Genomic and Proteomic Analyses of Bacterial Communities of Ixodes scapularis Ticks from Broome County, New York
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection
2.2. Tick Processing for Genomic Microbiome Screening
2.3. Tick Processing for Proteomic Screening
3. Results
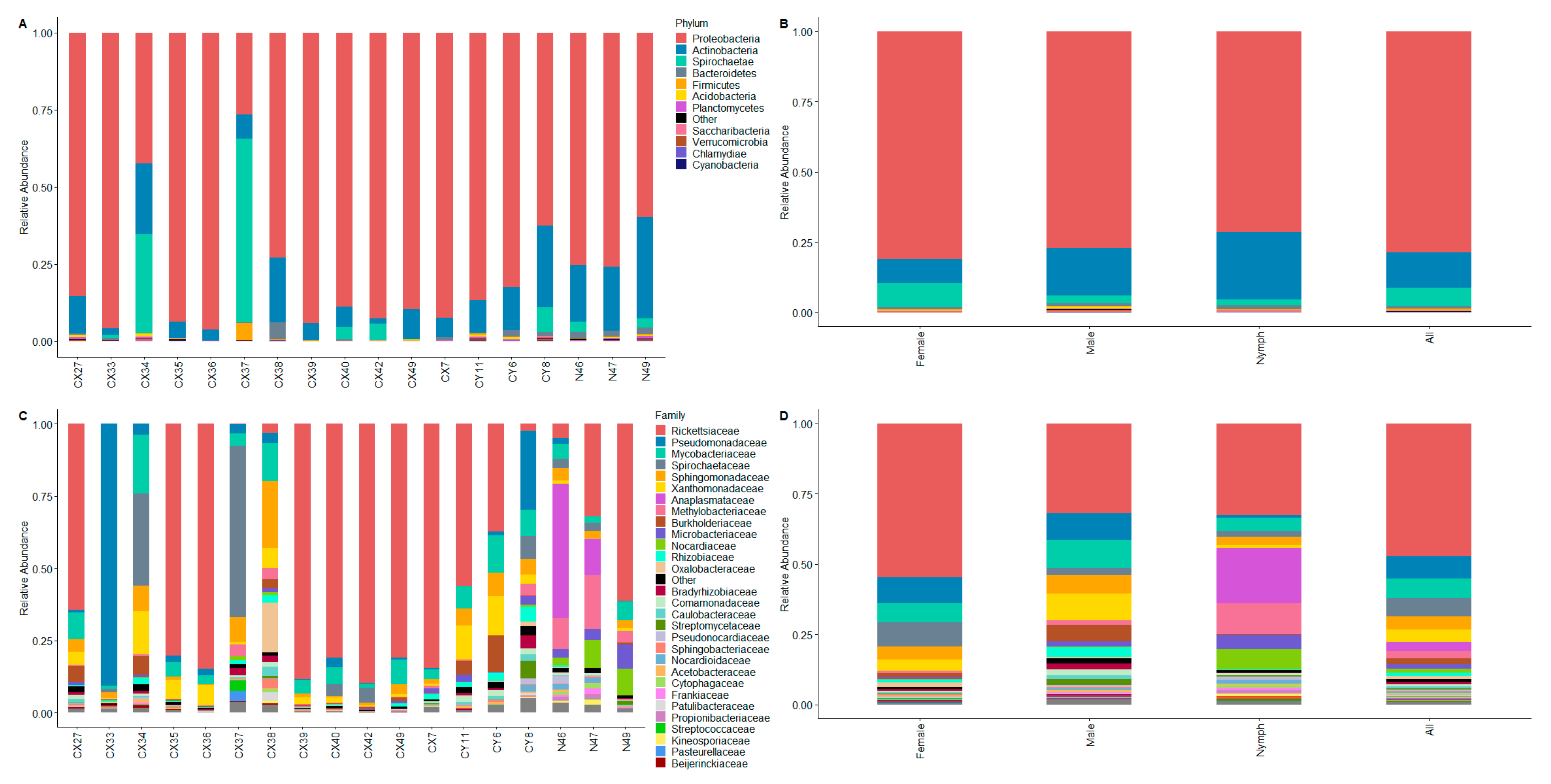
3.1. Composition of Ixodes scapularis Whole-Body Microbiomes
3.1.1. Beta Diversity
3.1.2. Detection of Endosymbiont Rickettsia in Male Ixodes scapularis Ticks
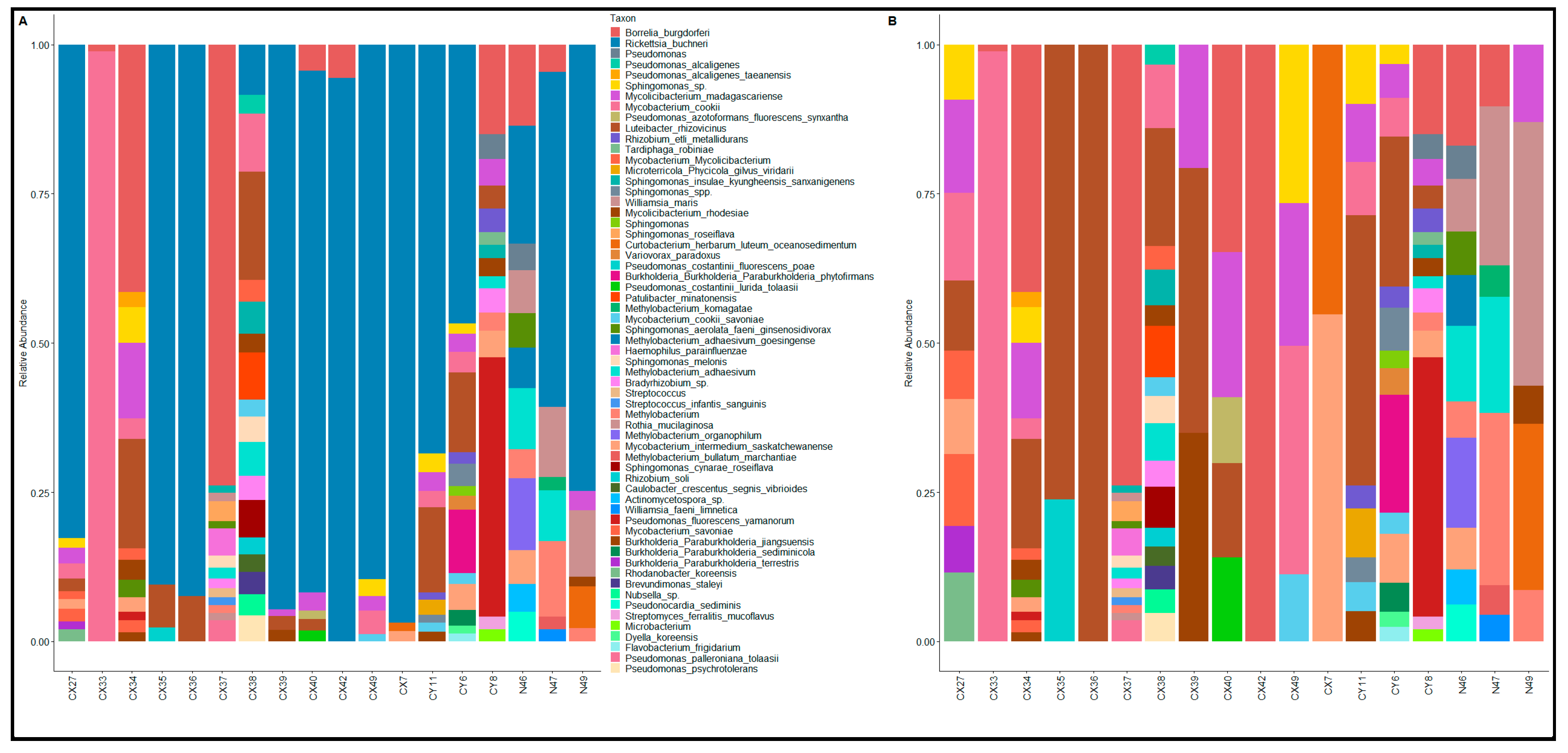
3.1.3. Tick-Borne Pathogens and Ixodes scapularis Microbiome
3.2. Proteome Profiling of Single Ticks and Microorganism Identification
4. Discussion
4.1. Endosymbiont Rickettsia
4.2. Microbiome-TBPs Interactions
4.3. “Core Microbiome” of I. scapularis
4.4. The Proteome Signature of Tick-Borne Pathogens in Ixodes scapularis Ticks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBD | Tick-borne Disease |
| TBP | Tick-borne Pathogen |
| Subsp | Subspecies |
| Spp. | Species |
References
- CDC. Blacklegged Tick Surveillance. Ticks. Available online: https://www.cdc.gov/ticks/data-research/facts-stats/blacklegged-tick-surveillance.html (accessed on 29 December 2024).
- Eisen, L. Tick Species Infesting Humans in the United States. Ticks Tick-Borne Dis. 2022, 13, 102025. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G.; Qiu, W. Borreliella. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- CDC. Lyme Disease Surveillance and Data. Lyme Disease. Available online: https://www.cdc.gov/lyme/data-research/facts-stats/index.html (accessed on 30 December 2024).
- Eisen, R.J.; Eisen, L. Evaluation of the Association between Climate Warming and the Spread and Proliferation of Ixodes scapularis in Northern States in the Eastern United States. Ticks Tick-Borne Dis. 2024, 15, 102286. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public. Health 2018, 15, 478. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of Land Use Changes and Habitat Fragmentation on the Eco-Epidemiology of Tick-Borne Diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef]
- Clow, K.M.; Ogden, N.H.; Lindsay, L.R.; Michel, P.; Pearl, D.L.; Jardine, C.M. The Influence of Abiotic and Biotic Factors on the Invasion of Ixodes scapularis in Ontario, Canada. Ticks Tick-Borne Dis. 2017, 8, 554–563. [Google Scholar] [CrossRef]
- Martin, A.M.; Buttke, D.; Raphael, J.; Taylor, K.; Maes, S.; Parise, C.M.; Ginsberg, H.S.; Cross, P.C. Deer Management Generally Reduces Densities of Nymphal Ixodes scapularis, but Not Prevalence of Infection with Borrelia Burgdorferi Sensu Stricto. Ticks Tick-Borne Dis. 2023, 14, 102202. [Google Scholar] [CrossRef]
- Deshpande, G.; Beetch, J.E.; Heller, J.G.; Naqvi, O.H.; Kuhn, K.G. Assessing the Influence of Climate Change and Environmental Factors on the Top Tick-Borne Diseases in the United States: A Systematic Review. Microorganisms 2024, 12, 50. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J. Changes in the Geographic Distribution of the Blacklegged Tick, Ixodes scapularis, in the United States. Ticks Tick-Borne Dis. 2023, 14, 102233. [Google Scholar] [CrossRef]
- Ripoche, M.; Irace-Cima, A.; Adam-Poupart, A.; Baron, G.; Bouchard, C.; Carignan, A.; Milord, F.; Ouhoummane, N.; Pilon, P.A.; Thivierge, K.; et al. Current and Future Burden from Lyme Disease in Québec as a Result of Climate Change. Can. Commun. Dis. Rep. 2023, 49, 446–456. [Google Scholar] [CrossRef]
- Hart, C.E.; Bhaskar, J.R.; Reynolds, E.; Hermance, M.; Earl, M.; Mahoney, M.; Martinez, A.; Petzlova, I.; Esterly, A.T.; Thangamani, S. Community Engaged Tick Surveillance and tickMAP as a Public Health Tool to Track the Emergence of Ticks and Tick-Borne Diseases in New York. PLoS Glob. Public Health 2022, 2, e0000215. [Google Scholar] [CrossRef]
- Lyme Disease and Other Diseases Carried by Ticks. Available online: https://www.health.ny.gov/diseases/communicable/lyme/ (accessed on 30 December 2024).
- Courtney, J.W.; Dryden, R.L.; Wyleto, P.; Schneider, B.S.; Massung, R.F. Characterization of Anaplasma Phagocytophila and Borrelia Burgdorferi Genotypes in Ixodes scapularis Ticks from Pennsylvania. Ann. N. Y. Acad. Sci. 2003, 990, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.A.; Singh, M.; Rodriguez, M. Human Babesiosis: Recent Advances and Future Challenges. Curr. Opin. Hematol. 2020, 27, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Molloy, P.J.; Telford, S.R.; Chowdri, H.R.; Lepore, T.J.; Gugliotta, J.L.; Weeks, K.E.; Hewins, M.E.; Goethert, H.K.; Berardi, V.P. Borrelia Miyamotoi Disease in the Northeastern United States: A Case Series. Ann. Intern. Med. 2015, 163, 91–98. [Google Scholar] [CrossRef]
- Piantadosi, A.; Solomon, I.H. Powassan Virus Encephalitis. Infect. Dis. Clin. North Am. 2022, 36, 671–688. [Google Scholar] [CrossRef]
- Xu, G.; Foster, E.; Ribbe, F.; Hojgaard, A.; Eisen, R.J.; Paull, S.; Rich, S.M. Detection of Ehrlichia Muris Eauclairensis in Blacklegged Ticks (Ixodes scapularis) and White-Footed Mice (Peromyscus leucopus) in Massachusetts. Vector Borne Zoonotic Dis. 2023, 23, 311–315. [Google Scholar] [CrossRef]
- Pritt, B.S.; Mead, P.S.; Johnson, D.K.H.; Neitzel, D.F.; Respicio-Kingry, L.B.; Davis, J.P.; Schiffman, E.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; et al. Identification of a Novel Pathogenic Borrelia Species Causing Lyme Borreliosis with Unusually High Spirochaetaemia: A Descriptive Study. Lancet Infect. Dis. 2016, 16, 556–564. [Google Scholar] [CrossRef]
- Milholland, M.T.; Xu, G.; Rich, S.M.; Machtinger, E.T.; Mullinax, J.M.; Li, A.Y. Pathogen Coinfections Harbored by Adult Ixodes scapularis from White-Tailed Deer Compared with Questing Adults Across Sites in Maryland, USA. Vector Borne Zoonotic Dis. Larchmt. N 2021, 21, 86–91. [Google Scholar] [CrossRef]
- Sanchez-Vicente, S.; Tokarz, R. Tick-Borne Co-Infections: Challenges in Molecular and Serologic Diagnoses. Pathog. Basel Switz. 2023, 12, 1371. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The Tick Microbiome: Why Non-Pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Morel, O.; Noël, V.; Buysse, M.; Binetruy, F.; Lancelot, R.; Loire, E.; Ménard, C.; Bouchez, O.; Vavre, F.; et al. Tick-Bacteria Mutualism Depends on B Vitamin Synthesis Pathways. Curr. Biol. CB 2018, 28, 1896–1902.e5. [Google Scholar] [CrossRef]
- Wu-Chuang, A.; Hodžić, A.; Mateos-Hernández, L.; Estrada-Peña, A.; Obregon, D.; Cabezas-Cruz, A. Current Debates and Advances in Tick Microbiome Research. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100036. [Google Scholar] [CrossRef] [PubMed]
- Neelakanta, G.; Sultana, H.; Fish, D.; Anderson, J.F.; Fikrig, E. Anaplasma phagocytophilum Induces Ixodes scapularis Ticks to Express an Antifreeze Glycoprotein Gene That Enhances Their Survival in the Cold. J. Clin. Invest. 2010, 120, 3179–3190. [Google Scholar] [CrossRef]
- Ross, B.D.; Hayes, B.; Radey, M.C.; Lee, X.; Josek, T.; Bjork, J.; Neitzel, D.; Paskewitz, S.; Chou, S.; Mougous, J.D. Ixodes scapularis Does Not Harbor a Stable Midgut Microbiome. ISME J. 2018, 12, 2596–2607. [Google Scholar] [CrossRef]
- Landesman, W.J.; Mulder, K.; Fredericks, L.P.; Allan, B.F. Cross-Kingdom Analysis of Nymphal-Stage Ixodes scapularis Microbial Communities in Relation to Borrelia Burgdorferi Infection and Load. FEMS Microbiol. Ecol. 2019, 95, fiz167. [Google Scholar] [CrossRef]
- Sperling, J.L.H.; Fitzgerald, D.; Sperling, F.A.H.; Magor, K.E. Microbiome Composition and Borrelia Detection in Ixodes scapularis Ticks at the Northwestern Edge of Their Range. Trop. Med. Infect. Dis. 2020, 5, 173. [Google Scholar] [CrossRef]
- Cull, B.; Burkhardt, N.Y.; Wang, X.-R.; Thorpe, C.J.; Oliver, J.D.; Kurtti, T.J.; Munderloh, U.G. The Ixodes scapularis Symbiont Rickettsia buchneri Inhibits Growth of Pathogenic Rickettsiaceae in Tick Cells: Implications for Vector Competence. Front. Vet. Sci. 2021, 8, 748427. [Google Scholar] [CrossRef]
- Wang, X.-R.; Cull, B.; Oliver, J.D.; Kurtti, T.J.; Munderloh, U.G. The Role of Autophagy in Tick-Endosymbiont Interactions: Insights from Ixodes scapularis and Rickettsia buchneri. Microbiol. Spectr. 2024, 12, e0108623. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Cabezas-Cruz, A.; Obregón, D. Behind Taxonomic Variability: The Functional Redundancy in the Tick Microbiome. Microorganisms 2020, 8, 1829. [Google Scholar] [CrossRef]
- Narasimhan, S.; Rajeevan, N.; Liu, L.; Zhao, Y.O.; Heisig, J.; Pan, J.; Eppler-Epstein, R.; Deponte, K.; Fish, D.; Fikrig, E. Gut Microbiota of the Tick Vector Ixodes scapularis Modulate Colonization of the Lyme Disease Spirochete. Cell Host Microbe 2014, 15, 58–71. [Google Scholar] [CrossRef]
- Cross, S.T.; Kapuscinski, M.L.; Perino, J.; Maertens, B.L.; Weger-Lucarelli, J.; Ebel, G.D.; Stenglein, M.D. Co-Infection Patterns in Individual Ixodes scapularis Ticks Reveal Associations between Viral, Eukaryotic and Bacterial Microorganisms. Viruses 2018, 10, 388. [Google Scholar] [CrossRef]
- Krupa, E.; Dziedziech, A.; Paul, R.; Bonnet, S. Update on Tick-Borne Pathogens Detection Methods Within Ticks. Curr. Res. Parasitol. Vector Borne Dis. 2024, 6, 100199. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.R.; Canessa, E.H.; Roy, R.; Spathis, R.; Pour, M.S.; Hathout, Y. A Single Tick Screening for Infectious Pathogens Using Targeted Mass Spectrometry. Anal. Bioanal. Chem. 2022, 414, 3791–3802. [Google Scholar] [CrossRef] [PubMed]
- Yssouf, A.; Almeras, L.; Terras, J.; Socolovschi, C.; Raoult, D.; Parola, P. Detection of Rickettsia Spp in Ticks by MALDI-TOF MS. PLoS Negl. Trop. Dis. 2015, 9, e0003473. [Google Scholar] [CrossRef] [PubMed]
- Fotso Fotso, A.; Mediannikov, O.; Diatta, G.; Almeras, L.; Flaudrops, C.; Parola, P.; Drancourt, M. MALDI-TOF Mass Spectrometry Detection of Pathogens in Vectors: The Borrelia Crocidurae/Ornithodoros Sonrai Paradigm. PLoS Negl. Trop. Dis. 2014, 8, e2984. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 30 December 2024).
- Wickham, H. Use R! In Ggplot2; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2024. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 30 December 2024).
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic Species Co-Occurrence Analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Dzieciatkowska, M.; Hill, R.; Hansen, K.C. GeLC-MS/MS Analysis of Complex Protein Mixtures. Methods Mol. Biol. Clifton NJ 2014, 1156, 53–66. [Google Scholar] [CrossRef]
- Paulson, A.R.; Lougheed, S.C.; Huang, D.; Colautti, R.I. Multiomics Reveals Symbionts, Pathogens, and Tissue-Specific Microbiome of Blacklegged Ticks (Ixodes scapularis) from a Lyme Disease Hot Spot in Southeastern Ontario, Canada. Microbiol. Spectr. 2023, 11, e0140423. [Google Scholar] [CrossRef]
- Thapa, S.; Zhang, Y.; Allen, M.S. Bacterial Microbiomes of Ixodes scapularis Ticks Collected from Massachusetts and Texas, USA. BMC Microbiol. 2019, 19, 138. [Google Scholar] [CrossRef]
- Wiesinger, A.; Wenderlein, J.; Ulrich, S.; Hiereth, S.; Chitimia-Dobler, L.; Straubinger, R.K. Revealing the Tick Microbiome: Insights into Midgut and Salivary Gland Microbiota of Female Ixodes Ricinus Ticks. Int. J. Mol. Sci. 2023, 24, 1100. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, E.C.; Hemmerich, C.; Rusch, D.B.; Fuqua, C.; Clay, K. Concordance of Bacterial Communities of Two Tick Species and Blood of Their Shared Rodent Host. Mol. Ecol. 2015, 24, 2566–2579. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.; Lam, V.; Glickman, L.; Hirsch, J.; Tampori, J.; Johnson, R.; Macher, J.; Garruto, R.; Shamoon-Pour, M. Prevalence of Human Pathogens in Ixodes scapularis Ticks of Southern Tier, NY. In Proceedings of the the Human Biology Association (HBA) 49th Annual Meetings, Los Angeles, CA, USA, 20–22 March 2024. [Google Scholar]
- Kumar, D.; Downs, L.P.; Adegoke, A.; Machtinger, E.; Oggenfuss, K.; Ostfeld, R.S.; Embers, M.; Karim, S. An Exploratory Study on the Microbiome of Northern and Southern Populations of Ixodes scapularis Ticks Predicts Changes and Unique Bacterial Interactions. Pathogens 2022, 11, 130. [Google Scholar] [CrossRef]
- Noden, B.H.; Gilliland, M.; Propst, J.; Slater, K.; Karpathy, S.E.; Paddock, C.D. Rickettsia Tillamookensis (Rickettsiales: Rickettsiaceae) in Ixodes scapularis (Acari: Ixodidae) in Oklahoma. J. Med. Entomol. 2024, 61, 257–260. [Google Scholar] [CrossRef]
- Van Treuren, W.; Ponnusamy, L.; Brinkerhoff, R.J.; Gonzalez, A.; Parobek, C.M.; Juliano, J.J.; Andreadis, T.G.; Falco, R.C.; Ziegler, L.B.; Hathaway, N.; et al. Variation in the Microbiota of Ixodes Ticks with Regard to Geography, Species, and Sex. Appl. Environ. Microbiol. 2015, 81, 6200–6209. [Google Scholar] [CrossRef]
- Kolo, A.O.; Raghavan, R. Impact of Endosymbionts on Tick Physiology and Fitness. Parasitology 2023, 150, 859–865. [Google Scholar] [CrossRef]
- Hussain, S.; Perveen, N.; Hussain, A.; Song, B.; Aziz, M.U.; Zeb, J.; Li, J.; George, D.; Cabezas-Cruz, A.; Sparagano, O. The Symbiotic Continuum Within Ticks: Opportunities for Disease Control. Front. Microbiol. 2022, 13, 854803. [Google Scholar] [CrossRef]
- Al-Khafaji, A.M.; Armstrong, S.D.; Varotto Boccazzi, I.; Gaiarsa, S.; Sinha, A.; Li, Z.; Sassera, D.; Carlow, C.K.S.; Epis, S.; Makepeace, B.L. Rickettsia buchneri, Symbiont of the Deer Tick Ixodes scapularis, Can Colonise the Salivary Glands of Its Host. Ticks Tick-Borne Dis. 2020, 11, 101299. [Google Scholar] [CrossRef]
- Hagen, R.; Verhoeve, V.I.; Gillespie, J.J.; Driscoll, T.P. Conjugative Transposons and Their Cargo Genes Vary across Natural Populations of Rickettsia buchneri Infecting the Tick Ixodes scapularis. Genome Biol. Evol. 2018, 10, 3218–3229. [Google Scholar] [CrossRef]
- Kurtti, T.J.; Felsheim, R.F.; Burkhardt, N.Y.; Oliver, J.D.; Heu, C.C.; Munderloh, U.G. Rickettsia buchneri Sp. Nov., a Rickettsial Endosymbiont of the Blacklegged Tick Ixodes scapularis. Int. J. Syst. Evol. Microbiol. 2015, 65, 965–970. [Google Scholar] [CrossRef]
- Simser, J.A.; Palmer, A.T.; Fingerle, V.; Wilske, B.; Kurtti, T.J.; Munderloh, U.G. Rickettsia Monacensis Sp. Nov., a Spotted Fever Group Rickettsia, from Ticks (Ixodes ricinus) Collected in a European City Park. Appl. Environ. Microbiol. 2002, 68, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mendoza, R.; Ángeles-Argáiz, R.; Lozano Aguirre-Beltrán, L.F.; Almaraz-Suárez, J.J.; Hernández-Oaxaca, D.; Ortiz-Lopez, I.; Perez-Moreno, J. Whole-Genome Sequence of Pseudomonas yamanorum OLsAu1 Isolated from the Edible Wild Ectomycorrhizal Mushroom lactarius Sp. Section Deliciosi. Microbiol. Resour. Announc. 2023, 12, e00843-23. [Google Scholar] [CrossRef] [PubMed]
- Arnau, V.G.; Sánchez, L.A.; Delgado, O.D. Pseudomonas yamanorum Sp. Nov., a Psychrotolerant Bacterium Isolated from a Subantarctic Environment. Int. J. Syst. Evol. Microbiol. 2015, 65, 424–431. [Google Scholar] [CrossRef]
- Steiner, F.E.; Pinger, R.R.; Vann, C.N.; Grindle, N.; Civitello, D.; Clay, K.; Fuqua, C. Infection and Co-Infection Rates of Anaplasma phagocytophilum Variants, Babesia Spp., Borrelia burgdorferi, and the Rickettsial Endosymbiont in Ixodes scapularis (Acari: Ixodidae) from Sites in Indiana, Maine, Pennsylvania, and Wisconsin. J. Med. Entomol. 2008, 45, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Fountain-Jones, N.M.; Khoo, B.S.; Rau, A.; Berman, J.D.; Burton, E.N.; Oliver, J.D. Positive Associations Matter: Microbial Relationships Drive Tick Microbiome Composition. Mol. Ecol. 2023, 32, 4078–4092. [Google Scholar] [CrossRef]
- Abraham, N.M.; Liu, L.; Jutras, B.L.; Yadav, A.K.; Narasimhan, S.; Gopalakrishnan, V.; Ansari, J.M.; Jefferson, K.K.; Cava, F.; Jacobs-Wagner, C.; et al. Pathogen-Mediated Manipulation of Arthropod Microbiota to Promote Infection. Proc. Natl. Acad. Sci. USA 2017, 114, E781–E790. [Google Scholar] [CrossRef]
- Mota, T.F.; Fukutani, E.R.; Martins, K.A.; Salgado, V.R.; Andrade, B.B.; Fraga, D.B.M.; Queiroz, A.T.L. Another Tick Bites the Dust: Exploring the Association of Microbial Composition with a Broad Transmission Competence of Tick Vector Species. Microbiol. Spectr. 2023, 11, e0215623. [Google Scholar] [CrossRef]
- Brinkerhoff, R.J.; Clark, C.; Ocasio, K.; Gauthier, D.T.; Hynes, W.L. Factors Affecting the Microbiome of Ixodes scapularis and Amblyomma Americanum. PLoS ONE 2020, 15, e0232398. [Google Scholar] [CrossRef]
- Tveten, A.-K.; Riborg, A.; Vadseth, H.T. DGGE Identification of Microorganisms Associated with Borrelia Burgdorferi Sensu Lato- or Anaplasma phagocytophilum-Infected Ixodes Ricinus Ticks from Northwest Norway. Int. J. Microbiol. 2013, 2013, 805456. [Google Scholar] [CrossRef]
- Portillo, A.; Palomar, A.M.; de Toro, M.; Santibáñez, S.; Santibáñez, P.; Oteo, J.A. Exploring the Bacteriome in Anthropophilic Ticks: To Investigate the Vectors for Diagnosis. PLoS ONE 2019, 14, e0213384. [Google Scholar] [CrossRef]
- Swei, A.; Kwan, J.Y. Tick Microbiome and Pathogen Acquisition Altered by Host Blood Meal. ISME J. 2017, 11, 813–816. [Google Scholar] [CrossRef]
- Dunaj, J.; Drewnowska, J.; Moniuszko-Malinowska, A.; Swiecicka, I.; Pancewicz, S. First Metagenomic Report of Borrelia Americana and Borrelia Carolinensis in Poland–a Preliminary Study. Ann. Agric. Environ. Med. AAEM 2021, 28, 49–55. [Google Scholar] [CrossRef]
- Petersen, A.; Rosenstierne, M.W.; Rasmussen, M.; Fuursted, K.; Nielsen, H.V.; O’Brien Andersen, L.; Bødker, R.; Fomsgaard, A. Field Samplings of Ixodes ricinus Ticks from a Tick-Borne Encephalitis Virus Micro-Focus in Northern Zealand, Denmark. Ticks Tick-Borne Dis. 2019, 10, 1028–1032. [Google Scholar] [CrossRef]
- Kazda, J.; Müller, H.-J.; Stackebrandt, E.; Daffe, M.; Müller, K.; Pitulle, C. Mycobacterium madagascariense Sp. Nov. Int. J. Syst. Evol. Microbiol. 1992, 42, 524–528. [Google Scholar] [CrossRef]
- Uskoković, V.; Wu, V.M. Altering Microbiomes with Hydroxyapatite Nanoparticles: A Metagenomic Analysis. Mater. Basel Switz. 2022, 15, 5824. [Google Scholar] [CrossRef]
- Li, C.; Tang, D.; Wang, Y.; Fan, Q.; Zhang, X.; Cui, X.; Yu, H. Endogenous Bacteria Inhabiting the Ophiocordyceps Highlandensis during Fruiting Body Development. BMC Microbiol. 2021, 21, 178. [Google Scholar] [CrossRef]
- Laudadio, I.; Fulci, V.; Palone, F.; Stronati, L.; Cucchiara, S.; Carissimi, C. Quantitative Assessment of Shotgun Metagenomics and 16S rDNA Amplicon Sequencing in the Study of Human Gut Microbiome. Omics, J. Integr. Biol. 2018, 22, 248–254. [Google Scholar] [CrossRef]
- Chakravorty, S.; Helb, D.; Burday, M.; Connell, N.; Alland, D. A Detailed Analysis of 16S Ribosomal RNA Gene Segments for the Diagnosis of Pathogenic Bacteria. J. Microbiol. Methods 2007, 69, 330–339. [Google Scholar] [CrossRef]
- Sakamoto, J.M.; Silva Diaz, G.E.; Wagner, E.A. Bacterial Communities of Ixodes scapularis from Central Pennsylvania, USA. Insects 2020, 11, 718. [Google Scholar] [CrossRef]
- Uchiyama, T. Tropism and Pathogenicity of Rickettsiae. Front. Microbiol. 2012, 3, 230. [Google Scholar] [CrossRef]
- Yang, D.C.H.; Abeykoon, A.H.; Choi, B.-E.; Ching, W.-M.; Chock, P.B. Outer Membrane Protein OmpB Methylation May Mediate Bacterial Virulence. Trends Biochem. Sci. 2017, 42, 936–945. [Google Scholar] [CrossRef]
- O’Connor, C.; Prusinski, M.A.; Jiang, S.; Russell, A.; White, J.; Falco, R.; Kokas, J.; Vinci, V.; Gall, W.; Tober, K.; et al. A Comparative Spatial and Climate Analysis of Human Granulocytic Anaplasmosis and Human Babesiosis in New York State (2013–2018). J. Med. Entomol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Kogut, S.J.; Thill, C.D.; Prusinski, M.A.; Lee, J.-H.; Backerson, P.B.; Coleman, J.L.; Anand, M.; White, D.J. Babesia Microti, Upstate New York. Emerg. Infect. Dis. 2005, 11, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Ruiz, L.P.; Neupane, S.; Park, Y.; Zurek, L. The bacterial community of the lone star tick (Amblyomma americanum). Parasites Vectors 2021, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, L.; Gonzalez, A.; Van Treuren, W.; Weiss, S.; Parobek, C.M.; Juliano, J.J.; Knight, R.; Roe, R.M.; Apperson, C.S.; Meshnick, S.R. Diversity of rickettsiales in the microbiome of the lone star tick, Amblyomma Americanum. Appl. Environ. Microbiol. 2014, 80, 354–359. [Google Scholar] [CrossRef]


| Microorganism | Identified Protein | Number of Positive Ticks | |
|---|---|---|---|
| Female (n = 22) | Male (n = 14) | ||
| Rickettsia buchneri | Adhesin (A0A8E1C0T9) | 10 | 0 |
| Adhesin A0A8E1C0I9) | 8 | 0 | |
| Outer membrane protein B (A0A8E0WKQ0) | 12 | 0 | |
| Cell surface antigen Sca1 (A0A8E0WLZ4) | 4 | 0 | |
| Porin domain-containing protein (A0A8E0WLM2) | 6 | 0 | |
| Peptidoglycan-associated lipoprotein (A0A8E1C013) | 3 | 0 | |
| Parvulin-like PPIase (A0A8E0WMR4) | 4 | 0 | |
| Spore protein SP21 (A0A8E1BZB2) | 7 | 0 | |
| Hsp20/alpha crystallin family protein (A0A8E0WKN8) | 10 | 0 | |
| Biotin synthase (A0A8E1BZ92) | 2 | 0 | |
| Transposase (A0A8E1BZI6) | 3 | 0 | |
| Putative adhesin (A0A0B7J122) | 5 | 0 | |
| Total number of ticks with R. buchneri proteins | 15 | 0 | |
| Borreliella burgdorferi | Outer surface protein A (P0CL66) | 2 | 0 |
| Flagellar filament 41 kDa core protein (P11089) | 3 | 0 | |
| Integral outer membrane protein P66 (A0A0H3C4D7) | 1 | 0 | |
| Total number of ticks with B. burgdorferi proteins | 3 | 0 | |
| Babesia microti | Uncharacterized protein (A0A1N6LY41) [KLVSDYCSLGR] | 4 | 0 |
| Uncharacterized protein (A0A1N6LY97) [TFGNFSLFEK] | 1 | 0 | |
| Total number of ticks with B. microti proteins | 4 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamoon-Pour, M.; Canessa, E.H.; Macher, J.; Fruitwala, A.; Draper, E.; Policriti, B.; Chin, M.; Nunez, M.; Puccio, P.; Fang, Y.; et al. Genomic and Proteomic Analyses of Bacterial Communities of Ixodes scapularis Ticks from Broome County, New York. Microorganisms 2025, 13, 258. https://doi.org/10.3390/microorganisms13020258
Shamoon-Pour M, Canessa EH, Macher J, Fruitwala A, Draper E, Policriti B, Chin M, Nunez M, Puccio P, Fang Y, et al. Genomic and Proteomic Analyses of Bacterial Communities of Ixodes scapularis Ticks from Broome County, New York. Microorganisms. 2025; 13(2):258. https://doi.org/10.3390/microorganisms13020258
Chicago/Turabian StyleShamoon-Pour, Michel, Emily H. Canessa, John Macher, Amaan Fruitwala, Emma Draper, Benjamin Policriti, Matthew Chin, Matthew Nunez, Paul Puccio, Yuan Fang, and et al. 2025. "Genomic and Proteomic Analyses of Bacterial Communities of Ixodes scapularis Ticks from Broome County, New York" Microorganisms 13, no. 2: 258. https://doi.org/10.3390/microorganisms13020258
APA StyleShamoon-Pour, M., Canessa, E. H., Macher, J., Fruitwala, A., Draper, E., Policriti, B., Chin, M., Nunez, M., Puccio, P., Fang, Y., Wang, X.-R., & Hathout, Y. (2025). Genomic and Proteomic Analyses of Bacterial Communities of Ixodes scapularis Ticks from Broome County, New York. Microorganisms, 13(2), 258. https://doi.org/10.3390/microorganisms13020258






