Enhancing Wastewater Treatment with Aerobic Granular Sludge: Impacts of Tetracycline Pressure on Microbial Dynamics and Structural Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Operating Conditions
2.2. Seed Sludge and Wastewater Composition
2.3. Analysis of Conventional Sludge Indicators
2.4. EPS Extraction and Analysis
2.4.1. Quantitative Analysis of EPS
2.4.2. Qualitative Analysis of EPS
2.5. Metagenomic Sequence, Assembly and Analysis
3. Results and Discussion
3.1. Effect of TC on AGS Formation and Performance
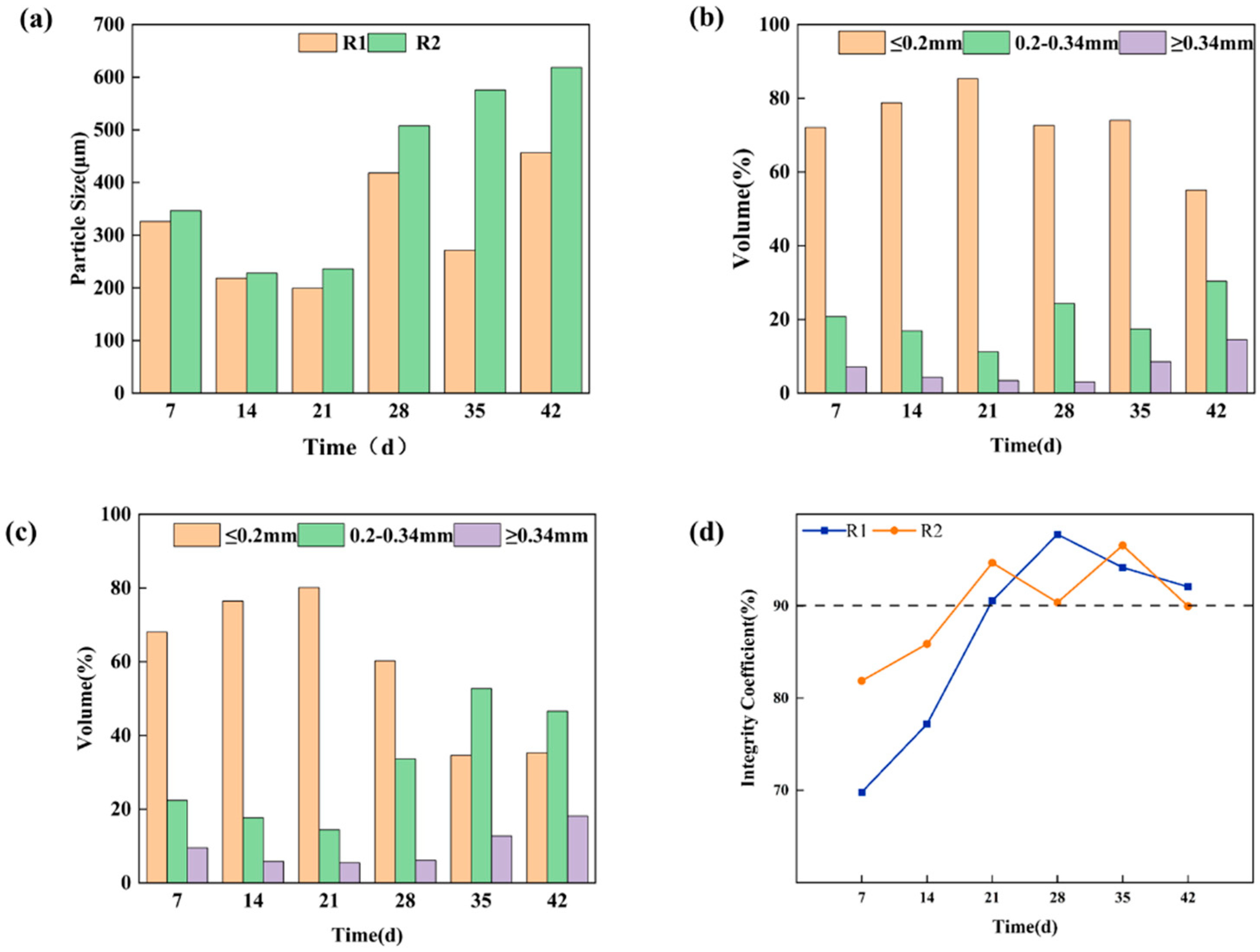
3.1.1. Morphological Evolution of Aerobic Granular Sludge
3.1.2. Performance of Aerobic Granular Sludge
Sludge Concentration
Removal Performance
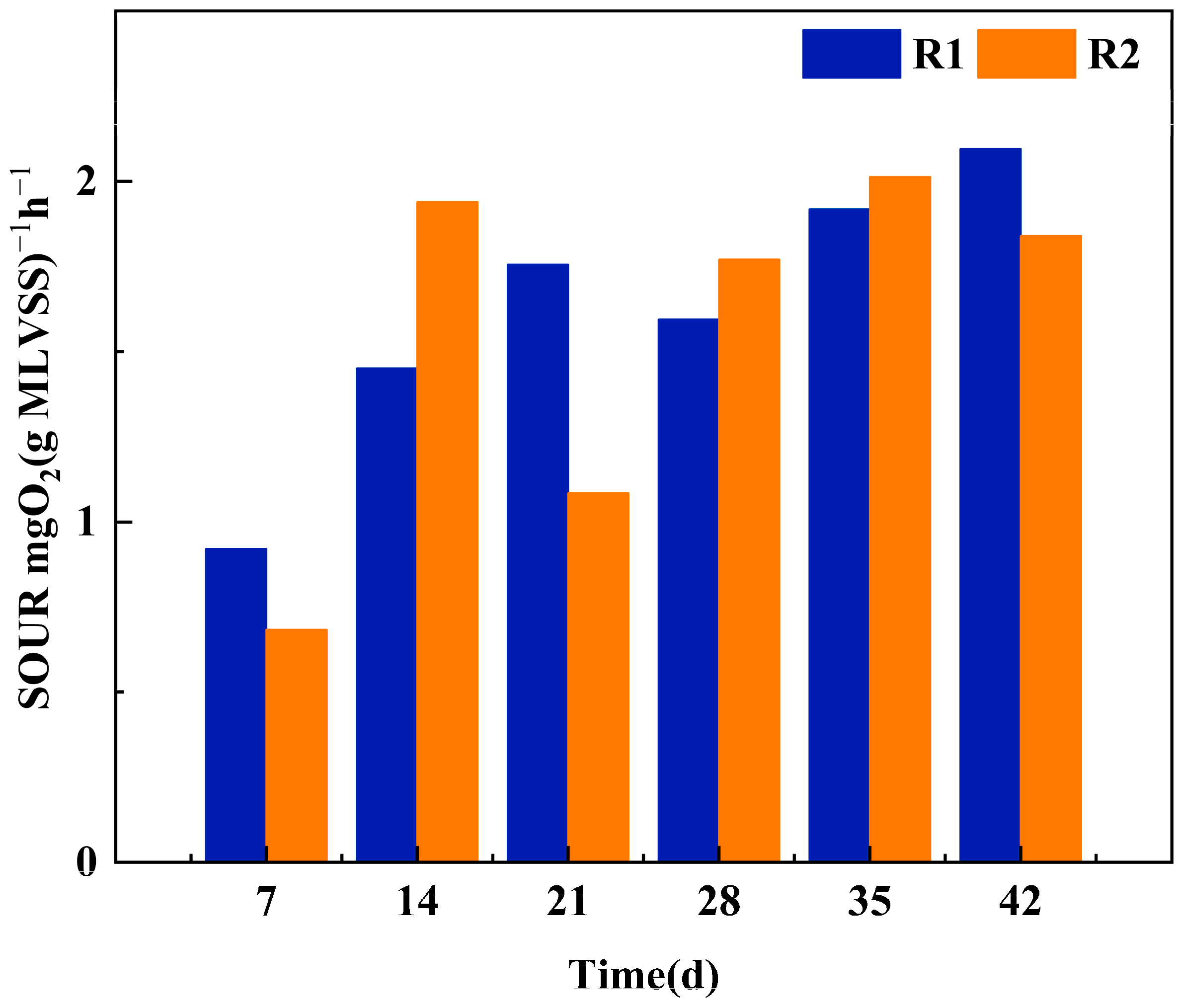
Sludge Activity
3.2. Effect of TC on EPS Characteristics of Aerobic Granular Sludge
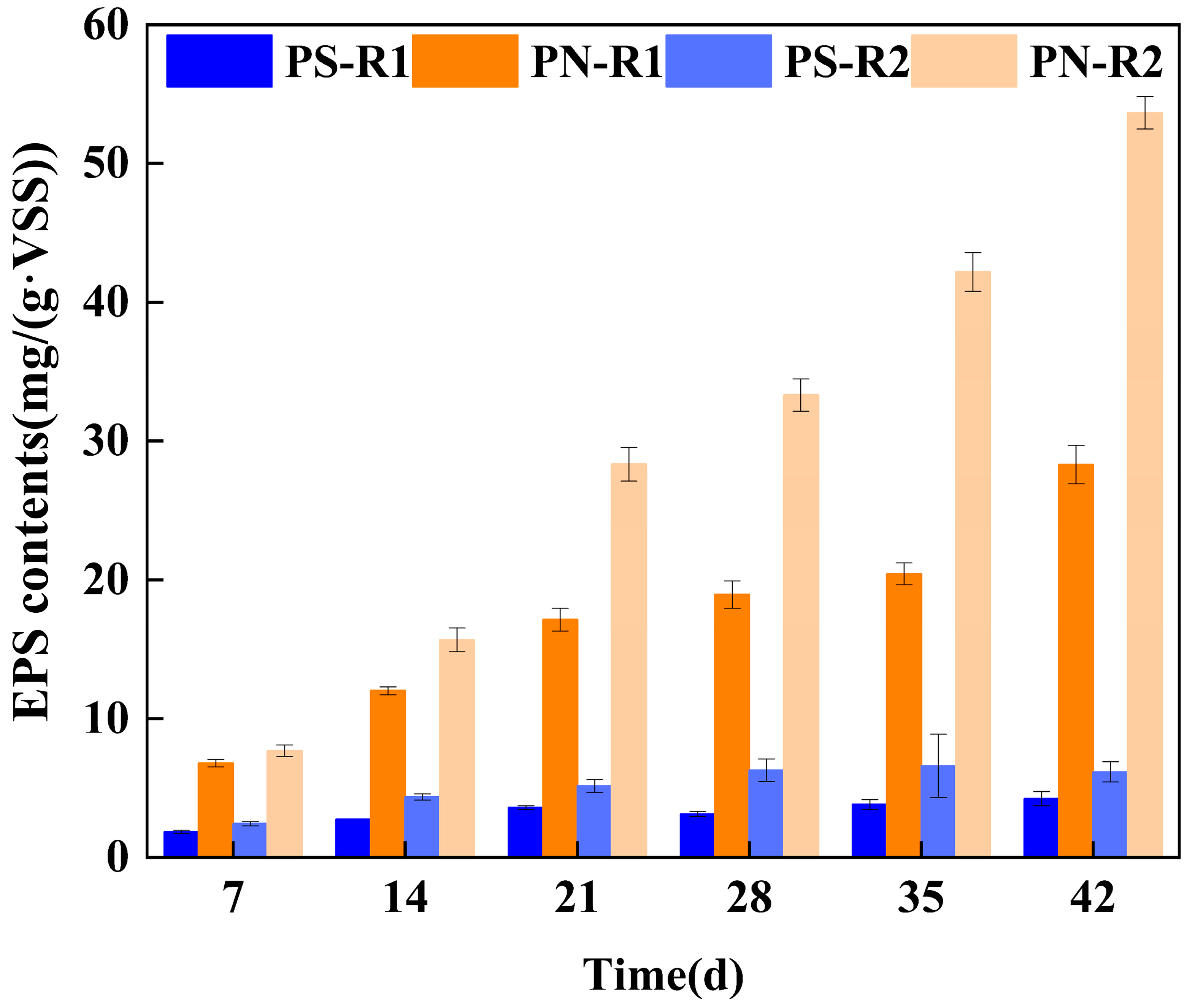
3.2.1. Quantitative Analysis of EPS of Aerobic Granular Sludge
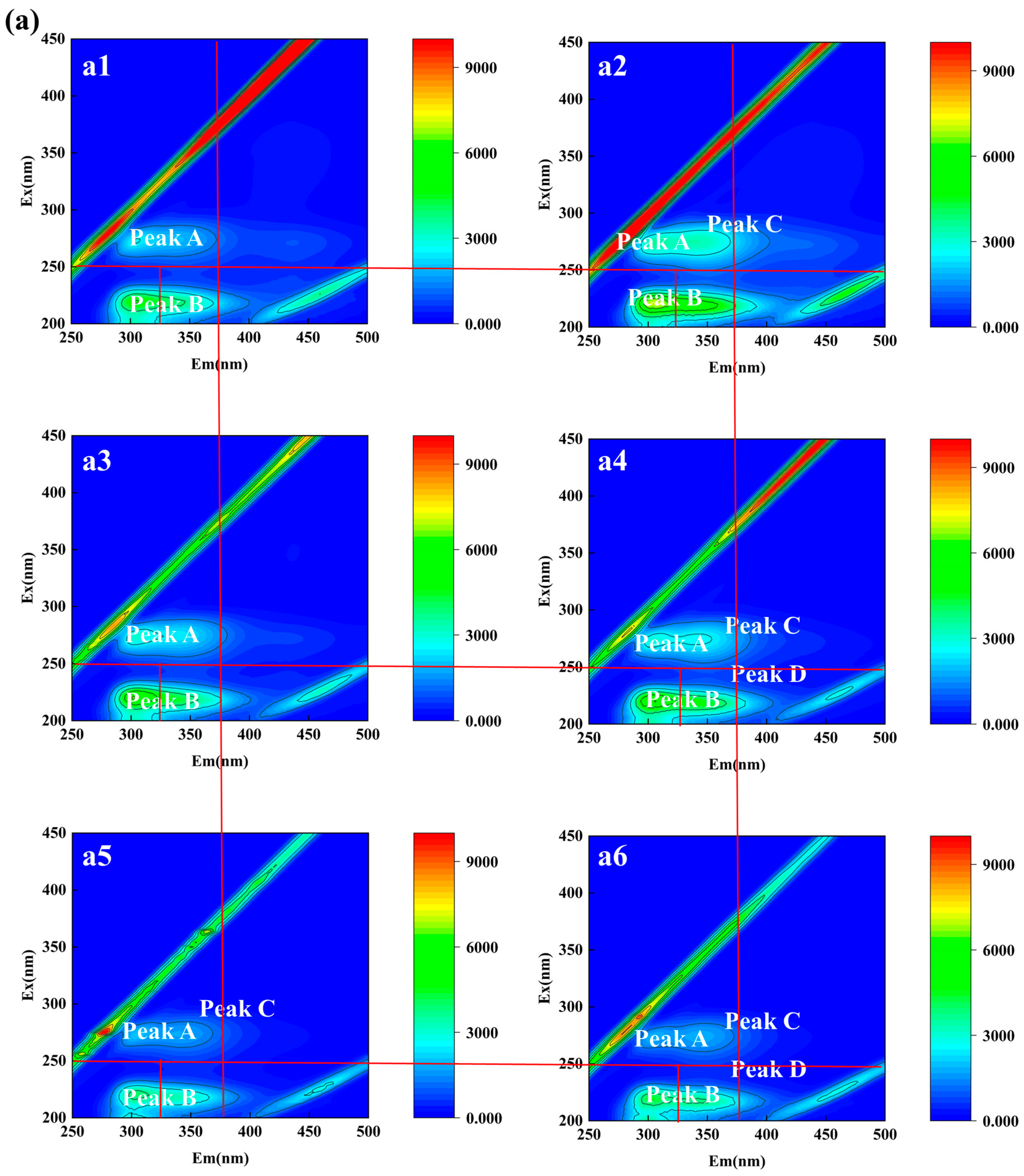
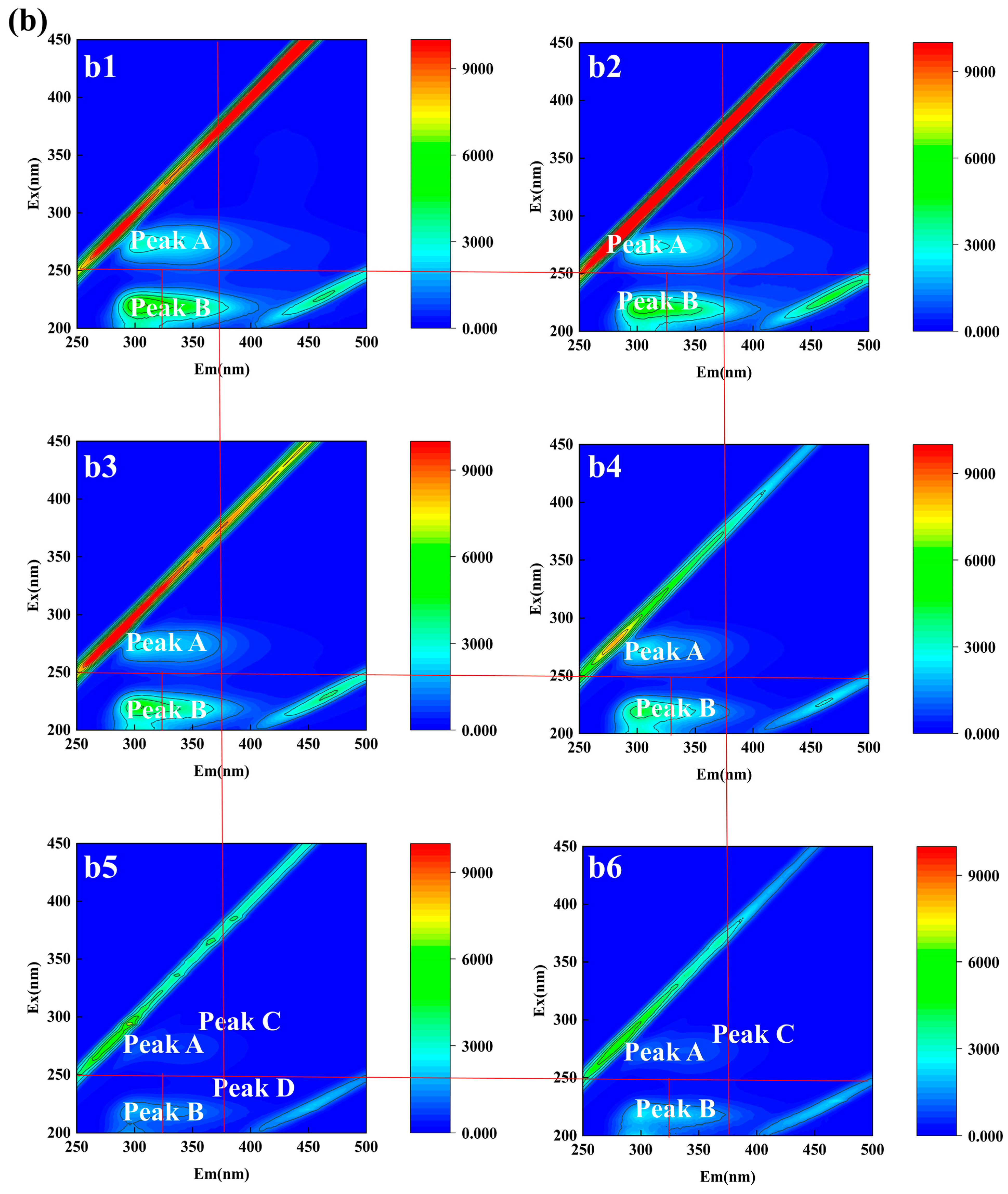
3.2.2. Qualitative Analysis of EPS of Aerobic Granular Sludge
3.3. Effect of TC on Microbial Community in Aerobic Granular Sludge
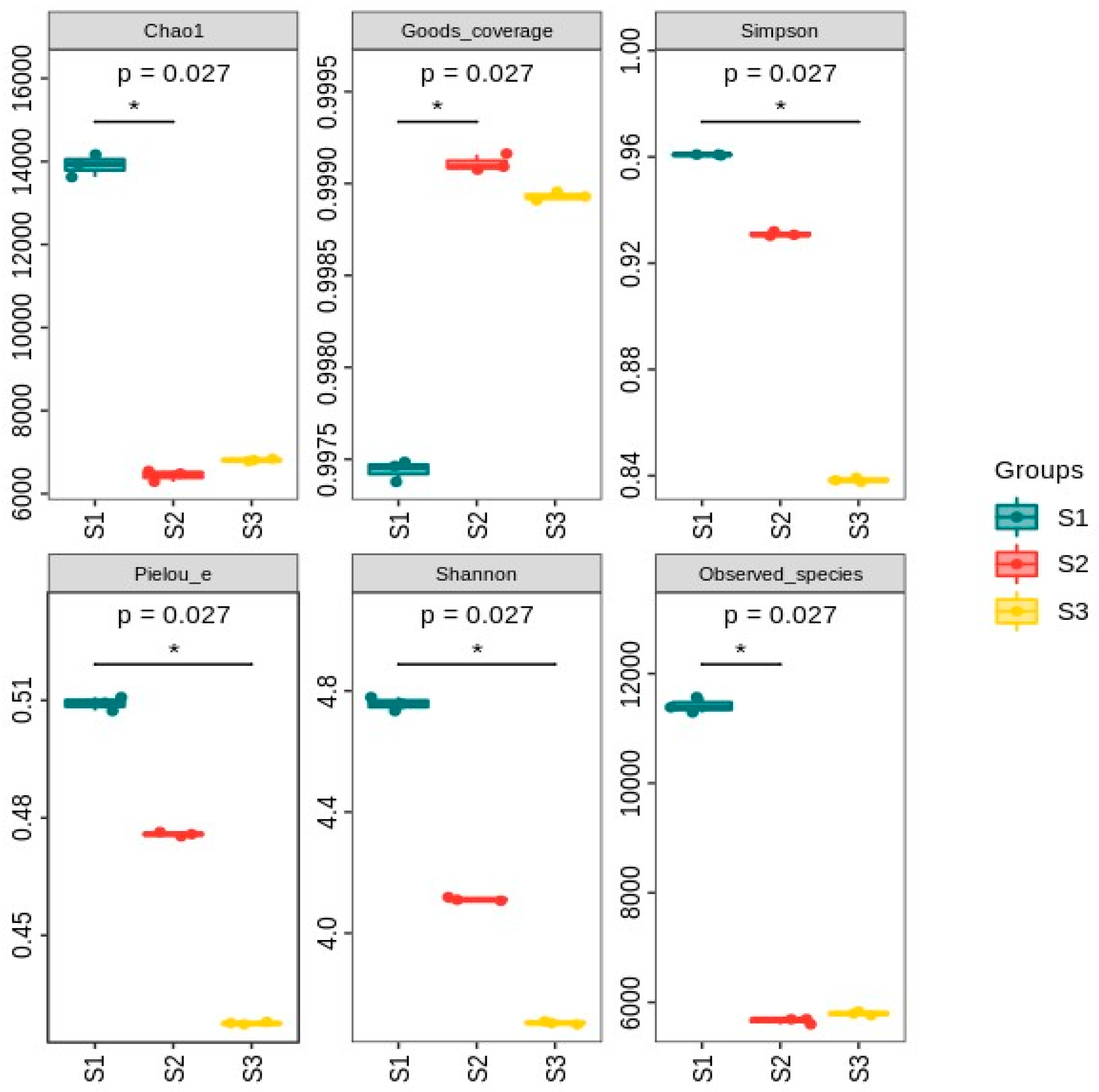
3.3.1. Alpha Diversity Analysis
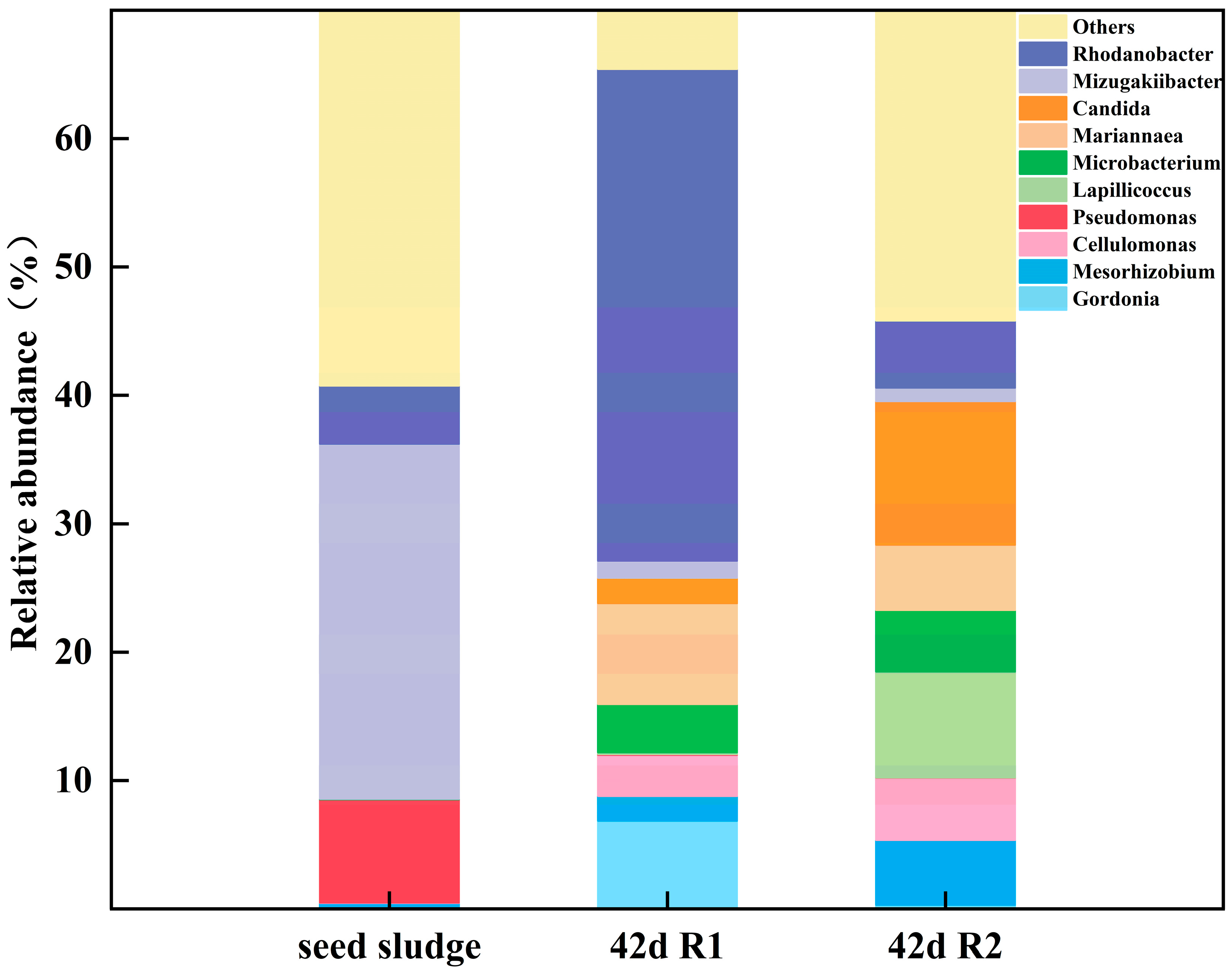
3.3.2. Structural Analysis of the Microbial Community
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into fate and removal of antibiotics in engineered biological treatment systems: A critical review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Q.; Yuan, L.; Li, Z.-H.; Zhang, H.C.; Sheng, G.P. Tetracycline exposure shifted microbial communities and enriched antibiotic resistance genes in the aerobic granular sludge. Environ. Int. 2019, 130, 104902. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Interaction between tetracycline and microorganisms during wastewater treatment: A review. Sci. Total Environ. 2021, 757, 143981. [Google Scholar] [CrossRef]
- Guo, T.; Pan, K.; Chen, Y.; Tian, Y.; Deng, J.; Li, J. When aerobic granular sludge faces emerging contaminants: A review. Sci. Total Environ. 2024, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Molinos-Senante, M.; Garrido-Baserba, M.; Reif, R.; Hernández-Sancho, F.; Poch, M. Assessment of wastewater treatment plant design for small communities: Environmental and economic aspects. Sci. Total Environ. 2012, 427–428, 11–18. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, S.; Wang, X.; Wang, S. Changes of the reactor performance and the properties of granular sludge under tetracycline (TC) stress. Bioresour. Technol. 2013, 139, 170–175. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, M.; Hu, Q.; Li, B.; Li, B.; Wang, W.; Abudi, Z.N.; Hu, Z. Aerobic granular sludge formation and stability in enhanced biological phosphorus removal system under antibiotics pressure: Performance, granulation mechanism, and microbial successions. J. Hazard. Mater. 2023, 454, 131472. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.; Brdjanovic, D. Anticipating the next century of wastewater treatment. Science 2014, 344, 1452–1453. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Sarvajith, M.; Mohan, T.V.K. Pilot-scale aerobic granular sludge reactors with granular activated carbon for effective nitrogen and phosphorus removal from domestic wastewater. Sci. Total Environ. 2023, 894, 164822. [Google Scholar] [CrossRef]
- Adav, S.S.; Lee, D.-J.; Lai, J.-Y. Proteolytic activity in stored aerobic granular sludge and structural integrity. Bioresour. Technol. 2009, 100, 68–73. [Google Scholar] [CrossRef]
- Liu, H.; Xie, Q.; Qiu, L.; Li, H.; Long, Y.; Hu, L.; Fang, C. Effects of antibiotic exposure on bioreactor treatment efficiency and dynamic migration of resistance genes in landfill leachate. J. Water Process Eng. 2023, 56, 104528. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Lee, D.-J.; Tay, J.H.; Zhang, Y.; Wan, C.L.; Chen, X.F. Recent advances on biosorption by aerobic granular sludge. J. Hazard. Mater. 2018, 357, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-F.; Zheng, P.; Ji, Q.-X.; Zhang, H.T.; Ji, J.Y.; Wang, L.; Ding, S.; Chen, T.T.; Zhang, J.Q.; Tang, C.J.; et al. The structure, density and settlability of anammox granular sludge in high-rate reactors. Bioresour. Technol. 2012, 123, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, J.; Lee, D.-J. Aerobic granular processes: Current research trends. Bioresour. Technol. 2016, 210, 74–80. [Google Scholar] [CrossRef]
- Lou, B.; Yang, Z.; Zheng, S.; Ou, D.; Hu, W.; Ai, N. Characteristics, performance and microbial response of aerobic granular sludge for treating tetracycline hypersaline pharmaceutical wastewater. Microorganisms 2024, 12, 1173. [Google Scholar] [CrossRef]
- Michael, S.G.; Drigo, B.; Michael-Kordatou, I.; Michael, C.; Jäger, T.; Aleer, S.C.; Schwartz, T.; Donner, E.; Fatta-Kassinos, D. The effect of ultrafiltration process on the fate of antibiotic-related microcontaminants, pathogenic microbes, and toxicity in urban wastewater. J. Hazard. Mater. 2022, 435, 128943. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.; Ning, A.; Hu, C.; Liu, Y. Metagenomics unraveled the characteristics and microbial response to hypersaline stress in salt-tolerant aerobic granular sludge. J. Environ. Manag. 2022, 321, 115950. [Google Scholar] [CrossRef]
- Corsino, S.F.; Capodici, M.; Torregrossa, M.; Viviani, G. Physical properties and Extracellular Polymeric Substances pattern of aerobic granular sludge treating hypersaline wastewater. Bioresour. Technol. 2017, 229, 152–159. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Ren, T.-T.; Liu, L.; Sheng, G.-P.; Liu, X.W.; Yu, H.Q.; Zhang, M.C.; Zhu, J.R. Calcium spatial distribution in aerobic granules and its effects on granule structure, strength and bioactivity. Water Res. 2008, 42, 3343–3352. [Google Scholar] [CrossRef]
- Beutler, M.; Wiltshire, K.; Meyer, B.; Moldaenke, C.; Luring, C.; Meyerhofer, M.; Hansen, U.P. APHA (2005), Standard Methods for the Examination of Water and Wastewater, Washington DC: American Public Health Association. Ahmad, SR, and DM Reynolds (1999), Monitoring of water quality using fluorescence technique: Prospect of on-line process control. Dissolved Oxyg. Dyn. Model. Case Study A Subtrop. Shallow Lake 2014, 217, 95. [Google Scholar]
- Chen, B.; Yang, Z.; Pan, J.; Ren, Y.; Wu, H.; Wei, C. Functional identification behind gravity-separated sludge in high concentration organic coking wastewater: Microbial aggregation, apoptosis-like decay and community. Water Res. 2019, 150, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Jiang, Y.; Su, H. Influence of an aniline supplement on the stability of aerobic granular sludge. J. Environ. Manag. 2015, 162, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ai, N.; Yang, Z.; Lou, B.; Yang, D.; Wang, Q.; Ou, D.; Hu, C. Impact of stepwisely reducing settling time on the formation and performance of aerobic granular sludge. J. Water Process Eng. 2024, 60, 105117. [Google Scholar] [CrossRef]
- Li, X.Y.; Yang, S.F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res 2007, 41, 1022–1030. [Google Scholar] [CrossRef]
- Gerhardt, P.; Murray, R.G.E.; Wood, W.A.; Krieg, N.R. Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Wang, W.; Gao, X.; Zhang, J.; Yang, T.; Li, R.; Sun, Y. Effect of SDS and neutral protease on the release of Extracellular Polymeric Substances (EPS) from mechanical dewatered sludge. Waste Biomass Valorization 2019, 10, 1053–1064. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, S.; Li, X. Physical and hydrodynamic properties of aerobic granules produced in sequencing batch reactors. Sep. Purif. Technol. 2008, 63, 634–641. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhu, N.; Chen, J. Role of extracellular protein in the formation and stability of aerobic granules. Enzym. Microb. Technol. 2007, 41, 551–557. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Yue, Z.-B. Production of extracellular polymeric substances from Rhodopseudomonas acidophila in the presence of toxic substances. Appl. Microbiol. Biotechnol. 2005, 69, 216–222. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Wang, Z.; She, Z.; Chang, Q.; Sun, C.; Zhang, J.; Ren, Y.; Yang, N. Effect of salinity on extracellular polymeric substances of activated sludge from an anoxic–aerobic sequencing batch reactor. Chemosphere 2013, 93, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Liang, H.; He, J.; Ma, J.; Wang, Z.; Yu, H.; Li, G. Characterization of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) of Microcystis aeruginosa and their impacts on UF membrane fouling. Water Res. 2012, 46, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhan, H.; Wang, Q.; Wu, G.; Cui, D. Enhanced aerobic granulation by inoculating dewatered activated sludge under short settling time in a sequencing batch reactor. Bioresour. Technol. 2019, 286, 121386. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, P.; Zhang, M.; Zeng, Z.; Wang, Z.; Ding, A.; Ding, K. Hydrophilicity/hydrophobicity of anaerobic granular sludge surface and their causes: An in situ research. Bioresour. Technol. 2016, 220, 117–123. [Google Scholar] [CrossRef]
- Wan, C.; Li, Z.; Deng, L.; Yuan, Y.; Wu, C. Microbial population properties in the hierarchically structured aerobic granular sludge: Phenotype and genotype. Sci. Total Environ. 2023, 867, 161164. [Google Scholar] [CrossRef]
- Szabó, E.; Liébana, R.; Hermansson, M.; Modin, O.; Persson, F.; Wilén, B.M. Microbial Population Dynamics and Ecosystem Functions of Anoxic/Aerobic Granular Sludge in Sequencing Batch Reactors Operated at Different Organic Loading Rates. Front. Microbiol. 2017, 8, 770. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Huang, J.; Chen, J.; Jia, X.; Peng, X. Dimorphism of Candida tropicalis and its effect on nitrogen and phosphorus removal and sludge settleability. Bioresour. Technol. 2023, 382, 129186. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, L.; Ning, S.; He, M. Reactor performance and microbial community dynamics during aerobic degradation and detoxification of Acid Red B with activated sludge bioaugmented by a yeast Candida tropicalis TL-F1 in MBR. Int. Biodeterior. Biodegrad. 2015, 104, 149–156. [Google Scholar] [CrossRef]
- He, Y.; Song, Z.; Dong, X.; Zheng, Q.; Peng, X.; Jia, X. Candida tropicalis prompted effectively simultaneous removal of carbon, nitrogen and phosphorus in activated sludge reactor: Microbial community succession and functional characteristics. Bioresour. Technol. 2022, 348, 126820. [Google Scholar] [CrossRef]



| Time (d) | Feeding (min/Cycle) | Anoxic Time (min/Cycle) | Aerobic Time (min/Cycle) | Settling Time (min/Cycle) | Discharge (min/Cycle) |
|---|---|---|---|---|---|
| 0–7 | 2 | 30 | 296 | 30 | 2 |
| 8–14 | 2 | 30 | 296 | 30 | 2 |
| 15–21 | 2 | 30 | 311 | 15 | 2 |
| 22–28 | 2 | 30 | 311 | 15 | 2 |
| 29–35 | 2 | 30 | 316 | 10 | 2 |
| 36–42 | 2 | 30 | 316 | 10 | 2 |
| Samples | Time | Peak A | Peak B | Peak C | Peak D | Peak E | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (d) | Ex/Em | Intensity | Ex/Em | Intensity | Ex/Em | Intensity | Ex/Em | Intensity | Ex/Em | Intensity | |
| R1 | 0–7 | 272/304 | 2572 | 220/306 | 5040 | - | - | - | - | 272/438 | 849.9 |
| 8–14 | 274/306 | 3677 | 220/310 | 7218 | 274/338 | 3378 | - | - | - | - | |
| 15–21 | 272/306 | 3095 | 220/302 | 6647 | - | - | - | - | - | - | |
| 22–28 | 274/306 | 2991 | 220/302 | 6353 | 274/342 | 2649 | 220/334 | 5013 | - | - | |
| 29–35 | 274/306 | 2041 | 220/302 | 4099 | 274/340 | 1707 | - | - | - | - | |
| 36–42 | 274/306 | 1865 | 220/304 | 4431 | 274/344 | 1776 | 218/334 | 3925 | - | - | |
| R2 | 0–7 | 274/306 | 3210 | 220/306 | 6460 | - | - | - | - | - | - |
| 8–14 | 274/306 | 3098 | 220/306 | 5826 | - | - | - | - | - | - | |
| 15–21 | 274/306 | 2663 | 220/306 | 5082 | - | - | - | - | - | - | |
| 22–28 | 272/304 | 2578 | 218/304 | 4580 | - | - | - | - | - | - | |
| 29–35 | 272/302 | 953.2 | 220/300 | 1795 | 274/342 | 630.8 | 218/330 | 1496 | - | - | |
| 36–42 | 274/304 | 1127 | 218/302 | 2449 | 274/336 | 853.3 | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Lou, B.; Yang, Z.; Ou, D.; Ai, N. Enhancing Wastewater Treatment with Aerobic Granular Sludge: Impacts of Tetracycline Pressure on Microbial Dynamics and Structural Stability. Microorganisms 2024, 12, 1913. https://doi.org/10.3390/microorganisms12091913
Zheng S, Lou B, Yang Z, Ou D, Ai N. Enhancing Wastewater Treatment with Aerobic Granular Sludge: Impacts of Tetracycline Pressure on Microbial Dynamics and Structural Stability. Microorganisms. 2024; 12(9):1913. https://doi.org/10.3390/microorganisms12091913
Chicago/Turabian StyleZheng, Shengyan, Bichen Lou, Zhonghui Yang, Dong Ou, and Ning Ai. 2024. "Enhancing Wastewater Treatment with Aerobic Granular Sludge: Impacts of Tetracycline Pressure on Microbial Dynamics and Structural Stability" Microorganisms 12, no. 9: 1913. https://doi.org/10.3390/microorganisms12091913
APA StyleZheng, S., Lou, B., Yang, Z., Ou, D., & Ai, N. (2024). Enhancing Wastewater Treatment with Aerobic Granular Sludge: Impacts of Tetracycline Pressure on Microbial Dynamics and Structural Stability. Microorganisms, 12(9), 1913. https://doi.org/10.3390/microorganisms12091913






