Coaggregation Occurs between a Piliated Unicellular Cyanobacterium, Thermosynechococcus, and a Filamentous Bacterium, Chloroflexus aggregans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Disruption of the pilB Gene in Thermosynechococcus sp. NK55a
2.3. Transmission Electron Microscopy (TEM) Imaging
2.4. Co-Cultivation of Thermosynechococcus sp. and C. aggregans
2.5. Bright-Field and Fluorescence Microscopy Imaging
2.6. Scanning Electron Microscopy (SEM) Imaging
2.7. DNA Extraction and Quantitative PCR (qPCR)
2.8. Total Protein Quantification after Size Fractionation
2.9. Statistical Analysis
3. Results
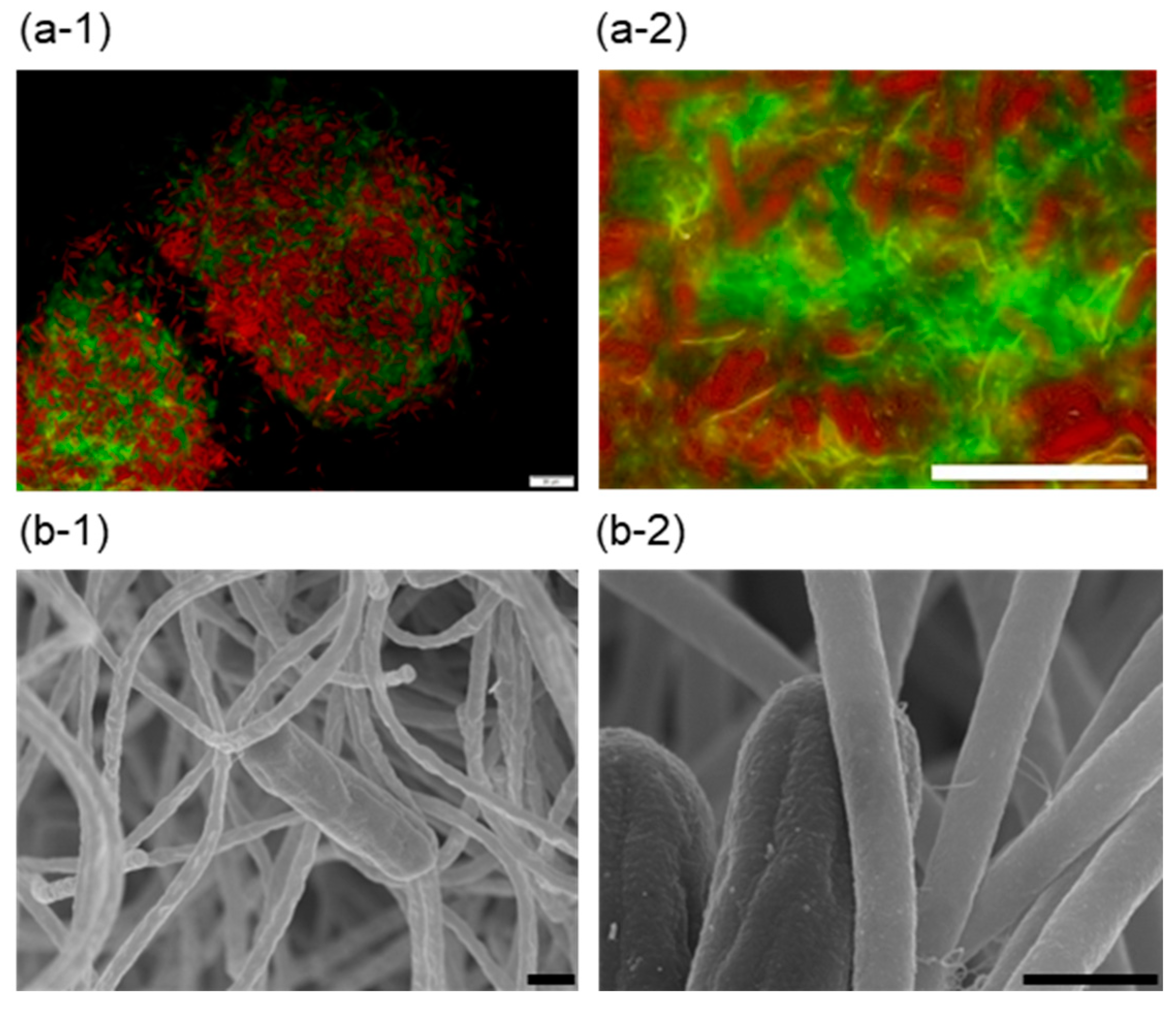
3.1. Cell Aggregate Formation in the Co-Culture of Thermosynechococcus sp. NK55a and C. aggregans NBF
3.2. Morphology of Co-Aggregates
3.3. Co-Cultivation of the ∆pilB Mutant of Thermosynechococcus sp. and C. aggregans NBF
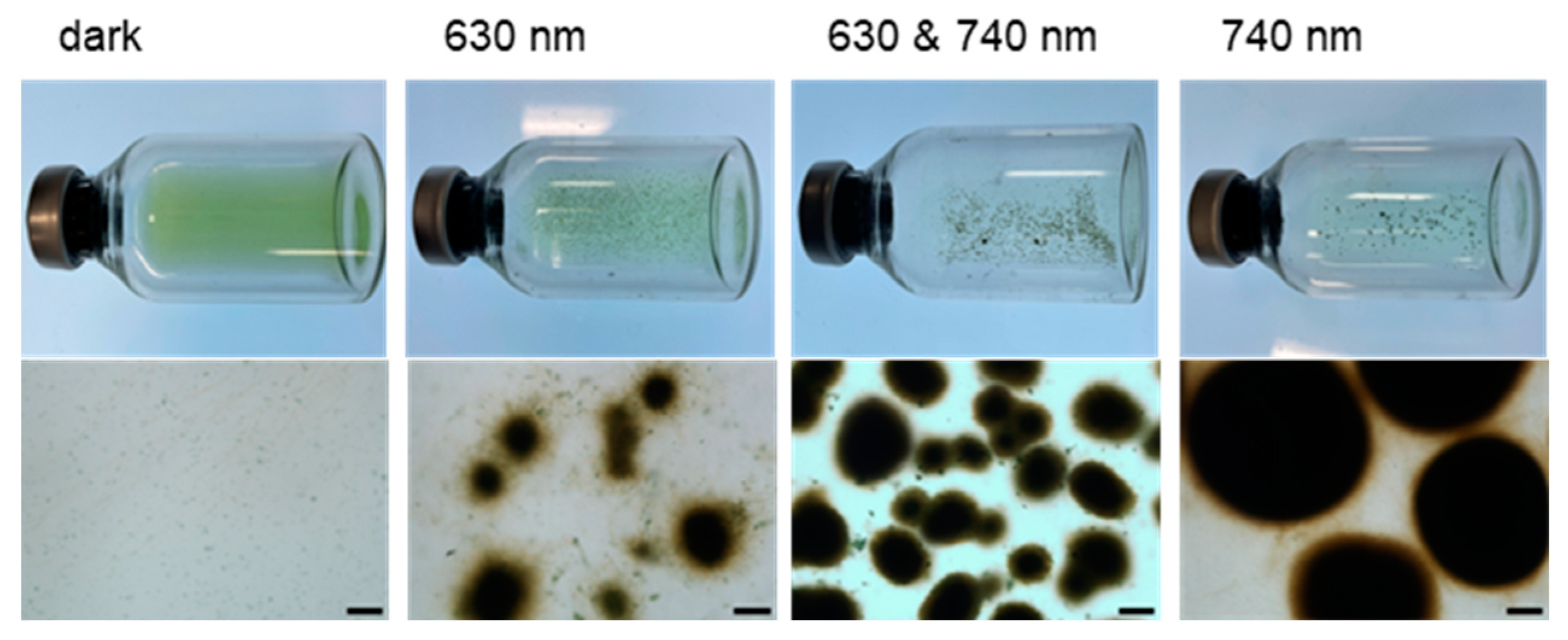
3.4. Effects of Illumination Wavelength on Cell Aggregate Formation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II; Whitton, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–13. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Schuergers, N.; Wilde, A. Appendages of the cyanobacterial cell. Life 2015, 5, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Conradi, F.D.; Mullineaux, C.W.; Wilde, A. The Role of the Cyanobacterial Type IV Pilus Machinery in Finding and Maintaining a Favourable Environment. Life 2020, 10, 252. [Google Scholar] [CrossRef]
- Bharti, A.; Velmourougane, K.; Prasanna, R. Phototrophic biofilms: Diversity, ecology and applications. J. Appl. Phycol. 2017, 29, 2729–2744. [Google Scholar] [CrossRef]
- Bernstein, H.C.; McClure, R.S.; Thiel, V.; Sadler, N.C.; Kim, Y.M.; Chrisler, W.B.; Hill, E.A.; Bryant, D.A.; Romine, M.F.; Jansson, J.K.; et al. Indirect Interspecies Regulation: Transcriptional and Physiological Responses of a Cyanobacterium to Heterotrophic Partnership. mSystems 2017, 2, e00181-16. [Google Scholar] [CrossRef]
- McClure, R.S.; Overall, C.C.; Hill, E.A.; Song, H.S.; Charania, M.; Bernstein, H.C.; McDermott, J.E.; Beliaev, A.S. Species-specific transcriptomic network inference of interspecies interactions. ISME J. 2018, 12, 2011–2023. [Google Scholar] [CrossRef]
- Jing, H.; Lacap, D.C.; Lau, C.Y.; Pointing, S.B. Community phylogenetic diversity of cyanobacterial mats associated with geothermal springs along a tropical intertidal gradient. Extremophiles 2006, 10, 159–163. [Google Scholar] [CrossRef]
- Klatt, C.G.; Wood, J.M.; Rusch, D.B.; Bateson, M.M.; Hamamura, N.; Heidelberg, J.F.; Grossman, A.R.; Bhaya, D.; Cohan, F.M.; Kuhl, M.; et al. Community ecology of hot spring cyanobacterial mats: Predominant populations and their functional potential. ISME J. 2011, 5, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Castenholz, R.W. Thermophilic Blue-Green Algae and the Thermal Environment. Bacteriol. Rev. 1969, 33, 476–504. [Google Scholar] [CrossRef]
- Castenholz, R.W. Portrait of a Geothermal Spring, Hunter’s Hot Springs, Oregon. Life 2015, 5, 332–347. [Google Scholar] [CrossRef]
- Katoh, H.; Itoh, S.; Shen, J.R.; Ikeuchi, M. Functional analysis of psbV and a novel c-type cytochrome gene psbV2 of the thermophilic cyanobacterium Thermosynechococcus elongatus strain BP-1. Plant Cell Physiol. 2001, 42, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Prondzinsky, P.; Berkemer, S.J.; Ward, L.M.; McGlynn, S.E. The Thermosynechococcus Genus: Wide Environmental Distribution, but a Highly Conserved Genomic Core. Microbes Environ. 2021, 36, ME20138. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.T.; Ramsing, N.B.; Bateson, M.M.; Ward, D.M. Geographical isolation in hot spring cyanobacteria. Environ. Microbiol. 2003, 5, 650–659. [Google Scholar] [CrossRef]
- Kamiya, N.; Shen, J.-R. Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-Å resolution. Proc. Natl. Acad. Sci. USA 2003, 100, 98–103. [Google Scholar] [CrossRef]
- Enomoto, G.; Ni-Ni-Win; Narikawa, R.; Ikeuchi, M. Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation. Proc. Natl. Acad. Sci. USA 2015, 112, 8082–8087. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Rockwell, N.C.; Nishiyama, K.; Narikawa, R.; Ukaji, Y.; Inomata, K.; Lagarias, J.C.; Ikeuchi, M. Green/red cyanobacteriochromes regulate complementary chromatic acclimation via a protochromic photocycle. Proc. Natl. Acad. Sci. USA 2013, 110, 4974–4979. [Google Scholar] [CrossRef]
- Loll, B.; Kern, J.; Zouni, A.; Saenger, W.; Biesiadka, J.; Irrgang, K. The antenna system of Photosystem II from Thermosynechococcus elongatus at 3.2 Å resolution. Photosynth. Res. 2005, 86, 175–184. [Google Scholar] [CrossRef]
- Çoruh, O.; Frank, A.; Tanaka, H.; Kawamoto, A.; El-Mohsnawy, E.; Kato, T.; Namba, K.; Gerle, C.; Nowaczyk, M.M.; Kurisu, G. Cryo-EM structure of a functional monomeric Photosystem I from Thermosynechococcus elongatus reveals red chlorophyll cluster. Commun. Biol. 2021, 4, 304. [Google Scholar] [CrossRef]
- Huang, G.; Xiao, Y.; Pi, X.; Zhao, L.; Zhu, Q.; Wang, W.; Kuang, T.; Han, G.; Sui, S.F.; Shen, J.R. Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus. Proc. Natl. Acad. Sci. USA 2021, 118, e2018053118. [Google Scholar] [CrossRef]
- Kawakami, K.; Nagao, R.; Tahara, Y.O.; Hamaguchi, T.; Suzuki, T.; Dohmae, N.; Kosumi, D.; Shen, J.R.; Miyata, M.; Yonekura, K.; et al. Structural implications for a phycobilisome complex from the thermophilic cyanobacterium Thermosynechococcus vulcanus. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148458. [Google Scholar] [CrossRef]
- Nakane, D.; Enomoto, G.; Bahre, H.; Hirose, Y.; Wilde, A.; Nishizaka, T. Thermosynechococcus switches the direction of phototaxis by a c-di-GMP-dependent process with high spatial resolution. eLife 2022, 11, e73405. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tang, J.; Luo, Y.; Kaczmarek, M.; Li, X.; Daroch, M. Thermosynechococcus as a thermophilic photosynthetic microbial cell factory for CO2 utilisation. Bioresour. Technol. 2019, 278, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Thiel, V.; Nakagawa, M.; Ayukawa, S.; Yamamura, M. Effect of light wavelength on hot spring microbial mat biodiversity. PLoS ONE 2018, 13, e0191650. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Martinez, J.N.; Lichtenberg, M.; Trampe, E.; Kuhl, M.; Tank, M.; Haruta, S.; Nishihara, A.; Hanada, S.; Thiel, V. In-Situ Metatranscriptomic Analyses Reveal the Metabolic Flexibility of the Thermophilic Anoxygenic Photosynthetic Bacterium Chloroflexus aggregans in a Hot Spring Cyanobacteria-Dominated Microbial Mat. Microorganisms 2021, 9, 652. [Google Scholar] [CrossRef]
- Martinez, J.N.; Nishihara, A.; Lichtenberg, M.; Trampe, E.; Kawai, S.; Tank, M.; Kühl, M.; Hanada, S.; Thiel, V. Vertical Distribution and Diversity of Phototrophic Bacteria within a Hot Spring Microbial Mat (Nakabusa Hot Springs, Japan). Microbes Environ. 2019, 34, 374–387. [Google Scholar] [CrossRef]
- Haruta, S. 6 Thermophilic photosynthesis-based microbial communities—Energy production and conversion. In Biotechnological Applications of Extremophilic Microorganisms; Lee, N.M., Ed.; De Gruyter: Berlin, Germany, 2020; pp. 153–162. [Google Scholar]
- Enomoto, G.; Okuda, Y.; Ikeuchi, M. Tlr1612 is the major repressor of cell aggregation in the light-color-dependent c-di-GMP signaling network of Thermosynechococcus vulcanus. Sci. Rep. 2018, 8, 5338. [Google Scholar] [CrossRef]
- Kawano, Y.; Saotome, T.; Ochiai, Y.; Katayama, M.; Narikawa, R.; Ikeuchi, M. Cellulose Accumulation and a Cellulose Synthase Gene are Responsible for Cell Aggregation in the Cyanobacterium Thermosynechococcus vulcanus RKN. Plant Cell Physiol. 2011, 52, 957–966. [Google Scholar] [CrossRef]
- Pierson, B.K.; Castenholz, R.W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch. Microbiol. 1974, 100, 5–24. [Google Scholar] [CrossRef]
- Hanada, S. The Phylum Chloroflexi, the Family Chloroflexaceae, and the Related Phototrophic Families Oscillochloridaceae and Roseiflexaceae. In The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 515–532. [Google Scholar]
- Hanada, S.; Hiraishi, A.; Shimada, K.; Matsuura, K. Chloroflexus aggregans sp. nov., a filamentous phototrophic bacterium which forms dense cell aggregates by active gliding movement. Int. J. Syst. Bacteriol. 1995, 45, 676–681. [Google Scholar] [CrossRef]
- Hanada, S.; Shimada, K.; Matsuura, K. Active and energy-dependent rapid formation of cell aggregates in the thermophilic photosynthetic bacterium Chloroflexus aggregans. FEMS Microbiol. Lett. 2002, 208, 275–279. [Google Scholar] [CrossRef]
- Stolyar, S.; Liu, Z.; Thiel, V.; Tomsho, L.P.; Pinel, N.; Nelson, W.C.; Lindemann, S.R.; Romine, M.F.; Haruta, S.; Schuster, S.C.; et al. Genome Sequence of the Thermophilic Cyanobacterium Thermosynechococcus sp. Strain NK55a. Genome Announc. 2014, 2, e01060-13. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, S.; Matsuura, K.; Haruta, S. Secreted protease mediates interspecies interaction and promotes cell aggregation of the photosynthetic bacterium Chloroflexus aggregans. FEMS Microbiol. Lett. 2015, 362, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.; Herdman, M.; Stanier, R. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Muramatsu, S.; Hirose, S.; Iino, T.; Ohkuma, M.; Hanada, S.; Haruta, S. Neotabrizicola shimadae gen. nov., sp. nov., an aerobic anoxygenic phototrophic bacterium harbouring photosynthetic genes in the family Rhodobacteraceae, isolated from a terrestrial hot spring. Antonie van Leeuwenhoek 2022, 115, 731–740. [Google Scholar] [CrossRef]
- Haruta, S.; Kakuhama, H.; Fukushima, S.; Morohoshi, S. Motility assay of Chloroflexus. In Bacterial and Archaeal Motility (Springer Protocols, Methods in Molecular Biology); Minamino, T., Miyata, M., Namba, K., Eds.; Humana: New York, NY, USA, 2023; pp. 383–390. [Google Scholar]
- Iwai, M.; Katoh, H.; Katayama, M.; Ikeuchi, M. Improved genetic transformation of the thermophilic cyanobacterium, Thermosynechococcus elongatus BP-1. Plant Cell Physiol. 2004, 45, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Griese, M.; Lange, C.; Soppa, J. Ploidy in cyanobacteria. FEMS Microbiol. Lett. 2011, 323, 124–131. [Google Scholar] [CrossRef]
- Riaz, S.; Xiao, M.; Chen, P.; Li, M.; Cui, Y.; Daroch, M. The Genome Copy Number of the Thermophilic Cyanobacterium Thermosynechococcus elongatus E542 Is Controlled by Growth Phase and Nutrient Availability. Appl. Environ. Microbiol. 2021, 87, e02993-20. [Google Scholar] [CrossRef]
- Piepenbrink, K.; Sundberg, E. Motility and adhesion through type IV pili in Gram-positive bacteria. Biochem. Soc. Trans. 2016, 44, 1659–1666. [Google Scholar] [CrossRef]
- Craig, L.; Forest, K.T.; Maier, B. Type IV pili: Dynamics, biophysics and functional consequences. Nat. Rev. Microbiol. 2019, 17, 429–440. [Google Scholar] [CrossRef]
- Yoshihara, S.; Ikeuchi, M. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. Sci. 2004, 3, 512–518. [Google Scholar] [CrossRef]
- Jakovljevic, V.; Leonardy, S.; Hoppert, M.; Sogaard-Andersen, L. PilB and PilT are ATPases acting antagonistically in type IV pilus function in Myxococcus xanthus. J. Bacteriol. 2008, 190, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Bhaya, D.; Bianco, N.R.; Bryant, D.; Grossman, A. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 2000, 37, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Geng, X.; Okamoto, S.; Yura, K.; Murata, T.; Go, M.; Ohmori, M.; Ikeuchi, M. Mutational Analysis of Genes Involved in Pilus Structure, Motility and Transformation Competency in the Unicellular Motile Cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001, 42, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Moon, Y.J.; Oh, H.W.; Kim, S.; Chung, Y.; Kweon, H.; Kim, Y. Observations of the Cyanobacteria Synechocystis sp. PCC 6803 using Cryo-Methods and Cryo-SEM. Korean J. Microsc. 2009, 39, 65–72. [Google Scholar]
- Nagar, E.; Zilberman, S.; Sendersky, E.; Simkovsky, R.; Shimoni, E.; Gershtein, D.; Herzberg, M.; Golden, S.; Schwarz, R. Type 4 pili are dispensable for biofilm development in the cyanobacterium Synechococcus elongatus. Environ. Microbiol. 2017, 19, 2862–2872. [Google Scholar] [CrossRef]
- Nakasugi, K.; Neilan, B.A. Identification of pilus-like structures and genes in Microcystis aeruginosa PCC7806. Appl. Environ. Microbiol. 2005, 71, 7621–7625. [Google Scholar] [CrossRef]
- Allen, R.; Rittmann, B.E.; Curtiss, R. Axenic Biofilm Formation and Aggregation by Synechocystis sp. Strain PCC 6803 Are Induced by Changes in Nutrient Concentration and Require Cell Surface Structures. Appl. Environ. Microbiol. 2019, 85, e02192-18. [Google Scholar] [CrossRef]
- Conradi, F.D.; Zhou, R.Q.; Oeser, S.; Schuergers, N.; Wilde, A.; Mullineaux, C.W. Factors Controlling Floc Formation and Structure in the Cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 2019, 201, e00344-19. [Google Scholar] [CrossRef]
- Peabody, C.R.; Chung, Y.J.; Yen, M.R.; Vidal-Ingigliardi, D.; Pugsley, A.P.; Saier, M.H. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 2003, 149, 3051–3072. [Google Scholar] [CrossRef]
- Black, W.P.; Wang, L.; Jing, X.; Saldaña, R.C.; Li, F.; Scharf, B.E.; Schubot, F.D.; Yang, Z. The type IV pilus assembly ATPase PilB functions as a signaling protein to regulate exopolysaccharide production in Myxococcus xanthus. Sci. Rep. 2017, 7, 7263. [Google Scholar] [CrossRef]
- Meissner, J.; Krauss, J.; Jürgens, U.; Weckesser, J. Absence of a Characteristic Cell Wall Lipopolysaccharide in the Phototrophic Bacterium Chloroflexus aurantiacus. J. Bacteriol. 1988, 170, 3213–3216. [Google Scholar] [CrossRef] [PubMed]
- Gaisin, V.A.; Kalashnikov, A.M.; Grouzdev, D.S.; Sukhacheva, M.V.; Kuznetsov, B.B.; Gorlenko, V.M. Chloroflexus islandicus sp. nov., a thermophilic filamentous anoxygenic phototrophic bacterium from a geyser. Int. J. Syst. Evol. Microbiol. 2017, 67, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Gaisin, V.A.; Kooger, R.; Grouzdev, D.S.; Gorlenko, V.M.; Pilhofer, M. Cryo-Electron Tomography Reveals the Complex Ultrastructural Organization of Multicellular Filamentous Chloroflexota (Chloroflexi) Bacteria. Front. Microbiol. 2020, 11, 1373. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Robinson, R.C.; Uyeda, T.Q.P.; Fukumori, Y.; Fukushima, S.I.; Haruta, S.; Homma, M.; Inaba, K.; Ito, M.; Kaito, C.; et al. Tree of motility—A proposed history of motility systems in the tree of life. Genes Cells 2020, 25, 6–21. [Google Scholar] [CrossRef]
- Kamagata, Y. Recent biofilm studies open a new door in microbial ecology. Microbes Environ. 2020, 35, ME3501rh. [Google Scholar] [CrossRef]
- Mittermeier, F.; Bäumler, M.; Arulrajah, P.; García Lima, J.J.; Hauke, S.; Stock, A.; Weuster-Botz, D. Artificial microbial consortia for bioproduction processes. Eng. Life Sci. 2022, 23, e2100152. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, J.; Yuan, X.; Jiang, X.; Wang, Y.; Zhong, C.; Xu, D.; Gu, T.; Wang, F. Bacterial biofilms as platforms engineered for diverse applications. Biotechnol. Adv. 2022, 57, 107932. [Google Scholar] [CrossRef]



| Product (Size) | Name | Sequence (5′–3′) |
|---|---|---|
| pUC19 vector backbone (2684 bp) | pUC19_1R1 | GGCTTCTTCCCTAGAGTGCAAGCTTGGCGTAA |
| pUC19_8F1 | CTGTTTTGGCTGACTACCGAGCTCGAATTCAC | |
| Chloramphenicol-resistant cassette of pHSG398 (1093 bp) | CmR_4F1 | TCGATCCCGAACTACGGAAGATCACTTCGCAG |
| CmR_5R1 | AAGTCTACACGTCCTCACATTAATTGCGTTGC | |
| Upstream region of pilB (2531 bp) | up NKpilB_2F1 | CCAAGCTTGCACTCTAGGGAAGAAGCCAGCG |
| up NKpilB_3R1 | GTGATCTTCCGTAGTTCGGGATCGAGGCAATC | |
| Downstream region of pilB (2607 bp) | dw NKpilB_6F1 | GCAACGCAATTAATGTGAGGACGTGTAGACTTC |
| dw NKpilB_7R1 | TTCGAGCTCGGTAGTCAGCCAAAACAGCGATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kono, M.; Haruta, S. Coaggregation Occurs between a Piliated Unicellular Cyanobacterium, Thermosynechococcus, and a Filamentous Bacterium, Chloroflexus aggregans. Microorganisms 2024, 12, 1904. https://doi.org/10.3390/microorganisms12091904
Kono M, Haruta S. Coaggregation Occurs between a Piliated Unicellular Cyanobacterium, Thermosynechococcus, and a Filamentous Bacterium, Chloroflexus aggregans. Microorganisms. 2024; 12(9):1904. https://doi.org/10.3390/microorganisms12091904
Chicago/Turabian StyleKono, Megumi, and Shin Haruta. 2024. "Coaggregation Occurs between a Piliated Unicellular Cyanobacterium, Thermosynechococcus, and a Filamentous Bacterium, Chloroflexus aggregans" Microorganisms 12, no. 9: 1904. https://doi.org/10.3390/microorganisms12091904






