McFarland Standards-Based Spectrophotometry Method for Calculating Approximate Multiplicity of Infection for an Obligate Intracellular Bacterium Anaplasma phagocytophilum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates, Cell Lines, and Infection
2.2. Preparation of McFarland Standards
2.3. DNA Extraction and Quantitative Real-Time PCR (QRT-PCR)
2.4. Fluorescence Imaging
2.5. Transmission Electron Microscopy (TEM)
2.6. Statistics
3. Results
3.1. Preparation of McFarland Standards
3.2. OD Measurement of A. phagocytophilum in Crude Extracts Generated from HL-60 Cells
3.3. Calculation of Approximate Number of Bacteria and Multiplicity of Infection (MOI) Using McFarland Standards
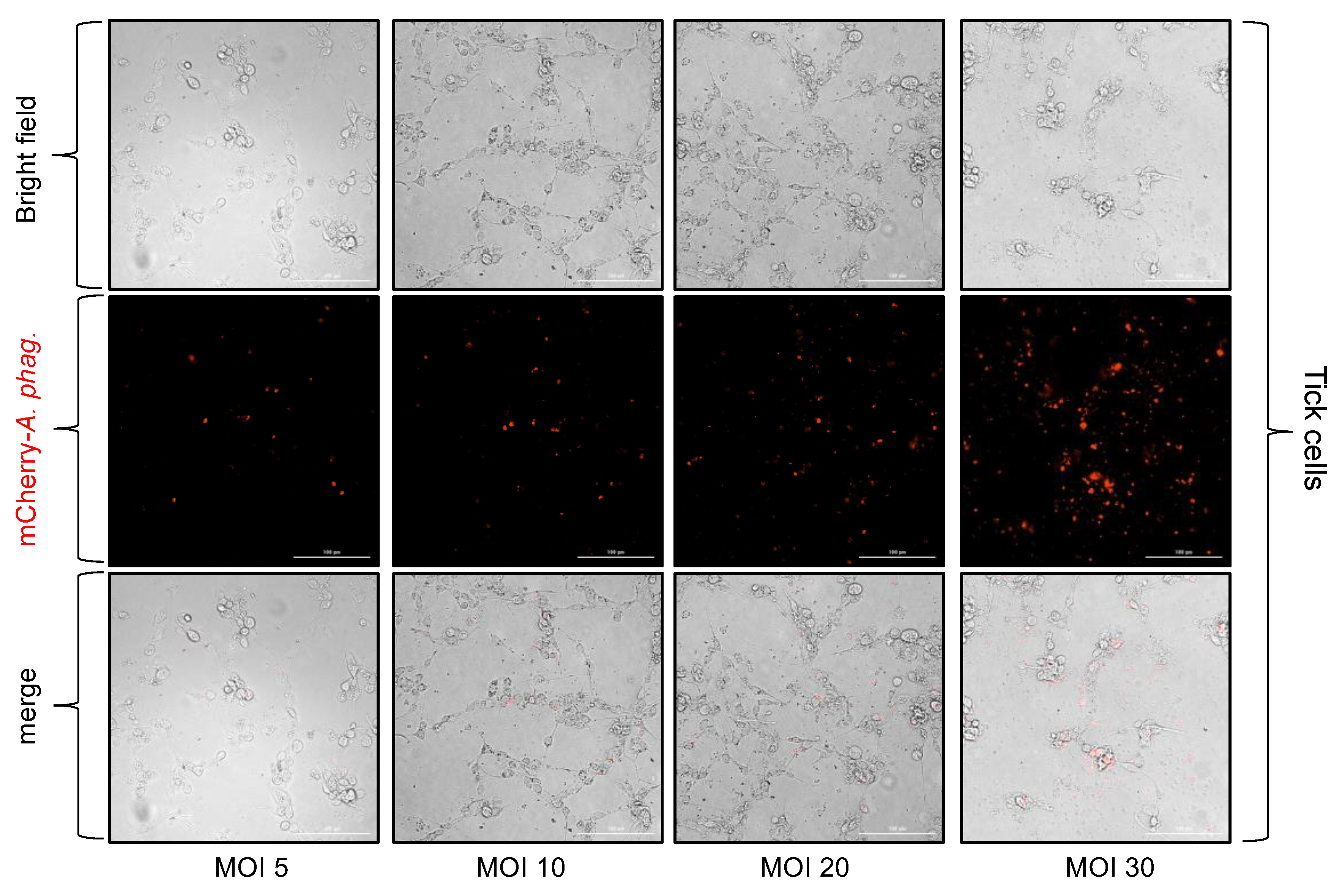
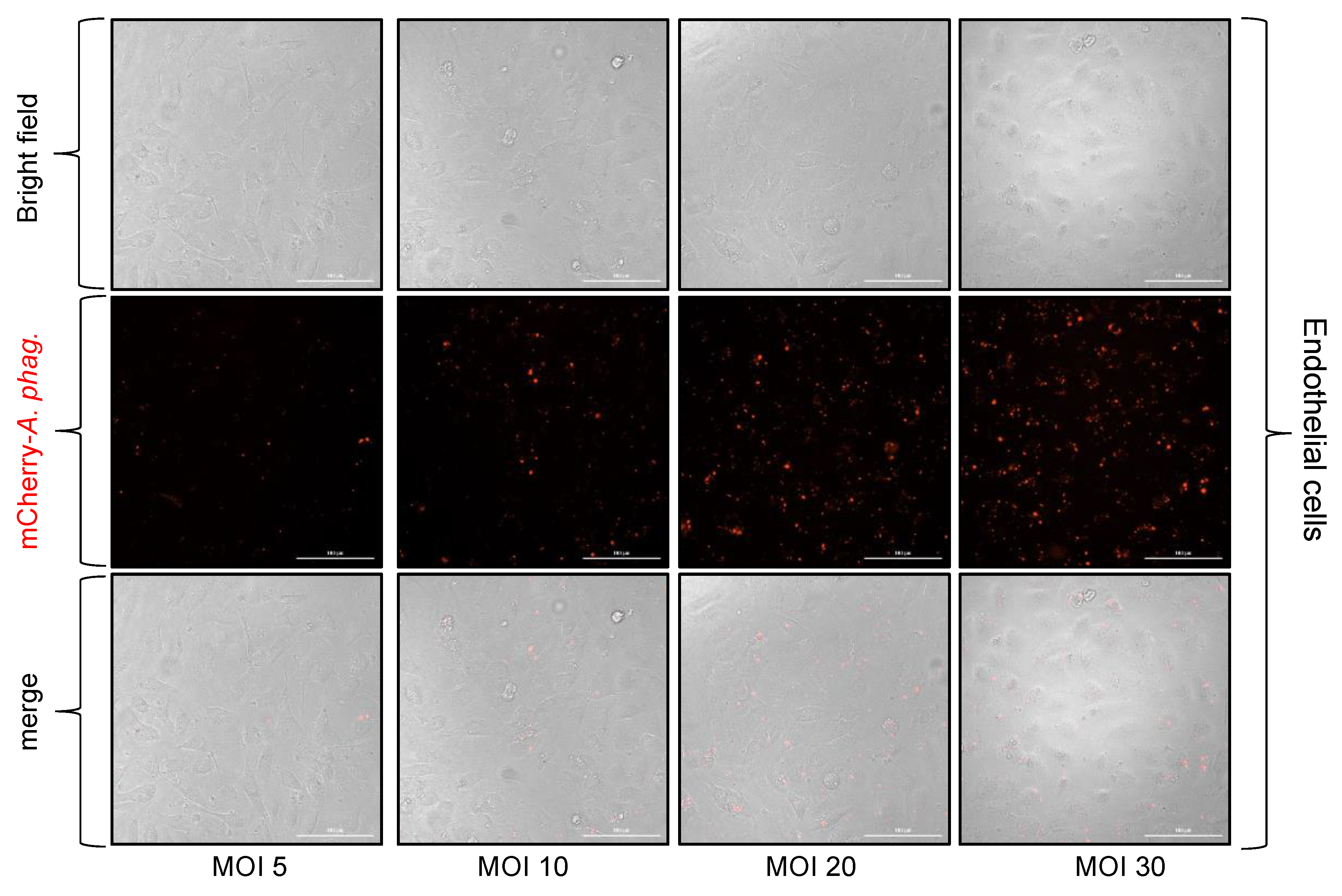
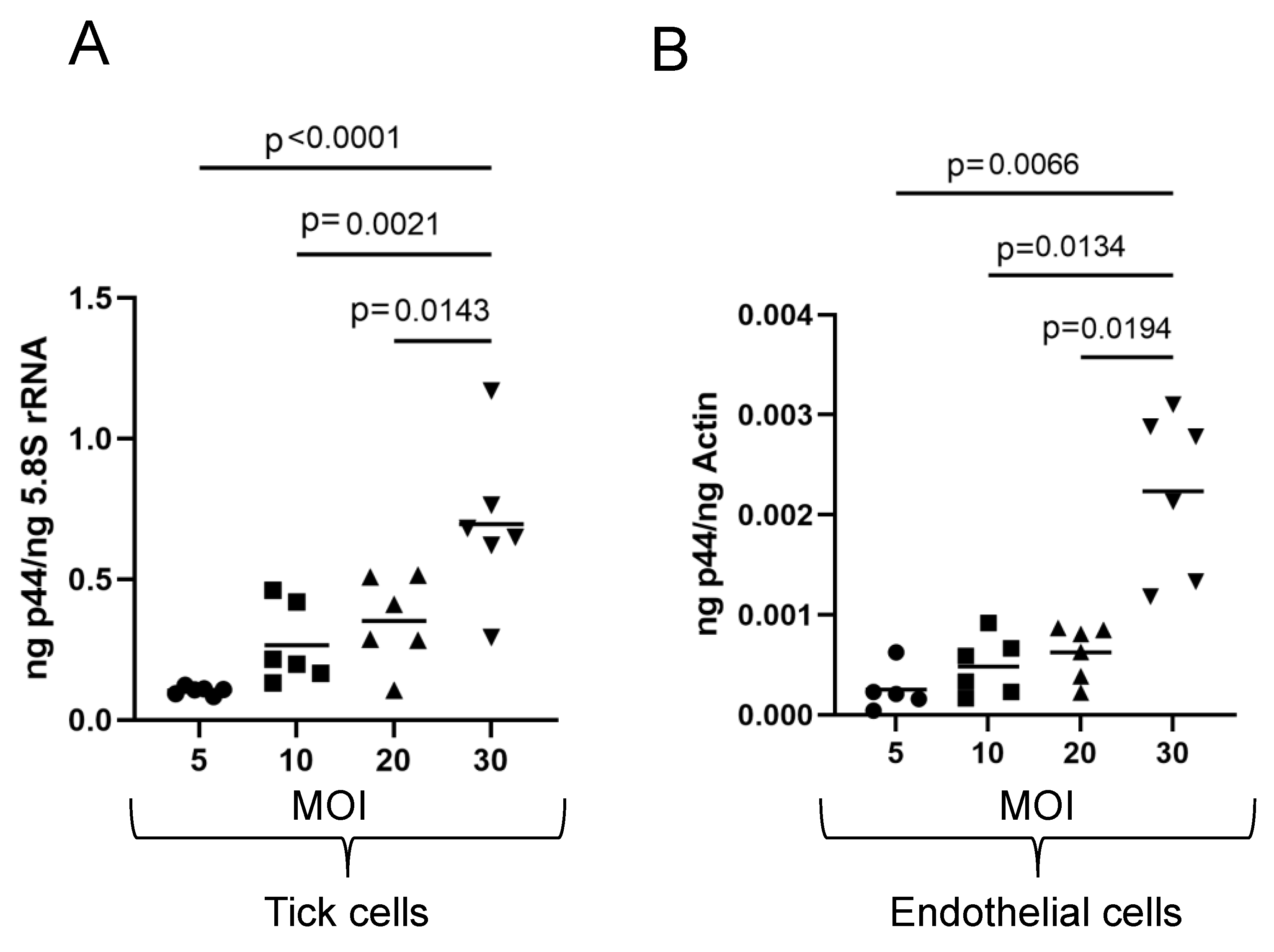
3.4. Anaplasma Phagocytophilum Infection of Tick and Human Endothelial Cells Based on the MOI Determined Using the McFarland Method
3.5. Infection of HL-60 Cells with A. phagocytophilum Based on the MOI Determined Using the McFarland Method
3.6. Consistency Noted with the Use of the McFarland Method in In Vitro Infection Studies with A. phagocytophilum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef]
- Dumler, J.S.; Choi, K.S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S.; Chen, S.M.; Eckman, M.R.; Van Etta, L.L.; Walker, D.H. Human granulocytic ehrlichiosis in the upper Midwest United States: A new species emerging? JAMA 1994, 272, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.J.; Brady, G.; Ackman, D.M.; Wong, S.J.; Jacquette, G.; Lloyd, E.E.; Birkhead, G.S. Human granulocytic ehrlichiosis in New York. Arch. Intern. Med. 1998, 158, 769–773. [Google Scholar] [CrossRef]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Pusterla, N.; Chae, J.S.; Kimsey, R.B.; Berger Pusterla, J.; DeRock, E.; Dumler, J.S.; Madigan, J.E. Transmission of Anaplasma phagocytophila (human granulocytic ehrlichiosis agent) in horses using experimentally infected ticks (Ixodes scapularis). J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 484–488. [Google Scholar] [CrossRef]
- Zeman, P.; Januska, J.; Orolinova, M.; Stuen, S.; Struhar, V.; Jebavy, L. High seroprevalence of granulocytic ehrlichiosis distinguishes sheep that were the source of an alimentary epidemic of tick-borne encephalitis. Wien. Klin. Wochenschr. 2004, 116, 614–616. [Google Scholar] [CrossRef]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L. The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef]
- Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef]
- Foley, J.; Nieto, N. Anaplasma phagocytophilum subverts tick salivary gland proteins. Trends Parasitol. 2007, 23, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.; Magnarelli, L.A. Biology of ticks. Infect. Dis. Clin. N. Am. 2008, 22, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: Subversive manipulators of host cells. Nat. Rev. Microbiol. 2010, 8, 328–339. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Jauron, S.D.; Fingerle, V.; Leitritz, L.; Hayes, S.F.; Hautman, J.M.; Nelson, C.M.; Huberty, B.W.; Kurtti, T.J.; Ahlstrand, G.G.; et al. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J. Clin. Microbiol. 1999, 37, 2518–2524. [Google Scholar] [CrossRef]
- Troese, M.J.; Carlyon, J.A. Anaplasma phagocytophilum dense-cored organisms mediate cellular adherence through recognition of human P-selectin glycoprotein ligand 1. Infect. Immun. 2009, 77, 4018–4027. [Google Scholar] [CrossRef]
- Hodzic, E.; Fish, D.; Maretzki, C.M.; De Silva, A.M.; Feng, S.; Barthold, S.W. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J. Clin. Microbiol. 1998, 36, 3574–3578. [Google Scholar] [CrossRef]
- Kim, H.Y.; Rikihisa, Y. Expression of interleukin-1beta, tumor necrosis factor alpha, and interleukin-6 in human peripheral blood leukocytes exposed to human granulocytic ehrlichiosis agent or recombinant major surface protein P44. Infect. Immun. 2000, 68, 3394–3402. [Google Scholar] [CrossRef]
- Kim, H.Y.; Rikihisa, Y. Roles of p38 mitogen-activated protein kinase, NF-kappaB, and protein kinase C in proinflammatory cytokine mRNA expression by human peripheral blood leukocytes, monocytes, and neutrophils in response to Anaplasma phagocytophila. Infect. Immun. 2002, 70, 4132–4141. [Google Scholar] [CrossRef]
- Taank, V.; Ramasamy, E.; Sultana, H.; Neelakanta, G. An efficient microinjection method to generate human anaplasmosis agent Anaplasma phagocytophilum-infected ticks. Sci. Rep. 2020, 10, 15994. [Google Scholar] [CrossRef]
- McFarland, J. The nephelometer—An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. 1907, 49, 1176–1178. [Google Scholar] [CrossRef]
- Sutton, S. Measurement of microbial cells by optical density. J. Valid. Technol. 2011, 17, 46–49. [Google Scholar]
- Leber, A.L. Preparation of Routine Media and Reagents Used in Antimicrobial Susceptibility Testing. In Clinical Microbiology Procedures Handbook; American Society for Microbiology: Washington, DC, USA, 2016; pp. 5.20.1.1–5.20.3.10. [Google Scholar]
- Mahesh, P.P.; Namjoshi, P.; Sultana, H.; Neelakanta, G. Immunization against arthropod protein impairs transmission of rickettsial pathogen from ticks to the vertebrate host. NPJ Vaccines 2023, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, M.; Anderson, J.F.; Sultana, H.; Neelakanta, G. Rickettsial pathogen uses arthropod tryptophan pathway metabolites to evade reactive oxygen species in tick cells. Cell Microbiol. 2020, 22, e13237. [Google Scholar] [CrossRef] [PubMed]
- Taank, V.; Dutta, S.; Dasgupta, A.; Steeves, T.K.; Fish, D.; Anderson, J.F.; Sultana, H.; Neelakanta, G. Human rickettsial pathogen modulates arthropod organic anion transporting polypeptide and tryptophan pathway for its survival in ticks. Sci. Rep. 2017, 7, 13256. [Google Scholar] [CrossRef]
- Namjoshi, P.; Dahmani, M.; Sultana, H.; Neelakanta, G. Rickettsial pathogen inhibits tick cell death through tryptophan metabolite mediated activation of p38 MAP kinase. iScience 2023, 26, 105730. [Google Scholar] [CrossRef]
- Kralik, P.; Beran, V.; Pavlik, I. Enumeration of Mycobacterium avium subsp. paratuberculosis by quantitative real-time PCR, culture on solid media and optical densitometry. BMC Res. Notes 2012, 5, 114. [Google Scholar] [CrossRef]
- Peñuelas-Urquides, K.; Villarreal-Treviño, L.; Silva-Ramírez, B.; Rivadeneyra-Espinoza, L.; Said-Fernández, S.; de León, M.B. Measuring of growth. A correlation of the optical measurements with colony forming units. Braz. J. Microbiol. 2013, 44, 287–290. [Google Scholar] [CrossRef]
- Mahesh, P.P.; Retnakumar, R.J.; Mundayoor, S. Downregulation of vimentin in macrophages infected with live Mycobacterium tuberculosis is mediated by Reactive Oxygen Species. Sci. Rep. 2016, 6, 21526. [Google Scholar] [CrossRef]
- Mira, P.; Yeh, P.; Hall, B.G. Estimating microbial population data from optical density. PLoS ONE 2022, 17, e0276040. [Google Scholar] [CrossRef]
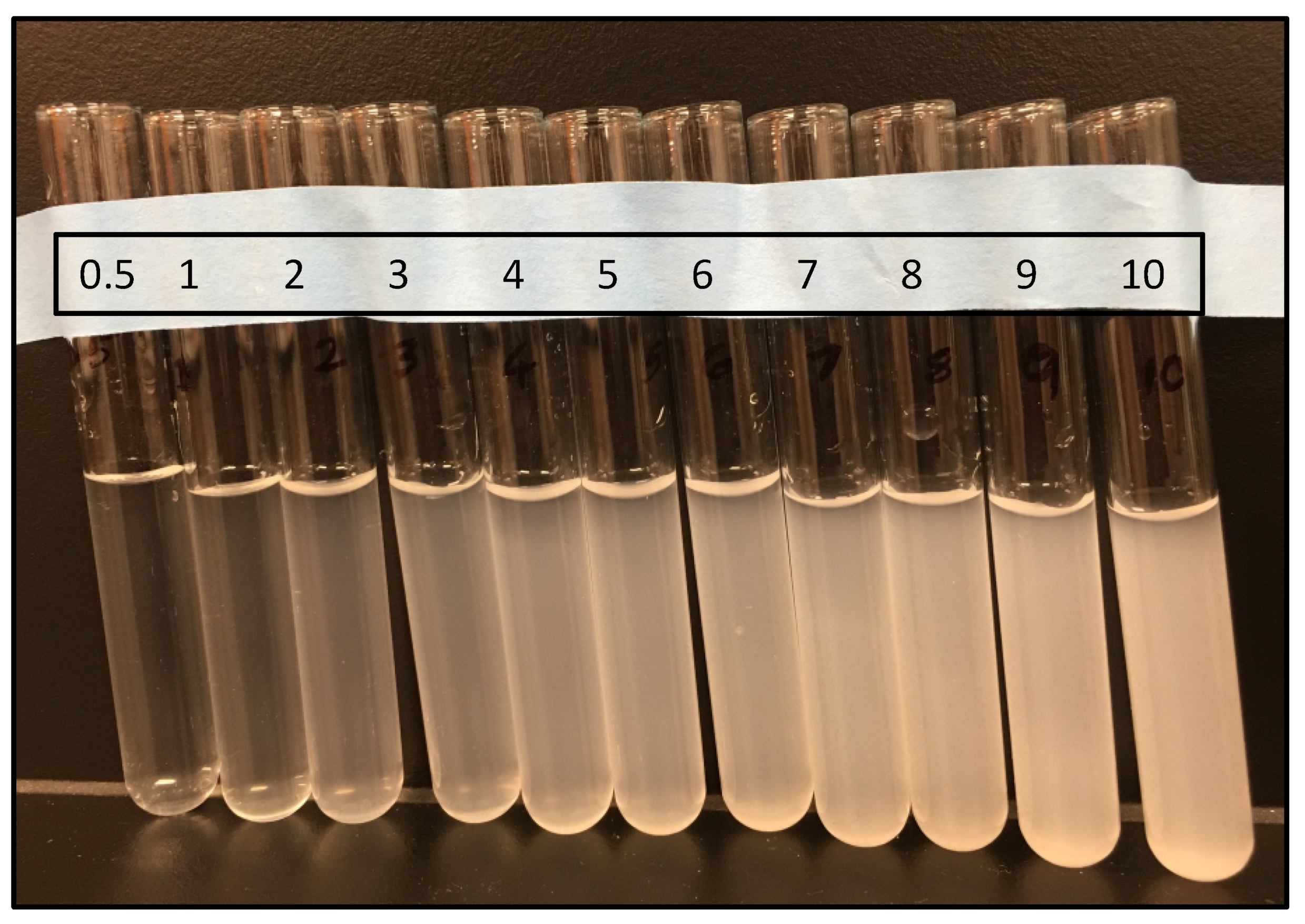


| McFarland Standard | 1% Barium Chloride (mL) | 1% Sulfuric Acid (mL) | Approximate Bacterial Number/mL | Corrected Blank 600 |
|---|---|---|---|---|
| 0.5 | 0.05 | 9.95 | 1.5 × 108 | 0.118 |
| 1 | 0.10 | 9.90 | 3.0 × 108 | 0.2615 |
| 2 | 0.20 | 9.80 | 6.0 × 108 | 0.4745 |
| 3 | 0.30 | 9.70 | 9.0 × 108 | 0.822 |
| 4 | 0.40 | 9.60 | 1.2 × 109 | 1.1555 |
| 5 | 0.50 | 9.50 | 1.5 × 109 | 1.4735 |
| 6 | 0.60 | 9.40 | 1.8 × 109 | 2.039 |
| 7 | 0.70 | 9.30 | 2.1 × 109 | 2.581 |
| 8 | 0.80 | 9.20 | 2.4 × 109 | 3.3545 |
| 9 | 0.90 | 9.10 | 2.7 × 109 | 4.188 |
| 10 | 1.00 | 9.00 | 3.0 × 109 | 5.2365 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahesh, P.P.; Kolape, J.; Sultana, H.; Neelakanta, G. McFarland Standards-Based Spectrophotometry Method for Calculating Approximate Multiplicity of Infection for an Obligate Intracellular Bacterium Anaplasma phagocytophilum. Microorganisms 2025, 13, 662. https://doi.org/10.3390/microorganisms13030662
Mahesh PP, Kolape J, Sultana H, Neelakanta G. McFarland Standards-Based Spectrophotometry Method for Calculating Approximate Multiplicity of Infection for an Obligate Intracellular Bacterium Anaplasma phagocytophilum. Microorganisms. 2025; 13(3):662. https://doi.org/10.3390/microorganisms13030662
Chicago/Turabian StyleMahesh, P. P., Jaydeep Kolape, Hameeda Sultana, and Girish Neelakanta. 2025. "McFarland Standards-Based Spectrophotometry Method for Calculating Approximate Multiplicity of Infection for an Obligate Intracellular Bacterium Anaplasma phagocytophilum" Microorganisms 13, no. 3: 662. https://doi.org/10.3390/microorganisms13030662
APA StyleMahesh, P. P., Kolape, J., Sultana, H., & Neelakanta, G. (2025). McFarland Standards-Based Spectrophotometry Method for Calculating Approximate Multiplicity of Infection for an Obligate Intracellular Bacterium Anaplasma phagocytophilum. Microorganisms, 13(3), 662. https://doi.org/10.3390/microorganisms13030662





