunol. 1992, 141, 233–242. [Google Scholar] [CrossRef]
- Ho, J.L.; He, S.H.; Jose, M.C.R.; Wick, E.A. Interleukin-4 Inhibits Human Macrophage Activation by Tumor Necrosis Factor, Granulocyte-Monocyte Colony-Stimulating Factor, and Interleukin-3 for Antileishmanial Activity and Oxidative Burst Capacity. J. Infect. Dis. 1992, 165, 344–351. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.; Alexandre-Pires, G.; Valério-Bolas, A.; Nunes, T.; Pereira da Fonseca, I.; Santos-Gomes, G. Kupffer Cells and Hepatocytes: A Key Relation in the Context of Canine Leishmaniasis. Microorganisms 2024, 12, 1887. https://doi.org/10.3390/microorganisms12091887
Rodrigues A, Alexandre-Pires G, Valério-Bolas A, Nunes T, Pereira da Fonseca I, Santos-Gomes G. Kupffer Cells and Hepatocytes: A Key Relation in the Context of Canine Leishmaniasis. Microorganisms. 2024; 12(9):1887. https://doi.org/10.3390/microorganisms12091887
Chicago/Turabian StyleRodrigues, Armanda, Graça Alexandre-Pires, Ana Valério-Bolas, Telmo Nunes, Isabel Pereira da Fonseca, and Gabriela Santos-Gomes. 2024. "Kupffer Cells and Hepatocytes: A Key Relation in the Context of Canine Leishmaniasis" Microorganisms 12, no. 9: 1887. https://doi.org/10.3390/microorganisms12091887
APA StyleRodrigues, A., Alexandre-Pires, G., Valério-Bolas, A., Nunes, T., Pereira da Fonseca, I., & Santos-Gomes, G. (2024). Kupffer Cells and Hepatocytes: A Key Relation in the Context of Canine Leishmaniasis. Microorganisms, 12(9), 1887. https://doi.org/10.3390/microorganisms12091887







