Thyme Essential Oil as a Potential Tool Against Common and Re-Emerging Foodborne Pathogens: Biocidal Effect on Bacterial Membrane Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Clinical Bacterial Strains and Growth Conditions
2.3. Agar Diffusion Assay
2.4. Broth Dilution Method
2.5. Disk-Volatilization Assay
2.6. Time-Kill Kinetics Assay
2.7. Membrane Permeability Assay
3. Results
3.1. In Vitro Antibacterial Activity of Thy-EO
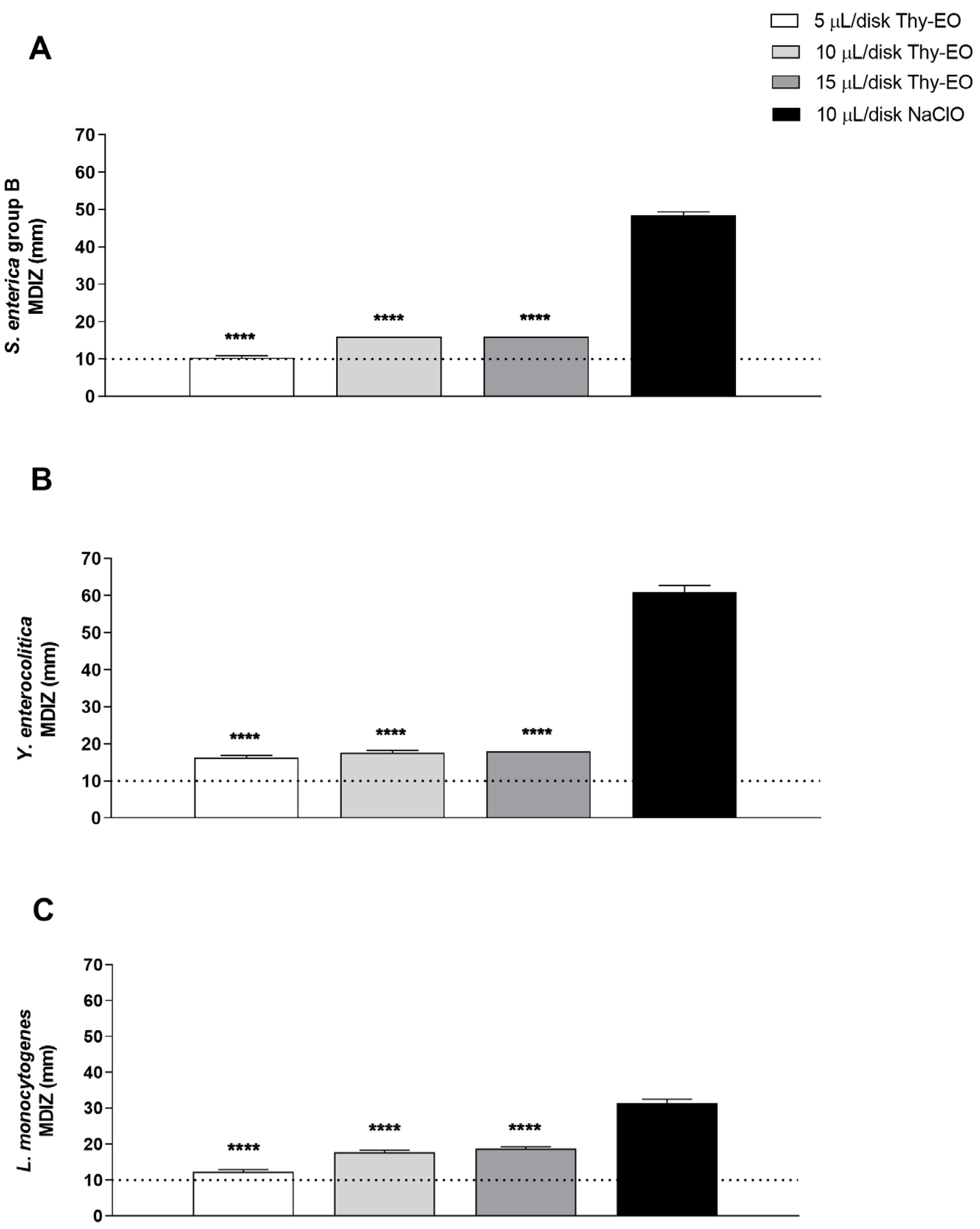
3.2. Evaluation of Antimicrobial Activity of Thy-EO Volatile Compounds
3.3. Evaluation of Antimicrobial Activity of Thy-EO by Time-Kill Kinetics Assay
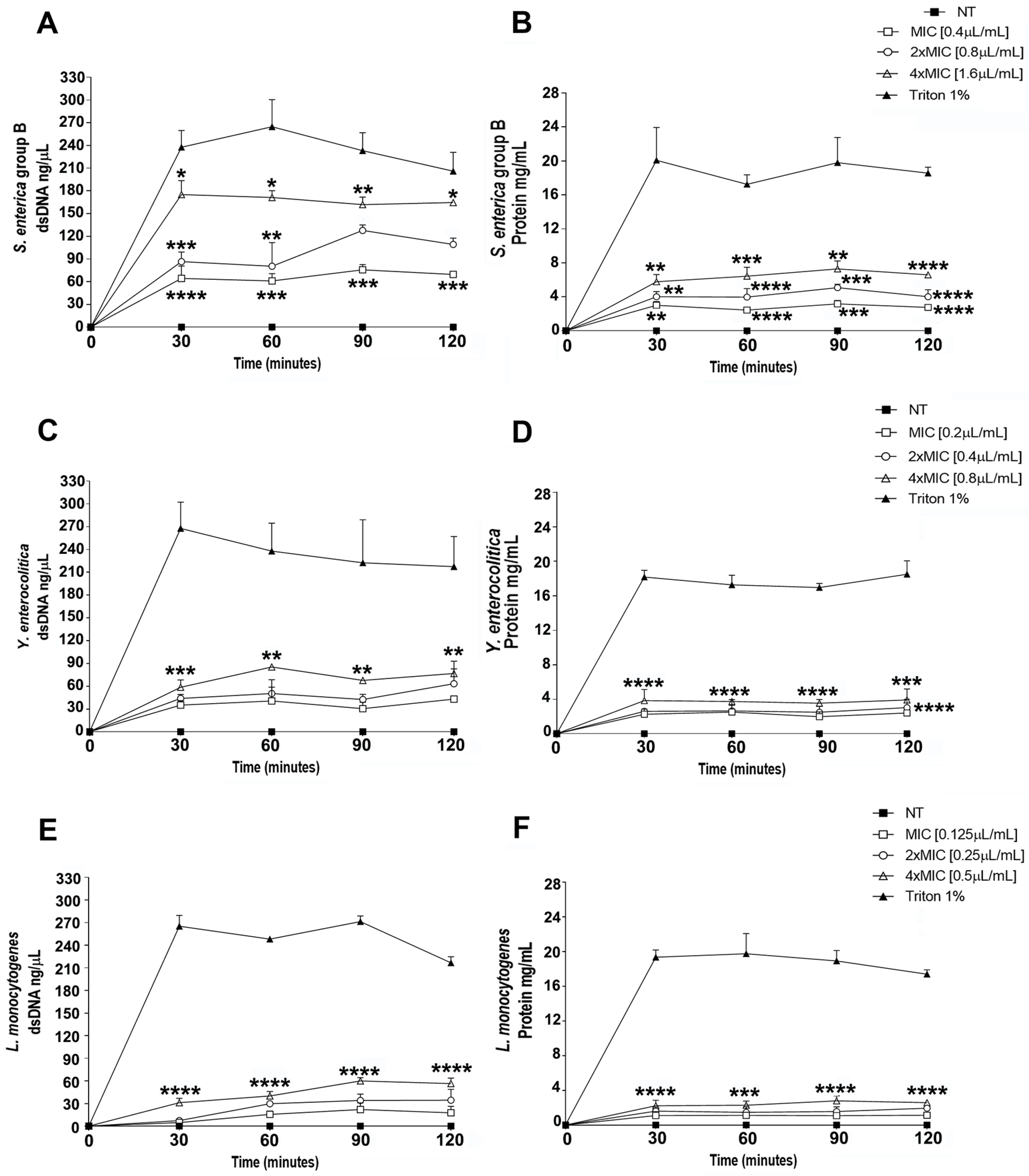
3.4. Evaluation of the Effect of Thy-EO on Bacterial Cell Membrane Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Braden, C.R.; Tauxe, R.V. Emerging trends in foodborne diseases. Infect. Dis. Clin. N. Am. 2013, 27, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Alsayeqh, A.F.; Baz, A.H.A.; Darwish, W.S. Antimicrobial-resistant foodborne pathogens in the Middle East: A systematic review. Environ. Sci. Pollut. Res. Int. 2021, 28, 68111–68133. [Google Scholar] [CrossRef]
- Antunes, P.; Novais, C.; Peixe, L. Food-to-Humans Bacterial Transmission. Microbiol. Spectr. 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural Phenolic Compounds for the Control of Oxidation, Bacterial Spoilage, and Foodborne Pathogens in Meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Oyarzabal, O.A. Emerging and Reemerging Foodborne Pathogens. In Microbial Food Safety. Food Science Text Series; Oyarzabal, O., Backert, S., Eds.; Springer: New York, NY, USA, 2012; pp. 3–12. [Google Scholar]
- Hassan, A.; Khan, M.K.I.; Fordos, S.; Hasan, A.; Khalid, S.; Naeem, M.Z.; Usman, A. Emerging Foodborne Pathogens: Challenges and Strategies for Ensuring Food Safety. Biol. Life Sci. Forum 2024, 31, 32. [Google Scholar]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial resistance: A concise update. Lancet Microbe 2024, 12, 100947. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Facchiano, S.; Boscaino, F.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Towards green strategies of food security: Antibacterial synergy of essential oils from Thymus vulgaris and Syzygium aromaticum to inhibit Escherichia coli and Staphylococcus aureus. pathogenic food isolates. Microorganisms 2022, 10, 2446. [Google Scholar] [CrossRef]
- Muthuvelu, K.S.; Ethiraj, B.; Pramnik, S.; Raj, N.K.; Venkataraman, S.; Rajendran, D.S.; Bharathi, P.; Palanisamy, E.; Narayanan, A.S.; Vaidyanathan, V.K.; et al. Biopreservative technologies of food: An alternative to chemical preservation and recent developments. Food Sci. Biotechnol. 2023, 32, 1337–1350. [Google Scholar] [CrossRef]
- Scaglione, E.; Sateriale, D.; Mantova, G.; Di Rosario, M.; Continisio, L.; Vitiello, M.; Pagliarulo, C.; Colicchio, R.; Pagliuca, C.; Salvatore, P. Antimicrobial efficacy of Punica granatum Lythraceae peel extract against pathogens belonging to the ESKAPE group. Front. Microbiol. 2024, 15, 1383027. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Antibacterial and antibiofilm efficacy of Thyme (Thymus vulgaris L.) essential oil against foodborne illness pathogens, Salmonella enterica subsp. enterica Serovar Typhimurium and Bacillus cereus. Antibiotic 2023, 12, 485. [Google Scholar] [CrossRef]
- Sateriale, D.; Forgione, G.; Di Rosario, M.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Vine-Winery byproducts as precious resource of natural antimicrobials: In vitro antibacterial and antibiofilm activity of grape pomace extracts against foodborne pathogens. Microorganisms 2024, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.K.; Roy, J. Antimicrobial and chemopreventive properties of herbs and spices. Curr. Med. Chem. 2004, 11, 1451–1460. [Google Scholar] [CrossRef]
- Khatri, P.; Rani, A.; Hameed, S.; Chandra, S.; Chang, C.-M.; Pandey, R.P. Current Understanding of the Molecular Basis of Spices for the Development of Potential Antimicrobial Medicine. Antibiotics 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for prevention and treatment of cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef]
- Basavegowda, N.; Patra, J.K.; Baek, K.H. Essential Oils and Mono/bi/tri-Metallic Nanocomposites as Alternative Sources of Antimicrobial Agents to Combat Multidrug-Resistant Pathogenic Microorganisms: An Overview. Molecules 2020, 25, 1058. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils and Science, Technology and Applications; Hüsnü Can Baser, K., Buchbauer, G., Eds.; CRC Press Taylor & Francis Group: London, UK, 2010; pp. 39–81. [Google Scholar]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Ahani, N.; Sangtarash, M.H.; Alipour Eskandani, M.; Houshmand, M. Zataria multiflora Boiss. Essential Oil Induce Apoptosis in Two Human Colon Cancer Cell Lines (HCT116 & SW48). Iran. J. Public Health 2020, 49, 753–762. [Google Scholar]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. Int. J. Mol. Sci. 2023, 24, 6936. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Chávez-González, M.L.; Silva, A.S.; Singh, P. Essential oils from the genus Thymus as antimicrobial food preservatives: Progress in their use as nanoemulsions-a new paradigm. Trends Food Sci. Technol. 2021, 11, 426–441. [Google Scholar] [CrossRef]
- Jevremovic, S.; Lazarevic, J.; Kostic, M.; Krnjajic, S.; Ugrenovic, V.; Radonjic, A.; Kostic, I. Contact application of Lamiaceae botanicals reduces bean weevil infestation in stored beans. Arch. Biol. Sci. 2019, 71, 665. [Google Scholar] [CrossRef]
- Perez, C.; Pauli, M.; Bazerque, P. An Antibiotic Assay by Agar Well Diffusion Method. Acta Biol. Et Med. Exp. 1990, 15, 113–115. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2022; ISBN 978-1-68440-134-5. [Google Scholar]
- Tyagi, A.K.; Malik, A. Antimicrobial action of essential oil vapours and negative air ions against Pseudomonas fluorescens. Int. J. Food Microbiol. 2010, 143, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Ma, X.; Lu, X.; Zhu, Y.; Abula, R.; Wu, T.; Bakri, M.; He, F.; Maiwulanjiang, M. Antimicrobial properties of essential oil extracted from Schizonepeta annua against methicillin-resistant Staphylococcus aureus via membrane disruption. Microb. Pathog. 2024, 196, 106975. [Google Scholar]
- Kowalczyk, A. Essential Oils against Candida auris-A Promising Approach for Antifungal Activity. Antibiotic 2024, 13, 568. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2020, 152, 104620. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G. New Tricks for Old Guys: Recent Developments in the Chemistry, Biochemistry, Applications and Exploitation of Selected Species from the Lamiaceae Family. Chem. Biodivers. 2020, 17, e1900677. [Google Scholar] [CrossRef]
- Alam, M.; Bano, N.; Ahmad, T.; Sharangi, A.B.; Upadhyay, T.K.; Alraey, Y.; Alabdallah, N.M.; Rauf, M.A.; Saeed, M. Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases. Antibiotics 2022, 11, 855. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Diniz, A.F.; Santos, B.; Nóbrega, L.M.M.O.; Santos, V.R.L.; Mariz, W.S.; Cruz, P.S.C.; Nóbrega, R.O.; Silva, R.L.; Paula, A.F.R.; Santos, J.R.D.A.; et al. Antibacterial activity of Thymus vulgaris (thyme) essential oil against strains of Pseudomonas aeruginosa, Klebsiella pneumoniae and Staphylococcus saprophyticus isolated from meat product. Braz. J. Biol. 2023, 83, e275306. [Google Scholar] [CrossRef]
- Yin, L.; Liang, C.; Wei, W.; Huang, S.; Ren, Y.; Geng, Y.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; et al. The Antibacterial Activity of Thymol Against Drug-Resistant Streptococcus iniae and Its Protective Effect on Channel Catfish (Ictalurus punctatus). Front. Microbiol. 2022, 13, 914868. [Google Scholar] [CrossRef]
- Wang, L.H.; Zhang, Z.H.; Zeng, X.A.; Gong, D.M.; Wang, M.S. Combination of microbiological, spectroscopic and molecular docking techniques to study the antibacterial mechanism of thymol against Staphylococcus aureus: Membrane damage and genomic DNA binding. Anal. Bioanal. Chem. 2017, 409, 1615, Erratum in: Anal. Bioanal. Chem. 2017, 409, 3055. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. Available online: https://www.efsa.europa.eu/en (accessed on 2017).
- Longenberger, A.H.; Gronostaj, M.P.; Yee, G.Y.; Johnson, L.M.; Lando, J.F.; Voorhees, R.E.; Waller, K.; Weltman, A.C.; Moll, M.; Lyss, S.B.; et al. Yersinia enterocolitica infections associated with improperly pasteurized milk products: Southwest Pennsylvania, March–August, 2011. Epidemiol. Infect. 2014, 142, 1640–1650. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Rivera-Chavira, B.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Differential antimicrobial effect of essential oils and their main components: Insights based on the cell membrane and external structure. Membranes 2021, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Omer Qader, K.; Malik Al-Saadi, S.A.A.; Hiwa Arif, H.; Al-Fekaiki, D.F. Antibacterial and Antioxidant Activity of Ziziphora clinopodioid Lam. (Lamiaceae) Essential Oil. Arch. Razi Inst. 2023, 78, 205–211. [Google Scholar] [PubMed]
- Schneider, G.; Steinbach, A.; Putics, Á.; Solti-Hodován, Á.; Palkovics, T. Potential of Essential Oils in the Control of Listeria monocytogenes. Microorganisms 2023, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Fathy, S.S.; Awad, E.I.; Abd-El Aal, S.F.A.; Abdelfatah, E.N.; Tahoun, A.B.M.B. Inhibitory effect of some probiotic strains and essential oils on the growth of some foodborne pathogens. Open Vet. J. 2024, 14, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Hulankova, R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro—A Review. Plants 2024, 13, 2784. [Google Scholar] [CrossRef] [PubMed]


| Antimicrobial Agent | ||||
|---|---|---|---|---|
| Thy-EO (µL/mL) | Gentamicin (μg/mL) | |||
| a MIC | b MBC | MIC | MBC | |
| S. enterica group B | 0.4 | 0.4/0.5 | 40 | 40 |
| Y. enterocolitica | 0.2 | 0.4/0.5 | 10 | 20 |
| L. monocytogenes | 0.125 | 0.2/0.3 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rosario, M.; Continisio, L.; Mantova, G.; Carraturo, F.; Scaglione, E.; Sateriale, D.; Forgione, G.; Pagliuca, C.; Pagliarulo, C.; Colicchio, R.; et al. Thyme Essential Oil as a Potential Tool Against Common and Re-Emerging Foodborne Pathogens: Biocidal Effect on Bacterial Membrane Permeability. Microorganisms 2025, 13, 37. https://doi.org/10.3390/microorganisms13010037
Di Rosario M, Continisio L, Mantova G, Carraturo F, Scaglione E, Sateriale D, Forgione G, Pagliuca C, Pagliarulo C, Colicchio R, et al. Thyme Essential Oil as a Potential Tool Against Common and Re-Emerging Foodborne Pathogens: Biocidal Effect on Bacterial Membrane Permeability. Microorganisms. 2025; 13(1):37. https://doi.org/10.3390/microorganisms13010037
Chicago/Turabian StyleDi Rosario, Martina, Leonardo Continisio, Giuseppe Mantova, Francesca Carraturo, Elena Scaglione, Daniela Sateriale, Giuseppina Forgione, Chiara Pagliuca, Caterina Pagliarulo, Roberta Colicchio, and et al. 2025. "Thyme Essential Oil as a Potential Tool Against Common and Re-Emerging Foodborne Pathogens: Biocidal Effect on Bacterial Membrane Permeability" Microorganisms 13, no. 1: 37. https://doi.org/10.3390/microorganisms13010037
APA StyleDi Rosario, M., Continisio, L., Mantova, G., Carraturo, F., Scaglione, E., Sateriale, D., Forgione, G., Pagliuca, C., Pagliarulo, C., Colicchio, R., Vitiello, M., & Salvatore, P. (2025). Thyme Essential Oil as a Potential Tool Against Common and Re-Emerging Foodborne Pathogens: Biocidal Effect on Bacterial Membrane Permeability. Microorganisms, 13(1), 37. https://doi.org/10.3390/microorganisms13010037






