Characterization of Conjunctival Microflora and Antibiotic Sensitivity Patterns in Patients Undergoing Cataract Surgery
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Paiva, C.S.; Leger, A.J.S.; Caspi, R.R. Mucosal immunology of the ocular surface. Mucosal. Immunol. 2022, 15, 1143–1157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.; Yang, B.; Li, W. Defining the normal core microbiome of conjunctival microbial communities. Clin. Microbiol. Infect. 2016, 22, 643.e7–643.e12. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Baudouin, C.; Del Castillo, J.M.B.; Messmer, E.; Barabino, S.; Merayo-Lloves, J.; Brignole-Baudouin, F.; Inferrera, L.; Rolando, M.; Mencucci, R.; et al. The ocular microbiome and microbiota and their effects on ocular surface pathophysiology and disorders. Surv. Ophthalmol. 2021, 66, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Nakashizuka, H. Cataract Surgery by Intraoperative Surface Irrigation with 0.25% Povidone-Iodine. J. Clin. Med. 2021, 10, 3611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawasaki, S.; Tasaka, Y.; Suzuki, T.; Zheng, X.; Shiraishi, A.; Uno, T.; Ohashi, Y. Influence of elevated intraocular pressure on the posterior chamber-anterior hyaloid membrane barrier during cataract operations. Arch Ophthalmol. 2011, 129, 751. [Google Scholar] [CrossRef] [PubMed]
- Soare, S.D.; Ilie, L.; Costeliu, O.; Ghiță, A.C.; Voinea, L.M.; Ghiță, A.M. The ocular surface bacterial contamination and its management in the prophylaxis of post cataract surgery endophthalmitis. Rom. J. Ophthalmol. 2021, 65, 2–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Academy of Ophthalmology. Cataract in the Adult Eye: Preferred Practice Pattern; American Academy of Ophthalmology: San Francisco, CA, USA, 2021; Available online: https://www.aaojournal.org/action/showPdf?pii=S0161-6420%2821%2900750-8. (accessed on 8 October 2024).
- Behndig, A.; Cochener-Lamard, B.; Güell, J.; Kodjikian, L.; Mencucci, R.; Nuijts, R.; Pleyer, U.; Rosen, P.; Szaflik, J.; Tassignon, M.J. Surgical, antiseptic, and antibiotic practice in cataract surgery: Results from the European Observatory in 2013. J. Cataract. Refract. Surg. 2015, 41, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.; Cordovés, L.; Gardner, S. ESCRS Guidelines for Prevention and Treatment of Endophthamitis Following Cataract Surgery: Prevention & Treatment Endophthalmitis. Available online: www.escrs.org (accessed on 12 September 2024).
- Behndig, A.; Cochener, B.; Güell, J.L.; Kodjikian, L.; Mencucci, R.; Nuijts, R.M.; Pleyer, U.; Rosen, P.; Szaflik, J.P.; Tassignon, M.J. Endophthalmitis prophylaxis in cataract surgery: Overview of current practice patterns in 9 European countries. J. Cataract. Refract. Surg. 2013, 39, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Comis, S.; Jannuzzi, V.; Camposampiero, D.; Ponzin, D.; Cambria, S.; Santocono, M.; Pallozzi Lavorante, N.; Del Noce, C.; Scorcia, V.; et al. Effect of Liposomal-Lactoferrin-Based Eye Drops on the Conjunctival Microflora of Patients Undergoing Cataract Surgery. Ophthalmol. Ther. 2023, 12, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NCCLS Document M2–A8 2003; Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard. 8th ed. National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2003.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 5.0. 2015. Available online: http://www.eucast.org (accessed on 11 October 2024).
- Wen, X.; Miao, L.; Deng, Y.; Bible, P.W.; Hu, X.; Zou, Y.; Liu, Y.; Guo, S.; Liang, J.; Chen, T.; et al. The Influence of Age and Sex on Ocular Surface Microbiota in Healthy Adults. Investig. Opthalmol. Vis. Sci. 2017, 58, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Rubio, E.F. Influence of age on conjunctival bacteria of patients undergoing cataract surgery. Eye 2005, 20, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Romano, M.R.; Iannetta, D.; Romano, V.; Gualdi, L.; D’Agostino, I.; Ripandelli, G. Cataract surgery practice patterns worldwide: A survey. BMJ Open Ophthalmol. 2021, 6, e000464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Callegan, M.C.; Engelbert, M.; Parke, D.W., 2nd; Jett, B.D.; Gilmore, M.S. Bacterial endophthalmitis: Epidemiology, therapeutics, and bacterium-host interactions. Clin. Microbiol. Rev. 2002, 15, 111–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, D.P.; Wisniewski, S.R.; Wilson, L.A.; Barza, M.; Vine, A.K.; Doft, B.H.; Kelsey, S.F. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 1996, 122, 1–17, Erratum in: Am. J. Ophthalmol. 1996, 122, 920. [Google Scholar] [CrossRef] [PubMed]
- Aaberg, T.M., Jr.; Flynn, H.W., Jr.; Schiffman, J.; Newton, J. Nosocomial acute-onset postoperative endophthalmitis survey. A 10-year review of incidence and outcomes. Ophthalmology 1998, 105, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Schwartz, S.G.; Matsuura, K.; Ong Tone, S.; Arshinoff, S.; Ng, J.Q.; Meyer, J.J.; Liu, W.; Jacob, S.; Packer, M.; et al. Endophthalmitis Prophylaxis in Cataract Surgery: Overview of Current Practice Patterns Around the World. Curr. Pharm. Des. 2017, 23, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Gower, E.W.; Lindsley, K.; Tulenko, S.E.; Nanji, A.A.; Leyngold, I.; McDonnell, P.J. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst. Rev. 2017, 2017, CD006364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, Y.; Usui, M.; Ohashi, Y.; Shiota, H.; Yamazaki, T.; Preoperative Disinfection Study Group. Preoperative disinfection of the conjunctival sac with antibiotics and iodine compounds: A prospective randomized multicenter study. Jpn. J. Ophthalmol. 2008, 52, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.K.; Melton, R.; Asbell, P.A. Antibiotic resistance among ocular pathogens: Current trends from the ARMOR surveillance study (2009–2016). Clin Optom. 2019, 11, 15–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friling, E.; Lundström, M.; Stenevi, U.; Montan, P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J. Cataract. Refract. Surg. 2013, 39, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Borgia, A.; Mazzuca, D.; Della Corte, M.; Gratteri, N.; Fossati, G.; Raimondi, R.; Pagano, L.; Scorcia, V.; Giannaccare, G. Prophylaxis of Ocular Infection in the Setting of Intraocular Surgery: Implications for Clinical Practice and Risk Management. Ophthalmol. Ther. 2023, 12, 721–734, Erratum in: Ophthalmol. Ther. 2023, 12, 735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimada, H.; Arai, S.; Nakashizuka, H.; Hattori, T.; Yuzawa, M. Reduction of anterior chamber contamination rate after cataract surgery by intraoperative surface irrigation with 0.25% povidone-iodine. Arch. Ophthalmol. 2011, 151, 11–17.e1. [Google Scholar] [CrossRef] [PubMed]
- Valdez-García, J.E.; Climent, A.; Chávez-Mondragón, E.; Lozano-Ramírez, J.F. Anterior chamber bacterial contamination in cataract surgery. BMC Ophthalmol. 2014, 14, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bandello, F.; Coassin, M.; Di Zazzo, A.; Rizzo, S.; Biagini, I.; Pozdeyeva, N.; Sinitsyn, M.; Verzin, A.; De Rosa, P.; Calabrò, F.; et al. One week of levofloxacin plus dexamethasone eye drops for cataract surgery: An innovative and rational therapeutic strategy. Eye 2020, 34, 2112–2122, Erratum in: Eye 2020, 34, 2150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papa, V.; Blanco, A.R.; Santocono, M. Ocular flora and their antibiotic susceptibility in patients having cataract surgery in Italy. J. Cataract. Refract. Surg. 2016, 42, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, D. Chloramphenicol Resurrected: A Journey from Antibiotic Resistance in Eye Infections to Biofilm and Ocular Microbiota. Microorganisms 2019, 7, 278. [Google Scholar] [CrossRef]
- Drago, L. Topical Antibiotic Therapy in the Ocular Environment: The Benefits of Using Moxifloxacin Eyedrops. Microorganisms 2024, 12, 649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, D.G. Fluoroquinolone resistance in ophthalmology and the potential role for newer ophthalmic fluoroquinolones. Surv. Ophthalmol. 2004, 49, S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Del-Castillo, J.; Verboven, Y.; Stroman, D.; Kodjikian, L. The role of topical moxifloxacin, a new antibacterial in Europe, in the treatment of bacterial conjunctivitis. Clin. Drug Investig. 2011, 31, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.R.; Sudano Roccaro, A.; Spoto, C.G.; Papa, V. Susceptibility of methicillin-resistant Staphylococci clinical isolates to netilmicin and other antibiotics commonly used in ophthalmic therapy. Curr. Eye Res. 2013, 38, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G.; Bianco, G.; Boattini, M.; Scalabrin, S.; Iannaccone, M.; Fea, A.; Cavallo, R.; Costa, C. Bacterial etiology and antimicrobial resistance trends in ocular infections: A 30-year study, Turin area, Italy. Eur. J. Ophthalmol. 2019, 31, 405–414. [Google Scholar] [CrossRef] [PubMed]
| Saprophytic Flora | Potential Pathogenic Flora |
|---|---|
| Staphylococcus epidermidis | Moraxella lacunata |
| Staphylococcus haemolyticus | Morganella morganii |
| Staphylococcus hyicus | Pseudomonas fluorescens |
| Staphylococcus intermedius | Ralstonia pickettii |
| Staphylococcus lugdunensis | Serratia marcescens |
| Staphylococcus simulans | Staphylococcus aureus |
| Staphylococcus warneri | Methicillin-resistant Staphylococcus aureus (MRSA) |
| Staphylococcus xylosus | Methicillin-resistant Staphylococcus epidermidis (MRSE) |
| Stenotrophomonas maltophilia |
| Bacteria Isolated | n (%) |
|---|---|
| Moraxella lacunata | 1 (1.1%) |
| Morganella morganii | 1 (1.1%) |
| Pseudomonas fluorescens | 2 (2.3%) |
| Ralstonia pickettii | 1 (1.1%) |
| Serratia marcescens | 1 (1.1%) |
| Staphylococcus aureus | 13 (14.8%) |
| Staphylococcus aureus MRSA | 7 (8.0%) |
| Staphylococcus epidermidis | 18 (20.5%) |
| Staphylococcus epidermidis MRSE | 6 (6.8%) |
| Staphylococcus haemolyticus | 5 (5.7%) |
| Staphylococcus hyicus | 1 (1.1%) |
| Staphylococcus intermedius | 16 (18.2%) |
| Staphylococcus lugdunensis | 6 (6.8%) |
| Staphylococcus simulans | 3 (3.4%) |
| Staphylococcus warneri | 2 (2.3%) |
| Staphylococcus xylosus | 9 (10.2%) |
| Stenotrophomonas maltophila | 2 (2.3%) |
| No growth | 1 (1.1%) |
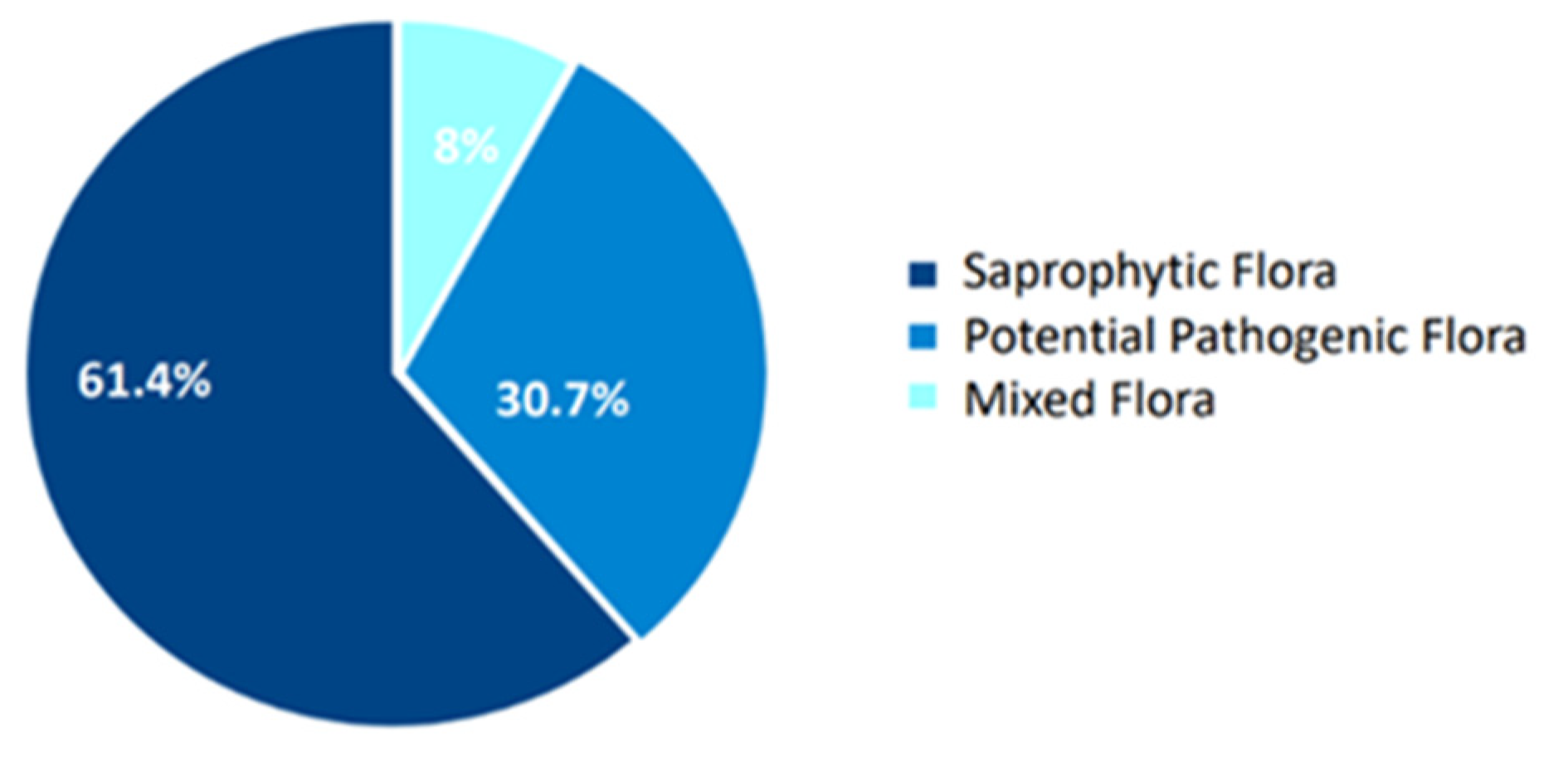
| Saprophytic flora | 54 (61.4%) |
| Pathogenic flora | 27 (30.7%) |
| Mixed flora | 7 (8%) |
| In Vitro Sensitivity (%) | OX | NET | TOB | C | OFX | LEV | MXF | CXM | AZM |
|---|---|---|---|---|---|---|---|---|---|
| Moraxella lacunata (1) | 1 (100) | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 1 (100) | 0 | 0 |
| Morganella morganii (1) | 0 | 1 (100) | 1 (100) | 1 (100) | 0 | 1 (100) | 1 (100) | 1 (100) | 0 |
| Pseudomonas fluorescens (2) | 0 | 2 (100) | 0 | 0 | 1 (50) | 1 (50) | 2 (100) | 0 | 0 |
| Ralstonia picketti (1) | 0 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 1 (100) | 0 | 0 |
| Serratia marcescens (1) | 0 | 1 (100) | 1 (100) | 1 (100) | 1(100) | 1 (100) | 1 (100) | 0 | 0 |
| Stenotroph. malthophilia (2) | 0 | 1 (50) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 2 (100) | 0 | 0 |
| Staph. aureus (13) | 12 (92.3) | 11 (84.6) | 3 (23.1) | 9 (69.2) | 5 (38.5) | 7 (53.8) | 12 (92.3) | 3 (23.1) | 1 (7.7) |
| Staph. aureus MRSA (7) | 0 | 6 (85.7) | 1 (14.3) | 6 (85.7) | 3 (42.9) | 3 (42.9) | 6 (85.7) | 1(14.3) | 1 (14.3) |
| Staph. epidermidis (16) | 16 (100) | 15 (93.8) | 10 (62.5) | 16 (100) | 9 (56.3) | 11(68.8) | 16 (100) | 9 (56.3) | 1 (6.3) |
| Staph. epidermidis MRSE (6) | 0 | 6 (100) | 0 | 4 (66.6) | 1 (16.7) | 2 (33.3) | 5 (83.3) | 2 (33.3) | 1 (16.7) |
| Staph. haemoliticus (4) | 4 (100) | 3 (75) | 1 (25) | 4 (100) | 2 (50) | 3 (75) | 4 (100) | 3 (75) | 3 (75) |
| Staph. intermedius (14) | 11 (78.6) | 10 (71.4) | 4 (28.6) | 12 (85.7) | 5 (35.7) | 5 (35.7) | 12 (85.7) | 6 (42.9) | 3 (21.4) |
| Staph. lugdunensis (6) | 5 (83.3) | 5(83.3) | 4 (66.7) | 5 (83.3) | 1 (16.7) | 3 (50) | 6 (100) | 1 (16.7) | 0 |
| Staph. simulans (3) | 2 (66.7) | 2 (66.7) | 2 (66.7) | 2 (66.7) | 0 | 1 (33.3) | 3 (100) | 0 | 0 |
| Staph. warneri (2) | 1 (50) | 1 (50) | 1 (50) | 2 (100) | 0 | 0 | 2 (100) | 1 (50) | 0 |
| Staph. xylosus (8) | 8 (100) | 6 (75) | 3 (42.9) | 8 (10) | 3 (42.9) | 4 (50) | 7 (87.5) | 2 (25) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagge, A.; Lixi, F.; Ponzin, D.; Del Noce, C.; Camposampiero, D.; Santocono, M.; Traverso, C.E.; Scorcia, V.; Giannaccare, G. Characterization of Conjunctival Microflora and Antibiotic Sensitivity Patterns in Patients Undergoing Cataract Surgery. Microorganisms 2025, 13, 227. https://doi.org/10.3390/microorganisms13020227
Vagge A, Lixi F, Ponzin D, Del Noce C, Camposampiero D, Santocono M, Traverso CE, Scorcia V, Giannaccare G. Characterization of Conjunctival Microflora and Antibiotic Sensitivity Patterns in Patients Undergoing Cataract Surgery. Microorganisms. 2025; 13(2):227. https://doi.org/10.3390/microorganisms13020227
Chicago/Turabian StyleVagge, Aldo, Filippo Lixi, Diego Ponzin, Chiara Del Noce, Davide Camposampiero, Marcello Santocono, Carlo Enrico Traverso, Vincenzo Scorcia, and Giuseppe Giannaccare. 2025. "Characterization of Conjunctival Microflora and Antibiotic Sensitivity Patterns in Patients Undergoing Cataract Surgery" Microorganisms 13, no. 2: 227. https://doi.org/10.3390/microorganisms13020227
APA StyleVagge, A., Lixi, F., Ponzin, D., Del Noce, C., Camposampiero, D., Santocono, M., Traverso, C. E., Scorcia, V., & Giannaccare, G. (2025). Characterization of Conjunctival Microflora and Antibiotic Sensitivity Patterns in Patients Undergoing Cataract Surgery. Microorganisms, 13(2), 227. https://doi.org/10.3390/microorganisms13020227








