The Structural, Biophysical, and Antigenic Characterization of the Goose Parvovirus Capsid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus-like Particle Production and Purification
2.2. Cryo-EM Data Collection
2.3. Particle Reconstruction
2.4. Model Building and Structure Refinement
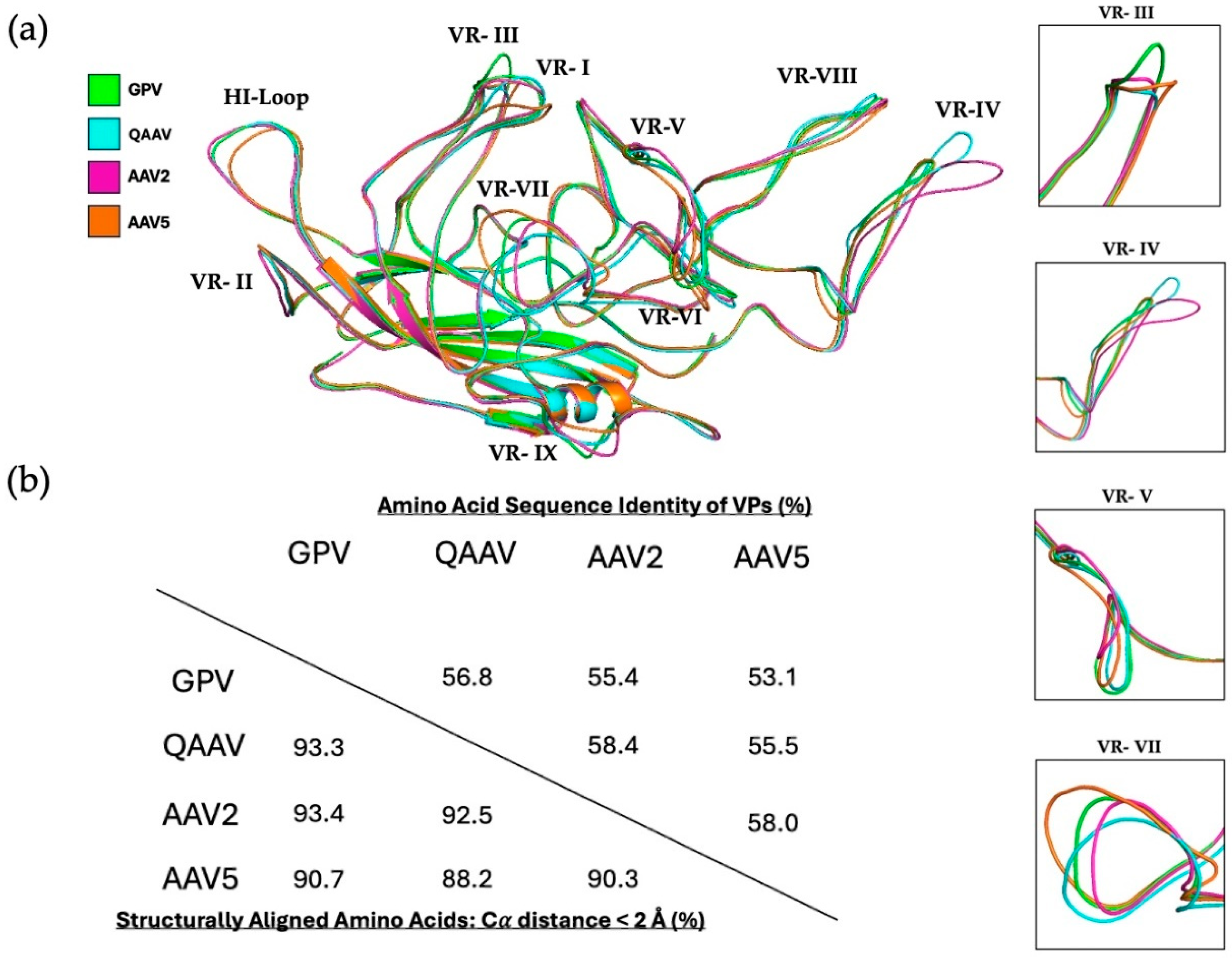
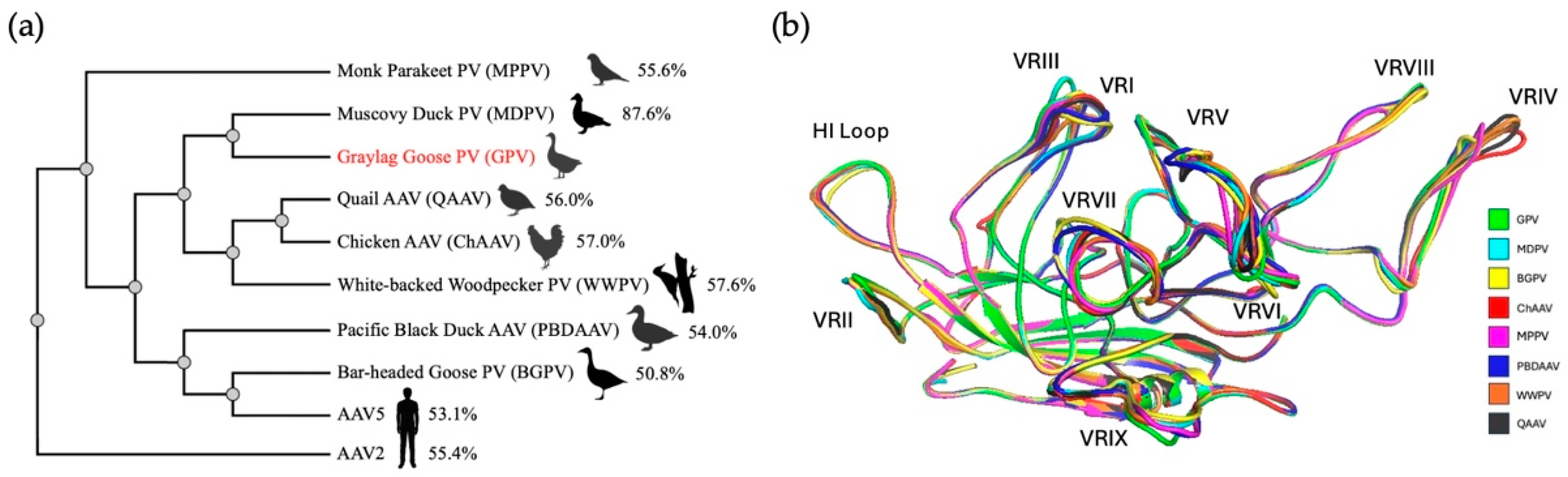
2.5. Sequence and Structure Comparisons
2.6. Biophysical Characterization of Capsid Using DSF
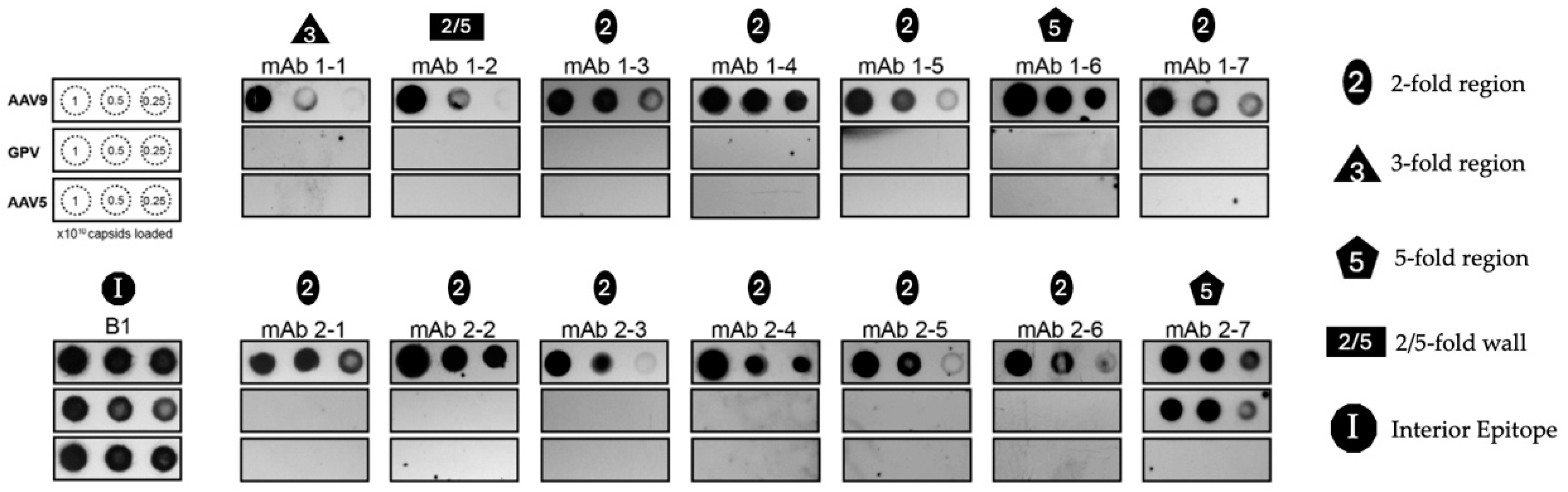
2.7. Native Immuno-Dot Blot Analysis
3. Results and Discussion
3.1. Producing VLPs of GPV and Determination of Its 3D Capsid Structure
3.2. GPV Shows Structural Variation from the AAVs in Several Variable Regions
3.3. Cross-Reacting Human mAbs Bind to GPV in Its Five-Fold Region
3.4. The GPV Capsid Has High Thermal Stability
3.5. Many Bird-Derived Dependoparvoviruses Are Structurally Similar to GPV
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bian, G.; Ma, H.; Luo, M.; Gong, F.; Li, B.; Wang, G.; Mohiuddin, M.; Liao, M.; Yuan, J. Identification and genomic analysis of two novel duck-origin GPV-related parvovirus in China. BMC Vet. Res. 2019, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.-M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Kailasan, S.; Agbandje-McKenna, M.; Parrish, C.R. Parvovirus Family Conundrum: What Makes a Killer? Annu. Rev. Virol. 2015, 2, 425–450. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2020, 29, 464. [Google Scholar] [CrossRef]
- Jager, M.C.; Tomlinson, J.E.; Lopez-Astacio, R.A.; Parrish, C.R.; Van de Walle, G.R. Small but mighty: Old and new parvoviruses of veterinary significance. Virol. J. 2021, 18, 210. [Google Scholar] [CrossRef]
- Zádori, Z.; Stefancsik, R.; Rauch, T.; Kisary, J. Analysis of the Complete Nucleotide Sequences of Goose and Muscovy Duck Pervoviruses Indicates Common Ancestral Origin with Adeno-Associated Virus 2. Virology 1995, 212, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, J.; Pintel, D.J. The Choice of Translation Initiation Site of the Rep Proteins from Goose Parvovirus P9-Generated mRNA Is Governed by Splicing and the Nature of the Excised Intron. J. Virol. 2009, 83, 10264. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Cheng, F.; Yoto, Y.; Zádori, Z.; Pintel, D. The Expression Strategy of Goose Parvovirus Exhibits Features of both the Dependovirus and Parvovirus Genera. J. Virol. 2005, 79, 11035. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Vandenberghe, L.H.; Alvira, M.R.; Lu, Y.; Calcedo, R.; Zhou, X.; Wilson, J.M. Clades of Adeno-Associated Viruses Are Widely Disseminated in Human Tissues. J. Virol. 2004, 78, 6381. [Google Scholar] [CrossRef]
- Gao, G.-P.; Alvira, M.R.; Wang, L.; Calcedo, R.; Johnston, J.; Wilson, J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 11854–11859. [Google Scholar] [CrossRef] [PubMed]
- Arbetman, A.E.; Lochrie, M.; Zhou, S.; Wellman, J.; Scallan, C.; Doroudchi, M.M.; Randlev, B.; Patarroyo-White, S.; Liu, T.; Smith, P.; et al. Novel Caprine Adeno-Associated Virus (AAV) Capsid (AAV-Go.1) Is Closely Related to the Primate AAV-5 and Has Unique Tropism and Neutralization Properties. J. Virol. 2005, 79, 15238. [Google Scholar] [CrossRef]
- Schmidt, M.; Katano, H.; Bossis, I.; Chiorini, J.A. Cloning and Characterization of a Bovine Adeno-Associated Virus. J. Virol. 2004, 78, 6509. [Google Scholar] [CrossRef]
- Kapgate, S.S.; Kumanan, K.; Vijayarani, K.; Barbuddhe, S.B. Avian parvovirus: Classification, phylogeny, pathogenesis and diagnosis. Avian Pathol. 2018, 47, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Chand, A.; Aviles, J.; Soule, G.; Auricchio, A.; Kobinger, G.P. Novel Adeno-associated Viruses Derived From Pig Tissues Transduce Most Major Organs in Mice. Sci. Rep. 2014, 4, 6644. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Liu, Y.; Shi, Z.; Liu, H.; Wei, Y.; Yang, L. Bat adeno-associated viruses as gene therapy vectors with the potential to evade human neutralizing antibodies. Gene Ther. 2019, 26, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Lochrie, M.A.; Tatsuno, G.P.; Arbetman, A.E.; Jones, K.; Pater, C.; Smith, P.H.; McDonnell, J.W.; Zhou, S.-Z.; Kachi, S.; Kachi, M.; et al. Adeno-associated virus (AAV) capsid genes isolated from rat and mouse liver genomic DNA define two new AAV species distantly related to AAV-5. Virology 2006, 353, 68–82. [Google Scholar] [CrossRef]
- Li, L.; Shan, T.; Wang, C.; Côté, C.; Kolman, J.; Onions, D.; Gulland, F.M.D.; Delwart, E. The Fecal Viral Flora of California Sea Lions. J. Virol. 2011, 85, 9909. [Google Scholar] [CrossRef] [PubMed]
- Pénzes, J.J.; Pham, H.T.; Benkö, M.; Tijssen, P. Novel parvoviruses in reptiles and genome sequence of a lizard parvovirus shed light on Dependoparvovirus genus evolution. J. Gen. Virol. 2015, 96, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Jose, A.; Chipman, P.; Bhattacharya, N.; Daneshparvar, N.; McKenna, R.; Agbandje-McKenna, M. Completion of the AAV Structural Atlas: Serotype Capsid Structures Reveals Clade-Specific Features. Viruses 2021, 13, 101. [Google Scholar] [CrossRef]
- Mietzsch, M.; Hull, J.A.; Makal, V.E.; Jimenez Ybargollin, A.; Yu, J.C.; McKissock, K.; Bennett, A.; Penzes, J.; Lins-Austin, B.; Yu, Q.; et al. Characterization of the Serpentine Adeno-Associated Virus (SAAV) Capsid Structure: Receptor Interactions and Antigenicity. J. Virol. 2022, 96, e00335-22. [Google Scholar] [CrossRef] [PubMed]
- Stagg, S.M.; Yoshioka, C.; Davulcu, O.; Chapman, M.S. Cryo-electron Microscopy of Adeno-associated Virus. Chem. Rev. 2022, 122, 14018–14054. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Pénzes, J.J.; Agbandje-McKenna, M. Twenty-Five Years of Structural Parvovirology. Viruses 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, L.; Padron, E.; McKenna, R.; Muzyczka, N.; Kaludov, N.; Chiorini, J.A.; Agbandje-McKenna, M. Structurally Mapping the Diverse Phenotype of Adeno-Associated Virus Serotype 4. J. Virol. 2006, 80, 11556–11570. [Google Scholar] [CrossRef] [PubMed]
- Derzsy, D. A viral disease of goslings. I. Epidemiological, clinical, pathological and aetiological studies. Acta Vet. Acad. Sci. Hung. 1967, 17, 443–448. [Google Scholar] [PubMed]
- Behboudi, S. Derzsy’s Disease. CABI Compendium. 2022. Available online: https://www.cabidigitallibrary.org/doi/abs/10.1079/cabicompendium.85757 (accessed on 21 November 2024). [CrossRef]
- Glávits, R.; Zolnai, A.; Szabó, E.; Ivanics, E.; Zarka, P.; Mató, T.; Palya, V. Comparative pathological studies on domestic geese (Anser anser domestica) and Muscovy ducks (Cairina moschata) experimentally infected with parvovirus strains of goose and Muscovy duck origin. Acta Vet. Hung. 2005, 53, 73–89. [Google Scholar] [CrossRef]
- Jansson, D.S.; Feinstein, R.; Kardi, V.; Mató, T.; Palya, V. Epidemiologic investigation of an outbreak of goose parvovirus infection in Sweden. Avian Dis. 2007, 51, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Takehara, K.; Nishio, T.; Hayashi, Y.; Kanda, J.; Sasaki, M.; Abe, N.; Hiraizumi, M.; Saito, S.; Yamada, T.; Haritani, M.; et al. An Outbreak of Goose Parvovirus Infection in Japan. J. Vet. Med. Sci. 1995, 57, 777–779. [Google Scholar] [CrossRef]
- Irvine, R.; Holmes, P. Diagnosis and control of goose parvovirus. Pract. 2010, 32, 382–386. [Google Scholar] [CrossRef]
- Kisary, J.; Derzsy, D.; Mészáros, J. Attenuation of the goose parvovirus strain B. laboratory and field trials of the attenuated mutant for vaccination against Derzsy’s disease. Avian Pathol. 1978, 7, 397–406. [Google Scholar] [CrossRef]
- Gough, R.E.; Spackman, D. Studies with a duck embryo adapted goose parvovirus vaccine. Avian Pathol. 1982, 11, 503–510. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Tseng, C.-H.; Chang, P.-C.; Mei, K.; Wang, S.-C. Genetic Variation of Viral Protein 1 Genes of Field Strains of Waterfowl Parvoviruses and Their Attenuated Derivatives. Avian Dis. 2004, 48, 512–521. [Google Scholar] [CrossRef]
- Shao, H.; Jiang, Y.; Yuan, H.; Ji, L.; Jin, W.; Qian, K.; Ye, J.; Qin, A. Generation and molecular characteristics of a highly attenuated GPV strain through adaptation in GEF cells. BMC Vet. Res. 2020, 16, 456. [Google Scholar] [CrossRef]
- Wang, J.; Duan, J.; Meng, X.; Gong, J.; Jiang, Z.; Zhu, G. Cloning of the genome of a goose parvovirus vaccine strain SYG61v and rescue of infectious virions from recombinant plasmid in embryonated goose eggs. J. Virol. Methods 2014, 200, 41–46. [Google Scholar] [CrossRef]
- Shien, J.-H.; Wang, Y.-S.; Chen, C.-H.; Shieh, H.K.; Hu, C.-C.; Chang, P.-C. Identification of sequence changes in live attenuated goose parvovirus vaccine strains developed in Asia and Europe. Avian Pathol. 2008, 37, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.A.; Erfan, A.M.; Samy, M.; Mahana, O.; Nasef, S.A. Detection of Novel Goose Parvovirus Disease Associated with Short Beak and Dwarfism Syndrome in Commercial Ducks. Animals 2020, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Yang, W.-T.; Shi, S.-H.; Li, Y.-J.; Zhao, L.; Shi, C.-W.; Zhou, F.-Y.; Jiang, Y.-L.; Hu, J.-T.; Gu, W.; et al. Immunogenicity of recombinant Lactobacillus plantarum NC8 expressing goose parvovirus VP2 gene in BALB/c mice. J. Vet. Sci. 2017, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Wei, N.; Wang, Q.; Wang, C.; Jing, Z.; Guo, L.; Liu, D.; Gao, M.; Ma, B.; Wang, J. Goose parvovirus structural proteins expressed by recombinant baculoviruses self-assemble into virus-like particles with strong immunogenicity in goose. Biochem. Biophys. Res. Commun. 2011, 409, 131–136. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Chang, W.-C.; Wu, M.-C.; Liaw, J.; Shiau, A.-L.; Chu, C.-Y. Oral DNA vaccine adjuvanted with cyclic peptide nanotubes induced a virus-specific antibody response in ducklings against goose parvovirus. Vet. Q. 2023, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Poterszman, A. Baculovirus expression: Old dog, new tricks. Bioengineered 2015, 6, 316–322. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Sun, Y.; Zhang, H.; Yang, R. Expression of VP2 protein of novel goose parvovirus in baculovirus and evaluation of its immune effect. Microb. Pathog. 2024, 195, 106751. [Google Scholar] [CrossRef]
- Ilyas, M.; Mietzsch, M.; Kailasan, S.; Väisänen, E.; Luo, M.; Chipman, P.; Smith, J.K.; Kurian, J.; Sousa, D.; McKenna, R.; et al. Atomic Resolution Structures of Human Bufaviruses Determined by Cryo-Electron Microscopy. Viruses 2018, 10, 22. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Palovcak, E.; Armache, J.-P.; Verba, K.A.; Cheng, Y.; Agard, D.A. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 2017, 14, 331–332. [Google Scholar] [CrossRef]
- Ho, P.T.; Montiel-Garcia, D.J.; Wong, J.J.; Carrillo-Tripp, M.; Brooks, C.L.; Johnson, J.E.; Reddy, V.S. VIPERdb: A Tool for Virus Research. Annu. Rev. Virol. 2018, 5, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.L.C. The PyMOL Molecular Graphics System, Version 2.5.7.; Schrödinger, LLC.: New York, NY, USA, 2021.
- Mietzsch, M.; Liu, W.; Ma, K.; Bennett, A.; Nelson, A.R.; Gliwa, K.; Chipman, P.; Fu, X.; Bechler, S.; McKenna, R.; et al. Production and characterization of an AAV1-VP3-only capsid: An analytical benchmark standard. Mol. Ther. Methods Clin. Dev. 2023, 29, 460–472. [Google Scholar] [CrossRef]
- Arriaga, I.; Navarro, A.; Etxabe, A.; Trigueros, C.; Samulski, R.J.; Moullier, P.; François, A.; Abrescia, N.G.A. Cellular and Structural Characterization of VP1 and VP2 Knockout Mutants of AAV3B Serotype and Implications for AAV Manufacturing. Hum. Gene Ther. 2022, 33, 1142–1156. [Google Scholar] [CrossRef]
- Ros, C.; Bieri, J.; Leisi, R. The VP1u of Human Parvovirus B19: A Multifunctional Capsid Protein with Biotechnological Applications. Viruses 2020, 12, 1463. [Google Scholar] [CrossRef]
- Venkatakrishnan, B.; Yarbrough, J.; Domsic, J.; Bennett, A.; Bothner, B.; Kozyreva, O.G.; Samulski, R.J.; Muzyczka, N.; McKenna, R.; Agbandje-McKenna, M. Structure and Dynamics of Adeno-Associated Virus Serotype 1 VP1-Unique N-Terminal Domain and Its Role in Capsid Trafficking. J. Virol. 2013, 87, 4974. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Hsi, J.; Mietzsch, M.; Chipman, P.; Afione, S.; Zeher, A.; Huang, R.; Chiorini, J.; McKenna, R. Structural and antigenic characterization of the avian adeno-associated virus capsid. J. Virol. 2023, 97, e00780-23. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, L.; DiMattia, M.A.; Gurda, B.L.; Halder, S.; McKenna, R.; Chiorini, J.A.; Muzyczka, N.; Zolotukhin, S.; Agbandje-McKenna, M. Structural Insights into Adeno-Associated Virus Serotype 5. J. Virol. 2013, 87, 11187. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Gargas, J.; Kansol, A.; Lewis, J.; Hsi, J.; Hull, J.; Mietzsch, M.; Tartaglia, L.; Muzyczka, N.; Bhattacharya, N.; et al. Structural and Biophysical Analysis of Adeno-Associated Virus Serotype 2 Capsid Assembly Variants. J. Virol. 2023, 97, e01772-22. [Google Scholar] [CrossRef]
- Afione, S.; DiMattia, M.A.; Halder, S.; Di Pasquale, G.; Agbandje-McKenna, M.; Chiorini, J.A. Identification and Mutagenesis of the Adeno-Associated Virus 5 Sialic Acid Binding Region. J. Virol. 2014, 89, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Opie, S.R.; Warrington, K.H., Jr.; Agbandje-McKenna, M.; Zolotukhin, S.; Muzyczka, N. Identification of Amino Acid Residues in the Capsid Proteins of Adeno-Associated Virus Type 2 That Contribute to Heparan Sulfate Proteoglycan Binding. J. Virol. 2003, 77, 6995–7006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cao, L.; Cui, M.; Sun, Z.; Hu, M.; Zhang, R.; Stuart, W.; Zhao, X.; Yang, Z.; Li, X.; et al. Adeno-associated virus 2 bound to its cellular receptor AAVR. Nat. Microbiol. 2019, 4, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, G.; Cao, L.; Sun, Z.; He, Y.; Cui, M.; Sun, Y.; Li, S.; Li, H.; Qin, L.; et al. Divergent engagements between adeno-associated viruses with their cellular receptor AAVR. Nat. Commun. 2019, 10, 3760. [Google Scholar] [CrossRef] [PubMed]
- Boutin, S.; Monteilhet, V.; Veron, P.; Leborgne, C.; Benveniste, O.; Montus, M.F.; Masurier, C. Prevalence of Serum IgG and Neutralizing Factors Against Adeno-Associated Virus (AAV) Types 1, 2, 5, 6, 8, and 9 in the Healthy Population: Implications for Gene Therapy Using AAV Vectors. Available online: https://www.liebertpub.com/doi/10.1089/hum.2009.182 (accessed on 21 November 2024).
- Earley, J.; Piletska, E.; Ronzitti, G.; Piletsky, S. Evading and overcoming AAV neutralization in gene therapy. Trends Biotechnol. 2023, 41, 836–845. [Google Scholar] [CrossRef]
- Kruzik, A.; Fetahagic, D.; Hartlieb, B.; Dorn, S.; Koppensteiner, H.; Horling, F.M.; Scheiflinger, F.; Reipert, B.M.; de la Rosa, M. Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors. Mol. Ther. Methods Clin. Dev. 2019, 14, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.J.; Mietzsch, M.; Khandekar, N.; D’Silva, A.; Anderson, D.; Mandwie, M.; Hsi, J.; Nelson, A.R.; Chipman, P.; Jackson, J.; et al. Structural and functional characterization of capsid binding by anti-AAV9 monoclonal antibodies from infants after SMA gene therapy. Mol. Ther. 2023, 31, 1979–1993. [Google Scholar] [CrossRef]
- Mietzsch, M.; Nelson, A.R.; Hsi, J.; Zachary, J.; Potts, L.; Chipman, P.; Ghanem, M.; Khandekar, N.; Alexander, I.E.; Logan, G.J.; et al. Structural characterization of antibody-responses from Zolgensma treatment provides the blueprint for the engineering of an AAV capsid suitable for redosing. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wobus, C.E.; Hügle-Dörr, B.; Girod, A.; Petersen, G.; Hallek, M.; Kleinschmidt, J.A. Monoclonal Antibodies against the Adeno-Associated Virus Type 2 (AAV-2) Capsid: Epitope Mapping and Identification of Capsid Domains Involved in AAV-2–Cell Interaction and Neutralization of AAV-2 Infection. J. Virol. 2000, 74, 9281–9293. [Google Scholar] [CrossRef] [PubMed]
- Rieser, R.; Penaud-Budloo, M.; Bouzelha, M.; Rossi, A.; Menzen, T.; Biel, M.; Büning, H.; Ayuso, E.; Winter, G.; Michalakis, S. Intrinsic Differential Scanning Fluorimetry for Fast and Easy Identification of Adeno-Associated Virus Serotypes. J. Pharm. Sci. 2020, 109, 854–862. [Google Scholar] [CrossRef]
- Bennett, A.; Patel, S.; Mietzsch, M.; Jose, A.; Lins-Austin, B.; Yu, J.C.; Bothner, B.; McKenna, R.; Agbandje-McKenna, M. Thermal Stability as a Determinant of AAV Serotype Identity. Mol. Ther. Methods Clin. Dev. 2017, 6, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Farner, D.S. The Hydrogen Ion Concentration in Avian Digestive Tracts. Poult. Sci. 1942, 21, 445–450. [Google Scholar] [CrossRef]
- Prinzinger, R.; Preßmar, A.; Schleucher, E. Body temperature in birds. Comp. Biochem. Physiol. Part. A Physiol. 1991, 99, 499–506. [Google Scholar] [CrossRef]
- Yates, V.J.; El Mishad, A.M.; McCormick, K.J.; Trentin, J.J. Isolation and Characterization of an Avian Adenovirus-Associated Virus. Infect. Immun. 1973, 7, 973–980. [Google Scholar] [CrossRef]
- Irvine, R.; Ceeraz, V.; Cox, B.; Twomey, F.; Young, S.; Bradshaw, J.; Featherstone, C.; Holmes, J.P.; Ainsworth, H.; Jones, R. Goose parvovirus in Great Britain. Vet. Rec. 2008, 163, 461. [Google Scholar] [CrossRef]



| Cryo-EM Data and Refinement Parameters | |
|---|---|
| Number of Micrographs | 1716 |
| Defocus Range (μm) | 0.4–4.7 |
| Electron Dose (e−/A2) | 50 |
| Frames/Micrograph | 50 |
| Pixel Size (Å/pixel) | 0.82 |
| Capsids Used for Final Map | 15,471 |
| Resolution of Final Map (Å) | 2.43 |
| PHENIX Model Refinement Statistics | |
| Map CC | 0.844 |
| RMSD Bonds (Å) | 0.01 |
| RMSD Angles (°) | 0.86 |
| All-Atom Clashscore | 6.82 |
| Ramachandran Plot | |
| Favored (%) | 97.5 |
| Allowed (%) | 2.5 |
| Outliers (%) | 0 |
| Rotamer Outliers (%) | 0 |
| C-β Deviations | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbari, K.; Mietzsch, M.; Hsi, J.; Chipman, P.; Qiu, J.; McKenna, R. The Structural, Biophysical, and Antigenic Characterization of the Goose Parvovirus Capsid. Microorganisms 2025, 13, 80. https://doi.org/10.3390/microorganisms13010080
Jabbari K, Mietzsch M, Hsi J, Chipman P, Qiu J, McKenna R. The Structural, Biophysical, and Antigenic Characterization of the Goose Parvovirus Capsid. Microorganisms. 2025; 13(1):80. https://doi.org/10.3390/microorganisms13010080
Chicago/Turabian StyleJabbari, Korosh, Mario Mietzsch, Jane Hsi, Paul Chipman, Jianming Qiu, and Robert McKenna. 2025. "The Structural, Biophysical, and Antigenic Characterization of the Goose Parvovirus Capsid" Microorganisms 13, no. 1: 80. https://doi.org/10.3390/microorganisms13010080
APA StyleJabbari, K., Mietzsch, M., Hsi, J., Chipman, P., Qiu, J., & McKenna, R. (2025). The Structural, Biophysical, and Antigenic Characterization of the Goose Parvovirus Capsid. Microorganisms, 13(1), 80. https://doi.org/10.3390/microorganisms13010080







