Viral Infection and Dissemination Through the Lymphatic System
Abstract
:1. Introduction
2. Anatomy of the Lymphatic System
2.1. Lymph Circulation Through Lymphatic Vessels
2.2. Lymph Nodes
3. Lymphatic Viral Dissemination
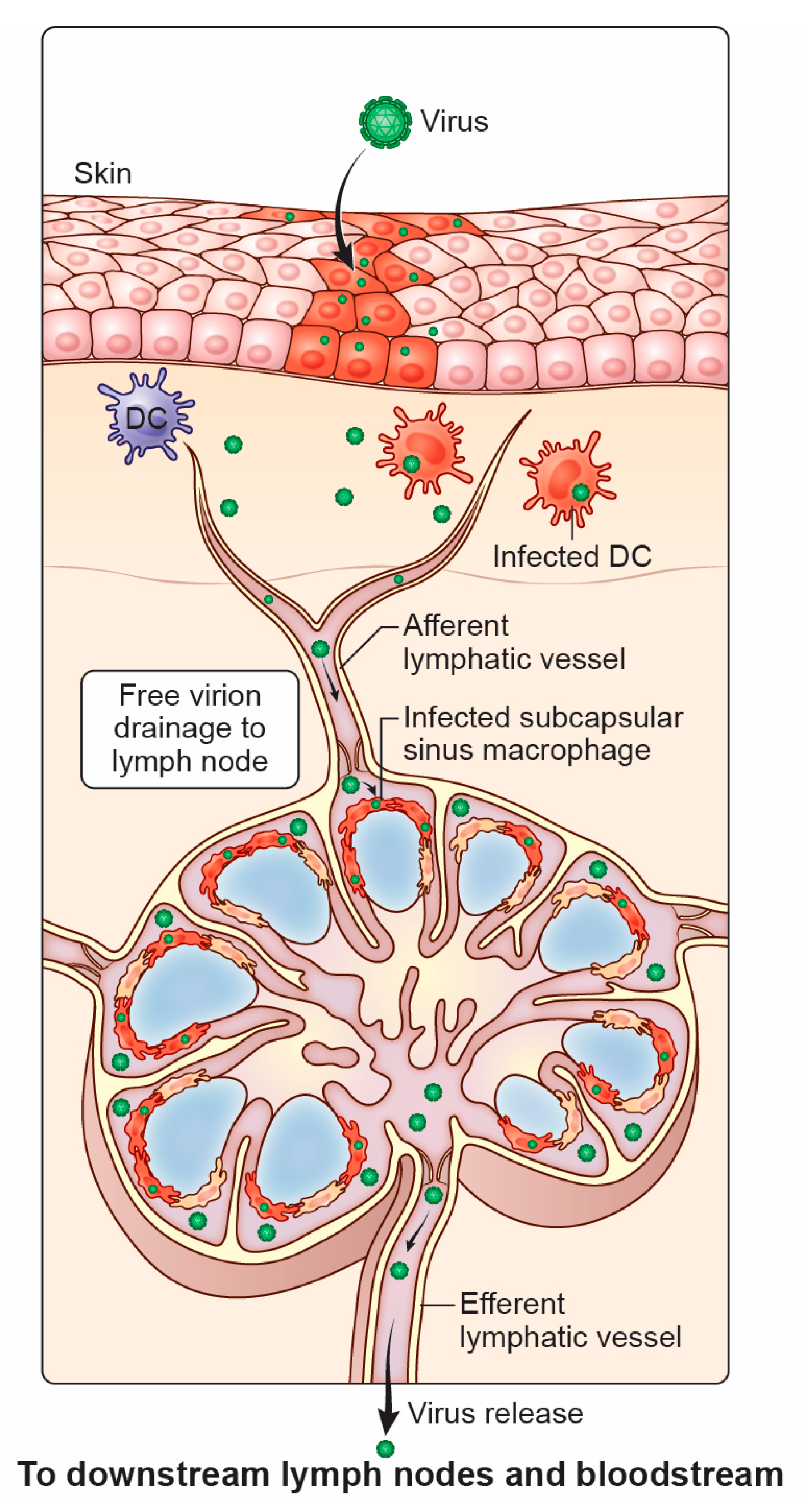
3.1. Viral Movement to the LN After Initial Entry
3.2. Lymphatic Contribution to Viral Spread
4. Lymph Node Protection
4.1. Activation of LN B Cells by SSMs
4.2. T Cell-Mediated Protection Against LN Viral Infection
4.3. Cytokine Production in Response to LN Infection
4.4. Cooperation Between Recruited Innate Immune Cells
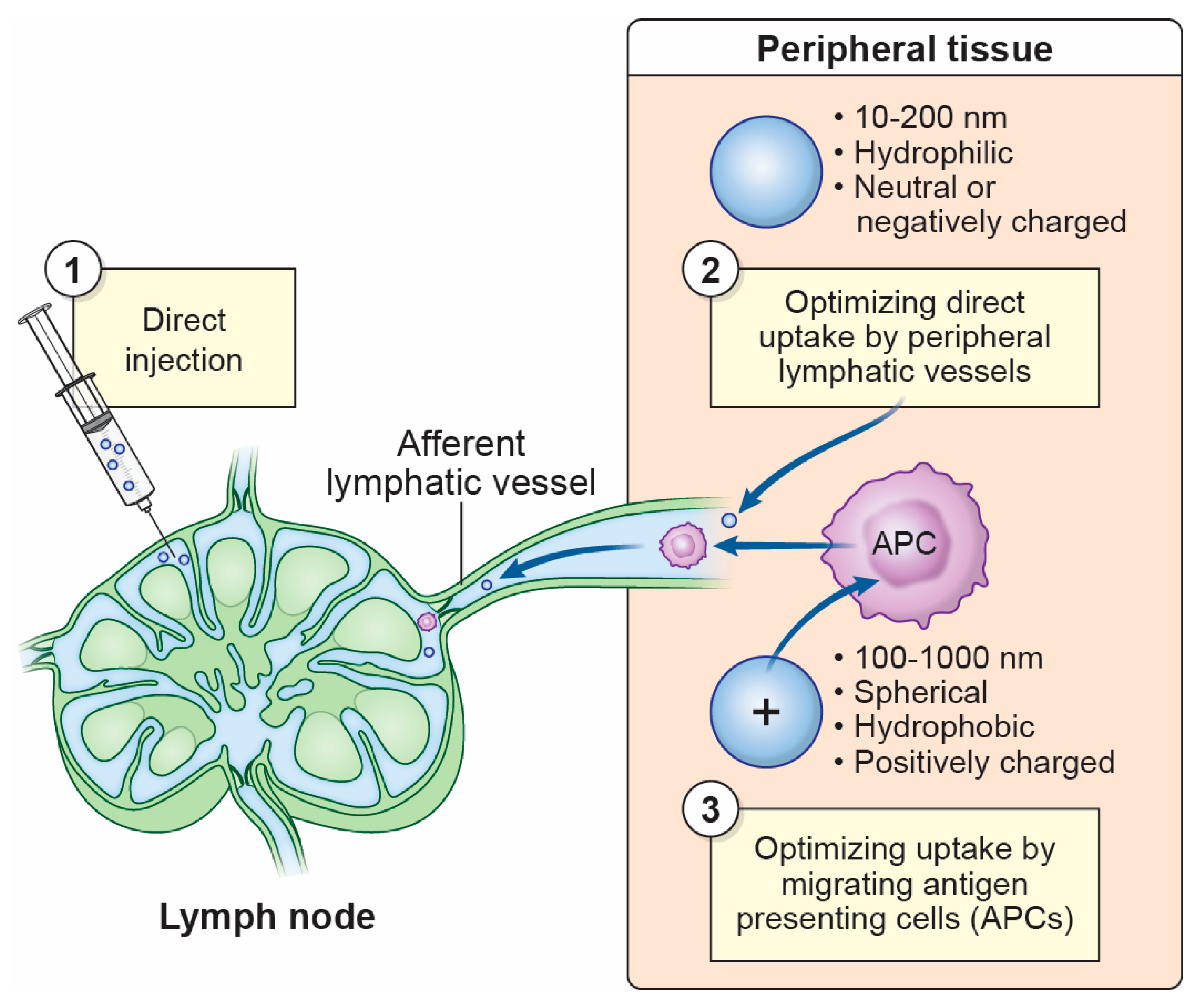
4.5. Harnessing Protection in the LN Through Vaccination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ab | Antibody |
| Ag | Antigen |
| APC | Antigen presenting cell |
| AdV | Adenovirus |
| BEC | Blood endothelial cell |
| CHIKV | Chikungunya virus |
| CLL | Clodronate liposome |
| CNS | Central nervous system |
| DC | Dendritic cell |
| ECTV | Ectromelia virus |
| FDC | Follicular dendritic cell |
| FRC | Fibroblast reticular cell |
| GC | Germinal center |
| HCMV | Human cytomegalovirus |
| HEV | High endothelial venule |
| HIV | Human immunodeficiency virus |
| IFN-g | Interferon gamma |
| IFN-I | Type I interferon |
| IFNAR | Interferon-a/b receptor |
| LCMV | Lymphocytic choriomeningitis virus |
| LEC | Lymphatic endothelial cell |
| LN | Lymph node |
| MCMV | Murine cytomegalovirus |
| mDC | Migratory dendritic cell |
| MS | Medullary sinus |
| MSM | Medullary sinus macrophage |
| MuHV-4 | Murid herpesvirus-4 |
| MVA | Modified vaccinia Ankara virus |
| NK cell | Natural killer cell |
| pDC | Plasmacytoid dendritic cell |
| PEG | Polyethylene glycol |
| PLN | Popliteal lymph node |
| PLVAP | Plasmalemma vesicle associated protein 1 |
| S1P | Spingosine-1-phosphate |
| SARS-CoV2 | Severe acute respiratory syndrome coronavirus 2 |
| SCS | Subcapsular sinus |
| Si-OH | Sianol |
| SPION | Superparamagnetic iron oxide nanoparticle |
| SSM | SCS macrophage |
| VACV | Vaccinia virus |
| VSV | Vesicular stomatitis virus |
| WNV | West Nile virus |
| ZIKV | Zika virus |
References
- Sainte-Marie, G. The lymph node revisited: Development, morphology, functioning, and role in triggering primary immune responses. Anat. Rec. 2010, 293, 320–337. [Google Scholar] [CrossRef]
- Grant, S.M.; Lou, M.; Yao, L.; Germain, R.N.; Radtke, A.J. The lymph node at a glance-how spatial organization optimizes the immune response. J. Cell Sci. 2020, 133, jcs241828. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. Blood, lymphoid tissues and haemopoiesis. In Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Standring, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–84. [Google Scholar]
- Davis, M.J.; Zawieja, S.D.; King, P.D. Transport and Immune Functions of the Lymphatic System. Annu. Rev. Physiol. 2024, 87, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J.; Ivanov, S.; Zinselmeyer, B.H.; Scallan, J.P. The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol. 2017, 35, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Scallan, J.; Huxley, V.H.; Korthius, R.J. The Lymphatic Vasculature. In Capillary Fluid Exchange: Regulation, Functions and Pathology; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Baish, J.W.; Padera, T.P.; Munn, L.L. The effects of gravity and compression on interstitial fluid transport in the lower limb. Sci. Rep. 2022, 12, 4890. [Google Scholar] [CrossRef]
- von der Weid, P.Y.; Zawieja, D.C. Lymphatic smooth muscle: The motor unit of lymph drainage. Int. J. Biochem. Cell Biol. 2004, 36, 1147–1153. [Google Scholar] [CrossRef]
- Guyton, A.C.; Coleman, T.G. Regulation on interstitial fluid volume and pressure. Ann. N. Y Acad. Sci. 1968, 150, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Clough, G.; Smaje, L.H. Simultaneous measurement of pressure in the interstitium and the terminal lymphatics of the cat mesentery. J. Physiol. 1978, 283, 457–468. [Google Scholar] [CrossRef]
- Zweifach, B.W.; Prather, J.W. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am. J. Physiol. 1975, 228, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Moriondo, A.; Mukenge, S.; Negrini, D. Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H263–H269. [Google Scholar] [CrossRef] [PubMed]
- Jamalian, S.; Jafarnejad, M.; Zawieja, S.D.; Bertram, C.D.; Gashev, A.A.; Zawieja, D.C.; Davis, M.J.; Moore, J.E., Jr. Demonstration and Analysis of the Suction Effect for Pumping Lymph from Tissue Beds at Subatmospheric Pressure. Sci. Rep. 2017, 7, 12080. [Google Scholar] [CrossRef] [PubMed]
- Horsnell, H.L.; Tetley, R.J.; De Belly, H.; Makris, S.; Millward, L.J.; Benjamin, A.C.; Heeringa, L.A.; de Winde, C.M.; Paluch, E.K.; Mao, Y.; et al. Lymph node homeostasis and adaptation to immune challenge resolved by fibroblast network mechanics. Nat. Immunol. 2022, 23, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Assen, F.P.; Abe, J.; Hons, M.; Hauschild, R.; Shamipour, S.; Kaufmann, W.A.; Costanzo, T.; Krens, G.; Brown, M.; Ludewig, B.; et al. Multitier mechanics control stromal adaptations in the swelling lymph node. Nat. Immunol. 2022, 23, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Franzeck, U.K.; Herrig, I.; Costanzo, U.; Wen, S.; Schiesser, M.; Hoffmann, U.; Bollinger, A. Flow velocity of single lymphatic capillaries in human skin. Am. J. Physiol. 1996, 270, H358–H363. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.U.; Hunziker, T.; Braathen, L.R. Isolation of human skin-derived lymph: Flow and output of cells following sodium lauryl sulphate-induced contact dermatitis. Arch. Dermatol. Res. 1992, 284, 123–126. [Google Scholar] [CrossRef]
- Drake, R.; Giesler, M.; Laine, G.; Gabel, J.; Hansen, T. Effect of outflow pressure on lung lymph flow in unanesthetized sheep. J Appl. Physiol. 1985, 58, 70–76. [Google Scholar] [CrossRef]
- Swartz, M.A.; Hubbell, J.A.; Reddy, S.T. Lymphatic drainage function and its immunological implications: From dendritic cell homing to vaccine design. Semin. Immunol. 2008, 20, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.G.; Pankova, V.; Krasny, L.; Singh, T.; Makris, S.; White, I.J.; Benjamin, A.C.; Dertschnig, S.; Horsnell, H.L.; Kriston-Vizi, J.; et al. Fibroblastic Reticular Cells Control Conduit Matrix Deposition during Lymph Node Expansion. Cell Rep. 2019, 29, 2810–2822.e5. [Google Scholar] [CrossRef]
- Thierry, G.R.; Kuka, M.; De Giovanni, M.; Mondor, I.; Brouilly, N.; Iannacone, M.; Bajenoff, M. The conduit system exports locally secreted IgM from lymph nodes. J. Exp. Med. 2018, 215, 2972–2983. [Google Scholar] [CrossRef]
- Fenner, F.; Bachmann, P.; Gibbs, E.; Murphy, F.; Studdert, M.; White, D. Pathogenesis: Infection and the Spread of Viruses in the Bodya. Vet. Virol. 2014, 133–152. [Google Scholar] [CrossRef]
- Aguilar, C.C.; Kalia, A.; Brisse, M.E.; Dowd, K.A.; Wise-Dent, O.; Burgomaster, K.E.; Droppo, J.; Pierson, T.C.; Hickman, H.D. Subcapsular sinus macrophages maximize germinal center development in non-draining lymph nodes during blood-borne viral infection. Sci. Immunol. 2024, 9, eadi4926. [Google Scholar] [CrossRef] [PubMed]
- Clement, C.C.; Wang, W.; Dzieciatkowska, M.; Cortese, M.; Hansen, K.C.; Becerra, A.; Thangaswamy, S.; Nizamutdinova, I.; Moon, J.Y.; Stern, L.J.; et al. Quantitative Profiling of the Lymph Node Clearance Capacity. Sci. Rep. 2018, 8, 11253. [Google Scholar] [CrossRef]
- Reynoso, G.V.; Gordon, D.N.; Kalia, A.; Aguilar, C.C.; Malo, C.S.; Aleshnick, M.; Dowd, K.A.; Cherry, C.R.; Shannon, J.P.; Vrba, S.M.; et al. Zika virus spreads through infection of lymph node-resident macrophages. Cell Rep. 2023, 42, 112126. [Google Scholar] [CrossRef]
- Harrell, M.I.; Iritani, B.M.; Ruddell, A. Lymph node mapping in the mouse. J. Immunol. Methods 2008, 332, 170–174. [Google Scholar] [CrossRef]
- Shao, L.; Takeda, K.; Kato, S.; Mori, S.; Kodama, T. Communication between lymphatic and venous systems in mice. J. Immunol. Methods 2015, 424, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ballou, B.; Ernst, L.A.; Andreko, S.; Harper, T.; Fitzpatrick, J.A.; Waggoner, A.S.; Bruchez, M.P. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjug. Chem. 2007, 18, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Hama, Y.; Koyama, Y.; Barrett, T.; Regino, C.A.; Urano, Y.; Choyke, P.L. Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett. 2007, 7, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Walling, M.A.; Novak, J.A.; Shepard, J.R.E. Quantum dots for live cell and in vivo imaging. Int. J. Mol. Sci. 2009, 10, 441–491. [Google Scholar] [CrossRef] [PubMed]
- Lohrberg, M.; Wilting, J. The lymphatic vascular system of the mouse head. Cell Tissue Res. 2016, 366, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Luo, J.; Hoeher, L.; Al-Maskari, R.; Horvath, I.; Chen, Y.; Kofler, F.; Piraud, M.; Paetzold, J.C.; Modamio, J.; et al. Whole-body cellular mapping in mouse using standard IgG antibodies. Nat. Biotechnol. 2024, 42, 617–627. [Google Scholar] [CrossRef]
- Yamaji, Y.; Akita, S.; Akita, H.; Miura, N.; Gomi, M.; Manabe, I.; Kubota, Y.; Mitsukawa, N. Development of a mouse model for the visual and quantitative assessment of lymphatic trafficking and function by in vivo imaging. Sci. Rep. 2018, 8, 5921. [Google Scholar] [CrossRef]
- Jacob, L.; de Brito Neto, J.; Lenck, S.; Corcy, C.; Benbelkacem, F.; Geraldo, L.H.; Xu, Y.; Thomas, J.M.; El Kamouh, M.R.; Spajer, M.; et al. Conserved meningeal lymphatic drainage circuits in mice and humans. J. Exp. Med. 2022, 219, e20220035. [Google Scholar] [CrossRef]
- Cupedo, T.; Coles, M.; Veiga-Fernandes, H. Development and Structure of Lymph Nodes in Humans and Mice. In Developmental Biology of Peripheral Lymphoid Organs; Balogh, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 59–74. [Google Scholar]
- Singhal, D.; Borner, K.; Chaikof, E.L.; Detmar, M.; Hollmen, M.; Iliff, J.J.; Itkin, M.; Makinen, T.; Oliver, G.; Padera, T.P.; et al. Mapping the lymphatic system across body scales and expertise domains: A report from the 2021 National Heart, Lung, and Blood Institute workshop at the Boston Lymphatic Symposium. Front. Physiol. 2023, 14, 1099403. [Google Scholar] [CrossRef]
- Reynolds, H.M.; Walker, C.G.; Dunbar, P.R.; O’Sullivan, M.J.; Uren, R.F.; Thompson, J.F.; Smith, N.P. Functional anatomy of the lymphatics draining the skin: A detailed statistical analysis. J. Anat. 2010, 216, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Cirocchi, R.; Metaj, G.; Cicoletti, M.; Arcangeli, F.; De Sol, A.; Poli, G.; Bruzzone, P.; Gioia, S.; Anagnostou, C.; Loreti, F.; et al. Analysis of the Different Lymphatic Drainage Patterns during Sentinel Lymph Node Biopsy for Skin Melanoma. J. Clin. Med. 2021, 10, 5544. [Google Scholar] [CrossRef] [PubMed]
- Schuenke, M.; Schulte, E.; Schumacher, U.; Ross, L.M.; Lamperti, E.D.; Voll, M.; Wesker, K. Neck and internal organs. In Thieme Atlas of Anatomy; Thieme: New York City, NY, USA, 2006; p. 136ff. [Google Scholar]
- Blumgart, E.I.; Uren, R.F.; Nielsen, P.M.; Nash, M.P.; Reynolds, H.M. Lymphatic drainage and tumour prevalence in the breast: A statistical analysis of symmetry, gender and node field independence. J. Anat. 2011, 218, 652–659. [Google Scholar] [CrossRef]
- Margaris, K.N.; Black, R.A. Modelling the lymphatic system: Challenges and opportunities. J. R. Soc. Interface 2012, 9, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.E.; Friedman, R.; Chen, A.; Bustos, V.; Aly, M.I.; Fleishman, A.; Hong, Y.K.; Tsai, L.; Parker, J.A.; Donohoe, K.; et al. The Upper Extremity Lymphatic System Is Not Symmetrical in Individuals: An Anatomic Study Utilizing ICG Lymphography and SPECT/CT Lymphoscintigraphy. Ann. Surg. 2024. [Google Scholar] [CrossRef]
- Houbaert, D.; Nikolakopoulos, A.P.; Jacobs, K.A.; Mece, O.; Roels, J.; Shankar, G.; Agrawal, M.; More, S.; Ganne, M.; Rillaerts, K.; et al. An autophagy program that promotes T cell egress from the lymph node controls responses to immune checkpoint blockade. Cell Rep. 2024, 43, 114020. [Google Scholar] [CrossRef] [PubMed]
- Rantakari, P.; Auvinen, K.; Jappinen, N.; Kapraali, M.; Valtonen, J.; Karikoski, M.; Gerke, H.; Iftakhar, E.K.I.; Keuschnigg, J.; Umemoto, E.; et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 2015, 16, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Hons, M.; Sixt, M. The lymph node filter revealed. Nat. Immunol. 2015, 16, 338–340. [Google Scholar] [CrossRef]
- Katakai, T.; Hara, T.; Lee, J.H.; Gonda, H.; Sugai, M.; Shimizu, A. A novel reticular stromal structure in lymph node cortex: An immuno-platform for interactions among dendritic cells, T cells and B cells. Int Immunol 2004, 16, 1133–1142. [Google Scholar] [CrossRef]
- Sixt, M.; Kanazawa, N.; Selg, M.; Samson, T.; Roos, G.; Reinhardt, D.P.; Pabst, R.; Lutz, M.B.; Sorokin, L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 2005, 22, 19–29. [Google Scholar] [CrossRef]
- Reynoso, G.V.; Weisberg, A.S.; Shannon, J.P.; McManus, D.T.; Shores, L.; Americo, J.L.; Stan, R.V.; Yewdell, J.W.; Hickman, H.D. Lymph node conduits transport virions for rapid T cell activation. Nat. Immunol. 2019, 20, 602–612. [Google Scholar] [CrossRef]
- Angel, C.E.; Chen, C.J.; Horlacher, O.C.; Winkler, S.; John, T.; Browning, J.; MacGregor, D.; Cebon, J.; Dunbar, P.R. Distinctive localization of antigen-presenting cells in human lymph nodes. Blood 2009, 113, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, Y.R.; Batista, F.D. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 2007, 27, 160–171. [Google Scholar] [CrossRef]
- Phan, T.G.; Grigorova, I.; Okada, T.; Cyster, J.G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007, 8, 992–1000. [Google Scholar] [CrossRef]
- Junt, T.; Moseman, E.A.; Iannacone, M.; Massberg, S.; Lang, P.A.; Boes, M.; Fink, K.; Henrickson, S.E.; Shayakhmetov, D.M.; Di Paolo, N.C.; et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 2007, 450, 110–114. [Google Scholar] [CrossRef]
- Gray, E.E.; Cyster, J.G. Lymph node macrophages. J. Innate Immun. 2012, 4, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, M.; Woodruff, M.C.; Zawieja, D.C.; Carroll, M.C.; Moore, J.E., Jr. Modeling Lymph Flow and Fluid Exchange with Blood Vessels in Lymph Nodes. Lymphat. Res. Biol. 2015, 13, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Steer, H.W.; Foot, R.A. Changes in the medulla of the parathymic lymph nodes of the rat during acute gastro-intestinal inflammation. J. Anat. 1987, 152, 23–36. [Google Scholar] [PubMed]
- Nossal, G.J.; Abbot, A.; Mitchell, J. Antigens in immunity. XIV. Electron microscopic radioautographic studies of antigen capture in the lymph node medulla. J. Exp. Med. 1968, 127, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Green, J.A.; Gray, E.E.; Xu, Y.; Cyster, J.G. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 2009, 10, 786–793. [Google Scholar] [CrossRef]
- Louie, D.A.P.; Liao, S. Lymph Node Subcapsular Sinus Macrophages as the Frontline of Lymphatic Immune Defense. Front. Immunol. 2019, 10, 347. [Google Scholar] [CrossRef]
- Phan, T.G.; Gray, E.E.; Cyster, J.G. The microanatomy of B cell activation. Curr. Opin. Immunol. 2009, 21, 258–265. [Google Scholar] [CrossRef]
- Fossum, S. The architecture of rat lymph nodes. IV. Distribution of ferritin and colloidal carbon in the draining lymph nodes after foot-pad injection. Scand. J. Immunol. 1980, 12, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Nossal, G.J.; Abbot, A.; Mitchell, J.; Lummus, Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J. Exp. Med. 1968, 127, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Tew, J.G.; Mandel, T.E.; Phipps, R.P.; Szakal, A.K. Tissue localization and retention of antigen in relation to the immune response. Am. J. Anat. 1984, 170, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Nakajima, S.; Kobayashi, D.; Tomii, K.; Li, N.J.; Watarai, T.; Suzuki, R.; Watanabe, S.; Kanda, Y.; Takeuchi, A.; et al. Micro- and Macro-Anatomical Frameworks of Lymph Nodes Indispensable for the Lymphatic System Filtering Function. Front. Cell. Dev. Biol. 2022, 10, 902601. [Google Scholar] [CrossRef] [PubMed]
- Bajenoff, M.; Egen, J.G.; Koo, L.Y.; Laugier, J.P.; Brau, F.; Glaichenhaus, N.; Germain, R.N. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006, 25, 989–1001. [Google Scholar] [CrossRef]
- Cyster, J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 2005, 23, 127–159. [Google Scholar] [CrossRef]
- Forster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Germain, R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 2009, 9, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.P.; Moussion, C.; Forster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 2012, 12, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Schnizlein, C.T.; Szakal, A.K.; Tew, J.G. Follicular dendritic cells in the regulation and maintenance of immune responses. Immunobiology 1984, 168, 391–402. [Google Scholar] [CrossRef]
- Heesters, B.A.; Myers, R.C.; Carroll, M.C. Follicular dendritic cells: Dynamic antigen libraries. Nat. Rev. Immunol. 2014, 14, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Heath, W.R.; Kato, Y.; Steiner, T.M.; Caminschi, I. Antigen presentation by dendritic cells for B cell activation. Curr. Opin. Immunol. 2019, 58, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans Cells-Programmed by the Epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef] [PubMed]
- Tew, J.G.; Mandel, T.E.; Burgess, A.W. Retention of intact HSA for prolonged periods in the popliteal lymph nodes of specifically immunized mice. Cell. Immunol. 1979, 45, 207–212. [Google Scholar] [CrossRef]
- Aung, A.; Cui, A.; Maiorino, L.; Amini, A.P.; Gregory, J.R.; Bukenya, M.; Zhang, Y.; Lee, H.; Cottrell, C.A.; Morgan, D.M.; et al. Low protease activity in B cell follicles promotes retention of intact antigens after immunization. Science 2023, 379, eabn8934. [Google Scholar] [CrossRef]
- Kalia, A.; Hickman, H.D. A vaccine sanctuary in the lymph node. Science 2023, 379, 332–333. [Google Scholar] [CrossRef]
- Qi, H.; Egen, J.G.; Huang, A.Y.; Germain, R.N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 2006, 312, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.F.; Lukacs-Kornek, V.; Kuligowski, M.P.; Pitcher, L.A.; Degn, S.E.; Kim, Y.A.; Cloninger, M.J.; Martinez-Pomares, L.; Gordon, S.; Turley, S.J.; et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat. Immunol. 2010, 11, 427–434. [Google Scholar] [CrossRef] [PubMed]
- El Shikh, M.E.; El Sayed, R.M.; Sukumar, S.; Szakal, A.K.; Tew, J.G. Activation of B cells by antigens on follicular dendritic cells. Trends Immunol. 2010, 31, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Heesters, B.A.; Herndon, C.N.; Groom, J.R.; Thomas, P.G.; Luster, A.D.; Turley, S.J.; Carroll, M.C. Trans-nodal migration of resident dendritic cells into medullary interfollicular regions initiates immunity to influenza vaccine. J. Exp. Med. 2014, 211, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Moran, I.; Nguyen, A.; Khoo, W.H.; Butt, D.; Bourne, K.; Young, C.; Hermes, J.R.; Biro, M.; Gracie, G.; Ma, C.S.; et al. Memory B cells are reactivated in subcapsular proliferative foci of lymph nodes. Nat. Commun. 2018, 9, 3372. [Google Scholar] [CrossRef]
- Zhang, Y.; Garcia-Ibanez, L.; Ulbricht, C.; Lok, L.S.C.; Pike, J.A.; Mueller-Winkler, J.; Dennison, T.W.; Ferdinand, J.R.; Burnett, C.J.M.; Yam-Puc, J.C.; et al. Recycling of memory B cells between germinal center and lymph node subcapsular sinus supports affinity maturation to antigenic drift. Nat. Commun. 2022, 13, 2460. [Google Scholar] [CrossRef] [PubMed]
- Hickman, H.D.; Takeda, K.; Skon, C.N.; Murray, F.R.; Hensley, S.E.; Loomis, J.; Barber, G.N.; Bennink, J.R.; Yewdell, J.W. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat. Immunol. 2008, 9, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hickman, H.D.; Li, L.; Reynoso, G.V.; Rubin, E.J.; Skon, C.N.; Mays, J.W.; Gibbs, J.; Schwartz, O.; Bennink, J.R.; Yewdell, J.W. Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J. Exp. Med. 2011, 208, 2511–2524. [Google Scholar] [CrossRef] [PubMed]
- Gerner, M.Y.; Torabi-Parizi, P.; Germain, R.N. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 2015, 42, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Reis e Sousa, C.; Germain, R.N. Analysis of adjuvant function by direct visualization of antigen presentation in vivo: Endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J. Immunol. 1999, 162, 6552–6561. [Google Scholar] [CrossRef]
- Allan, R.S.; Waithman, J.; Bedoui, S.; Jones, C.M.; Villadangos, J.A.; Zhan, Y.; Lew, A.M.; Shortman, K.; Heath, W.R.; Carbone, F.R. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 2006, 25, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.; Labios, R.J.; Fenton, C.; Knopper, K.; Jobin, K.; Imdahl, F.; Golda, G.; Hoh, K.; Grafen, A.; Kaisho, T.; et al. Lymph node medulla regulates the spatiotemporal unfolding of resident dendritic cell networks. Immunity 2023, 56, 1778–1793 e1710. [Google Scholar] [CrossRef] [PubMed]
- Mempel, T.R.; Henrickson, S.E.; Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004, 427, 154–159. [Google Scholar] [CrossRef]
- Miller, M.J.; Safrina, O.; Parker, I.; Cahalan, M.D. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 2004, 200, 847–856. [Google Scholar] [CrossRef]
- Hugues, S.; Fetler, L.; Bonifaz, L.; Helft, J.; Amblard, F.; Amigorena, S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat. Immunol. 2004, 5, 1235–1242. [Google Scholar] [CrossRef]
- Lee, H.K.; Zamora, M.; Linehan, M.M.; Iijima, N.; Gonzalez, D.; Haberman, A.; Iwasaki, A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J. Exp. Med. 2009, 206, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kastenmuller, W.; Torabi-Parizi, P.; Subramanian, N.; Lammermann, T.; Germain, R.N. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 2012, 150, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Bousso, P. T-cell activation by dendritic cells in the lymph node: Lessons from the movies. Nat. Rev. Immunol. 2008, 8, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.C.; Teijeira, A.; Halin, C. T Cell Trafficking through Lymphatic Vessels. Front. Immunol. 2016, 7, 613. [Google Scholar] [CrossRef]
- Gasteiger, G.; Ataide, M.; Kastenmuller, W. Lymph node-an organ for T-cell activation and pathogen defense. Immunol. Rev. 2016, 271, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Li, L.; Paluskievicz, C.; Kasinath, V.; Bean, A.; Abdi, R.; Jewell, C.M.; Bromberg, J.S. Role of lymph node stroma and microenvironment in T cell tolerance. Immunol. Rev. 2019, 292, 9–23. [Google Scholar] [CrossRef]
- Cording, S.; Wahl, B.; Kulkarni, D.; Chopra, H.; Pezoldt, J.; Buettner, M.; Dummer, A.; Hadis, U.; Heimesaat, M.; Bereswill, S.; et al. The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol. 2014, 7, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Pasztoi, M.; Pezoldt, J.; Beckstette, M.; Lipps, C.; Wirth, D.; Rohde, M.; Paloczi, K.; Buzas, E.I.; Huehn, J. Mesenteric lymph node stromal cell-derived extracellular vesicles contribute to peripheral de novo induction of Foxp3(+) regulatory T cells. Eur. J. Immunol. 2017, 47, 2142–2152. [Google Scholar] [CrossRef]
- Pezoldt, J.; Pasztoi, M.; Zou, M.; Wiechers, C.; Beckstette, M.; Thierry, G.R.; Vafadarnejad, E.; Floess, S.; Arampatzi, P.; Buettner, M.; et al. Neonatally imprinted stromal cell subsets induce tolerogenic dendritic cells in mesenteric lymph nodes. Nat. Commun. 2018, 9, 3903. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, C.; Zou, M.; Galvez, E.; Beckstette, M.; Ebel, M.; Strowig, T.; Huehn, J.; Pezoldt, J. The microbiota is dispensable for the early stages of peripheral regulatory T cell induction within mesenteric lymph nodes. Cell. Mol. Immunol. 2021, 18, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Esterhazy, D.; Canesso, M.C.C.; Mesin, L.; Muller, P.A.; de Castro, T.B.R.; Lockhart, A.; ElJalby, M.; Faria, A.M.C.; Mucida, D. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 2019, 569, 126–130. [Google Scholar] [CrossRef]
- Brown, H.; Komnick, M.R.; Brigleb, P.H.; Dermody, T.S.; Esterhazy, D. Lymph node sharing between pancreas, gut, and liver leads to immune crosstalk and regulation of pancreatic autoimmunity. Immunity 2023, 56, 2070–2085 e2011. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, S.I.; Ahrendt, M.; Bode, U.; Wahl, B.; Kremmer, E.; Forster, R.; Pabst, O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 2008, 205, 2483–2490. [Google Scholar] [CrossRef]
- Ferguson, A.R.; Engelhard, V.H. CD8 T cells activated in distinct lymphoid organs differentially express adhesion proteins and coexpress multiple chemokine receptors. J. Immunol. 2010, 184, 4079–4086. [Google Scholar] [CrossRef] [PubMed]
- Barreto de Albuquerque, J.; Altenburger, L.M.; Abe, J.; von Werdt, D.; Wissmann, S.; Martinez Magdaleno, J.; Francisco, D.; van Geest, G.; Ficht, X.; Iannacone, M.; et al. Microbial uptake in oral mucosa-draining lymph nodes leads to rapid release of cytotoxic CD8(+) T cells lacking a gut-homing phenotype. Sci. Immunol. 2022, 7, eabf1861. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.C.; Malide, D.; Gibbs, J.S.; Bennink, J.R.; Yewdell, J.W. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 2002, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.L.; Parekh, N.J.; Kaminsky, L.W.; Soni, C.; Reider, I.E.; Krouse, T.E.; Fischer, M.A.; van Rooijen, N.; Rahman, Z.S.M.; Norbury, C.C. A systemic macrophage response is required to contain a peripheral poxvirus infection. PLoS Pathog. 2017, 13, e1006435. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.E.; Davis-Poynter, N.; Bruce, K.; Lawler, C.; Dolken, L.; Mach, M.; Stevenson, P.G. Lymph Node Macrophages Restrict Murine Cytomegalovirus Dissemination. J. Virol. 2015, 89, 7147–7158. [Google Scholar] [CrossRef]
- Frederico, B.; Chao, B.; Lawler, C.; May, J.S.; Stevenson, P.G. Subcapsular sinus macrophages limit acute gammaherpesvirus dissemination. J. Gen. Virol. 2015, 96, 2314–2327. [Google Scholar] [CrossRef]
- Winkelmann, E.R.; Widman, D.G.; Xia, J.; Johnson, A.J.; van Rooijen, N.; Mason, P.W.; Bourne, N.; Milligan, G.N. Subcapsular sinus macrophages limit dissemination of West Nile virus particles after inoculation but are not essential for the development of West Nile virus-specific T cell responses. Virology 2014, 450–451, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Iannacone, M.; Moseman, E.A.; Tonti, E.; Bosurgi, L.; Junt, T.; Henrickson, S.E.; Whelan, S.P.; Guidotti, L.G.; von Andrian, U.H. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 2010, 465, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.C.; Lucas, C.J.; Brisse, M.E.; Ware, B.C.; Hickman, H.D.; Morrison, T.E.; Diamond, M.S. Ly6C(+) monocytes in the skin promote systemic alphavirus dissemination. Cell Rep. 2024, 43, 113876. [Google Scholar] [CrossRef] [PubMed]
- Gaya, M.; Castello, A.; Montaner, B.; Rogers, N.; Reis e Sousa, C.; Bruckbauer, A.; Batista, F.D. Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science 2015, 347, 667–672. [Google Scholar] [CrossRef]
- Hickman, H.D. Immunology. There goes the macrophage neighborhood. Science 2015, 347, 609–610. [Google Scholar] [CrossRef]
- Sagoo, P.; Garcia, Z.; Breart, B.; Lemaitre, F.; Michonneau, D.; Albert, M.L.; Levy, Y.; Bousso, P. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat. Med. 2016, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Chatziandreou, N.; Farsakoglu, Y.; Palomino-Segura, M.; D’Antuono, R.; Pizzagalli, D.U.; Sallusto, F.; Lukacs-Kornek, V.; Uguccioni, M.; Corti, D.; Turley, S.J.; et al. Macrophage Death following Influenza Vaccination Initiates the Inflammatory Response that Promotes Dendritic Cell Function in the Draining Lymph Node. Cell Rep. 2017, 18, 2427–2440. [Google Scholar] [CrossRef]
- Mondor, I.; Baratin, M.; Lagueyrie, M.; Saro, L.; Henri, S.; Gentek, R.; Suerinck, D.; Kastenmuller, W.; Jiang, J.X.; Bajenoff, M. Lymphatic Endothelial Cells Are Essential Components of the Subcapsular Sinus Macrophage Niche. Immunity 2019, 50, 1453–1466 e1454. [Google Scholar] [CrossRef] [PubMed]
- Hufert, F.T.; van Lunzen, J.; Janossy, G.; Bertram, S.; Schmitz, J.; Haller, O.; Racz, P.; von Laer, D. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS 1997, 11, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Maric, I.; Bryant, R.; Abu-Asab, M.; Cohen, J.I.; Vivero, A.; Jaffe, E.S.; Raffeld, M.; Tsokos, M.; Banks, P.M.; Pittaluga, S. Human herpesvirus-6-associated acute lymphadenitis in immunocompetent adults. Mod. Pathol. 2004, 17, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Yamanishi, K. HHV-6A, 6B, and 7: Pathogenesis, host response, and clinical disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Johnson, K.E.; Tarakanova, V.L. Gammaherpesviruses and B Cells: A Relationship That Lasts a Lifetime. Viral Immunol. 2020, 33, 316–326. [Google Scholar] [CrossRef]
- Fiorentini, S.; Luganini, A.; Dell’Oste, V.; Lorusso, B.; Cervi, E.; Caccuri, F.; Bonardelli, S.; Landolfo, S.; Caruso, A.; Gribaudo, G. Human cytomegalovirus productively infects lymphatic endothelial cells and induces a secretome that promotes angiogenesis and lymphangiogenesis through interleukin-6 and granulocyte-macrophage colony-stimulating factor. J. Gen. Virol. 2011, 92, 650–660. [Google Scholar] [CrossRef]
- Carpentier, K.S.; Sheridan, R.M.; Lucas, C.J.; Davenport, B.J.; Li, F.S.; Lucas, E.D.; McCarthy, M.K.; Reynoso, G.V.; May, N.A.; Tamburini, B.A.J.; et al. MARCO(+) lymphatic endothelial cells sequester arthritogenic alphaviruses to limit viremia and viral dissemination. EMBO J. 2021, 40, e108966. [Google Scholar] [CrossRef]
- Dai, L.; Qin, Z. Role of lymphatic endothelium specific hyaluronan receptor 1 in virus infection and associated diseases. J. Med. Virol. 2024, 96, e29457. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Grosso, R.A.; Takeda, A.; Pan, J.; Bekkhus, T.; Brulois, K.; Dermadi, D.; Nordling, S.; Vanlandewijck, M.; Jalkanen, S.; et al. A Single-Cell Transcriptional Roadmap of the Mouse and Human Lymph Node Lymphatic Vasculature. Front. Cardiovasc. Med. 2020, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Aggio, J.B.; Krmeska, V.; Ferguson, B.J.; Wowk, P.F.; Rothfuchs, A.G. Vaccinia Virus Infection Inhibits Skin Dendritic Cell Migration to the Draining Lymph Node. J. Immunol. 2021, 206, 776–784. [Google Scholar] [CrossRef]
- Churchill, M.J.; du Bois, H.; Heim, T.A.; Mudianto, T.; Steele, M.M.; Nolz, J.C.; Lund, A.W. Infection-induced lymphatic zippering restricts fluid transport and viral dissemination from skin. J. Exp. Med. 2022, 219, e20211830. [Google Scholar] [CrossRef] [PubMed]
- Heim, T.A.; Schultz, A.C.; Delclaux, I.; Cristaldi, V.; Churchill, M.J.; Ventre, K.S.; Lund, A.W. Lymphatic vessel transit seeds cytotoxic resident memory T cells in skin draining lymph nodes. Sci. Immunol. 2024, 9, eadk8141. [Google Scholar] [CrossRef] [PubMed]
- Melo-Silva, C.R.; Sigal, L.J. Innate and adaptive immune responses that control lymph-borne viruses in the draining lymph node. Cell Mol. Immunol. 2024, 21, 999–1007. [Google Scholar] [CrossRef]
- Xu, R.H.; Wong, E.B.; Rubio, D.; Roscoe, F.; Ma, X.; Nair, S.; Remakus, S.; Schwendener, R.; John, S.; Shlomchik, M.; et al. Sequential Activation of Two Pathogen-Sensing Pathways Required for Type I Interferon Expression and Resistance to an Acute DNA Virus Infection. Immunity 2015, 43, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Fang, M.; Klein-Szanto, A.; Sigal, L.J. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proc. Natl. Acad. Sci. USA 2007, 104, 10992–10997. [Google Scholar] [CrossRef]
- Fang, M.; Siciliano, N.A.; Hersperger, A.R.; Roscoe, F.; Hu, A.; Ma, X.; Shamsedeen, A.R.; Eisenlohr, L.C.; Sigal, L.J. Perforin-dependent CD4+ T-cell cytotoxicity contributes to control a murine poxvirus infection. Proc. Natl. Acad. Sci. USA 2012, 109, 9983–9988. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Lanier, L.L.; Sigal, L.J. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathog. 2008, 4, e30. [Google Scholar] [CrossRef]
- Wong, E.; Montoya, B.; Stotesbury, C.; Ferez, M.; Xu, R.H.; Sigal, L.J. Langerhans Cells Orchestrate the Protective Antiviral Innate Immune Response in the Lymph Node. Cell Rep. 2019, 29, 3047–3059.e3. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Xu, R.H.; Rubio, D.; Lev, A.; Stotesbury, C.; Fang, M.; Sigal, L.J. Migratory Dendritic Cells, Group 1 Innate Lymphoid Cells, and Inflammatory Monocytes Collaborate to Recruit NK Cells to the Virus-Infected Lymph Node. Cell Rep. 2018, 24, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Roscoe, F.; Sigal, L.J. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J. Exp. Med. 2010, 207, 2369–2381. [Google Scholar] [CrossRef]
- Stotesbury, C.; Wong, E.B.; Tang, L.; Montoya, B.; Knudson, C.J.; Melo-Silva, C.R.; Sigal, L.J. Defective early innate immune response to ectromelia virus in the draining lymph nodes of aged mice due to impaired dendritic cell accumulation. Aging Cell 2020, 19, e13170. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Gmyrek, G.B.; Govero, J.; Tu, Y.; van der Windt, G.J.; Metcalf, T.U.; Haddad, E.K.; Textor, J.; Miller, M.J.; Diamond, M.S. Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection. PLoS Pathog. 2015, 11, e1005027. [Google Scholar] [CrossRef] [PubMed]
- Cruz de Casas, P.; Knopper, K.; Dey Sarkar, R.; Kastenmuller, W. Same yet different-how lymph node heterogeneity affects immune responses. Nat. Rev. Immunol. 2024, 24, 358–374. [Google Scholar] [CrossRef]
- Reed, H.O.; Wang, L.; Sonett, J.; Chen, M.; Yang, J.; Li, L.; Aradi, P.; Jakus, Z.; D’Armiento, J.; Hancock, W.W.; et al. Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage. J. Clin. Investig. 2019, 129, 2514–2526. [Google Scholar] [CrossRef] [PubMed]
- Stritt, S.; Koltowska, K.; Makinen, T. Homeostatic maintenance of the lymphatic vasculature. Trends Mol. Med. 2021, 27, 955–970. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, B.A.J.; Finlon, J.M.; Gillen, A.E.; Kriss, M.S.; Riemondy, K.A.; Fu, R.; Schuyler, R.P.; Hesselberth, J.R.; Rosen, H.R.; Burchill, M.A. Chronic Liver Disease in Humans Causes Expansion and Differentiation of Liver Lymphatic Endothelial Cells. Front. Immunol. 2019, 10, 1036. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Gorman, M.J.; Caine, E.A.; Zaitsev, K.; Begley, M.C.; Weger-Lucarelli, J.; Uccellini, M.B.; Tripathi, S.; Morrison, J.; Yount, B.L.; Dinnon, K.H., 3rd; et al. An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 2018, 23, 672–685.e6. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and macrophages as viral targets and reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 2008, 321, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, M.; Lau, L.T.; Lu, J.; Gao, Z.; Liu, J.; Yu, A.C.; Cao, Q.; Ye, J.; McNutt, M.A.; et al. Neutrophils may be a vehicle for viral replication and dissemination in human H5N1 avian influenza. Clin. Infect. Dis. 2008, 47, 1575–1578. [Google Scholar] [CrossRef]
- Bai, F.; Kong, K.F.; Dai, J.; Qian, F.; Zhang, L.; Brown, C.R.; Fikrig, E.; Montgomery, R.R. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J. Infect. Dis. 2010, 202, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Galani, I.E.; Andreakos, E. Neutrophils in viral infections: Current concepts and caveats. J. Leukoc. Biol. 2015, 98, 557–564. [Google Scholar] [CrossRef]
- Huang, S.; Ziegler, C.G.K.; Austin, J.; Mannoun, N.; Vukovic, M.; Ordovas-Montanes, J.; Shalek, A.K.; von Andrian, U.H. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 2021, 184, 441–459 e425. [Google Scholar] [CrossRef] [PubMed]
- Embretson, J.; Zupancic, M.; Ribas, J.L.; Burke, A.; Racz, P.; Tenner-Racz, K.; Haase, A.T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 1993, 362, 359–362. [Google Scholar] [CrossRef]
- Beck, S.E.; Veenhuis, R.T.; Blankson, J.N. Does B Cell Follicle Exclusion of CD8+ T Cells Make Lymph Nodes Sanctuaries of HIV Replication? Front. Immunol. 2019, 10, 2362. [Google Scholar] [CrossRef]
- Pantaleo, G.; Graziosi, C.; Demarest, J.F.; Butini, L.; Montroni, M.; Fox, C.H.; Orenstein, J.M.; Kotler, D.P.; Fauci, A.S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 1993, 362, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Horiike, M.; Iwami, S.; Kodama, M.; Sato, A.; Watanabe, Y.; Yasui, M.; Ishida, Y.; Kobayashi, T.; Miura, T.; Igarashi, T. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology 2012, 423, 107–118. [Google Scholar] [CrossRef]
- Zhang, J.; Perelson, A.S. Contribution of follicular dendritic cells to persistent HIV viremia. J. Virol. 2013, 87, 7893–7901. [Google Scholar] [CrossRef] [PubMed]
- Heesters, B.A.; Lindqvist, M.; Vagefi, P.A.; Scully, E.P.; Schildberg, F.A.; Altfeld, M.; Walker, B.D.; Kaufmann, D.E.; Carroll, M.C. Follicular Dendritic Cells Retain Infectious HIV in Cycling Endosomes. PLoS Pathog. 2015, 11, e1005285. [Google Scholar] [CrossRef] [PubMed]
- Baiyegunhi, O.O.; Mann, J.; Khaba, T.; Nkosi, T.; Mbatha, A.; Ogunshola, F.; Chasara, C.; Ismail, N.; Ngubane, T.; Jajbhay, I.; et al. CD8 lymphocytes mitigate HIV-1 persistence in lymph node follicular helper T cells during hyperacute-treated infection. Nat. Commun. 2022, 13, 4041. [Google Scholar] [CrossRef] [PubMed]
- Windmann, S.; Otto, L.; Hrycak, C.P.; Malyshkina, A.; Bongard, N.; David, P.; Gunzer, M.; Dittmer, U.; Bayer, W. Infection of B Cell Follicle-Resident Cells by Friend Retrovirus Occurs during Acute Infection and Is Maintained during Viral Persistence. mBio 2019, 10, 10-1128. [Google Scholar] [CrossRef]
- Vinton, C.L.; Magaziner, S.J.; Dowd, K.A.; Robertson, S.J.; Amaro-Carambot, E.; Karmele, E.P.; Ortiz, A.M.; Starke, C.E.; Mudd, J.C.; Whitehead, S.S.; et al. Simian Immunodeficiency Virus Infection of Rhesus Macaques Results in Delayed Zika Virus Clearance. mBio 2019, 10, e00004-19. [Google Scholar] [CrossRef] [PubMed]
- Aid, M.; Abbink, P.; Larocca, R.A.; Boyd, M.; Nityanandam, R.; Nanayakkara, O.; Martinot, A.J.; Moseley, E.T.; Blass, E.; Borducchi, E.N.; et al. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell 2017, 169, 610–620.e14. [Google Scholar] [CrossRef]
- Chang, C.J.; Sanchez, L.M.; Vageesh, A.; Popkov, A.J.; Chandrasekaran, A.; Moore, B.B.; Weinberg, J.B. Mouse Adenovirus Type 1 Persistence Exacerbates Inflammation Induced by Allogeneic Bone Marrow Transplantation. J. Virol. 2022, 96, e0170621. [Google Scholar] [CrossRef]
- Boggiatto, P.M.; Buckley, A.; Cassmann, E.D.; Seger, H.; Olsen, S.C.; Palmer, M.V. Persistence of viral RNA in North American elk experimentally infected with an ancestral strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Sci. Rep. 2024, 14, 11171. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Heesters, B.A.; van der Poel, C.E.; Das, A.; Carroll, M.C. Antigen Presentation to B Cells. Trends Immunol. 2016, 37, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Lytle, A.G.; Shen, S.; McGettigan, J.P. Lymph node but not intradermal injection site macrophages are critical for germinal center formation and antibody responses to rabies vaccination. J. Virol. 2015, 89, 2842–2848. [Google Scholar] [CrossRef]
- Purtha, W.E.; Chachu, K.A.; Virgin, H.W.; Diamond, M.S. Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. J. Virol. 2008, 82, 10964–10974. [Google Scholar] [CrossRef] [PubMed]
- Buerki, H.; Kraft, R.; Hess, M.W.; Laissue, J.; Cottier, H.; Stoner, R.D. Germinal center kinetics in lymph nodes of primed mice stimulated with complexed as opposed to free antigen. Immunol. Lett. 1989, 23, 87–94. [Google Scholar] [CrossRef] [PubMed]
- DeFranco, A.L. The germinal center antibody response in health and disease. F1000Research 2016, 5, 999. [Google Scholar] [CrossRef]
- de Carvalho, R.V.H.; Ersching, J.; Barbulescu, A.; Hobbs, A.; Castro, T.B.R.; Mesin, L.; Jacobsen, J.T.; Phillips, B.K.; Hoffmann, H.H.; Parsa, R.; et al. Clonal replacement sustains long-lived germinal centers primed by respiratory viruses. Cell 2023, 186, 131–146 e113. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Mylvaganam, G.H.; Rios, D.; Abdelaal, H.M.; Iyer, S.; Tharp, G.; Mavigner, M.; Hicks, S.; Chahroudi, A.; Ahmed, R.; Bosinger, S.E.; et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc. Natl. Acad. Sci. USA 2017, 114, 1976–1981. [Google Scholar] [CrossRef]
- Allie, S.R.; Randall, T.D. Resident Memory B Cells. Viral Immunol. 2020, 33, 282–293. [Google Scholar] [CrossRef]
- Inoue, T.; Kurosaki, T. Memory B cells. Nat. Rev. Immunol. 2024, 24, 5–17. [Google Scholar] [CrossRef]
- Shaabani, N.; Duhan, V.; Khairnar, V.; Gassa, A.; Ferrer-Tur, R.; Häussinger, D.; Recher, M.; Zelinskyy, G.; Liu, J.; Dittmer, U.; et al. CD169+ macrophages regulate PD-L1 expression via type I interferon and thereby prevent severe immunopathology after LCMV infection. Cell Death Dis. 2016, 7, e2446. [Google Scholar] [CrossRef] [PubMed]
- Hogquist, K.A.; Tomlinson, A.J.; Kieper, W.C.; McGargill, M.A.; Hart, M.C.; Naylor, S.; Jameson, S.C. Identification of a naturally occurring ligand for thymic positive selection. Immunity 1997, 6, 389–399. [Google Scholar] [CrossRef]
- Halle, S.; Keyser, K.A.; Stahl, F.R.; Busche, A.; Marquardt, A.; Zheng, X.; Galla, M.; Heissmeyer, V.; Heller, K.; Boelter, J.; et al. In Vivo Killing Capacity of Cytotoxic T Cells Is Limited and Involves Dynamic Interactions and T Cell Cooperativity. Immunity 2016, 44, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Regoes, R.R.; Yates, A.; Antia, R. Mathematical models of cytotoxic T-lymphocyte killing. Immunol. Cell Biol. 2007, 85, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Elemans, M.; Seich Al Basatena, N.K.; Asquith, B. The efficiency of the human CD8+ T cell response: How should we quantify it, what determines it, and does it matter? PLoS Comput. Biol. 2012, 8, e1002381. [Google Scholar] [CrossRef]
- Chen, M.L.; Pittet, M.J.; Gorelik, L.; Flavell, R.A.; Weissleder, R.; von Boehmer, H.; Khazaie, K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Mempel, T.R.; Pittet, M.J.; Khazaie, K.; Weninger, W.; Weissleder, R.; von Boehmer, H.; von Andrian, U.H. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 2006, 25, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Guarda, G.; Hons, M.; Soriano, S.F.; Huang, A.Y.; Polley, R.; Martin-Fontecha, A.; Stein, J.V.; Germain, R.N.; Lanzavecchia, A.; Sallusto, F. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat. Immunol. 2007, 8, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harbor Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Brewitz, A.; Eickhoff, S.; Dahling, S.; Quast, T.; Bedoui, S.; Kroczek, R.A.; Kurts, C.; Garbi, N.; Barchet, W.; Iannacone, M.; et al. CD8(+) T Cells Orchestrate pDC-XCR1(+) Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity 2017, 46, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Cucak, H.; Yrlid, U.; Reizis, B.; Kalinke, U.; Johansson-Lindbom, B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity 2009, 31, 491–501. [Google Scholar] [CrossRef]
- Jergovic, M.; Coplen, C.P.; Uhrlaub, J.L.; Besselsen, D.G.; Cheng, S.; Smithey, M.J.; Nikolich-Zugich, J. Infection-induced type I interferons critically modulate the homeostasis and function of CD8(+) naive T cells. Nat. Commun. 2021, 12, 5303. [Google Scholar] [CrossRef] [PubMed]
- Havenar-Daughton, C.; Kolumam, G.A.; Murali-Krishna, K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J. Immunol. 2006, 176, 3315–3319. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, M.; Cutillo, V.; Giladi, A.; Sala, E.; Maganuco, C.G.; Medaglia, C.; Di Lucia, P.; Bono, E.; Cristofani, C.; Consolo, E.; et al. Spatiotemporal regulation of type I interferon expression determines the antiviral polarization of CD4(+) T cells. Nat. Immunol. 2020, 21, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Kuka, M.; De Giovanni, M.; Iannacone, M. The role of type I interferons in CD4(+) T cell differentiation. Immunol. Lett. 2019, 215, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Zhang, H.; Moseman, E.A.; Alvarez, D.; Iannacone, M.; Henrickson, S.E.; de la Torre, J.C.; Groom, J.R.; Luster, A.D.; von Andrian, U.H. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell 2012, 150, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Marrack, P.; Kappler, J.; Mitchell, T. Type I interferons keep activated T cells alive. J. Exp. Med. 1999, 189, 521–530. [Google Scholar] [CrossRef]
- Perez-Shibayama, C.; Islander, U.; Lutge, M.; Cheng, H.W.; Onder, L.; Ring, S.S.; De Martin, A.; Novkovic, M.; Colston, J.; Gil-Cruz, C.; et al. Type I interferon signaling in fibroblastic reticular cells prevents exhaustive activation of antiviral CD8(+) T cells. Sci. Immunol. 2020, 5, eabb7066. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.D.; Tamburini, B.A.J. Lymph Node Lymphatic Endothelial Cell Expansion and Contraction and the Programming of the Immune Response. Front. Immunol. 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.D.; Finlon, J.M.; Burchill, M.A.; McCarthy, M.K.; Morrison, T.E.; Colpitts, T.M.; Tamburini, B.A.J. Type 1 IFN and PD-L1 Coordinate Lymphatic Endothelial Cell Expansion and Contraction during an Inflammatory Immune Response. J. Immunol. 2018, 201, 1735–1747. [Google Scholar] [CrossRef]
- Bennett, A.K.; Richner, M.; Mun, M.D.; Richner, J.M. Type I IFN stimulates lymph node stromal cells from adult and old mice during a West Nile virus infection. Aging Cell 2023, 22, e13796. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, E.; Junt, T.; Waibler, Z.; Forster, R.; Kalinke, U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 2006, 108, 3253–3261. [Google Scholar] [CrossRef]
- Phillips, M.B.; Dina Zita, M.; Howells, M.A.; Weinkopff, T.; Boehme, K.W. Lymphatic Type 1 Interferon Responses Are Critical for Control of Systemic Reovirus Dissemination. J. Virol. 2021, 95, 10-1128. [Google Scholar] [CrossRef]
- Moseman, E.A.; Iannacone, M.; Bosurgi, L.; Tonti, E.; Chevrier, N.; Tumanov, A.; Fu, Y.X.; Hacohen, N.; von Andrian, U.H. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 2012, 36, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Ramirez, N.P.; Gibson, G.; Kline, C.; Watkins, S.; Ambrose, Z.; Gummuluru, S. Interferon-Inducible CD169/Siglec1 Attenuates Anti-HIV-1 Effects of Alpha Interferon. J. Virol. 2017, 91, 10-1128. [Google Scholar] [CrossRef]
- Han, S.J.; Melichar, H.J.; Coombes, J.L.; Chan, S.W.; Koshy, A.A.; Boothroyd, J.C.; Barton, G.M.; Robey, E.A. Internalization and TLR-dependent type I interferon production by monocytes in response to Toxoplasma gondii. Immunol. Cell Biol. 2014, 92, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Sammicheli, S.; Kuka, M.; Di Lucia, P.; de Oya, N.J.; De Giovanni, M.; Fioravanti, J.; Cristofani, C.; Maganuco, C.G.; Fallet, B.; Ganzer, L.; et al. Inflammatory monocytes hinder antiviral B cell responses. Sci. Immunol. 2016, 1, eaah6789. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.K.; Reynoso, G.V.; Winkler, E.S.; Mack, M.; Diamond, M.S.; Hickman, H.D.; Morrison, T.E. MyD88-dependent influx of monocytes and neutrophils impairs lymph node B cell responses to chikungunya virus infection via Irf5, Nos2 and Nox2. PLoS Pathog. 2020, 16, e1008292. [Google Scholar] [CrossRef] [PubMed]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Cao, W.; Liu, Y.-J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef]
- Ali, S.; Mann-Nuttel, R.; Schulze, A.; Richter, L.; Alferink, J.; Scheu, S. Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver’s Seat. Front. Immunol. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Matsuno, K.; Toda, E.; Nishiwaki, T.; Matsuo, N.; Nakano, A.; Narumi, S.; Lu, B.; Gerard, C.; Ishikawa, S.; et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 2005, 202, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.B.; Sevick-Muraca, E.M. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine 2013, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.L.; Greenwood, D.L.; Han, X.; Scheerlinck, J.P. Inflammatory cytokines IL-6 and TNF-alpha regulate lymphocyte trafficking through the local lymph node. Vet Immunol. Immunopathol. 2011, 144, 95–103. [Google Scholar] [CrossRef]
- Cahill, R.N.; Frost, H.; Trnka, Z. The effects of antigen on the migration of recirculating lymphocytes through single lymph nodes. J. Exp. Med. 1976, 143, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.R.; Marston, W.; Dudler, L. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur. J. Immunol. 1992, 22, 2205–2210. [Google Scholar] [CrossRef]
- Basic, M.; Peppermuller, P.P.; Bolsega, S.; Bleich, A.; Bornemann, M.; Bode, U.; Buettner, M. Lymph Node Stromal Cells From Different Draining Areas Distinctly Regulate the Development of Chronic Intestinal Inflammation. Front. Immunol. 2020, 11, 549473. [Google Scholar] [CrossRef]
- Gregory, J.L.; Walter, A.; Alexandre, Y.O.; Hor, J.L.; Liu, R.; Ma, J.Z.; Devi, S.; Tokuda, N.; Owada, Y.; Mackay, L.K.; et al. Infection Programs Sustained Lymphoid Stromal Cell Responses and Shapes Lymph Node Remodeling upon Secondary Challenge. Cell Rep. 2017, 18, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.L.; Hunt, P.W.; Reilly, C.S.; Hatano, H.; Beilman, G.J.; Khoruts, A.; Jasurda, J.S.; Somsouk, M.; Thorkelson, A.; Russ, S.; et al. Lymphoid fibrosis occurs in long-term nonprogressors and persists with antiretroviral therapy but may be reversible with curative interventions. J. Infect. Dis. 2015, 211, 1068–1075. [Google Scholar] [CrossRef]
- Tang, H.L.; Cyster, J.G. Chemokine Up-regulation and activated T cell attraction by maturing dendritic cells. Science 1999, 284, 819–822. [Google Scholar] [CrossRef]
- Rapp, M.; Wintergerst, M.W.M.; Kunz, W.G.; Vetter, V.K.; Knott, M.M.L.; Lisowski, D.; Haubner, S.; Moder, S.; Thaler, R.; Eiber, S.; et al. CCL22 controls immunity by promoting regulatory T cell communication with dendritic cells in lymph nodes. J. Exp. Med. 2019, 216, 1170–1181. [Google Scholar] [CrossRef]
- Malhotra, D.; Fletcher, A.L.; Astarita, J.; Lukacs-Kornek, V.; Tayalia, P.; Gonzalez, S.F.; Elpek, K.G.; Chang, S.K.; Knoblich, K.; Hemler, M.E.; et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 2012, 13, 499–510. [Google Scholar] [CrossRef]
- Tomei, A.A.; Siegert, S.; Britschgi, M.R.; Luther, S.A.; Swartz, M.A. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 2009, 183, 4273–4283. [Google Scholar] [CrossRef] [PubMed]
- Mazzaglia, C.; Munir, H.; Le, I.M.; Gerigk, M.; Huang, Y.Y.S.; Shields, J.D. Modelling Structural Elements and Functional Responses to Lymphatic-Delivered Cues in a Murine Lymph Node on a Chip. Adv. Healthc. Mater. 2024, 13, e2303720. [Google Scholar] [CrossRef] [PubMed]
- Ulvmar, M.H.; Werth, K.; Braun, A.; Kelay, P.; Hub, E.; Eller, K.; Chan, L.; Lucas, B.; Novitzky-Basso, I.; Nakamura, K.; et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014, 15, 623–630. [Google Scholar] [CrossRef]
- Saeki, H.; Moore, A.M.; Brown, M.J.; Hwang, S.T. Cutting edge: Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 1999, 162, 2472–2475. [Google Scholar] [CrossRef]
- Randolph, G.J.; Angeli, V.; Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005, 5, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, R.H.; Karnezis, T.; Maciburko, S.J.; Mueller, S.N.; Stacker, S.A. The Interplay Between Lymphatic Vessels and Chemokines. Front. Immunol. 2019, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; De Giovanni, M.; Xu, Y.; An, J.; Kirthivasan, N.; Lu, E.; Jiang, K.; Brooks, S.; Ranucci, S.; Yang, J.; et al. Inflammation switches the chemoattractant requirements for naive lymphocyte entry into lymph nodes. Cell 2024. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.V.; Khakoo, S.I.; Blunt, M.D. NK Cells in the Lymph Nodes and Their Role in Anti-Tumour Immunity. Biomedicines 2024, 12, 1667. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Watt, S.V.; Andrews, D.M.; Takeda, K.; Smyth, M.J.; Hayakawa, Y. IFN-gamma-dependent recruitment of mature CD27(high) NK cells to lymph nodes primed by dendritic cells. J. Immunol. 2008, 181, 5323–5330. [Google Scholar] [CrossRef]
- Bajenoff, M.; Breart, B.; Huang, A.Y.; Qi, H.; Cazareth, J.; Braud, V.M.; Germain, R.N.; Glaichenhaus, N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J. Exp. Med. 2006, 203, 619–631. [Google Scholar] [CrossRef]
- Ferlazzo, G.; Thomas, D.; Lin, S.L.; Goodman, K.; Morandi, B.; Muller, W.A.; Moretta, A.; Munz, C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J. Immunol. 2004, 172, 1455–1462. [Google Scholar] [CrossRef]
- Martin-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Huot, N.; Jacquelin, B.; Garcia-Tellez, T.; Rascle, P.; Ploquin, M.J.; Madec, Y.; Reeves, R.K.; Derreudre-Bosquet, N.; Muller-Trutwin, M. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat. Med. 2017, 23, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; He, Y.; Liu, B.; Zhang, X.; Song, C.; Wu, Y.; Hu, W.; Yan, Y.; Chen, N.; Ding, Y.; et al. Single-cell RNA sequencing reveals the dynamics and heterogeneity of lymph node immune cells during acute and chronic viral infections. Front. Immunol. 2024, 15, 1341985. [Google Scholar] [CrossRef]
- Leal, J.M.; Huang, J.Y.; Kohli, K.; Stoltzfus, C.; Lyons-Cohen, M.R.; Olin, B.E.; Gale, M., Jr.; Gerner, M.Y. Innate cell microenvironments in lymph nodes shape the generation of T cell responses during type I inflammation. Sci. Immunol. 2021, 6, eabb9435. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, A.; Bracero, S.; Chaluvadi, V.S.; Khodadadi-Jamayran, A.; Cammer, M.; Schwab, S.R. Monocyte-derived S1P in the lymph node regulates immune responses. Nature 2021, 592, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Cheong, C.; Matos, I.; Choi, J.H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Leiriao, P.; del Fresno, C.; Ardavin, C. Monocytes as effector cells: Activated Ly-6C(high) mouse monocytes migrate to the lymph nodes through the lymph and cross-present antigens to CD8+ T cells. Eur. J. Immunol. 2012, 42, 2042–2051. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Lee, P.; Snively, J.; Boswell, W.; Ounpraseuth, S.; Lee, S.; Hickingbottom, B.; Smith, J.; Johnson, D.; Weber, J.S. Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma. Cancer 2003, 98, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Boswell, W.; Smith, J.; Hersh, E.; Snively, J.; Diaz, M.; Miles, S.; Liu, X.; Obrocea, M.; Qiu, Z.; et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J. Immunother. 2008, 31, 215–223. [Google Scholar] [CrossRef]
- Jewell, C.M.; Lopez, S.C.; Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 2011, 108, 15745–15750. [Google Scholar] [CrossRef]
- Bialkowski, L.; van Weijnen, A.; Van der Jeught, K.; Renmans, D.; Daszkiewicz, L.; Heirman, C.; Stange, G.; Breckpot, K.; Aerts, J.L.; Thielemans, K. Intralymphatic mRNA vaccine induces CD8 T-cell responses that inhibit the growth of mucosally located tumours. Sci Rep 2016, 6, 22509. [Google Scholar] [CrossRef]
- Jonuleit, H.; Giesecke-Tuettenberg, A.; Tuting, T.; Thurner-Schuler, B.; Stuge, T.B.; Paragnik, L.; Kandemir, A.; Lee, P.P.; Schuler, G.; Knop, J.; et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int. J. Cancer 2001, 93, 243–251. [Google Scholar] [CrossRef]
- Nair, S.K.; Heiser, A.; Boczkowski, D.; Majumdar, A.; Naoe, M.; Lebkowski, J.S.; Vieweg, J.; Gilboa, E. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat. Med. 2000, 6, 1011–1017. [Google Scholar] [CrossRef]
- Okada, H.; Kalinski, P.; Ueda, R.; Hoji, A.; Kohanbash, G.; Donegan, T.E.; Mintz, A.H.; Engh, J.A.; Bartlett, D.L.; Brown, C.K.; et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with alpha-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011, 29, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H. Lymph node targeting for immunotherapy. Immunooncol. Technol. 2023, 20, 100395. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, L.; Strand, S.E.; Persson, B.R. Particle sizing and biokinetics of interstitial lymphoscintigraphic agents. Semin. Nucl. Med. 1983, 13, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A. Lymphatic and nonlymphatic pathways of peritoneal absorption in mice: Physiology versus pathology. Blood Purif. 1992, 10, 148–162. [Google Scholar] [CrossRef]
- Khullar, O.V.; Griset, A.P.; Gibbs-Strauss, S.L.; Chirieac, L.R.; Zubris, K.A.; Frangioni, J.V.; Grinstaff, M.W.; Colson, Y.L. Nanoparticle migration and delivery of Paclitaxel to regional lymph nodes in a large animal model. J. Am. Coll. Surg. 2012, 214, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Oussoren, C.; Zuidema, J.; Crommelin, D.J.; Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid compostion and lipid dose. Biochim. Biophys. Acta 1997, 1328, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Rehor, A.; Schmoekel, H.G.; Hubbell, J.A.; Swartz, M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control Release 2006, 112, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, N.L.; Kaminskas, L.M.; Porter, C.J. From sewer to saviour-targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 2015, 14, 781–803. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Porter, C.J. Targeting the lymphatics using dendritic polymers (dendrimers). Adv. Drug Deliv. Rev. 2011, 63, 890–900. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Poh, M.Z.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of particles in the extracellular matrix: The effect of repulsive electrostatic interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef]
- Howard, G.P.; Verma, G.; Ke, X.; Thayer, W.M.; Hamerly, T.; Baxter, V.K.; Lee, J.E.; Dinglasan, R.R.; Mao, H.Q. Critical Size Limit of Biodegradable Nanoparticles for Enhanced Lymph Node Trafficking and Paracortex Penetration. Nano Res. 2019, 12, 837–844. [Google Scholar] [CrossRef]
- Ryan, G.M.; Kaminskas, L.M.; Porter, C.J. Nano-chemotherapeutics: Maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J. Control Release 2014, 193, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Noh, Y.W.; Kang, T.H.; Kim, J.E.; Kim, S.; Um, S.H.; Oh, D.B.; Park, Y.M.; Lim, Y.T. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials 2017, 130, 56–66. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Z.; Jaklenec, A.; Hu, Q. Vaccine delivery systems toward lymph nodes. Adv. Drug Deliv. Rev. 2021, 179, 113914. [Google Scholar] [CrossRef] [PubMed]
- Louten, J. Virus Structure and Classification. Essent. Human Virol. 2016, 19–29. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Sun, X. Lymph node targeting strategies to improve vaccination efficacy. J. Control Release 2017, 267, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Swartz, M.A. Interstitial fluid and lymph formation and transport: Physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef]
- Kaminskas, L.M.; Kota, J.; McLeod, V.M.; Kelly, B.D.; Karellas, P.; Porter, C.J. PEGylation of polylysine dendrimers improves absorption and lymphatic targeting following SC administration in rats. J. Control Release 2009, 140, 108–116. [Google Scholar] [CrossRef]
- Nicolas, J.F.; Guy, B. Intradermal, epidermal and transcutaneous vaccination: From immunology to clinical practice. Expert Rev. Vaccines 2008, 7, 1201–1214. [Google Scholar] [CrossRef]
- Waku, T.; Nishigaki, S.; Kitagawa, Y.; Koeda, S.; Kawabata, K.; Kunugi, S.; Kobori, A.; Tanaka, N. Effect of the Hydrophilic-Hydrophobic Balance of Antigen-Loaded Peptide Nanofibers on Their Cellular Uptake, Cellular Toxicity, and Immune Stimulatory Properties. Int. J. Mol. Sci. 2019, 20, 3781. [Google Scholar] [CrossRef] [PubMed]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kroger, M.; Liu, W.K. Shape effect in cellular uptake of PEGylated nanoparticles: Comparison between sphere, rod, cube and disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef]
- Neek, M.; Kim, T.I.; Wang, S.W. Protein-based nanoparticles in cancer vaccine development. Nanomedicine 2019, 15, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Picece, V.; Ou, B.S.; Luo, W.; Pulendran, B.; Appel, E.A. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 2022, 7, 174–195. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-Based Vaccines Against Respiratory Viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Wu, N.; Liu, Y.; Miao, C.; Yu, Z.; Ma, G.; Wu, J. Enhancing the Deformability of the Adjuvant: Achieving Lymph Node Targeted Delivery to Elicit a Long-Lasting Immune Protection. Adv. Healthc. Mater. 2024, 14, e2401520. [Google Scholar] [CrossRef]
- Cirelli, K.M.; Carnathan, D.G.; Nogal, B.; Martin, J.T.; Rodriguez, O.L.; Upadhyay, A.A.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; et al. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 2019, 177, 1153–1171 e1128. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.H.; Melo, M.B.; Kang, M.; Pelet, J.M.; Ruda, V.M.; Foley, M.H.; Hu, J.K.; Kumari, S.; Crampton, J.; Baldeon, A.D.; et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. USA 2016, 113, E6639–E6648. [Google Scholar] [CrossRef]
- Lee, J.H.; Sutton, H.J.; Cottrell, C.A.; Phung, I.; Ozorowski, G.; Sewall, L.M.; Nedellec, R.; Nakao, C.; Silva, M.; Richey, S.T.; et al. Long-primed germinal centres with enduring affinity maturation and clonal migration. Nature 2022, 609, 998–1004. [Google Scholar] [CrossRef]
- Jiang, W.; Maldeney, A.R.; Yuan, X.; Richer, M.J.; Renshaw, S.E.; Luo, W. Ipsilateral immunization after a prior SARS-CoV-2 mRNA vaccination elicits superior B cell responses compared to contralateral immunization. Cell Rep. 2024, 43, 113665. [Google Scholar] [CrossRef]
- Kuraoka, M.; Yeh, C.H.; Bajic, G.; Kotaki, R.; Song, S.; Windsor, I.; Harrison, S.C.; Kelsoe, G. Recall of B cell memory depends on relative locations of prime and boost immunization. Sci. Immunol. 2022, 7, eabn5311. [Google Scholar] [CrossRef]
- Ziegler, L.; Klemis, V.; Schmidt, T.; Schneitler, S.; Baum, C.; Neumann, J.; Becker, S.L.; Gartner, B.C.; Sester, U.; Sester, M. Differences in SARS-CoV-2 specific humoral and cellular immune responses after contralateral and ipsilateral COVID-19 vaccination. EBioMedicine 2023, 95, 104743. [Google Scholar] [CrossRef]
- Iro, M.A.; Khatami, A.; Marshall, A.S.; Pace, D.; Voysey, M.; McKenna, J.; Campbell, D.; Attard-Montalto, S.; Finn, A.; White, C.; et al. Immunological effect of administration of sequential doses of Haemophilus influenzae type b and pneumococcal conjugate vaccines in the same versus alternating limbs in the routine infant immunisation schedule: An open-label randomised controlled trial. Lancet Infect Dis. 2015, 15, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Liang, C.Y.; Desai, P.; Scheaffer, S.M.; Elbashir, S.M.; Edwards, D.K.; Thackray, L.B.; Diamond, M.S. Ipsilateral or contralateral boosting of mice with mRNA vaccines confers equivalent immunity and protection against a SARS-CoV-2 Omicron strain. J. Virol. 2024, 98, e0057424. [Google Scholar] [CrossRef]
- Oussoren, C.; Storm, G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection: III. Influence of surface modification with poly(ethyleneglycol). Pharm. Res. 1997, 14, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Yang, Q.; Bagby, T.R.; Forrest, M.L. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hawley, A.E.; Christy, N.M.; Gray, T.; Illum, L.; Davis, S.S. Surface engineered nanospheres with enhanced drainage into lymphatics and uptake by macrophages of the regional lymph nodes. FEBS Lett. 1994, 344, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Zhong, X.; Du, G.; Hou, Y.; Zhang, Y.; Zhang, Z.; Gong, T.; Zhang, L.; Sun, X. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci. Adv. 2020, 6, eaaz4462. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, J.; Lee, Y.M.; Kim, J.; Choi, H.W.; Lee, J.; Park, H.; Kang, Y.; Kim, I.S.; Lee, B.H.; et al. Poly-paclitaxel/cyclodextrin-SPION nano-assembly for magnetically guided drug delivery system. J. Control Release 2016, 231, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, F.; Hu, J.; Long, J.; Wang, C.; Fu, D.; Ni, Q. Hydrophilic multi-walled carbon nanotubes decorated with magnetite nanoparticles as lymphatic targeted drug delivery vehicles. Chem. Commun. 2009, 4447–4449. [Google Scholar] [CrossRef]
- Jin, H.; Qian, Y.; Dai, Y.; Qiao, S.; Huang, C.; Lu, L.; Luo, Q.; Chen, J.; Zhang, Z. Magnetic Enrichment of Dendritic Cell Vaccine in Lymph Node with Fluorescent-Magnetic Nanoparticles Enhanced Cancer Immunotherapy. Theranostics 2016, 6, 2000–2014. [Google Scholar] [CrossRef]
- Yang, F.; Jin, C.; Yang, D.; Jiang, Y.; Li, J.; Di, Y.; Hu, J.; Wang, C.; Ni, Q.; Fu, D. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur. J. Cancer 2011, 47, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- McCright, J.; Yarmovsky, J.; Maisel, K. Para- and Transcellular Transport Kinetics of Nanoparticles across Lymphatic Endothelial Cells. Mol. Pharm. 2023, 21, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Archer, P.A.; Thomas, S.N. Innovations in lymph node targeting nanocarriers. Semin. Immunol. 2021, 56, 101534. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kawamoto, S.; Bernardo, M.; Brechbiel, M.W.; Knopp, M.V.; Choyke, P.L. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: Comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J. Control Release 2006, 111, 343–351. [Google Scholar] [CrossRef]
| Virus | LN Cell Type | Productive Infection? |
|---|---|---|
| Adenovirus | SSMs | Yes |
| Chikungunya virus | SSMs, LECs | Yes (SSMs), unknown (LECs) |
| Ectromelia virus | B cells, myeloid cells | Yes |
| Gammaherpes viruses | B cells | Yes |
| Human Cytomegalovirus | LECs | Yes |
| Human herpesvirus 6 | CD4 T cells | Yes |
| Human Immunodeficiency virus | CD4 T cells | Yes |
| Murine Cytomegalovirus | SSMs | Yes |
| Murid herpesvirus-4 | SSMs | Yes |
| West Nile virus | SSMs | Yes |
| Zika Virus | SSMs | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brisse, M.E.; Hickman, H.D. Viral Infection and Dissemination Through the Lymphatic System. Microorganisms 2025, 13, 443. https://doi.org/10.3390/microorganisms13020443
Brisse ME, Hickman HD. Viral Infection and Dissemination Through the Lymphatic System. Microorganisms. 2025; 13(2):443. https://doi.org/10.3390/microorganisms13020443
Chicago/Turabian StyleBrisse, Morgan E., and Heather D. Hickman. 2025. "Viral Infection and Dissemination Through the Lymphatic System" Microorganisms 13, no. 2: 443. https://doi.org/10.3390/microorganisms13020443
APA StyleBrisse, M. E., & Hickman, H. D. (2025). Viral Infection and Dissemination Through the Lymphatic System. Microorganisms, 13(2), 443. https://doi.org/10.3390/microorganisms13020443





