Investigation of Virulence-Related Markers in Atypical Strains of Toxoplasma gondii from Brazil
Abstract
1. Introduction
2. Materials and Methods
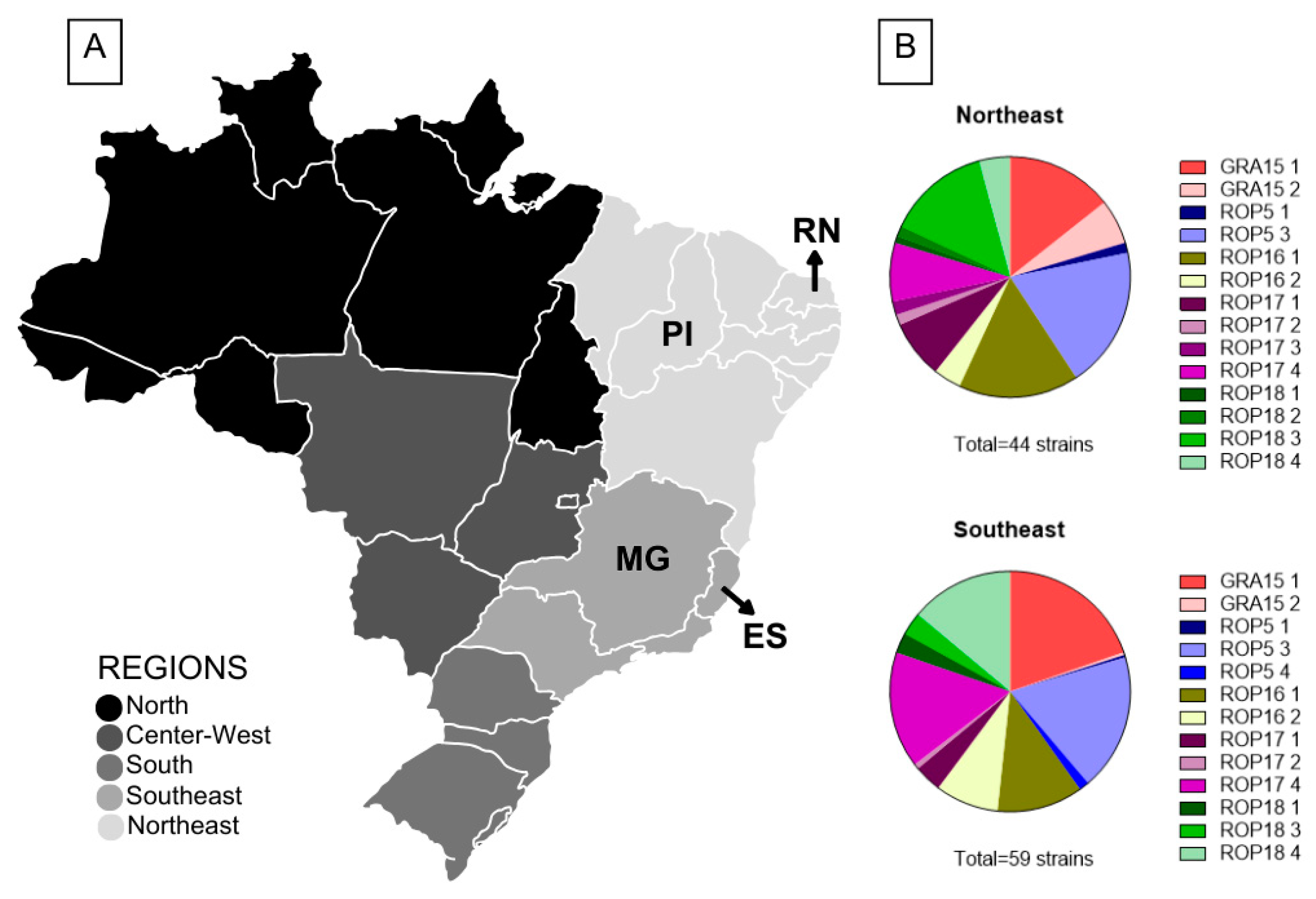
2.1. Toxoplasma gondii Strains
2.2. PCR-RFLP Genotyping of T. gondii
2.3. Parasite Virulence Determination
2.4. Statistical Analyses
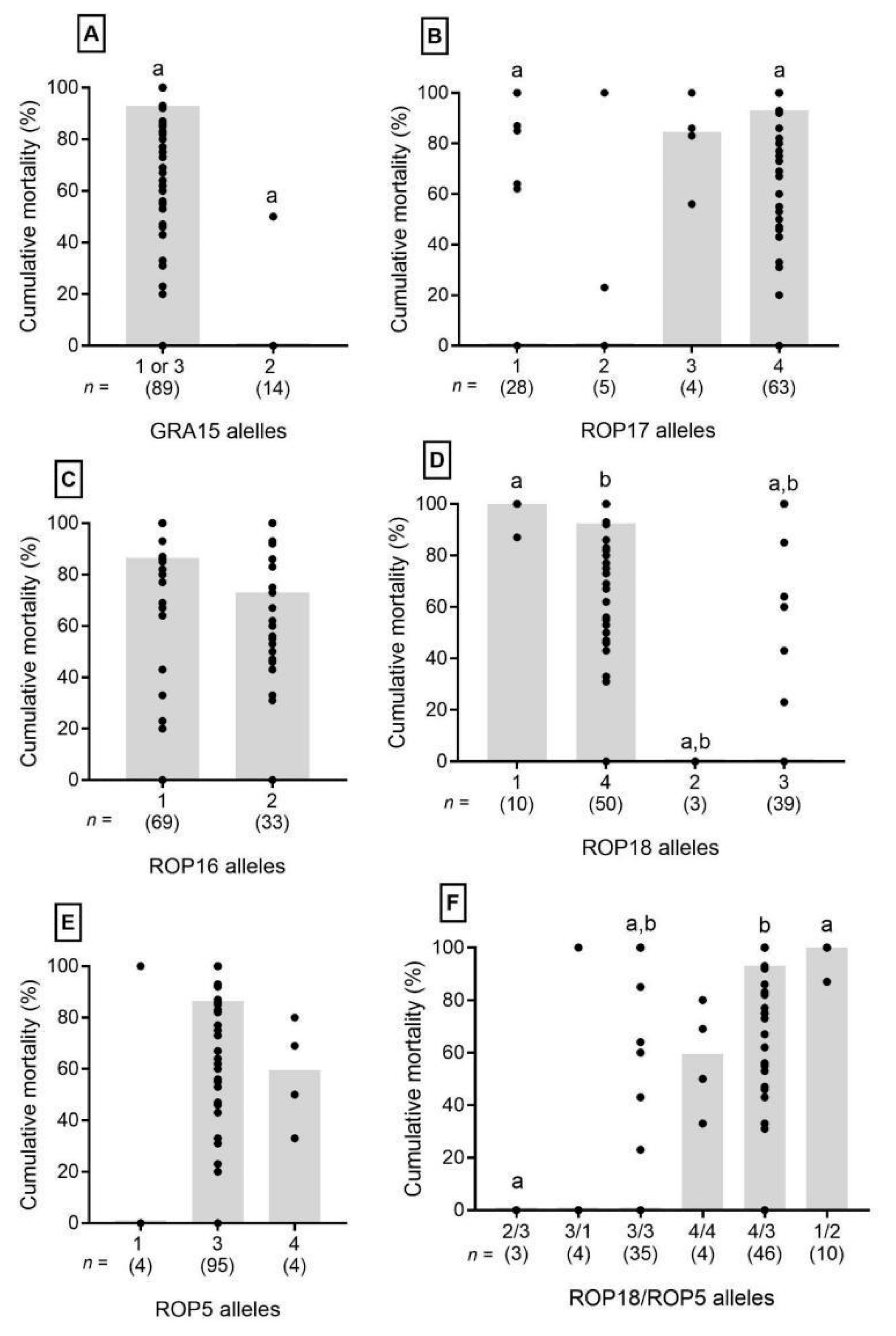
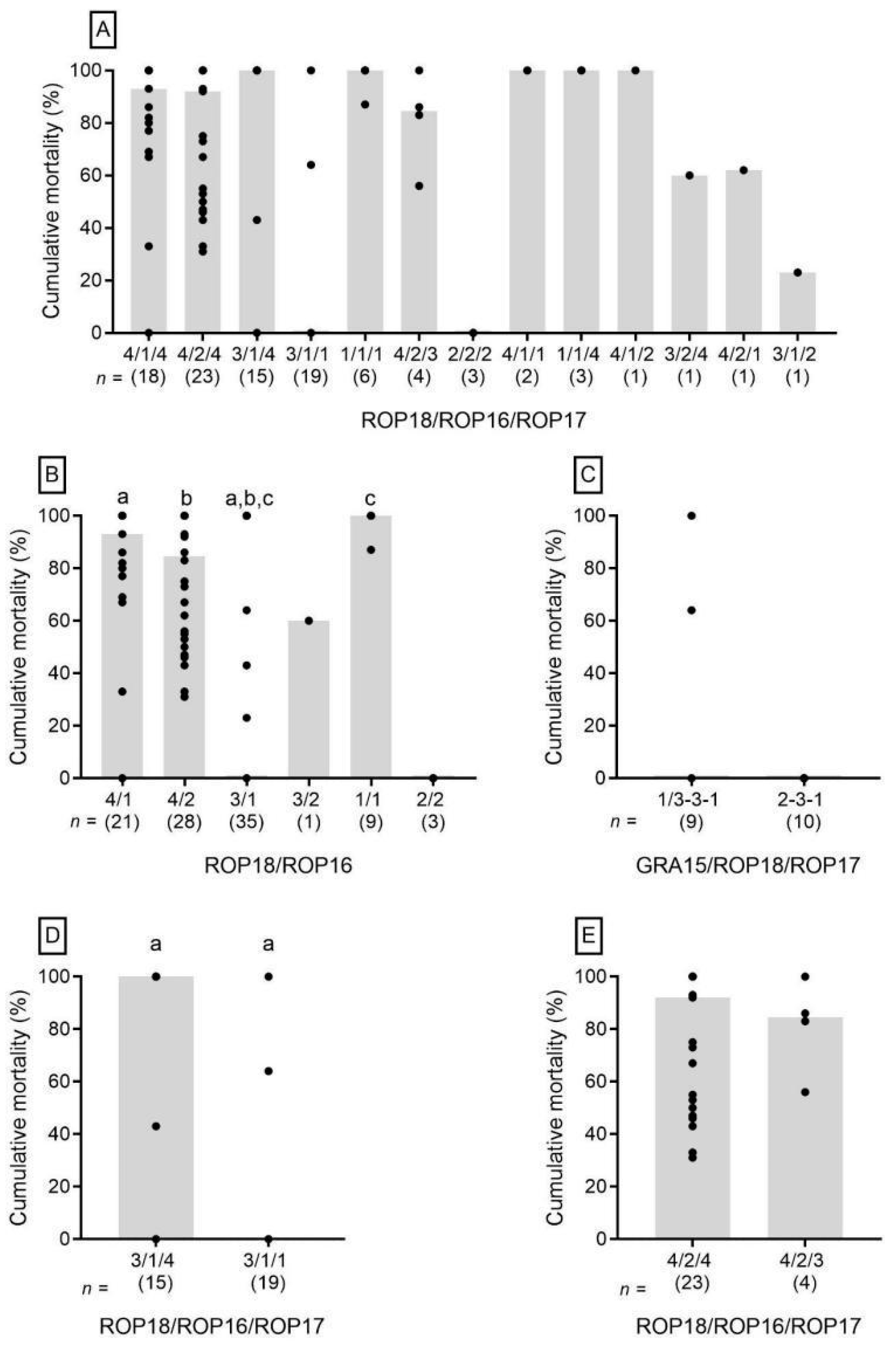
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flegr, J. How and Why Toxoplasma Makes Us Crazy. Trends Parasitol. 2013, 29, 156–163. [Google Scholar] [CrossRef]
- Deganich, M.; Boudreaux, C.; Benmerzouga, I. Toxoplasmosis Infection during Pregnancy. Trop. Med. Infect. Dis. 2022, 8, 3. [Google Scholar] [CrossRef]
- Layton, J.; Theiopoulou, D.-C.; Rutenberg, D.; Elshereye, A.; Zhang, Y.; Sinnott, J.; Kim, K.; Montoya, J.G.; Contopoulos-Ioannidis, D.G. Clinical Spectrum, Radiological Findings, and Outcomes of Severe Toxoplasmosis in Immunocompetent Hosts: A Systematic Review. Pathogens 2023, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Pardini, L.; Bernstein, M.; Carral, L.A.; Kaufer, F.J.; Dellarupe, A.; Gos, M.L.; Campero, L.M.; Moré, G.; Messina, M.T.; Schneider, M.V.; et al. Congenital Human Toxoplasmosis Caused by Non-Clonal Toxoplasma gondii Genotypes in Argentina. Parasitol. Int. 2019, 68, 48–52. [Google Scholar] [CrossRef]
- Shwab, E.K.; Jiang, T.; Pena, H.F.J.; Gennari, S.M.; Dubey, J.P.; Su, C. The ROP18 and ROP5 Gene Allele Types Are Highly Predictive of Virulence in Mice across Globally Distributed Strains of Toxoplasma gondii. Int. J. Parasitol. 2016, 46, 141–146. [Google Scholar] [CrossRef]
- Rêgo, W.M.F.; Costa, J.G.L.; Baraviera, R.C.A.; Pinto, L.V.; Bessa, G.L.; Lopes, R.E.N.; Vitor, R.W.A. Association of ROP18 and ROP5 Was Efficient as a Marker of Virulence in Atypical Isolates of Toxoplasma gondii Obtained from Pigs and Goats in Piauí, Brazil. Vet. Parasitol. 2017, 247, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Saraf, P.; Shwab, E.K.; Dubey, J.P.; Su, C. On the Determination of Toxoplasma gondii Virulence in Mice. Exp. Parasitol. 2017, 174, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Van Why, K.; Verma, S.K.; Choudhary, S.; Kwok, O.C.H.; Khan, A.; Behinke, M.S.; Sibley, L.D.; Ferreira, L.R.; Oliveira, S.; et al. Genotyping Toxoplasma gondii from Wildlife in Pennsylvania and Identification of Natural Recombinants Virulent to Mice. Vet. Parasitol. 2014, 200, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.C.A.V.; Andrade, G.M.; Costa, J.G.L.; Pinheiro, B.V.; Vasconcelos-Santos, D.V.; Ferreira, A.M.; Su, C.; Januário, J.N.; Vitor, R.W.A. Genetic Characterization of Toxoplasma gondii Revealed Highly Diverse Genotypes for Isolates from Newborns with Congenital Toxoplasmosis in Southeastern Brazil. J. Clin. Microbiol. 2013, 51, 901–907. [Google Scholar] [CrossRef]
- Brandão, G.P.; Ferreira, A.M.; Melo, M.N.; Vitor, R.W.A. Characterization of Toxoplasma gondii from Domestic Animals from Minas Gerais, Brazil. Parasite 2006, 13, 143–149. [Google Scholar] [CrossRef][Green Version]
- Ferreira, A.d.M.; Vitor, R.W.A.; Gazzinelli, R.T.; Melo, M.N. Genetic Analysis of Natural Recombinant Brazilian Toxoplasma gondii Strains by Multilocus PCR-RFLP. Infect. Genet. Evol. 2006, 6, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Clementino Andrade, M.M.; Pinheiro, B.V.; Cunha, M.M.; Carneiro, A.C.a.V.; Andrade Neto, V.F.; Vitor, R.W.A. New Gentotypes of Toxoplasma gondii Obtained from Farm Animals in Northeast Brazil. Res. Vet. Sci. 2013, 94, 587–589. [Google Scholar] [CrossRef]
- Ferreira, T.C.R.; Buery, J.C.; Moreira, N.I.B.; Santos, C.B.; Costa, J.G.L.; Pinto, L.V.; Baraviera, R.C.d.A.; Vitor, R.W.A.; Fux, B. Toxoplasma gondii: Isolation, Biological and Molecular Characterisation of Samples from Free-Range Gallus gallus Domesticus from Countryside Southeast Brazil. Rev. Bras. Parasitol. Vet. 2018, 27, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, W.M.F.; Costa, J.G.L.; Baraviera, R.C.A.; Pinto, L.V.; Bessa, G.L.; Lopes, R.E.N.; Silveira, J.a.G.; Vitor, R.W.A. Genetic Diversity of Toxoplasma gondii Isolates Obtained from Free-Living Wild Birds Rescued in Southeastern Brazil. Int. J. Parasitol. Parasites Wildl. 2018, 7, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.V.; Noviello, M.d.L.M.; Cunha, M.M.; Tavares, A.T.; Carneiro, A.C.A.V.; Arantes, R.M.E.; Vitor, R.W.A. Pathological Changes in Acute Experimental Toxoplasmosis with Toxoplasma gondii Strains Obtained from Human Cases of Congenital Disease. Exp. Parasitol. 2015, 156, 87–94. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, C.; Xu, T.; Liu, F.; Pappoe, F.; Luo, Q.; Xu, Y.; Lu, F.; Shen, J. Genotyping of Polymorphic Effectors of Toxoplasma gondii Isolates from China. Parasit. Vectors 2017, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.D.C.; Camejo, A.; Melo, M.B.; Cordeiro, C.; Julien, L.; Grotenbreg, G.M.; Frickel, E.-M.; Ploegh, H.L.; Young, L.; Saeij, J.P.J. Toxoplasma gondii Superinfection and Virulence during Secondary Infection Correlate with the Exact ROP5/ROP18 Allelic Combination. mBio 2015, 6, e02280. [Google Scholar] [CrossRef]
- Kim, E.W.; Nadipuram, S.M.; Tetlow, A.L.; Barshop, W.D.; Liu, P.T.; Wohlschlegel, J.A.; Bradley, P.J. The Rhoptry Pseudokinase ROP54 Modulates Toxoplasma gondii Virulence and Host GBP2 Loading. mSphere 2016, 1, e00045-16. [Google Scholar] [CrossRef]
- Saeij, J.P.J.; Boyle, J.P.; Coller, S.; Taylor, S.; Sibley, L.D.; Brooke-Powell, E.T.; Ajioka, J.W.; Boothroyd, J.C. Polymorphic Secreted Kinases Are Key Virulence Factors in Toxoplasmosis. Science 2006, 314, 1780–1783. [Google Scholar] [CrossRef]
- de Melo, R.P.B.; Wanderley, F.S.; Porto, W.J.N.; Pedrosa, C.d.M.; Hamilton, C.M.; de Oliveira, M.H.G.S.; Ribeiro-Andrade, M.; Rêgo, R.C.d.S.; Katzer, F.; Mota, R.A. Description of an Atypical Toxoplasma gondii Isolate from a Case of Congenital Toxoplasmosis in Northeastern Brazil. Parasitol. Res. 2020, 119, 2727–2731. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.; Rudzinski, M.; Schneider, V.; Messina, M.; Gos, M.L.; Helman, E.; Dellarupe, A.; Unzaga, J.M.; Venturini, M.C.; Moré, G.; et al. Genetic Characterization of Toxoplasma gondii from Human and Chicken Isolates from Argentina. Parasitol. Res. 2024, 123, 129. [Google Scholar] [CrossRef]
- Calero-Bernal, R.; Fernández-Escobar, M.; Katzer, F.; Su, C.; Ortega-Mora, L.M. Unifying Virulence Evaluation in Toxoplasma gondii: A Timely Task. Front. Cell. Infect. Microbiol. 2022, 12, 868727. [Google Scholar] [CrossRef]
- Etheridge, R.D.; Alaganan, A.; Tang, K.; Lou, H.J.; Turk, B.E.; Sibley, L.D. The Toxoplasma Pseudokinase ROP5 Forms Complexes with ROP18 and ROP17 Kinases That Synergize to Control Acute Virulence in Mice. Cell Host Microbe 2014, 15, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.M.; Black, L.; Oliveira, S.; Burrells, A.; Bartley, P.M.; Melo, R.P.B.; Chianini, F.; Palarea-Albaladejo, J.; Innes, E.A.; Kelly, P.J.; et al. Comparative Virulence of Caribbean, Brazilian and European Isolates of Toxoplasma gondii. Parasit. Vectors 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.; Pardini, L.; Bello Pede Castro, B.; Unzaga, J.M.; Venturini, M.C.; Moré, G. ROP18 and ROP5 Alleles Combinations Are Related with Virulence of T. gondii Isolates from Argentina. Parasitol. Int. 2021, 83, 102328. [Google Scholar] [CrossRef]
- Rosowski, E.E.; Lu, D.; Julien, L.; Rodda, L.; Gaiser, R.A.; Jensen, K.D.C.; Saeij, J.P.J. Strain-Specific Activation of the NF-kappaB Pathway by GRA15, a Novel Toxoplasma gondii Dense Granule Protein. J. Exp. Med. 2011, 208, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Niedelman, W.; Gold, D.A.; Rosowski, E.E.; Sprokholt, J.K.; Lim, D.; Farid Arenas, A.; Melo, M.B.; Spooner, E.; Yaffe, M.B.; Saeij, J.P.J. The Rhoptry Proteins ROP18 and ROP5 Mediate Toxoplasma gondii Evasion of the Murine, but Not the Human, Interferon-Gamma Response. PLoS Pathog. 2012, 8, e1002784. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Freeman, K.; Lago, E.G.; Bahia-Oliveira, L.M.G.; Tan, H.K.; Wallon, M.; Buffolano, W.; Stanford, M.R.; Petersen, E. European Multicentre Study on Congenital Toxoplasmosis (EMSCOT) Ocular Sequelae of Congenital Toxoplasmosis in Brazil Compared with Europe. PLoS Negl. Trop. Dis. 2008, 2, e277. [Google Scholar] [CrossRef]
- Khan, A.; Jordan, C.; Muccioli, C.; Vallochi, A.L.; Rizzo, L.V.; Belfort, R.; Vitor, R.W.A.; Silveira, C.; Sibley, L.D. Genetic Divergence of Toxoplasma gondii Strains Associated with Ocular Toxoplasmosis, Brazil. Emerg. Infect. Dis. 2006, 12, 942–949. [Google Scholar] [CrossRef] [PubMed]
- de Lima Bessa, G.; de Almeida Vitor, R.W.; Dos Santos Martins-Duarte, E. Toxoplasma gondii in South America: A Differentiated Pattern of Spread, Population Structure and Clinical Manifestations. Parasitol. Res. 2021, 120, 3065–3076. [Google Scholar] [CrossRef]
- Switaj, K.; Master, A.; Borkowski, P.K.; Skrzypczak, M.; Wojciechowicz, J.; Zaborowski, P. Association of Ocular Toxoplasmosis with Type I Toxoplasma gondii Strains: Direct Genotyping from Peripheral Blood Samples. J. Clin. Microbiol. 2006, 44, 4262–4264. [Google Scholar] [CrossRef]
- Vallochi, A.L.; Muccioli, C.; Martins, M.C.; Silveira, C.; Belfort, R.; Rizzo, L.V. The Genotype of Toxoplasma gondii Strains Causing Ocular Toxoplasmosis in Humans in Brazil. Am. J. Ophthalmol. 2005, 139, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Hernández-de-Los-Ríos, A.; Murillo-Leon, M.; Mantilla-Muriel, L.E.; Arenas, A.F.; Vargas-Montes, M.; Cardona, N.; de-la-Torre, A.; Sepúlveda-Arias, J.C.; Gómez-Marín, J.E. Influence of Two Major Toxoplasma gondii Virulence Factors (ROP16 and ROP18) on the Immune Response of Peripheral Blood Mononuclear Cells to Human Toxoplasmosis Infection. Front. Cell. Infect. Microbiol. 2019, 9, 413. [Google Scholar] [CrossRef]
- Torres-Morales, E.; Taborda, L.; Cardona, N.; De-la-Torre, A.; Sepulveda-Arias, J.C.; Patarroyo, M.A.; Gomez-Marin, J.E. Th1 and Th2 Immune Response to P30 and ROP18 Peptides in Human Toxoplasmosis. Med. Microbiol. Immunol. 2014, 203, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, V.; de-la-Torre, A.; Gómez-Marín, J.E. Characterization of ROP18 Alleles in Human Toxoplasmosis. Parasitol. Int. 2014, 63, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Rico-Torres, C.P.; Valenzuela-Moreno, L.F.; Luna-Pastén, H.; Cedillo-Peláez, C.; Correa, D.; Morales-Salinas, E.; Martínez-Maya, J.J.; Alves, B.F.; Pena, H.F.J.; Caballero-Ortega, H. Genotyping of Toxoplasma gondii Isolates from México Reveals Non-Archetypal and Potentially Virulent Strains for Mice. Infect. Genet. Evol. 2023, 113, 105473. [Google Scholar] [CrossRef] [PubMed]
| Lethal Strains | Non-Lethal Strains | |||
|---|---|---|---|---|
| GRA15 | n (f) | n (f) | Total | p value * |
| 1 or 3 | 75 (98.7%) | 14 (51.9%) | 89 (86.4%) | <0.001 |
| 2 | 1 (1.3%) | 13 (48.1%) | 14 (13.6%) | |
| Total | 76 | 27 | 103 | |
| ROP5 | ||||
| 1 | 1 (1.3%) | 3 (11.1%) | 4 (3.9%) | 0.04 |
| 3 | 71 (93.4%) | 24 (88.9%) | 95 (92.2%) | |
| 4 | 4 (5.3%) | 0 (0.0%) | 4 (3.9%) | |
| Total | 76 | 27 | 103 | |
| ROP16 | ||||
| 1 | 46 (60.5%) | 23 (88.5%) | 69 (67.6%) | 0.007 |
| 2 | 30 (39.5%) | 3 (11.5%) | 33 (32.4%) | |
| Total | 76 | 26 | 102 a | |
| ROP17 | ||||
| 1 | 11 (15.1%) | 17 (63.0%) | 28 (28.0%) | <0.001 |
| 2 | 2 (2.7%) | 3 (11.1%) | 5 (5.0%) | |
| 3 | 4 (5.5%) | 0 (0.0%) | 4 (4.0%) | |
| 4 | 56 (76.7%) | 7 (25.9%) | 63 (63.0%) | |
| Total | 73 | 27 | 100 b | |
| ROP18 | ||||
| 1 | 10 (13.3%) | 0 (0.0%) | 10 (9.8%) | <0.001 |
| 2 | 0 (0.0%) | 3 (11.1%) | 3 (2.9%) | |
| 3 | 17 (22.7%) | 22 (81.5%) | 39 (38.2%) | |
| 4 | 48 (64.0%) | 2 (7.4%) | 50 (49.0%) | |
| Total | 75 | 27 | 102 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.G.L.; Martins-Duarte, É.S.; Pinto, L.V.; Baraviera, R.A.d.C.; do Rego, W.M.F.; Vitor, R.W.d.A. Investigation of Virulence-Related Markers in Atypical Strains of Toxoplasma gondii from Brazil. Microorganisms 2025, 13, 301. https://doi.org/10.3390/microorganisms13020301
Costa JGL, Martins-Duarte ÉS, Pinto LV, Baraviera RAdC, do Rego WMF, Vitor RWdA. Investigation of Virulence-Related Markers in Atypical Strains of Toxoplasma gondii from Brazil. Microorganisms. 2025; 13(2):301. https://doi.org/10.3390/microorganisms13020301
Chicago/Turabian StyleCosta, Júlia Gatti Ladeia, Érica Santos Martins-Duarte, Lorena Velozo Pinto, Ramon Araujo de Castro Baraviera, Wagner Martins Fontes do Rego, and Ricardo Wagner de Almeida Vitor. 2025. "Investigation of Virulence-Related Markers in Atypical Strains of Toxoplasma gondii from Brazil" Microorganisms 13, no. 2: 301. https://doi.org/10.3390/microorganisms13020301
APA StyleCosta, J. G. L., Martins-Duarte, É. S., Pinto, L. V., Baraviera, R. A. d. C., do Rego, W. M. F., & Vitor, R. W. d. A. (2025). Investigation of Virulence-Related Markers in Atypical Strains of Toxoplasma gondii from Brazil. Microorganisms, 13(2), 301. https://doi.org/10.3390/microorganisms13020301





