Soil Fungal Community Diversity, Co-Occurrence Networks, and Assembly Processes under Diverse Forest Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Soil Physicochemical Properties Determination
2.4. Illumina MiSeq Sequencing and Bioinformatics Processing
2.5. Bioinformatics Analysis
2.6. Statistical Analyses
3. Results
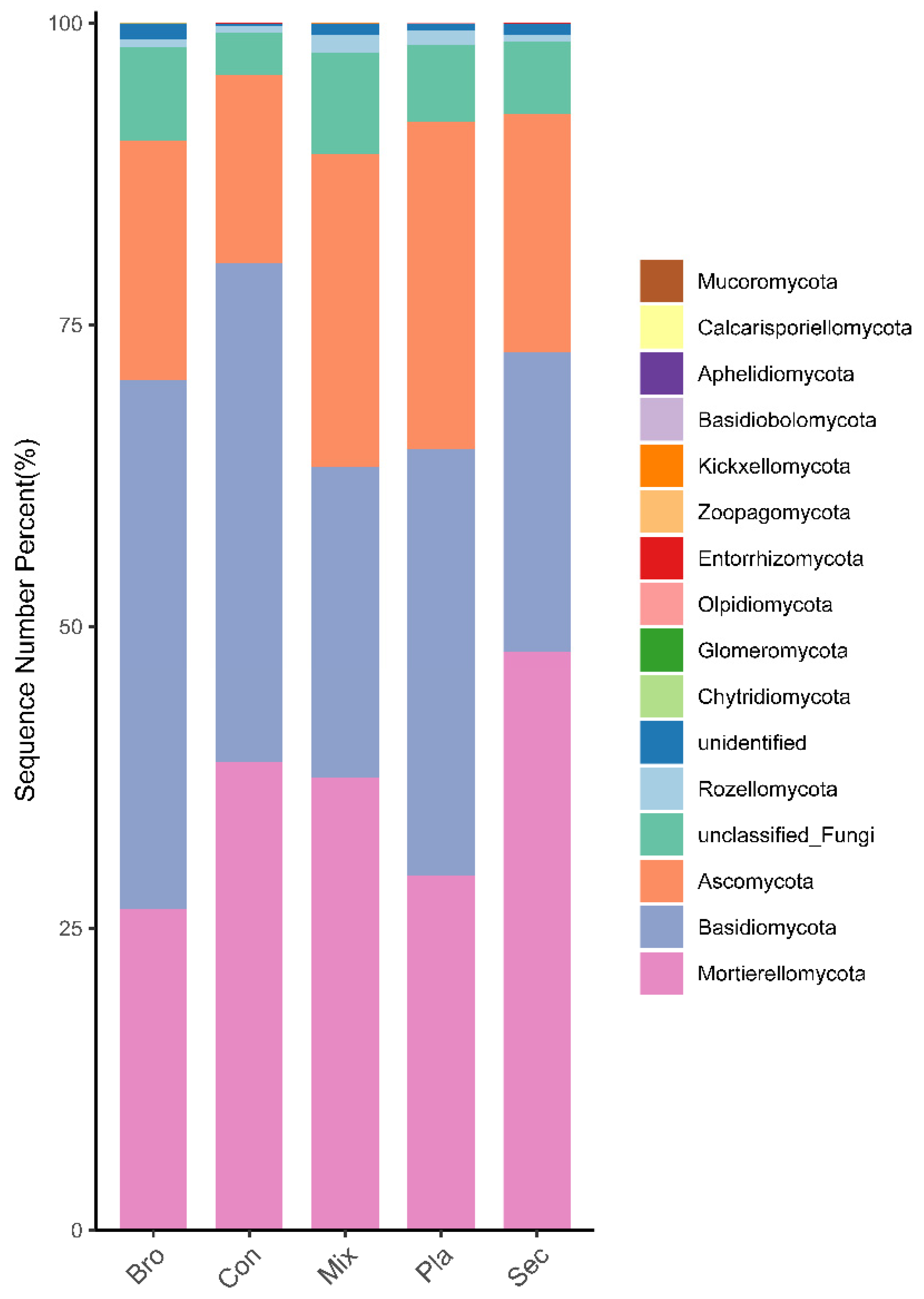
3.1. Soil Fungal Community Composition
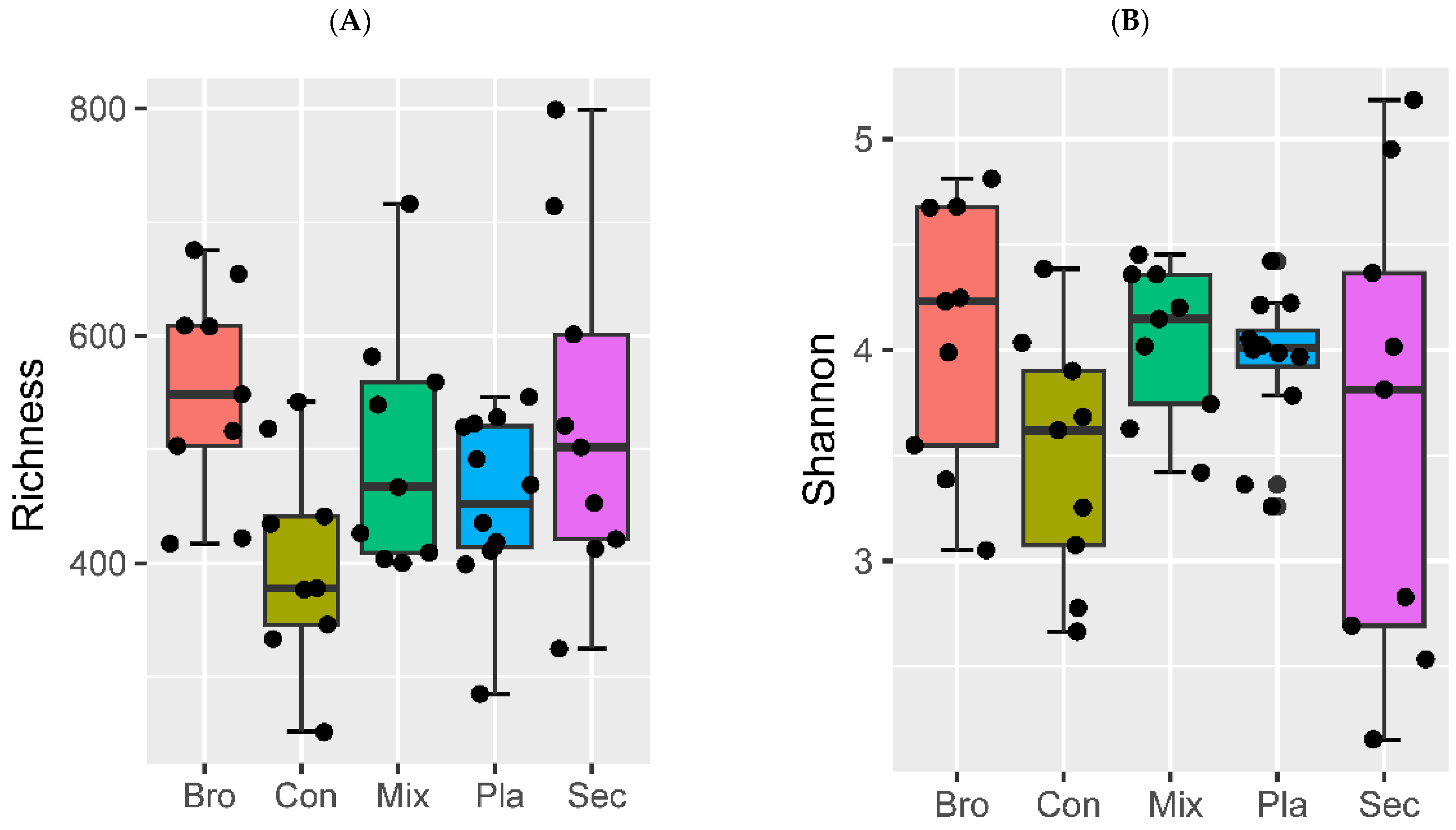
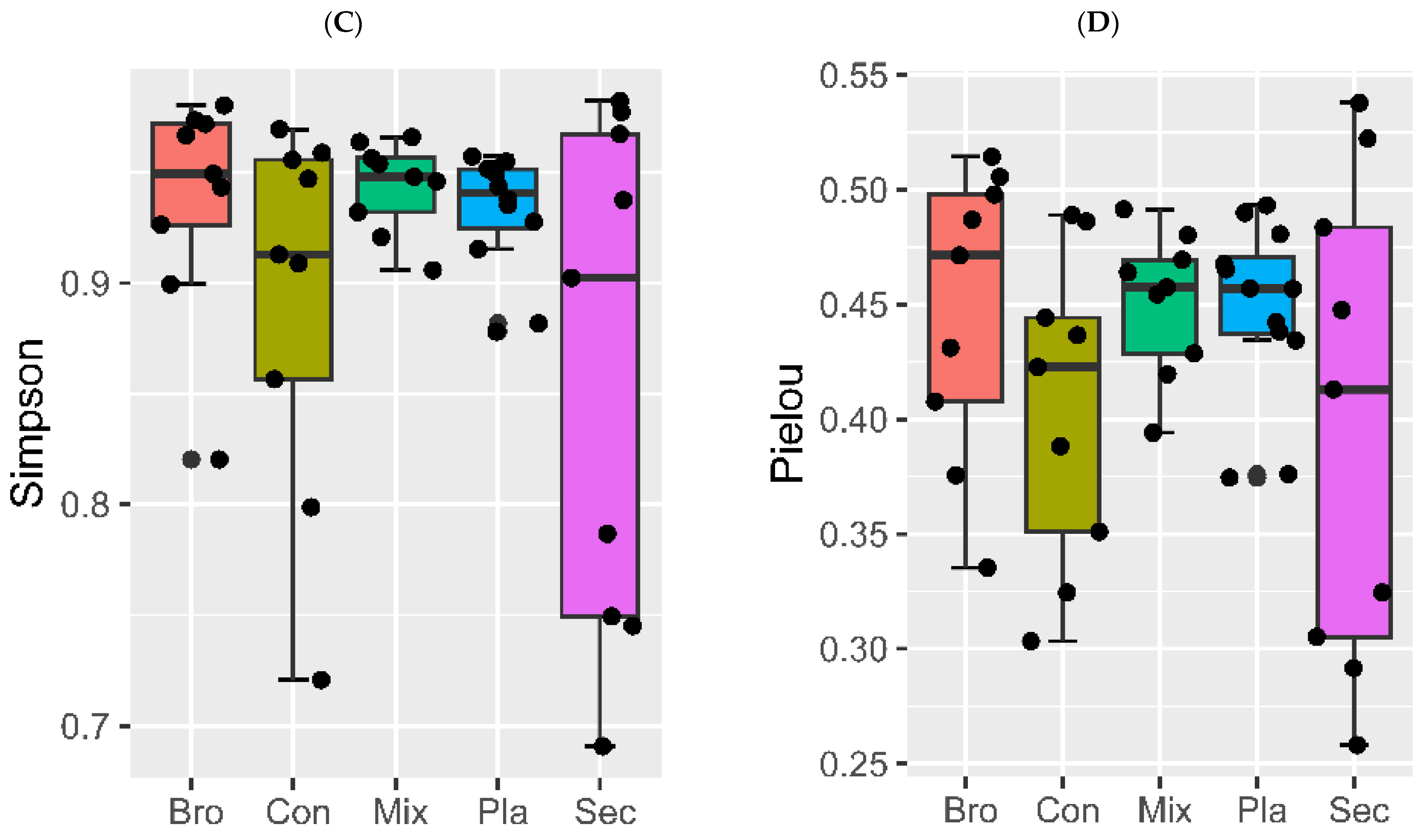
3.2. Soil Fungal Community Diversity
3.2.1. Alpha Diversity
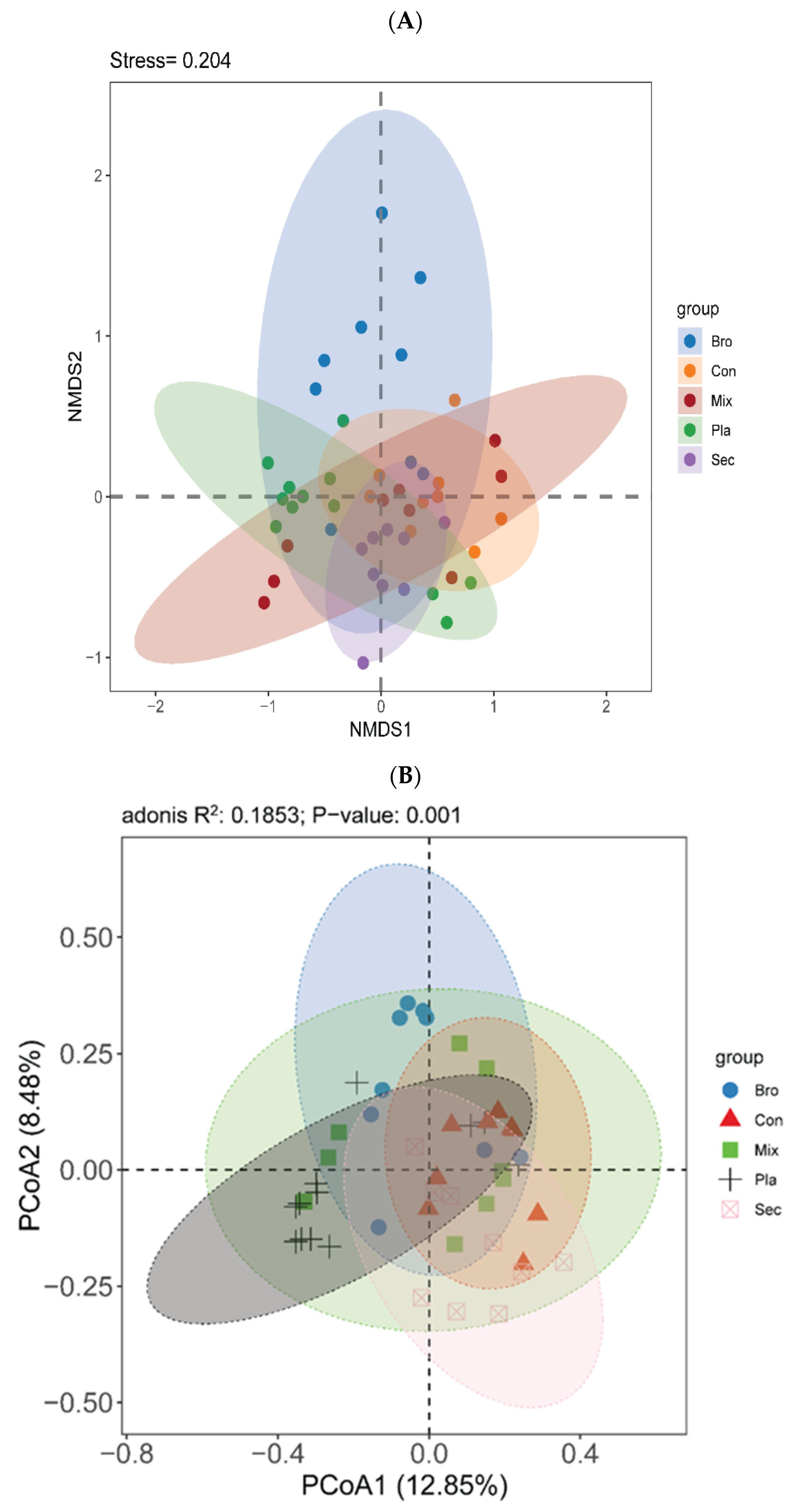
3.2.2. Beta Diversity
3.2.3. Indicator Species
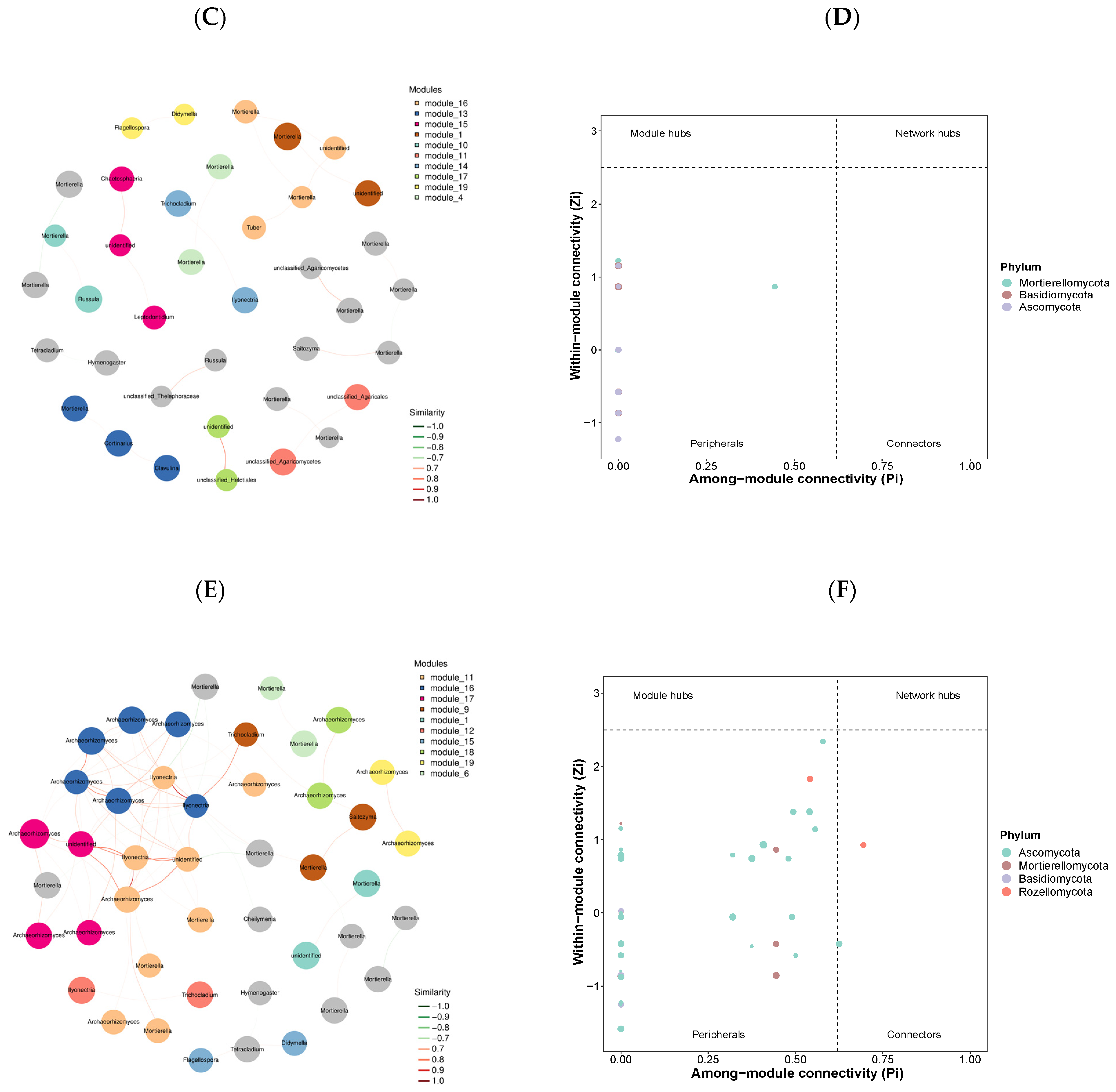
3.3. Co-Occurrence Networks
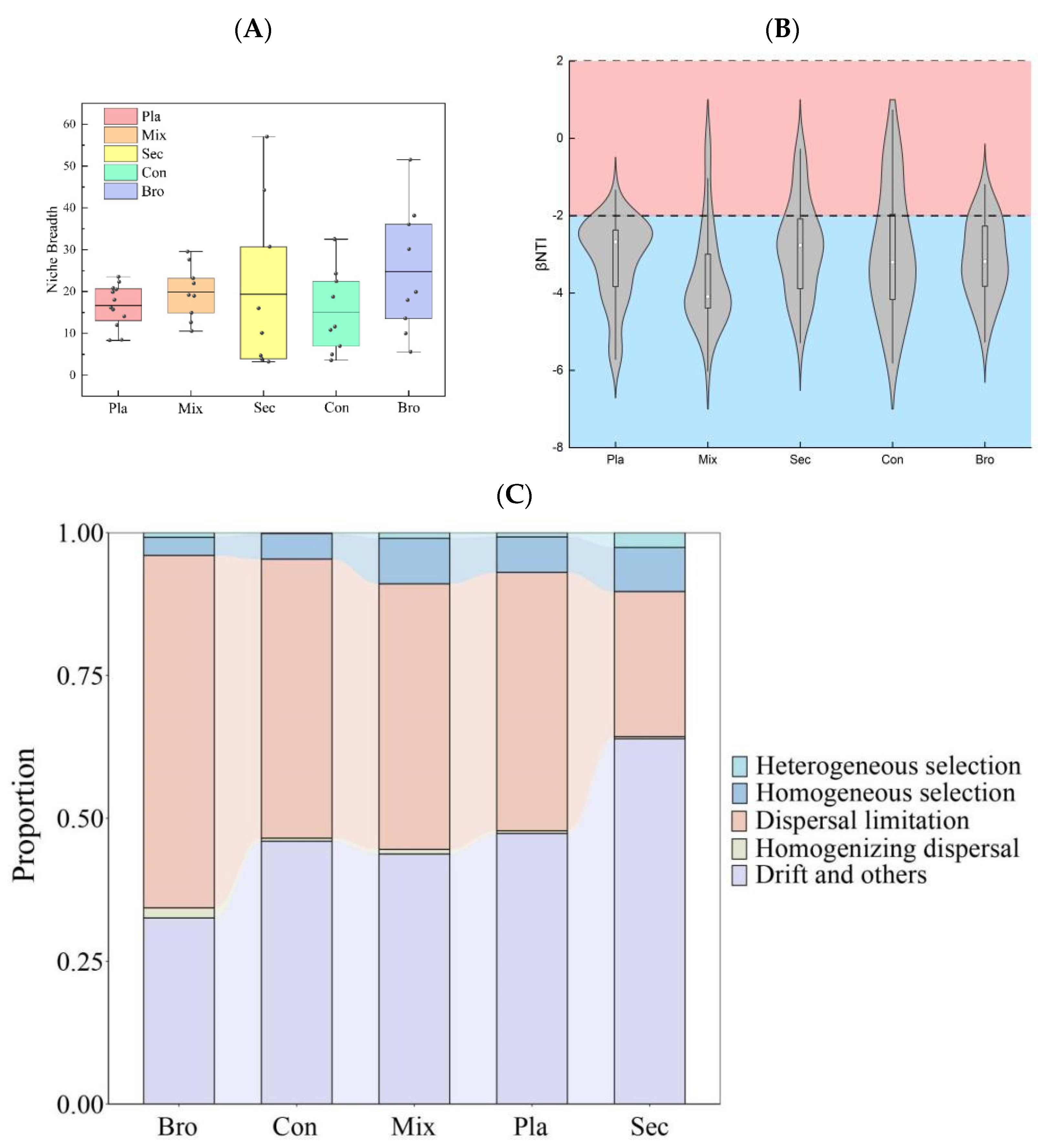
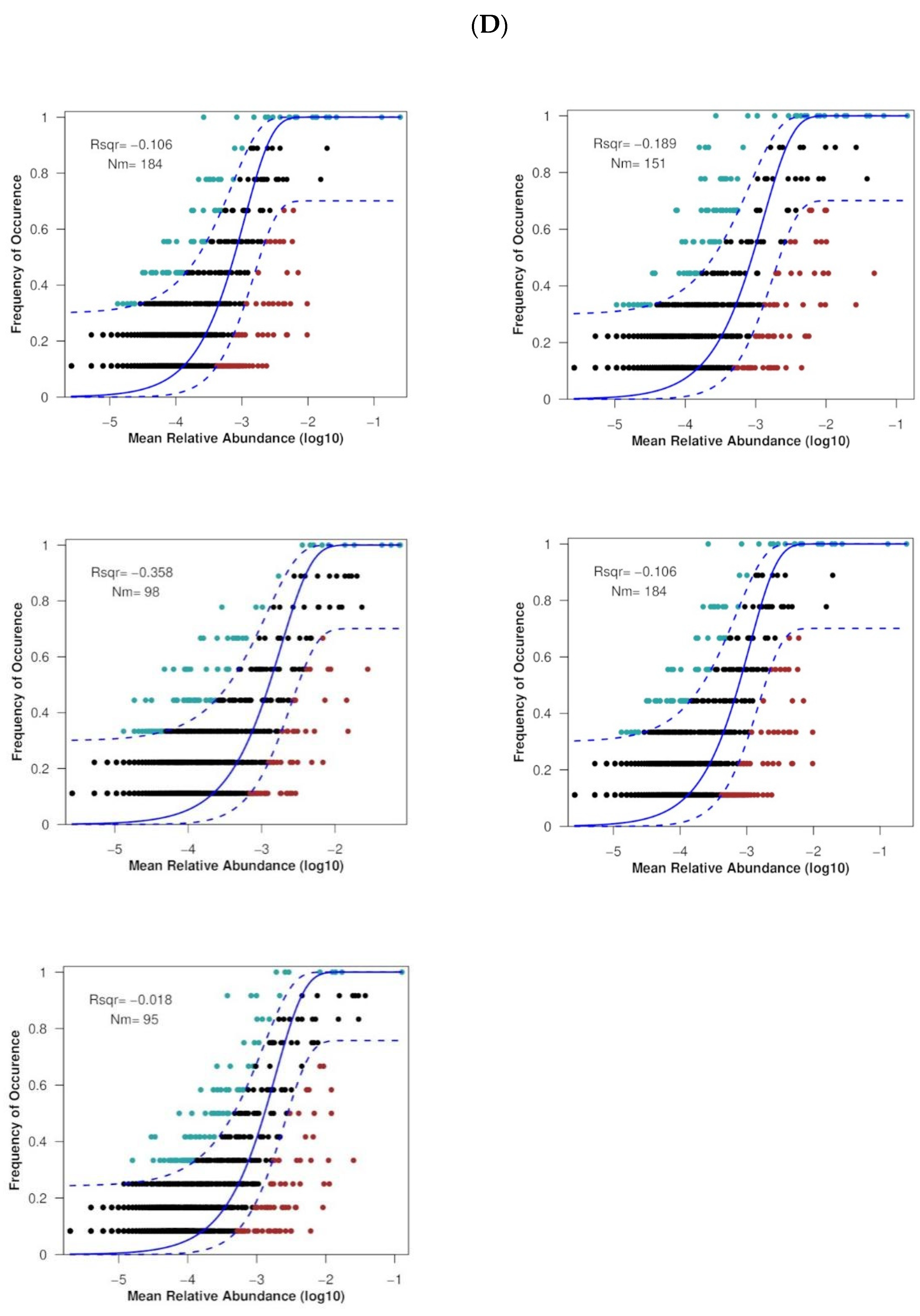
3.4. Soil Fungal Community Assembly Processes
4. Discussion
4.1. Relative Abundances of Soil Fungi at Various Levels
4.2. Soil Fungal Community Diversity across Distinct Vegetation Types
4.3. Co-Occurrence Network Patterns
4.4. Soil Fungal Community Assembly Processes
4.5. Implications for Forest Ecosystem Conservation and Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Lekberg, Y.; Arnillas, C.A.; Borer, E.T.; Bullington, L.S.; Fierer, N.; Kennedy, P.G.; Leff, J.W.; Luis, A.D.; Seabloom, E.W.; Henning, J.A. Nitrogen and phosphorus fertilization consistently favor pathogenic over mutualistic fungi in grassland soils. Nat. Commun. 2021, 12, 3484. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Anthony, M.A.; Tedersoo, L.; De Vos, B.; Croisé, L.; Meesenburg, H.; Wagner, M.; Andreae, H.; Jacob, F.; Lech, P.; Kowalska, A.; et al. Fungal community composition predicts forest carbon storage at a continental scale. Nat. Commun. 2024, 15, 2385. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Qu, Z.; Liu, B.; Huang, L.; Ming, A.; Sun, H. Mixed and continuous cropping eucalyptus plantation facilitated soil carbon cycling and fungal community diversity after a 14-year field trail. Ind. Crops Prod. 2024, 210, 118157. [Google Scholar] [CrossRef]
- Xie, L.; Yin, C. Seasonal variations of soil fungal diversity and communities in subalpine coniferous and broadleaved forests. Sci. Total Environ. 2022, 846, 157409. [Google Scholar] [CrossRef]
- Huang, K.; Guo, Z.; Zhao, W.; Song, C.; Wang, H.; Li, J.; Mumin, R.; Sun, Y.; Cui, B. Response of fungal communities to afforestation and its indication for forest restoration. For. Ecosyst. 2023, 10, 100125. [Google Scholar] [CrossRef]
- Sweeney, C.J.; de Vries, F.T.; van Dongen, B.E.; Bardgett, R.D. Root traits explain rhizosphere fungal community composition among temperate grassland plant species. New Phytol. 2020, 229, 1492–1507. [Google Scholar] [CrossRef]
- Guo, Q.; Gong, L. Compared with pure forest, mixed forest alters microbial diversity and increases the complexity of interdomain networks in arid areas. Microbiol. Spectr. 2024, 12, e02623–e02642. [Google Scholar] [CrossRef]
- Vannier, N.; Bittebiere, A.-K.; Mony, C.; Vandenkoornhuyse, P. Root endophytic fungi impact host plant biomass and respond to plant composition at varying spatio-temporal scales. Fungal Ecol. 2020, 44, 100907. [Google Scholar] [CrossRef]
- Bittebiere, A.K.; Vandenkoornhuyse, P.; Maluenda, E.; Gareil, A.; Dheilly, A.; Coudouel, S.; Bahin, M.; Mony, C.; Singh, B. Past spatial structure of plant communities determines arbuscular mycorrhizal fungal community assembly. J. Ecol. 2019, 108, 546–560. [Google Scholar] [CrossRef]
- Liang, M.; Johnson, D.; Burslem, D.F.R.P.; Yu, S.; Fang, M.; Taylor, J.D.; Taylor, A.F.S.; Helgason, T.; Liu, X. Soil fungal networks maintain local dominance of ectomycorrhizal trees. Nat. Commun. 2020, 11, 2636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, X.; Howard, M.M.; Kou, Y.; Liu, Q. Functional shifts in soil fungal communities regulate differential tree species establishment during subalpine forest succession. Sci. Total Environ. 2023, 861, 160616. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2020, 461, 219–244. [Google Scholar] [CrossRef]
- Branco, S.; Schauster, A.; Liao, H.L.; Ruytinx, J. Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol. 2022, 235, 2158–2175. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A Critical Review on Communication Mechanism within Plant-Endophytic Fungi Interactions to Cope with Biotic and Abiotic Stresses. J. Fungi 2021, 7, 719. [Google Scholar] [CrossRef]
- Fontana, D.C.; de Paula, S.; Torres, A.G.; de Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic Fungi: Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef]
- Guo, S.; Xia, L.; Xia, D.; Li, M.; Xu, W.; Liu, L. Enhancing plant resilience: Arbuscular mycorrhizal fungi’s role in alleviating drought stress in vegetation concrete. Front. Plant Sci. 2024, 15, 1401050. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Auer, L.; Buée, M.; Fauchery, L.; Lombard, V.; Barry, K.W.; Clum, A.; Copeland, A.; Daum, C.; Foster, B.; LaButti, K.; et al. Metatranscriptomics sheds light on the links between the functional traits of fungal guilds and ecological processes in forest soil ecosystems. New Phytol. 2023, 242, 1676–1690. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, F.; Wang, Y.; Wang, J.; Li, J.; Zhang, Z. Variation and drivers of soil fungal and functional groups among different forest types in warm temperate secondary forests. Glob. Ecol. Conserv. 2023, 45, e02523. [Google Scholar] [CrossRef]
- Šnajdr, J.; Dobiášová, P.; Větrovský, T.; Valášková, V.; Alawi, A.; Boddy, L.; Baldrian, P. Saprotrophic basidiomycete mycelia and their interspecific interactions affect the spatial distribution of extracellular enzymes in soil. FEMS Microbiol. Ecol. 2011, 78, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Chen, W.; Liu, Y.; Cahill, J.F. Local Community Assembly Mechanisms and the Size of Species Pool Jointly Explain the Beta Diversity of Soil Fungi. Microb. Ecol. 2024, 87, 58. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Ramos, J.C. Characterization, Restoration, and Assembly of Fungal Communities in Lodgepole Pine Forests Impacted by Recent Disturbances. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canaada, 2021. [Google Scholar]
- Rodriguez-Ramos, J.C.; Cale, J.A.; Cahill, J.F.; Simard, S.W.; Karst, J.; Erbilgin, N. Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytol. 2020, 229, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Talbot, J.M.; Bruns, T.D.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Peay, K.G. Independent roles of ectomycorrhizal and saprotrophic communities in soil organic matter decomposition. Soil Biol. Biochem. 2013, 57, 282–291. [Google Scholar] [CrossRef]
- Bödeker, I.T.M.; Lindahl, B.D.; Olson, Å.; Clemmensen, K.E.; Treseder, K. Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct. Ecol. 2016, 30, 1967–1978. [Google Scholar] [CrossRef]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, 772. [Google Scholar] [CrossRef]
- Likulunga, L.E.; Rivera Pérez, C.A.; Schneider, D.; Daniel, R.; Polle, A. Tree species composition and soil properties in pure and mixed beech-conifer stands drive soil fungal communities. For. Ecol. Manag. 2021, 502, 119709. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, M.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 345–357. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Liu, Y.; He, H.; Kou, Y.; Liu, Q. Dominant plant species and soil properties drive differential responses of fungal communities and functions in the soils and roots during secondary forest succession in the subalpine region. Rhizosphere 2022, 21, 100483. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Prieto-Rubio, J.; Garrido, J.L.; Pérez-Izquierdo, L.; Alcántara, J.M.; Azcón-Aguilar, C.; López-García, A.; Rincón, A. Scale dependency of ectomycorrhizal fungal community assembly processes in Mediterranean mixed forests. Mycorrhiza 2022, 32, 315–325. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Graco-Roza, C.; Soininen, J. Scale dependency of community assembly differs between coastal marine bacteria and fungi. Ecography 2023, 2024, e06863. [Google Scholar] [CrossRef]
- Zinger, L.; Taberlet, P.; Schimann, H.; Bonin, A.; Boyer, F.; De Barba, M.; Gaucher, P.; Gielly, L.; Giguet-Covex, C.; Iribar, A.; et al. Body size determines soil community assembly in a tropical forest. Mol. Ecol. 2019, 28, 528–543. [Google Scholar] [CrossRef]
- Ge, Y.; Jing, Z.; Diao, Q.; He, J.Z.; Liu, Y.J. Host species and geography differentiate honeybee gut bacterial communities by changing the relative contribution of community assembly processes. mBio 2021, 12, 1110–1128. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Huo, Q.-B.; Wen, T.; Wang, X.-Y.; Zhao, M.-Y.; Du, Y.-Z. Mechanisms of fungal community assembly in wild stoneflies moderated by host characteristics and local environment. npj Biofilms Microbiomes 2022, 8, 31. [Google Scholar] [CrossRef]
- Niu, X.; Wang, H.; Wang, T.; Zhang, P.; Zhang, H.; Wang, H.; Kong, X.; Xie, S.; Xu, J. The combination of multiple environmental stressors strongly alters microbial community assembly in aquatic ecosystems. J. Environ. Manag. 2024, 350, 119594. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Cline, L.C.; Hobbie, S.E.; Madritch, M.D.; Buyarski, C.R.; Tilman, D.; Cavender-Bares, J.M. Resource availability underlies the plant-fungal diversity relationship in a grassland ecosystem. Ecology 2018, 99, 204–216. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Nguyen, N.H.; Stefanski, A.; Han, Y.; Hobbie, S.E.; Montgomery, R.A.; Reich, P.B.; Kennedy, P.G. Ectomycorrhizal fungal response to warming is linked to poor host performance at the boreal-temperate ecotone. Glob. Chang. Biol. 2016, 23, 1598–1609. [Google Scholar] [CrossRef]
- Jangid, K.; Whitman, W.B.; Condron, L.M.; Turner, B.L.; Williams, M.A. Soil bacterial community succession during long-term ecosystem development. Mol. Ecol. 2013, 22, 3415–3424. [Google Scholar] [CrossRef]
- Chen, J.; Shi, Z.; Liu, S.; Zhang, M.; Cao, X.; Chen, M.; Xu, G.; Xing, H.; Li, F.; Feng, Q. Altitudinal Variation Influences Soil Fungal Community Composition and Diversity in Alpine–Gorge Region on the Eastern Qinghai–Tibetan Plateau. J. Fungi 2022, 8, 807. [Google Scholar] [CrossRef]
- Qiang, W.; Gunina, A.; Kuzyakov, Y.; Luo, R.; Zhang, Y.; Liu, B.; Pang, X. Shifts of understory vegetation induced by thinning drive the expansion of soil rare fungi. J. Environ. Manag. 2023, 342, 118119. [Google Scholar] [CrossRef]
- Sui, X.; Zeng, X.; Li, M.; Weng, X.; Frey, B.; Yang, L.; Li, M. Influence of Different Vegetation Types on Soil Physicochemical Parameters and Fungal Communities. Microorganisms 2022, 10, 829. [Google Scholar] [CrossRef]
- Deng, J.; Yin, Y.; Luo, J.; Zhu, W.; Zhou, Y. Different revegetation types alter soil physical-chemical characteristics and fungal community in the Baishilazi Nature Reserve. PeerJ 2019, 6, e6251. [Google Scholar] [CrossRef]
- Sun, H.; Terhonen, E.; Kovalchuk, A.; Tuovila, H.; Chen, H.; Oghenekaro, A.O.; Heinonsalo, J.; Kohler, A.; Kasanen, R.; Vasander, H.; et al. Dominant Tree Species and Soil Type Affect the Fungal Community Structure in a Boreal Peatland Forest. Appl. Environ. Microbiol. 2016, 82, 2632–2643. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Öpik, M.; Adholeya, A.; Ainsaar, L.; Bâ, A.; Burla, S.; Diedhiou, A.G.; Hiiesalu, I.; Jairus, T.; et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 2015, 349, 970–973. [Google Scholar] [CrossRef]
- Morrison, E.S.; Thomas, P.; Ogram, A.; Kahveci, T.; Turner, B.L.; Chanton, J.P. Characterization of Bacterial and Fungal Communities Reveals Novel Consortia in Tropical Oligotrophic Peatlands. Microb. Ecol. 2020, 82, 188–201. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, L.; Zhao, Y.; Feng, Q.; Li, C.; Wei, Y. Shift of soil fungal communities under afforestation in Nanliu River Basin, southwest China. J. Environ. Manag. 2022, 302, 114130. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C.; Ye, Z.; Li, J.; Feng, Y.; Lu, Q. Relationships Between Fungal and Plant Communities Differ Between Desert and Grassland in a Typical Dryland Region of Northwest China. Front. Microbiol. 2018, 9, 2327. [Google Scholar] [CrossRef]
- Yang, B.; Feng, W.; Zhou, W.; He, K.; Yang, Z. Association between Soil Physicochemical Properties and Bacterial Community Structure in Diverse Forest Ecosystems. Microorganisms 2024, 12, 728. [Google Scholar] [CrossRef]
- Deng, J.; Bai, X.; Zhou, Y.; Zhu, W.; Yin, Y. Variations of soil microbial communities accompanied by different vegetation restoration in an open-cut iron mining area. Sci. Total Environ. 2020, 704, 135243. [Google Scholar] [CrossRef]
- Lee, J.; Zhou, X.; Seo, Y.O.; Lee, S.T.; Yun, J.; Yang, Y.; Kim, J.; Kang, H. Effects of vegetation shift from needleleaf to broadleaf species on forest soil CO2 emission. Sci. Total Environ. 2023, 856, 158907. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Zanne, A.E.; Abarenkov, K.; Afkhami, M.E.; Aguilar-Trigueros, C.A.; Bates, S.; Bhatnagar, J.M.; Busby, P.E.; Christian, N.; Cornwell, W.K.; Crowther, T.W.; et al. Fungal functional ecology: Bringing a trait-based approach to plant-associated fungi. Biol. Rev. 2019, 95, 409–433. [Google Scholar] [CrossRef]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; Baumbach, J.; Haller, D.; List, M. Network analysis methods for studying microbial communities: A mini review. Comput. Struct. Biotechnol. 2021, 19, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Alm, E.J. Inferring correlation networks from genomic survey data. PLOS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef] [PubMed]
- von Mering, C.; Kurtz, Z.D.; Müller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and Compositionally Robust Inference of Microbial Ecological Networks. PLoS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef]
- Xin, Y.; Shi, Y.; He, W.-M. A shift from inorganic to organic nitrogen-dominance shapes soil microbiome composition and co-occurrence networks. Front. Microbiol. 2022, 13, 1074064. [Google Scholar] [CrossRef]
- Csárdi, G.; Nepusz, T. The igraph software package for complex network research. Comput. Sci. Eng. 2006, 1695, 1–9. [Google Scholar]
- Ji, L.; Yang, Y.; Yang, N.; Khan, A.; Yang, L. Seasonal variation of diversity and co-occurrence patterns of arbuscular mycorrhizal fungal communities in mixed broadleaf-conifer forests. Appl. Soil Ecol. 2021, 158, 103782. [Google Scholar] [CrossRef]
- Dawud, S.M.; Raulund-Rasmussen, K.; Ratcliffe, S.; Domisch, T.; Finér, L.; Joly, F.X.; Hättenschwiler, S.; Vesterdal, L.; Ostertag, R. Tree species functional group is a more important driver of soil properties than tree species diversity across major European forest types. Funct. Ecol. 2017, 31, 1153–1162. [Google Scholar] [CrossRef]
- Augusto, L.; De Schrijver, A.; Vesterdal, L.; Smolander, A.; Prescott, C.; Ranger, J. Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol. Rev. 2014, 90, 444–466. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Deng, Y.; Umbach, A.K.; Neufeld, J.D. Nonparametric richness estimators Chao1 and ACE must not be used with amplicon sequence variant data. ISME J. 2024, 18, wrae106. [Google Scholar] [CrossRef] [PubMed]
- Voříšková, J.; Brabcová, V.; Cajthaml, T.; Baldrian, P. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2013, 201, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Heděnec, P.; Zheng, H.; Pessanha Siqueira, D.; Lin, Q.; Peng, Y.; Kappel Schmidt, I.; Guldberg Frøslev, T.; Kjøller, R.; Rousk, J.; Vesterdal, L. Tree species traits and mycorrhizal association shape soil microbial communities via litter quality and species mediated soil properties. For. Ecol. Manag. 2023, 527, 120608. [Google Scholar] [CrossRef]
- Prada-Salcedo, L.D.; Prada-Salcedo, J.P.; Heintz-Buschart, A.; Buscot, F.; Goldmann, K. Effects of Tree Composition and Soil Depth on Structure and Functionality of Belowground Microbial Communities in Temperate European Forests. Front. Microbiol. 2022, 13, 920618. [Google Scholar] [CrossRef]
- Sheng, Y.; Cong, J.; Lu, H.; Yang, L.; Liu, Q.; Li, D.; Zhang, Y. Broad-leaved forest types affect soil fungal community structure and soil organic carbon contents. Microbiologyopen 2019, 8, e874. [Google Scholar] [CrossRef]
- Wang, M.; Sui, X.; Wang, X.; Zhang, X.; Zeng, X. Soil Fungal Community Differences in Manual Plantation Larch Forest and Natural Larch Forest in Northeast China. Microorganisms 2024, 12, 1322. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, G.; Liu, J.; Ni, J. Effects of vegetation type, season, and soil properties on soil microbial community in subtropical forests. Appl. Soil Ecol. 2021, 158, 103813. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Li, B.; Li, Y.; Du, X.; Sun, Y.; Li, Q.; Martijn Bezemer, T. Soil addition improves multifunctionality of degraded grasslands through increasing fungal richness and network complexity. Geoderma 2023, 437, 116607. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B.; et al. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef] [PubMed]
- Santolini, M.; Barabási, A.-L. Predicting perturbation patterns from the topology of biological networks. Proc. Natl. Acad. Sci. USA 2018, 115, E6375–E6383. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Hu, L.; Li, Q.; Yan, J.; Liu, C.; Zhong, J. Vegetation restoration facilitates belowground microbial network complexity and recalcitrant soil organic carbon storage in southwest China karst region. Sci. Total Environ. 2022, 820, 153137. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Hu, W.; Liu, C.-Q.; Niu, M.; Jin, R.; Deng, J.; Wu, L.; Li, P.; Yan, Z.; Zhu, Y.-G.; et al. Characteristics and assembly mechanisms of bacterial and fungal communities in soils from Chinese forests across different climatic zones. Catena 2024, 245, 108306. [Google Scholar] [CrossRef]
- Chase, J.M. Drought mediates the importance of stochastic community assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 17430–17434. [Google Scholar] [CrossRef]
- Logares, R.; Lindström, E.S.; Langenheder, S.; Logue, J.B.; Paterson, H.; Laybourn-Parry, J.; Rengefors, K.; Tranvik, L.; Bertilsson, S. Biogeography of bacterial communities exposed to progressive long-term environmental change. ISME J. 2012, 7, 937–948. [Google Scholar] [CrossRef]
- Lustenhouwer, N.; Maynard, D.S.; Bradford, M.A.; Lindner, D.L.; Oberle, B.; Zanne, A.E.; Crowther, T.W. A trait-based understanding of wood decomposition by fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 11551–11558. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Su, J. Selective logging enhances ecosystem multifunctionality via increase of functional diversity in a Pinus yunnanensis forest in Southwest China. For. Ecosyst. 2020, 7, 55. [Google Scholar] [CrossRef]



| Object | Adonis2 | ||
|---|---|---|---|
| F | R2 | Adjusted-P | |
| Among Groups | 2.8721 | 0.2231 | 0.001 |
| Mix vs. Sec | 2.5124 | 0.1357 | 0.001 |
| Mix vs. Con | 2.0193 | 0.1121 | 0.025 |
| Mix vs. Bro | 1.9657 | 0.1094 | 0.044 |
| Sec vs. Con | 2.0201 | 0.1121 | 0.008 |
| Sec vs. Bro | 3.0971 | 0.1622 | 0.001 |
| Con vs. Bro | 2.2155 | 0.1216 | 0.001 |
| Pla vs. Mix | 3.1503 | 0.1645 | 0.001 |
| Pla vs. Sec | 4.6003 | 0.2233 | 0.001 |
| Pla vs. Con | 4.5123 | 0.2200 | 0.001 |
| Pla vs. Bro | 3.2643 | 0.1694 | 0.001 |
| Variable | PCoA1 | PCoA2 | R2 |
|---|---|---|---|
| pH | −0.89511 | −0.44584 | 0.2815 |
| AP | 0.99812 | 0.06127 | 0.0205 |
| SOC | −0.47273 | 0.88121 | 0.1621 |
| TP | 0.48448 | −0.8748 | 0.0202 |
| TN | 0.01229 | 0.99992 | 0.2495 |
| AN | −0.00816 | 0.99997 | 0.1985 |
| SWC | −0.22794 | 0.97368 | 0.2782 |
| C/N | −0.81507 | −0.57937 | 0.1093 |
| C/P | −0.36499 | 0.93101 | 0.2254 |
| N/P | −0.02049 | 0.99979 | 0.3836 |
| Type | Objective | Bro | Con | Mix | Sec | Pla |
|---|---|---|---|---|---|---|
| Empirical network | Nodes | 69 | 63 | 87 | 60 | 89 |
| Edges | 87 | 39 | 121 | 52 | 121 | |
| Network diameter | 4.662 | 2.675 | 5.994 | 2.676 | 5.99 | |
| Modularity | 0.658 | 0.942 | 0.583 | 0.841 | 0.576 | |
| Graph density | 0.037 | 0.020 | 0.032 | 0.029 | 0.031 | |
| ANND | 4.225 | 1.408 | 4.883 | 2.477 | 4.785 | |
| Average path length | 2.683 | 1.458 | 3.023 | 1.845 | 3.07 | |
| Between centrality | 10,950 | 477 | 28,708 | 1541 | 31,012 | |
| Closeness centrality | 0.388 | 0.055 | 0.458 | 0.141 | 0.474 | |
| Degree centralization | 861 | 111 | 1150 | 316 | 1182 | |
| Random network | Network diameter | 9.10 ± 0.88 | 8.60 ± 2.01 | 9.40 ± 1.35 | 12.30 ± 2.11 | 9.50 ± 1.08 |
| Modularity | 0.575 ± 0.014 | 0.795 ± 0.039 | 0.557 ± 0.022 | 0.666 ± 0.030 | 0.580 ± 0.020 | |
| ANND | 3.451 ± 0.203 | 2.145 ± 0.170 | 3.771 ± 0.158 | 2.737 ± 0.210 | 3.608 ± 0.121 | |
| Average path length | 4.160 ± 0.214 | 3.528 ± 0.688 | 4.136 ± 0.193 | 5.197± 0.688 | 4.292 ± 0.231 | |
| Between centrality | 27,114.22 ± 8738.24 | 6092.80 ± 2835.92 | 36,778.26 ± 11,019.72 | 23,167.14 ± 6406.49 | 52,255.11 ± 1152.88 | |
| Closeness centrality | 1.349 ± 0.394 | 0.251 ± 0.105 | 1.436 ± 0.371 | 0.723 ± 0.092 | 1.559 ± 0.421 | |
| Degree centralization | 288.300 ± 80.006 | 192.900 ± 30.431 | 367.000 ± 100.460 | 232.00 ± 50.596 | 389.900 ± 97.945 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Yang, Z.; He, K.; Zhou, W.; Feng, W. Soil Fungal Community Diversity, Co-Occurrence Networks, and Assembly Processes under Diverse Forest Ecosystems. Microorganisms 2024, 12, 1915. https://doi.org/10.3390/microorganisms12091915
Yang B, Yang Z, He K, Zhou W, Feng W. Soil Fungal Community Diversity, Co-Occurrence Networks, and Assembly Processes under Diverse Forest Ecosystems. Microorganisms. 2024; 12(9):1915. https://doi.org/10.3390/microorganisms12091915
Chicago/Turabian StyleYang, Bing, Zhisong Yang, Ke He, Wenjia Zhou, and Wanju Feng. 2024. "Soil Fungal Community Diversity, Co-Occurrence Networks, and Assembly Processes under Diverse Forest Ecosystems" Microorganisms 12, no. 9: 1915. https://doi.org/10.3390/microorganisms12091915
APA StyleYang, B., Yang, Z., He, K., Zhou, W., & Feng, W. (2024). Soil Fungal Community Diversity, Co-Occurrence Networks, and Assembly Processes under Diverse Forest Ecosystems. Microorganisms, 12(9), 1915. https://doi.org/10.3390/microorganisms12091915






