A Review on Recent Trends in Bacteriophages for Post-Harvest Food Decontamination
Abstract
1. Introduction
2. Available Methods to Reduce the Microbial Burden in Food
2.1. Thermal Methods
2.2. Non-Thermal Methods
2.2.1. Chemical Methods
Non-Natural Approaches
Natural Approaches
2.2.2. Physical Methods
2.2.3. Biological Methods
3. Phages Against Foodborne Bacterial Pathogens
3.1. Use of Phages in the Food Industry at the Post-Harvest Stage
3.1.1. Approved Phages for Direct Post-Harvest Applications in Food
3.1.2. Recent Studies on Direct Post-Harvest Phage Treatment in Food
Phages for Biocontrol of Salmonella enterica
Phages for the Biocontrol of Escherichia coli
Phages for the Biocontrol of Listeria monocytogenes
Phages for Biocontrol of Campylobacter spp.
3.1.3. Factors Affecting the Effectiveness of Phage Treatment Directly in Food
3.1.4. Phages in Active Food Packaging Systems
Incorporated Phages Targeting Escherichia coli
Incorporated Phages Targeting Salmonella spp.
Incorporated Phages Targeting Listeria monocytogenes
4. Summary and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Food Safety Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 16 January 2025).
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting non-thermal food processing and preservation methods—Action mechanisms, pros and cons: A technological update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef]
- Deng, L.Z.; Sutar, P.P.; Mujumdar, A.S.; Tao, Y.; Pan, Z.; Liu, Y.H.; Xiao, H.W. Thermal decontamination technologies for microorganisms and mycotoxins in low-moisture foods. Annu. Rev. Food Sci. Technol. 2021, 12, 287–305. [Google Scholar] [CrossRef]
- Mendoza, I.C.; Luna, E.O.; Pozo, M.D.; Vásquez, M.V.; Montoya, D.C.; Moran, G.C.; Romero, L.G.; Yépez, X.; Salazar, R.; Romero-Peña, M.; et al. Conventional and non-conventional disinfection methods to prevent microbial contamination in minimally processed fruits and vegetables. LWT—Food Sci. Technol. 2022, 165, 113714. [Google Scholar] [CrossRef]
- Rendueles, C.; Duarte, A.C.; Escobedo, S.; Fernández, L.; Rodríguez, A.; García, P.; Martínez, B. Combined use of bacteriocins and bacteriophages as food biopreservatives. A review. Int. J. Food Microbiol. 2022, 368, 109611. [Google Scholar] [CrossRef]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012, 2012, 863945. [Google Scholar] [CrossRef]
- Deka, D.; Annapure, U.S.; Shirkole, S.S.; Thorat, B.N. Bacteriophages: An organic approach to food decontamination. J. Food Process. Preserv. 2021, 46, e16101. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Zhan, W.; Li, Z.; Zou, L.; Zhao, Q. Challenges for the application of bacteriophages as effective antibacterial agents in the food industry. J. Sci. Food Agric. 2022, 102, 461–471. [Google Scholar] [CrossRef]
- Alomari, M.M.M.; Dec, M.; Urban-Chmiel, R. Bacteriophages as an alternative method for control of zoonotic and foodborne pathogens. Viruses 2021, 13, 2348. [Google Scholar] [CrossRef]
- Endersen, L.; Coffey, A. The use of bacteriophages for food safety. Curr. Opin. Food Sci. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Ge, H.; Fu, S.; Guo, H.; Hu, M.; Xu, Z.; Zhou, X. Application and challenge of bacteriophage in the food protection. Int. J. Food Microbiol. 2022, 380, 109872. [Google Scholar] [CrossRef]
- Hyla, K.; Dusza, I.; Skaradzińska, A. Recent advances in the application of bacteriophages against common foodborne pathogens. Antibiotics 2022, 11, 1536. [Google Scholar] [CrossRef]
- Lavilla, M.; Domingo-Calap, P.; Sevilla-Navarro, S.; Lasagabaster, A. Natural Killers: Opportunities and challenges for the use of bacteriophages in microbial food safety from the one health perspective. Foods 2023, 12, 552. [Google Scholar] [CrossRef]
- Puligundla, P.; Lim, S. Biocontrol approaches against Escherichia coli O157:H7 in foods. Foods 2022, 11, 756. [Google Scholar] [CrossRef]
- Almutairi, M.; Imam, M.; Alammari, N.; Hafiz, R.; Patel, F.; Alajel, S. Using phages to reduce Salmonella prevalence in chicken meat: A systematic review. PHAGE Ther. Appl. Res. 2022, 3, 15–27. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of phage-based inhibition of Listeria monocytogenes in food products and food processing environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- Ushanov, L.; Lasareishvili, B.; Janashia, I.; Zautner, A.E. Application of Campylobacter jejuni phages: Challenges and perspectives. Animals 2020, 10, 279. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Alsayeqh, A.F.; Aqib, A.I. Role of bacteriophages for optimized health and production of poultry. Animals 2022, 12, 3378. [Google Scholar] [CrossRef]
- Islam, M.R.; Martinez-Soto, C.E.; Lin, J.T.; Khursigara, C.M.; Barbut, S.; Anany, H. A systematic review from basics to omics on bacteriophage applications in poultry production and processing. Crit. Rev. Food Sci. Nutr. 2023, 63, 3097–3129. [Google Scholar] [CrossRef]
- Shahdadi, M.; Safarirad, M.; Berizi, E.; Hosseinzadeh, S. Investigating the effect of phage on reducing Salmonella spp. in poultry meat: A systematic review and meta-analysis. Food Control 2024, 160, 110380. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepulveda, D.R.; Zamudio-Flores, P.B.; Acosta-Muñiz, C.H. Bacteriophages as additives in edible films and coatings. Trends Food Sci. Technol. 2023, 132, 150–161. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Sabu, S.; Sasidharan, A.; Hlaing, M.T.; George, J. Recent trends in bacterial decontamination of food products by hurdle technology: A synergistic approach using thermal and non-thermal processing techniques. Food Res. Int. 2021, 147, 110514. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Liu, C.; Law, C.L.; Mujumdar, A.S.; Xiao, H.W.; Zhang, C. Superheated steam processing: An emerging technology to improve food quality and safety. Crit. Rev. Food Sci. Nutr. 2022, 63, 8720–8736. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Cullen, P.J.; Muhammad, A.I.; Jiang, Z.; Ye, X.; Liu, D.; Ding, T. Cold plasma–based hurdle interventions: New strategies for improving food safety. Food Eng. Rev. 2020, 12, 321–332. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Tang, J.; Adhikari, B.; Cao, P. Innovative technologies for producing and preserving intermediate moisture foods: A review. Food Res. Int. 2019, 116, 90–102. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.H. Hurdle technology: A novel approach for enhanced food quality and safety—A review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Alfy, A.; Kiran, B.V.; Jeevitha, G.C.; Hebbar, H.U. Recent developments in superheated steam processing of foods—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2191–2208. [Google Scholar] [CrossRef]
- Rifna, E.J.; Singh, S.K.; Chakraborty, S.; Dwivedi, M. Effect of thermal and non-thermal techniques for microbial safety in food powder: Recent advances. Food Res. Int. 2019, 126, 108654. [Google Scholar] [CrossRef]
- Aboud, S.A.; Altemimi, A.B.; Al-HiIphy, A.R.S.; Yi-Chen, L.; Cacciola, F. A comprehensive review on infrared heating applications in food processing. Molecules 2019, 24, 4125. [Google Scholar] [CrossRef]
- Manyatsi, T.S.; Al-Hilphy, A.R.; Majzoobi, M.; Farahnaky, A.; Gavahian, M. Effects of infrared heating as an emerging thermal technology on physicochemical properties of foods. Crit. Rev. Food Sci. Nutr. 2022, 63, 6840–6859. [Google Scholar] [CrossRef]
- Khampakool, A.; Soisungwan, S.; Park, S.H. Potential application of infrared-assisted freeze drying (IRAFD) for banana snacks: Drying kinetics, energy consumption, and texture. LWT—Food Sci. Technol. 2019, 99, 355–363. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Kashaninejad, M.; Ziaiifar, A.M.; Ghorbani, M. Peeling of kiwifruit using infrared heating technology: A feasibility and optimization study. LWT—Food Sci. Technol. 2019, 99, 128–137. [Google Scholar] [CrossRef]
- Smith, D.L.; Atungulu, G.G.; Wilson, S.; Shad, Z.M. Deterrence of Aspergillus flavus regrowth and aflatoxin accumulation on shelled corn using infrared heat treatments. Appl. Eng. Agric. 2020, 36, 151–158. [Google Scholar] [CrossRef]
- Wilson, S.A.; Mohammadi Shad, Z.; Oduola, A.A.; Zhou, Z.; Jiang, H.; Carbonero, F.; Atungulu, G.G. Decontamination of mycotoxigenic fungi on shelled corn using selective infrared heating technique. Cereal Chem. 2021, 98, 31–43. [Google Scholar] [CrossRef]
- Alkaya, G.B.; Erdogdu, F.; Ekiz, H.I. Comparison of conventional far-infrared (IR) heating to continuous IR heating-cooling for surface pasteurization of shell eggs contaminated by Salmonella enterica serotype Enteritidis. J. Food Process. Preserv. 2022, 46, e16168. [Google Scholar] [CrossRef]
- Rosenthal, I.; Rosen, B.; Bernstein, S. Surface pasteurization of cottage cheese. Milchwissenschaft-Milk Sci. Int. 1996, 51, 198–201. [Google Scholar]
- Krishnamurthy, K.; Jun, S.; Irudayaraj, J.; Demirci, A. Efficacy of infrared heat treatment for inactivation of Staphylococcus aureus in milk. J. Food Process Eng. 2008, 31, 798–816. [Google Scholar] [CrossRef]
- Lee, E.-H. A review on applications of infrared heating for food processing in comparison to other industries. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier: Amsterdam, The Netherlands, 2021; pp. 431–455. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Koidis, A. Suitability, efficiency, and microbiological safety of novel physical technologies for the processing of ready-to-eat meals, meats, and pumpable products. Int. J. Food Sci. Technol. 2015, 50, 1283–1302. [Google Scholar] [CrossRef]
- Verma, T.; Subbiah, J. Conical twin-screw extrusion is an effective inactivation process for Salmonella in low-moisture foods at temperatures above 65 °C. LWT—Food Sci. Technol. 2019, 114, 108369. [Google Scholar] [CrossRef]
- Goodburn, C.; Wallace, C.A. The microbiological efficacy of decontamination methodologies for fresh produce: A review. Food Control 2013, 32, 418–427. [Google Scholar] [CrossRef]
- Smaoui, S.; Agriopoulou, S.; D’Amore, T.; Tavares, L.; Mousavi Khaneghah, A. The control of Fusarium growth and decontamination of produced mycotoxins by lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2022, 63, 11125–11152. [Google Scholar] [CrossRef] [PubMed]
- Trias, R.; Bañeras, L.; Badosa, E.; Montesinos, E. Bioprotection of golden delicious apples and iceberg lettuce against foodborne bacterial pathogens by lactic acid bacteria. Int. J. Food Microbiol. 2008, 123, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.N.R.; Ferreira, C.D. Essential oil for the control of fungi, bacteria, yeasts and viruses in food: An overview. Crit. Rev. Food Sci. Nutr. 2022, 63, 8960–8974. [Google Scholar] [CrossRef] [PubMed]
- Kaavya, R.; Pandiselvam, R.; Abdullah, S.; Sruthi, N.U.; Jayanath, Y.; Ashokkumar, C.; Chandra Khanashyam, A.; Kothakota, A.; Ramesh, S.V. Emerging non-thermal technologies for decontamination of Salmonella in food. Trends Food Sci. Technol. 2021, 112, 400–418. [Google Scholar] [CrossRef]
- Gracy, T.K.R.; Sharanyakanth, P.S.; Radhakrishnan, M. Non-thermal technologies: Solution for hazardous pesticides reduction in fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2022, 62, 1782–1799. [Google Scholar] [CrossRef]
- Mandal, R.; Singh, A.; Pratap Singh, A. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Turantaş, F.; Kiliç, G.B.; Kiliç, B. Ultrasound in the meat industry: General applications and decontamination efficiency. Int. J. Food Microbiol. 2015, 198, 59–69. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Díaz-Lima, M.C.; Ávila-Sosa, R.; Ruiz-López, I.I.; Corona-Jiménez, E.; Hernández-Carranza, P.; López-Malo, A.; Guerrero-Beltrán, J.A. Effect of UV-C light on Lactobacillus rhamnosus, Salmonella Typhimurium, and Saccharomyces cerevisiae kinetics in inoculated coconut water: Survival and residual effect. J. Food Eng. 2018, 223, 255–261. [Google Scholar] [CrossRef]
- Mandal, R.; Mohammadi, X.; Wiktor, A.; Singh, A.; Singh, A.P. Applications of pulsed light decontamination technology in food processing: An overview. Appl. Sci. 2020, 10, 3606. [Google Scholar] [CrossRef]
- Kuan, Y.H.; Bhat, R.; Patras, A.; Karim, A.A. Radiation processing of food proteins—A review on the recent developments. Trends Food Sci. Technol. 2013, 30, 105–120. [Google Scholar] [CrossRef]
- Harper, D.R.; Parracho, H.M.R.T.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Bolton, D.; McAuliffe, O.; Coffey, A. Bacteriophages in food applications: From foe to friend. Annu. Rev. Food Sci. Technol. 2019, 10, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Mao, J.; Xie, J. Bacteriophage polysaccharide depolymerases and biomedical applications. BioDrugs 2014, 28, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Ha, J.W.; Kang, D.H. Investigation of optimum ohmic heating conditions for inactivation of Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes in apple juice. BMC Microbiol. 2017, 17, 117. [Google Scholar] [CrossRef]
- Yildiz, H.; Bozkurt, H.; Icier, F. Ohmic and conventional heating of pomegranate juice: Effects on rheology, color, and total phenolics. Food Sci. Technol. Int. 2009, 15, 503–512. [Google Scholar] [CrossRef]
- Alkanan, Z.T.; Altemimi, A.B.; Al-Hilphy, A.R.S.; Watson, D.G.; Pratap-Singh, A. Ohmic heating in the food industry: Developments in concepts and applications during 2013–2020. Appl. Sci. 2021, 11, 2507. [Google Scholar] [CrossRef]
- Michalak, J.; Czarnowska-Kujawska, M.; Klepacka, J.; Gujska, E.Z. Effect of microwave heating on the acrylamide formation in foods. Molecules 2020, 25, 4140. [Google Scholar] [CrossRef]
- Tavman, S.; Otles, S.; Glaue, S.; Gogus, N. Food preservation technologies. In Saving Food: Production, Supply Chain, Food Waste and Food Consumption; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 117–140. [Google Scholar] [CrossRef]
- Lazou, A.E. Food extrusion: An advanced process for innovation and novel product development. Crit. Rev. Food Sci. Nutr. 2022, 64, 4532–4560. [Google Scholar] [CrossRef]
- Praeger, U.; Herppich, W.B.; Hassenberg, K. Aqueous chlorine dioxide treatment of horticultural produce: Effects on microbial safety and produce quality—A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 318–333. [Google Scholar] [CrossRef]
- Deng, L.Z.; Mujumdar, A.S.; Pan, Z.; Vidyarthi, S.K.; Xu, J.; Zielinska, M.; Xiao, H.W. Emerging chemical and physical disinfection technologies of fruits and vegetables: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2481–2508. [Google Scholar] [CrossRef]
- Reddy, S.V.R.; Rao, D.V.S.; Sharma, R.R.; Preethi, P.; Pandiselvam, R. Role of ozone in post-harvest disinfection and processing of horticultural crops: A review. Ozone Sci. Eng. 2021, 44, 127–146. [Google Scholar] [CrossRef]
- Xiang, Q.; Fan, L.; Li, Y.; Dong, S.; Li, K.; Bai, Y. A review on recent advances in plasma-activated water for food safety: Current applications and future trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 2250–2268. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.A.T.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Banerjee, R.; Dwivedi, H.P.; Juneja, V.K. Bacteriocins: Potential in food preservation. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 180–186. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef]
- López-Cuellar, M.R.; Rodríguez-Hernández, A.I.; Chavarría-Hernández, N. LAB bacteriocin applications in the last decade. Biotechnol. Biotechnol. Equip. 2016, 30, 1039–1050. [Google Scholar] [CrossRef]
- Da Costa, R.J.; Voloski, F.L.S.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J. Food Qual. 2019, 2019, 4726510. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Li, Y.X.; Erhunmwunsee, F.; Liu, M.; Yang, K.; Zheng, W.; Tian, J. Antimicrobial mechanisms of spice essential oils and application in food industry. Food Chem. 2022, 382, 132312. [Google Scholar] [CrossRef]
- Salanță, L.C.; Cropotova, J. An update on effectiveness and practicability of plant essential oils in the food industry. Plants 2022, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Dini, H.; Fallah, A.A.; Bonyadian, M.; Abbasvali, M.; Soleimani, M. Effect of edible composite film based on chitosan and cumin essential oil-loaded nanoemulsion combined with low-dose gamma irradiation on microbiological safety and quality of beef loins during refrigerated storage. Int. J. Biol. Macromol. 2020, 164, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Sabia, C.; de Niederhäusern, S.; Pellati, F.; Benvenuti, S.; Tardugno, R.; Bondi, M.; Messi, P. Antibacterial activity of Rosmarinus officinalis L. and Thymus vulgaris L. essential oils and their combination against food-borne pathogens and spoilage bacteria in ready-to-eat vegetables. Nat. Prod. Res. 2019, 33, 3568–3572. [Google Scholar] [CrossRef] [PubMed]
- Ozdikmenli, S.; Demirel Zorba, N.N. Evaluation of usage of essential oils instead of spices in meat ball formulation for controlling Salmonella spp. Food Sci. Technol. Int. 2016, 22, 93–101. [Google Scholar] [CrossRef]
- Hernández, H.; Fraňková, A.; Klouček, P.; Banout, J. The effect of the application of thyme essential oil on microbial load during meat drying. J. Vis. Exp. 2018, 2018, e57054. [Google Scholar] [CrossRef]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var. pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef]
- Roobab, U.; Abida, A.; Chacha, J.S.; Athar, A.; Madni, G.M.; Ranjha, M.M.A.N.; Rusu, A.V.; Zeng, X.A.; Aadil, R.M.; Trif, M. Applications of innovative non-thermal pulsed electric field technology in developing safer and healthier fruit juices. Molecules 2022, 27, 4031. [Google Scholar] [CrossRef]
- Mohamed, M.E.A.; Eissa, A.H.A. Pulsed electric fields for food processing technology. In Structure and Function of Food Engineering; Eissa, A.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 275–306. [Google Scholar] [CrossRef]
- Alirezalu, K.; Munekata, P.E.S.; Parniakov, O.; Barba, F.J.; Witt, J.; Toepfl, S.; Wiktor, A.; Lorenzo, J.M. Pulsed electric field and mild heating for milk processing: A review on recent advances. J. Sci. Food Agric. 2020, 100, 16–24. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Song, Q.; Li, R.; Song, X.; Clausen, M.P.; Orlien, V.; Giacalone, D. The effect of high-pressure processing on sensory quality and consumer acceptability of fruit juices and smoothies: A review. Food Res. Int. 2022, 157, 111250. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.A. Inactivation behaviors of foodborne microorganisms in multi-frequency power ultrasound-treated orange juice. Food Control 2014, 46, 189–196. [Google Scholar] [CrossRef]
- Moosavi, M.H.; Khaneghah, A.M.; Javanmardi, F.; Hadidi, M.; Hadian, Z.; Jafarzadeh, S.; Huseyn, E.; Sant’Ana, A.S. A review of recent advances in the decontamination of mycotoxin and inactivation of fungi by ultrasound. Ultrason. Sonochem. 2021, 79, 105755. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.H.M.C.; Silva, E.K.; Alvarenga, V.O.; Moraes, J.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Effects of ultrasound energy density on the non-thermal pasteurization of chocolate milk beverage. Ultrason. Sonochem. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Techathuvanan, C.; D’Souza, D.H. High intensity ultrasound for Salmonella Enteritidis inactivation in culture and liquid whole eggs. J. Food Sci. 2018, 83, 1733–1739. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Barut Gök, S.; Yüksel, A.N.; Tekgül, Y.; Koç, G.Ç.; Kothakota, A. Evaluation of the impact of UV radiation on rheological and textural properties of food. J. Texture Stud. 2022, 53, 800–808. [Google Scholar] [CrossRef]
- Dhar, R.; Basak, S.; Chakraborty, S. Pasteurization of fruit juices by pulsed light treatment: A review on the microbial safety, enzymatic stability, and kinetic approach to process design. Compr. Rev. Food Sci. Food Saf. 2022, 21, 499–540. [Google Scholar] [CrossRef]
- Cheigh, C.I.; Hwang, H.J.; Chung, M.S. Intense pulsed light (IPL) and UV-C treatments for inactivating Listeria monocytogenes on solid medium and seafoods. Food Res. Int. 2013, 54, 745–752. [Google Scholar] [CrossRef]
- Ganan, M.; Hierro, E.; Hospital, X.F.; Barroso, E.; Fernández, M. Use of pulsed light to increase the safety of ready-to-eat cured meat products. Food Control 2013, 32, 512–517. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Charles, F.; Renard, C.M.G.C.; Page, D.; Carlin, F. Pulsed light effects on surface decontamination, physical qualities and nutritional composition of tomato fruit. Postharvest Biol. Technol. 2013, 86, 29–36. [Google Scholar] [CrossRef]
- Salehi, F. Application of pulsed light technology for fruits and vegetables disinfection: A review. J. Appl. Microbiol. 2022, 132, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Agüero, M.V.; Jagus, R.J.; Martín-Belloso, O.; Soliva-Fortuny, R. Surface decontamination of spinach by intense pulsed light treatments: Impact on quality attributes. Postharvest Biol. Technol. 2016, 121, 118–125. [Google Scholar] [CrossRef]
- Kasahara, I.; Carrasco, V.; Aguilar, L. Inactivation of Escherichia coli in goat milk using pulsed ultraviolet light. J. Food Eng. 2015, 152, 43–49. [Google Scholar] [CrossRef]
- Sharifi-Yazdi, M.K.; Darghahi, H. Inactivation of pathogenic bacteria using pulsed UV-light and its application in water disinfection and quality control. Acta Med. Iran. 2006, 44, 305–308. [Google Scholar]
- Fernandes, Â.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effect of gamma and electron beam irradiation on the physico-chemical and nutritional properties of mushrooms: A review. Food Chem. 2012, 135, 641–650. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Rodrigues, B.L.; Bernardes, P.C.; Conte-Junior, C.A. Principles and applications of non-thermal technologies and alternative chemical compounds in meat and fish. Crit. Rev. Food Sci. Nutr. 2021, 61, 1163–1183. [Google Scholar] [CrossRef]
- Ranathunga, N.S.; Wijayasekara, K.N.; Abeyrathne, E.D.N.S. Application of bio-preservation to enhance food safety: A review. Korean J. Food Preserv. 2023, 30, 179–189. [Google Scholar] [CrossRef]
- Sankar, K.; Baranitharan, M.; Shreyasi, E.; Keerthish, P.N.; Swethaa, R. Biopreservative technologies of food: An alternative to chemical preservation and recent developments. Food Sci. Biotechnol. 2023, 32, 1337–1350. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of lactic acid bacteria in food preservation and safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Muccilli, S.; Restuccia, C. Bioprotective role of yeasts. Microorganisms 2015, 3, 588–611. [Google Scholar] [CrossRef]
- Ge, H.; Lin, C.; Xu, Y.; Hu, M.; Xu, Z.; Geng, S.; Jiao, X.; Chen, X. A phage for the controlling of Salmonella in poultry and reducing biofilms. Vet. Microbiol. 2022, 269, 109432. [Google Scholar] [CrossRef] [PubMed]
- Poojari, K.; Akhila, D.S.; Raj, M.J.R.; Santhosh, K.S.; Kenjar, A.; Ashwath, P. Biocontrol of Escherichia coli and Salmonella in poultry meat using phage cocktail. Iran. J. Vet. Res. 2022, 23, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Witte, S.; Huijboom, L.; Klamert, S.; van de Straat, L.; Hagens, S.; Fieseler, L.; de Vegt, B.T.; van Mierlo, J.T. Application of bacteriophages EP75 and EP335 efficiently reduces viable cell counts of Escherichia coli O157 on beef and vegetables. Food Microbiol. 2022, 104, 103978. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Tokman, J.I.; Woolston, J.; Sulakvelidze, A. Phage biocontrol improves food safety by significantly reducing the level and prevalence of Escherichia coli O157:H7 in various foods. J. Food Prot. 2020, 83, 668–676. [Google Scholar] [CrossRef]
- Wang, L.; Qu, K.; Li, X.; Cao, Z.; Wang, X.; Li, Z.; Song, Y.; Xu, Y. Use of bacteriophages to control Escherichia coli O157:H7 in domestic ruminants, meat products, and fruits and vegetables. Foodborne Pathog. Dis. 2017, 14, 483–493. [Google Scholar] [CrossRef]
- Lopes, R.P.; Mota, M.J.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. Application of high pressure with homogenization, temperature, carbon dioxide, and cold plasma for the inactivation of bacterial spores: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 532–555. [Google Scholar] [CrossRef]
- Kuchment, A. The fading of phage therapy. In The Forgotten Cure: The Past and Future of Phage Therapy, 1st ed.; Springer: New York, NY, USA, 2012; pp. 35–42. [Google Scholar] [CrossRef]
- Ranveer, S.A.; Dasriya, V.; Ahmad, F.; Dhillon, H.S.; Samtiya, M.; Shama, E. Positive and negative aspects of bacteriophages and their immense role in the food chain. npj Sci. Food 2024, 8, 1. [Google Scholar] [CrossRef]
- Nanda, A.M.; Thormann, K.; Frunzke, J. Impact of spontaneous prophage induction on the fitness of bacterial populations and host-microbe interactions. J. Bacteriol. 2015, 197, 410–419. [Google Scholar] [CrossRef]
- Chen, Q.; Dharmaraj, T.; Cai, P.C.; Burgener, E.B.; Haddock, N.L.; Spakowitz, A.J.; Bollyky, P.L. Bacteriophage and bacterial susceptibility, resistance, and tolerance to antibiotics. Pharmaceutics 2022, 14, 1425. [Google Scholar] [CrossRef]
- Harada, L.K.; Silva, E.C.; Campos, W.F.; Del Fiol, F.S.; Vila, M.; Dąbrowska, K.; Krylov, V.N.; Balcão, V.M. Biotechnological applications of bacteriophages: State of the art. Microbiol. Res. 2018, 212–213, 38–58. [Google Scholar] [CrossRef]
- Pereira, C.; Costa, P.; Duarte, J.; Balcão, V.M.; Almeida, A. Phage therapy as a potential approach in the biocontrol of pathogenic bacteria associated with shellfish consumption. Int. J. Food Microbiol. 2021, 338, 108995. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V.; Vila, M.M.D.C.; Teixeira, J.A.; Balcão, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M. Bacteriophages and the one health approach to combat multidrug resistance: Is this the way? Antibiotics 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Navarro, S.; Catalá-Gregori, P.; García, C.; Cortés, V.; Marin, C. Salmonella Infantis and Salmonella Enteritidis specific bacteriophages isolated from poultry faeces as a complementary tool for cleaning and disinfection against Salmonella. Compar. Immunol. Microbiol. Infect. Dis. 2020, 68, 101405. [Google Scholar] [CrossRef]
- Tang, S.S.; Biswas, S.K.; Tan, W.S.; Saha, A.K.; Leo, B.F. Efficacy and potential of phage therapy against multidrug resistant Shigella spp. PeerJ 2019, 7, e6225. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage biocontrol applications in food production and processing. Curr. Issues Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, M.T. Considerations for using bacteriophages for plant disease control. Bacteriophage 2012, 2, e23857. [Google Scholar] [CrossRef]
- Almeida, A.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; Costa, L.; Faustino, M.A.F. Phage therapy and photodynamic therapy: Low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar. Drugs 2009, 7, 268–313. [Google Scholar] [CrossRef]
- Jassim, S.A.A.; Limoges, R.G. Bacteriophages: Practical Applications for Nature’s Biocontrol; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Ruban, S.; Kannan, P.; Quintoil, M. Bacteriophage: A novel biocontrol agent against food pathogens: Myth or reality. In Applied Food Science and Engineering with Industrial Applications, 1st ed.; Aguilar, C.N., Carvajal-Millan, E., Eds.; Apple Academic Press: New York, NY, USA, 2019; pp. 1–15. [Google Scholar]
- Guenther, S.; Herzig, O.; Fieseler, L.; Klumpp, J.; Loessner, M.J. Biocontrol of Salmonella Typhimurium in RTE foods with the virulent bacteriophage FO1-E2. Int. J. Food Microbiol. 2012, 154, 66–72. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Costa, A.R.; Kluskens, L.D.; Azeredo, J. Revisiting phage therapy: New applications for old resources. Trends Microbiol. 2015, 23, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages contribute to the spread of antibiotic resistance genes among foodborne pathogens of the Enterobacteriaceae family—A review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef] [PubMed]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of environmental factors on phage-bacteria interaction and on the efficacy and infectivity of phage P100. Front. Microbiol. 2016, 7, 1152. [Google Scholar] [CrossRef] [PubMed]
- Iriarte, F.B.; Balogh, B.; Momol, M.T.; Smith, L.M.; Wilson, M.; Jones, J.B. Factors affecting survival of bacteriophage on tomato leaf surfaces. Appl. Environ. Microbiol. 2007, 73, 1704–1711. [Google Scholar] [CrossRef]
- Jonczyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages-review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef]
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phage therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef]
- Wessels, K.; Rip, D.; Gouws, P. The effect of spray parameters on the survival of bacteriophages. Processes 2022, 10, 673. [Google Scholar] [CrossRef]
- De Melo, A.G.; Levesque, S.; Moineau, S. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 2018, 49, 185–190. [Google Scholar] [CrossRef]
- Yen, M.; Cairns, L.S.; Camilli, A. A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nat. Commun. 2017, 8, 14187. [Google Scholar] [CrossRef]
- Garvey, M. Bacteriophages and food production: Biocontrol and bio-preservation options for food safety. Antibiotics 2022, 11, 1324. [Google Scholar] [CrossRef]
- Phothaworn, P.; Supokaivanich, R.; Lim, J.; Klumpp, J.; Imam, M.; Kutter, E.; Galyov, E.E.; Dunne, M.; Korbsrisate, S. Development of a broad-spectrum Salmonella phage cocktail containing Viunalike and Jerseylike viruses isolated from Thailand. Food Microbiol. 2020, 92, 103586. [Google Scholar] [CrossRef] [PubMed]
- Tomat, D.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of a novel cocktail of six lytic bacteriophages against Shiga toxin-producing Escherichia coli in broth, milk and meat. Food Microbiol. 2018, 76, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Komora, N.; Maciel, C.; Pinto, C.A.; Ferreira, V.; Brandão, T.R.S.; Saraiva, J.M.A.; Castro, S.M.; Teixeira, P. Non-thermal approach to Listeria monocytogenes inactivation in milk: The combined effect of high pressure, pediocin PA-1 and bacteriophage P100. Food Microbiol. 2020, 86, 103315. [Google Scholar] [CrossRef] [PubMed]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.; Castro, S.M.; Saraiva, J.A.; Teixeira, P. Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model—High pressure processing assisted by bacteriophage P100 and bacteriocinogenic Pediococcus acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef]
- Komora, N.; Bruschi, C.; Ferreira, V.; Maciel, C.; Brandão, T.R.S.; Fernandes, R.; Saraiva, J.A.; Castro, S.M.; Teixeira, P. The protective effect of food matrices on Listeria lytic bacteriophage P100 application towards high pressure processing. Food Microbiol. 2018, 76, 416–425. [Google Scholar] [CrossRef]
- Ishaq, A.; Syed, Q.A.; Ebner, P.D.; Ubaid ur Rahman, H. Multiple hurdle technology to improve microbial safety, quality and oxidative stability of refrigerated raw beef. LWT—Food Sci. Technol. 2021, 138, 110529. [Google Scholar] [CrossRef]
- Monferrer, E.; Domingo-Calap, P. Virus-host coevolution as a tool for controlling bacterial resistance to phage therapy. J. Biotechnol. Biomed. 2019, 2, 96–104. [Google Scholar] [CrossRef][Green Version]
- Peng, H.; Chen, I.A.; Qimron, U. Engineering phages to fight multidrug-resistant bacteria. Chem. Rev. 2025, 125, 933–971. [Google Scholar] [CrossRef]
- Cristobal-Cueto, P.; García-Quintanilla, A.; Esteban, J.; García-Quintanilla, M. Phages in food industry biocontrol and bioremediation. Antibiotics 2021, 10, 786. [Google Scholar] [CrossRef]
- Suja, E.; Gummadi, S.N. Advances in the applications of bacteriophages and phage products against food-contaminating bacteria. Crit. Rev. Microbiol. 2023, 50, 702–727. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Salmonella. Available online: https://www.cdc.gov/salmonella/general/index.html#two (accessed on 16 January 2025).
- Popa, G.L.; Popa, M.I. Salmonella spp. Infection—A continuous threat worldwide. Germs 2021, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Trampel, D.W.; Holder, T.G.; Gast, R.K. Integrated farm management to prevent Salmonella Enteritidis contamination of eggs. J. Appl. Poultry Res. 2014, 23, 353–365. [Google Scholar] [CrossRef]
- Paudyal, N.; Anihouvi, V.; Hounhouigan, J.; Matsheka, M.I.; Sekwati-Monang, B.; Amoa-Awua, W.; Atter, A.; Ackah, N.B.; Mbugua, S.; Asagbra, A.; et al. Prevalence of foodborne pathogens in food from selected African countries—A meta-analysis. Int. J. Food Microbiol. 2017, 249, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Al-Hindi, R.R.; Alharbi, M.G.; Alotibi, I.; Azhari, S.A.; Algothmi, K.M.; Esmael, A. Application of a novel lytic Jerseyvirus phage LPSent1 for the biological control of the multidrug-resistant Salmonella Enteritidis in foods. Front. Microbiol. 2023, 14, 1135806. [Google Scholar] [CrossRef] [PubMed]
- Brenner, T.; Schultze, D.M.; Mahoney, D.; Wang, S. Reduction of nontyphoidal Salmonella enterica in broth and on raw chicken breast by a broad-spectrum bacteriophage cocktail. J. Food Prot. 2024, 87, 100207. [Google Scholar] [CrossRef]
- Han, S.; Choi, M.W.; Byun, K.H.; Kim, B.H.; Song, M.S.; Kang, I.; Ha, S.D. Characterization of Salmonella ser. Enteritidis-specific bacteriophages and biocontrol strategy to reduce S. Enteritidis on egg products using bacteriophages and essential oil compounds. Food Control 2024, 160, 110304. [Google Scholar] [CrossRef]
- He, J.; Wong, C.W.Y.; Schultze, D.M.; Wang, S. Inactivation of Salmonella Enteritidis in liquid egg yolk and egg white using bacteriophage cocktails. Curr. Res. Food Sci. 2024, 8, 100703. [Google Scholar] [CrossRef]
- Kokab, A.; Sheikh, A.A.; Rabbani, M.; Shehzad, W.; Riaz, M.I.; Raza, S.; Durrani, R.H. Physiological profiling of lytic bacteriophages and their efficiency against Salmonella Enteritidis on chicken breast cuts. Pak. J. Zool. 2024, 56, 375–384. [Google Scholar] [CrossRef]
- Choi, Y.; Kwak, M.J.; Kang, M.G.; Kang, A.N.; Lee, W.; Mun, D.; Choi, H.; Park, J.; Eor, J.Y.; Song, M.; et al. Molecular characterization and environmental impact of newly isolated lytic phage SLAM_phiST1N3 in the Cornellvirus genus for biocontrol of a multidrug-resistant Salmonella Typhimurium in the swine industry chain. Sci. Total Environ. 2024, 922, 171208. [Google Scholar] [CrossRef]
- Evran, E.; Dasan, B.G.; Tayyarcan, E.K.; Boyaci, I.H. Effect of sequential treatment of plasma activated water and bacteriophage on decontamination of Salmonella Typhimurium in lettuce. Food Bioprocess Technol. 2024, 17, 3790–3799. [Google Scholar] [CrossRef]
- Jung, S.J.; Ashrafudoulla, M.; Kang, I.; Ha, S.D. Isolation and characterization of multidrug-resistant Salmonella-specific bacteriophages and their antibacterial efficiency in chicken breast. Poultry Sci. 2023, 102, 103073. [Google Scholar] [CrossRef] [PubMed]
- Unverdi, A.; Erol, H.B.; Kaskatepe, B.; Babacan, O. Characterization of Salmonella phages isolated from poultry coops and its effect with nisin on food bio-control. Food Sci. Nutr. 2024, 12, 2760–2771. [Google Scholar] [CrossRef] [PubMed]
- Gonmei, L.; Inbaraj, S.; Geyi, D.; Prakashan, L.; Dhiman, H.; Athira, V.; Thomas, P. Evaluation of bacteriophage cocktail as biopreservatives against Salmonella enterica serovar Typhimurium in chicken meat. Food Biosci. 2023, 56, 103290. [Google Scholar] [CrossRef]
- Pan, H.; Shu, M.; Li, T.; Shen, K.; Zhao, Y.; Liao, N.; Zhong, C.; Wu, G. Isolation and characterization of two lytic phages against multidrug-resistant Salmonella and their application as a cocktail for biocontrol in foods. LWT—Food Sci. Technol. 2023, 184, 115184. [Google Scholar] [CrossRef]
- Torkashvand, N.; Kamyab, H.; Reza, A.; Mohammad, S.; Khoshayand, R. Isolation, characterization, and genome analysis of a broad host range Salmonella phage vB_SenS_TUMS_E4: A candidate bacteriophage for biocontrol. Vet. Res. Commun. 2023, 47, 1493–1503. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, Y.; Wang, C.; Zayda, M.; Thida, A.; Noor, T.; Minh, H.; Yu, P.; Ma, M.; Gong, D.; et al. Biocontrol of Salmonella Typhimurium in milk, lettuce, raw pork meat and ready-to-eat steamed-chicken breast by using a novel bacteriophage with broad host range. Int. J. Food Microbiol. 2023, 402, 110295. [Google Scholar] [CrossRef]
- Erol, H.B.; Kaskatepe, B. Effect of phage and rhamnolipid on Salmonella Infantis biofilm removal and biological control of phage on food deterioration. Int. J. Food Sci. Technol. 2024, 59, 120–128. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Kim, H.J.; Her, E.; Shaila, S.; Park, S.H.; Ha, S.D. Characterization of Salmonella Thompson-specific bacteriophages and their preventive application against Salmonella Thompson biofilm on eggshell as a promising antimicrobial agent in the food industry. Food Control 2023, 154, 110008. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Islam, M.S.; Yan, T.; Zhou, Y.; Liang, L.; Connerton, I.F.; Deng, K.; Li, J. Application of a novel phage vB_SalS-LPSTLL for the biological control of Salmonella in foods. Food Res. Int. 2021, 147, 110492. [Google Scholar] [CrossRef]
- Lu, Z.; Marchant, J.; Thompson, S.; Melgarejo, H.; Ignatova, D.; Kopić, S.; Damaj, R.; Trejo, H.; Paramo, R.; Reed, A.; et al. Bacteriophages isolated from turkeys infecting diverse Salmonella serovars. Front. Microbiol. 2022, 13, 933751. [Google Scholar] [CrossRef]
- Thung, T.Y.; Lee, E.; Mahyudin, N.A.; Anuradha, K.; Mazlan, N.; Kuan, C.H.; Pui, C.F.; Ghazali, F.M.; Mahmud Ab Rashid, N.K.; Rollon, W.D.; et al. Evaluation of a lytic bacteriophage for bio-control of Salmonella Typhimurium in different food matrices. LWT—Food Sci. Technol. 2019, 105, 211–214. [Google Scholar] [CrossRef]
- Li, T.; Chen, H.; Zhao, J.; Tao, Z.; Lan, W.; Zhao, Y.; Sun, X. Characterization of phage vB_SalM_SPJ41 and the reduction of risk of antibiotic-resistant Salmonella enterica contamination in two ready-to-eat foods. Antibiotics 2023, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, Y.; Wang, L.; Yao, L.; Li, F.; Zhai, Y.; Zhang, Y. Biocontrol of Salmonella Typhimurium in raw salmon fillets and scallop adductors by using bacteriophage SLMP1. J. Food Prot. 2018, 81, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Le, T.S.; Southgate, P.C.; O’Connor, W.; Poole, S.; Kurtbӧke, D.I. Bacteriophages as biological control agents of enteric bacteria contaminating edible oysters. Curr. Microbiol. 2018, 75, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Esmael, A.; Azab, E.; Gobouri, A.A.; Nasr-Eldin, M.A.; Moustafa, M.M.A.; Mohamed, S.A.; Badr, O.A.M.; Abdelatty, A.M. Isolation and characterization of two lytic bacteriophages infecting a multi-drug resistant Salmonella typhimurium and their efficacy to combat salmonellosis in ready-to-use foods. Microorganisms 2021, 9, 423. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.; Park, M.K. Isolation, characterization, and application of a novel, lytic phage vB_SalA_KFSST3 with depolymerase for the control of Salmonella and its biofilm on cantaloupe under cold temperature. Food Res. Int. 2023, 172, 113062. [Google Scholar] [CrossRef]
- Wong, C.W.Y.; Delaquis, P.; Goodridge, L.; Lévesque, R.C.; Fong, K.; Wang, S. Inactivation of Salmonella enterica on post-harvest cantaloupe and lettuce by a lytic bacteriophage cocktail. Curr. Res. Food Sci. 2020, 2, 25–32. [Google Scholar] [CrossRef]
- Azari, R.; Yousefi, M.H.; Taghipour, Z.; Wagemans, J.; Lavigne, R.; Hosseinzadeh, S.; Mazloomi, S.M.; Vallino, M.; Khalatbari-Limaki, S.; Berizi, E. Application of the lytic bacteriophage Rostam to control Salmonella enteritidis in eggs. Int. J. Food Microbiol. 2023, 389, 110097. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.D.; Nan, Y.; Stanford, K.; Holley, R.; McAllister, T.; Narváez-Bravo, C. SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int. J. Food Microbiol. 2019, 305, 108250. [Google Scholar] [CrossRef]
- Li, S.; Konoval, H.M.; Marecek, S.; Lathrop, A.A.; Pokharel, S. Salmonella spp. response to lytic bacteriophage and lactic acid on marinated and tenderized raw pork loins. Foods 2022, 11, 879. [Google Scholar] [CrossRef]
- Shebs-Maurine, E.L.; Giotto, F.M.; Laidler, S.T.; de Mello, A.S. Effects of bacteriophages and peroxyacetic acid applications on beef contaminated with Salmonella during different grinding stages. Meat Sci. 2021, 173, 108407. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Li, S.; Strauss, H.; Pokharel, S. Salmonella Typhimurium DT 104 response to lytic bacteriophage and lactobionic acid on raw chicken breast. Food Microbiol. 2021, 100, 103862. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Ravindran, V.B.; Surapaneni, A.; Mantri, N.; Ball, A.S. Review: Trends in point-of-care diagnosis for Escherichia coli O157:H7 in food and water. Int. J. Food Microbiol. 2021, 349, 109233. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Wang, L.; Ding, P.; Yang, P.; Yang, B. Transcriptional activator OvrA encoded in O island 19 modulates virulence gene expression in enterohemorrhagic Escherichia coli O157:H7. J. Infect. Dis. 2020, 221, 820–829. [Google Scholar] [CrossRef]
- Li, Y.K.; Chen, H.; Shu, M.; Zhong, C.; Bi, Y.; Yang, H.H.; Wu, G.P. Isolation, characterization and application of an alkaline resistant virulent bacteriophage JN01 against Escherichia coli O157:H7 in milk and beef. LWT—Food Sci. Technol. 2021, 144, 111266. [Google Scholar] [CrossRef]
- Salaheen, S.; Kim, S.W.; Karns, J.S.; Hovingh, E.; Haley, B.J.; Van Kessel, J.A.S. Metagenomic analysis of the fecal microbiomes from Escherichia coli O157:H7-shedding and non-shedding cows on a single dairy farm. Food Control 2019, 102, 76–80. [Google Scholar] [CrossRef]
- Kintz, E.; Byrne, L.; Jenkins, C.; McCarthy, N.; Vivancos, R.; Hunter, P. Outbreaks of Shiga toxin–producing Escherichia coli linked to sprouted seeds, salad, and leafy greens: A systematic review. J. Food Prot. 2019, 82, 1950–1958. [Google Scholar] [CrossRef]
- Mok, J.H.; Niu, Y.; Yousef, A.; Zhao, Y.; Sastry, S.K. Spatial persistence of Escherichia coli O157:H7 flowing on micropatterned structures inspired by stomata and microgrooves of leafy greens. Innov. Food Sci. Emerg. Technol. 2022, 75, 102889. [Google Scholar] [CrossRef]
- Yeni, F.; Yavaş, S.; Alpas, H.; Soyer, Y. Most common foodborne pathogens and mycotoxins on fresh produce: A review of recent outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef]
- Choo, K.W.; Mao, L.; Mustapha, A. CAM-21, a novel lytic phage with high specificity towards Escherichia coli O157:H7 in food products. Int. J. Food Microbiol. 2023, 386, 110026. [Google Scholar] [CrossRef]
- Ding, Y.; Nan, Y.; Qiu, Y.; Niu, D.; Holley, R.; McAllister, T.; Narváez-Bravo, C.; Stanford, K. Use of a phage cocktail to reduce the numbers of seven Escherichia coli strains belonging to different STEC serogroups applied to fresh produce and seeds. J. Food Saf. 2023, 43, e13044. [Google Scholar] [CrossRef]
- Li, S.; Konoval, H.M.; Marecek, S.; Lathrop, A.A.; Feng, S.; Pokharel, S. Control of Escherichia coli O157:H7 using lytic bacteriophage and lactic acid on marinated and tenderized raw pork loins. Meat Sci. 2023, 196, 109030. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Cevallos-Urena, A.; Kim, B.S. Bacteriophages PECP14, PECP20, and their endolysins as effective biocontrol agents for Escherichia coli O157:H7 and other foodborne pathogens. Int. J. Food Microbiol. 2024, 409, 110460. [Google Scholar] [CrossRef] [PubMed]
- Yesil, M.; Kasler, D.R.; Huang, E.; Yousef, A.E. Lytic Escherichia phage OSYSP acts additively and synergistically with gaseous ozone against Escherichia coli O157:H7 on spinach leaves. Sci. Rep. 2023, 13, 10706. [Google Scholar] [CrossRef]
- Artawinata, P.C.; Lorraine, S.; Waturangi, D.E. Isolation and characterization of bacteriophages from soil against food spoilage and foodborne pathogenic bacteria. Sci. Rep. 2023, 13, 9282. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, Y.; Wu, J.; Wang, X.; Xue, F.; Ren, J.; Dai, J.; Tang, F. Sterilizing effect of phage cocktail against Shiga toxin-producing Escherichia coli O157:H7 in foods. Food Biosci. 2023, 51, 102282. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Shu, M.; Yang, W.Y.; Pan, H.; Tang, M.X.; Zhao, Y.Y.; Zhong, C.; Wu, G.P. Isolation and characterization of a novel Salmonella bacteriophage JNwz02 capable of lysing Escherichia coli O157:H7 and its antibacterial application in foods. LWT—Food Sci. Technol. 2023, 173, 114251. [Google Scholar] [CrossRef]
- L.A, L.A.; Waturangi, D.E. Application of BI-EHEC and BI-EPEC bacteriophages to control enterohemorrhagic and enteropathogenic Escherichia coli on various food surfaces. BMC Res. Notes 2023, 16, 102. [Google Scholar] [CrossRef]
- El-Nour, S.A.A.; Hammad, A.A.; Fathy, R.; Eid, A.S. Application of coliphage as biocontrol agent in combination with gamma irradiation to eliminate multi-drug-resistant E. coli in minimally processed vegetables. Environ. Sci. Pollut. Res. Int. 2023, 30, 123907–123924. [Google Scholar] [CrossRef]
- Kuek, M.; Mclean, S.K.; Palombo, E.A. Control of Escherichia coli in fresh-cut mixed vegetables using a combination of bacteriophage and carvacrol. Antibiotics 2023, 12, 1579. [Google Scholar] [CrossRef]
- Fawzy, S.; El-shenawy, M.A.; Senna, A.S.A.; El-shibiny, A.; Industries, F.; Therapy, P. The application of bacteriophage ZCEC5 to control multi-drug resistant E. coli in dairy products. Egypt. J. Chem. 2023, 66, 369–382. [Google Scholar] [CrossRef]
- Duc, H.M.; Son, H.M.; Yi, H.P.S.; Sato, J.; Ngan, P.H.; Masuda, Y.; Honjoh, K.-i.; Miyamoto, T. Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157:H7 in different food matrices. Food Res. Int. 2020, 131, 108977. [Google Scholar] [CrossRef] [PubMed]
- Dewanggana, M.N.; Evangeline, C.; Ketty, M.D.; Waturangi, D.E.; Yogiara; Magdalena, S. Isolation, characterization, molecular analysis and application of bacteriophage DW-EC to control Enterotoxigenic Escherichia coli on various foods. Sci. Rep. 2022, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hou, Y.; Huang, X.; Wang, S.; Xie, R.; Yang, J.; Lv, Q.; Hua, L.; Liang, W.; Peng, Z.; et al. Isolation of three coliphages and the evaluation of their phage cocktail for biocontrol of Shiga toxin-producing Escherichia coli O157 in milk. Curr. Microbiol. 2022, 79, 216. [Google Scholar] [CrossRef]
- Shebs, E.L.; Lukov, M.J.; Giotto, F.M.; Torres, E.S.; de Mello, A.S. Efficacy of bacteriophage and organic acids in decreasing STEC O157:H7 populations in beef kept under vacuum and aerobic conditions: A simulated high event period scenario. Meat Sci. 2020, 162, 108023. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Grant, I.R. Evaluation of the efficacy of multiple physical, biological and natural antimicrobial interventions for control of pathogenic Escherichia coli on beef. Food Microbiol. 2018, 76, 209–218. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, Q.; Bao, H.; Guo, Y.; Zhu, S.; Zhang, H.; Pang, M.; Wang, R. Application of a novel lytic phage vB_EcoM_SQ17 for the biocontrol of enterohemorrhagic Escherichia coli O157:H7 and enterotoxigenic E. coli in food matrices. Front. Microbiol. 2022, 13, 929005. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Listeria Infection (Listeriosis). Available online: https://www.cdc.gov/listeria/index.html (accessed on 16 January 2025).
- Barancelli, G.V.; Camargo, T.M.; Reis, C.M.F.; Porto, E.; Hofer, E.; Oliveira, C.A.F. Incidence of Listeria monocytogenes in cheese manufacturing plants from the northeast region of São Paulo, Brazil. J. Food Prot. 2011, 74, 816–819. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Garner, D.; Kathariou, S. Fresh produce-associated listeriosis outbreaks, sources of concern, teachable moments, and insights. J. Food Prot. 2016, 79, 337–344. [Google Scholar] [CrossRef]
- Kurpas, M.; Wieczorek, K.; Osek, J. Ready-to-eat meat products as a source of Listeria monocytogenes. J. Vet. Res. 2018, 62, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; García-Díez, J.; da Conceição Fontes, M.; Esteves, A. Modeling the behavior of Listeria monocytogenes in meat. In Listeria Monocytogenes, 1st ed.; Nyila, M.A., Ed.; InTech: Rijeka, Croatia, 2018; pp. 154–196. [Google Scholar] [CrossRef]
- Ahmadi, H.; Barbut, S.; Lim, L.T.; Balamurugan, S. Examination of the use of bacteriophage as an additive and determining its best application method to control Listeria monocytogenes in a cooked-meat model system. Front. Microbiol. 2020, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, N.D.; Onaran, B.; Cufaoglu, G.; Goncuoglu, M.; Ormanci, F.S.; Erol, I. Prevalence and characterization of Listeria monocytogenes isolated from beef and sheep carcasses in Turkey with characterization of locally isolated Listeriophages as a control measure. J. Food Prot. 2018, 81, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.H.; Han, S.H.; Choi, M.W.; Kim, B.H.; Ha, S.D. Efficacy of disinfectant and bacteriophage mixture against planktonic and biofilm state of Listeria monocytogenes to control in the food industry. Int. J. Food Microbiol. 2024, 413, 110587. [Google Scholar] [CrossRef]
- Colás-Medà, P.; Viñas, I.; Alegre, I. Evaluation of commercial anti-Listerial Products for improvement of food safety in ready-to-eat meat and dairy products. Antibiotics 2023, 12, 414. [Google Scholar] [CrossRef]
- Hluchanova, L.; Korena, K.; Juricova, H. Vacuum-packed steak tartare: Prevalence of Listeria monocytogenes and evaluation of efficacy of ListexTM P100. Foods 2022, 11, 533. [Google Scholar] [CrossRef]
- Ishaq, A.; Ebner, P.D.; Syed, Q.A.; Ubaid ur Rahman, H. Employing list-shield bacteriophage as a bio-control intervention for Listeria monocytogenes from raw beef surface and maintain meat quality during refrigeration storage. LWT—Food Sci. Technol. 2020, 132, 109784. [Google Scholar] [CrossRef]
- Byun, K.H.; Han, S.H.; Choi, M.W.; Park, S.H.; Ha, S.D. Isolation, characterization, and application of bacteriophages to reduce and inhibit Listeria monocytogenes in celery and enoki mushroom. Food Control 2022, 135, 108826. [Google Scholar] [CrossRef]
- Gómez-Galindo, M.; Truchado, P.; Allende, A.; Gil, M.I. Optimization of the use of a commercial phage-based product as a control strategy of Listeria monocytogenes in the fresh-cut industry. Foods 2023, 12, 3171. [Google Scholar] [CrossRef]
- Lewis, R.; Bolocan, A.S.; Draper, L.A.; Ross, P.; Hill, C. The effect of a commercially available bacteriophage and bacteriocin on Listeria monocytogenes in coleslaw. Viruses 2019, 11, 977. [Google Scholar] [CrossRef]
- Stone, E.; Lhomet, A.; Neve, H.; Grant, I.R.; Campbell, K.; McAuliffe, O. Isolation and characterization of Listeria monocytogenes phage vB_LmoH_P61, a phage with biocontrol potential on different food matrices. Front. Sustain. Food Syst. 2020, 4, 521645. [Google Scholar] [CrossRef]
- Truchado, P.; Elsser-Gravesen, A.; Gil, M.I.; Allende, A. Post-process treatments are effective strategies to reduce Listeria monocytogenes on the surface of leafy greens: A pilot study. Int. J. Food Microbiol. 2020, 313, 108390. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, L.; Bjerke, G.A.; McLeod, A.; Berget, I.; Holck, A.L. Growth behavior of Listeria monocytogenes in a traditional Norwegian fermented fish product (Rakfisk), and its inhibition through bacteriophage addition. Foods 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Heir, E.; Jensen, M.R.; Aasli, A.W.; Berget, I.; Holck, A.L. Reduction and growth inhibition of Listeria monocytogenes by use of anti-listerial nisin, P100 phages and buffered dry vinegar fermentates in standard and sodium-reduced cold-smoked salmon. Foods 2023, 12, 4391. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, M.; Wang, Y.; Yang, Z.; Ye, M.; Wu, L.; Bao, H.; Pang, M.; Zhou, Y.; Wang, R.; et al. Broad host range phage vB-LmoM-SH3-3 reduces the risk of Listeria contamination in two types of ready-to-eat food. Food Control 2020, 108, 106830. [Google Scholar] [CrossRef]
- Henderson, L.O.; Cabrera-Villamizar, L.A.; Skeens, J.; Kent, D.; Murphy, S.; Wiedmann, M.; Guariglia-Oropeza, V. Environmental conditions and serotype affect Listeria monocytogenes susceptibility to phage treatment in a laboratory cheese model. J. Dairy Sci. 2019, 102, 9674–9688. [Google Scholar] [CrossRef]
- Schamp, C.N.; Dhowlaghar, N.; Hudson, L.K.; Bryan, D.W.; Zhong, Q.; Fozo, E.M.; Gaballa, A.; Wiedmann, M.; Denes, T.G. Selection of mutant Listeria phages under food-relevant conditions can enhance application potential. Appl. Environ. Microbiol. 2023, 89, e01007-23. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 16 January 2025).
- Deng, W.; Dittoe, D.K.; Pavilidis, H.O.; Chaney, W.E.; Yang, Y.; Ricke, S.C. Current perspectives and potential of probiotics to limit foodborne Campylobacter in poultry. Front. Microbiol. 2020, 11, 583429. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Lis, L.; Lasagabaster, A.; Nafarrate, I.; Ferrocino, I.; Cocolin, L.; Rantsiou, K. Campylobacter spp. prevalence and mitigation strategies in the broiler production chain. Food Microbiol. 2022, 104, 103998. [Google Scholar] [CrossRef]
- Umar, S.; Maiyah, A.T.; Mushtaq, A. Campylobacter infections in poultry: Update on challenges and potential immune interventions. World’s Poult. Sci. J. 2016, 72, 381–390. [Google Scholar] [CrossRef]
- Thung, T.Y.; Lee, E.; Mahyudin, N.A.; Wan Mohamed Radzi, C.W.J.; Mazlan, N.; Tan, C.W.; Radu, S. Partial characterization and in vitro evaluation of a lytic bacteriophage for biocontrol of Campylobacter jejuni in mutton and chicken meat. J. Food Saf. 2020, 40, e12770. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, M.; Zhou, Q.; Lu, J.; Zhang, H.; Tang, X.; Ma, L.; Zhang, J.; Chen, D.; Gao, Y. A broad host phage, CP6, for combating multidrug-resistant Campylobacter prevalent in poultry meat. Poult. Sci. 2024, 103, 103548. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, C.; Hammerl, J.A.; Hertwig, S. Campylobacter phage isolation and characterization: What we have learned so far. Methods Protoc. 2019, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- García-Anaya, M.C.; Sepúlveda, D.R.; Rios-Velasco, C.; Zamudio-Flores, P.B.; Sáenz-Mendoza, A.I.; Acosta-Muñiz, C.H. The role of food compounds and emerging technologies on phage stability. Innov. Food Sci. Emerg. Technol. 2020, 64, 102436. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepulveda, D.R.; Sáenz-Mendoza, A.I.; Rios-Velasco, C.; Zamudio-Flores, P.B.; Acosta-Muñiz, C.H. Phages as biocontrol agents in dairy products. Trends Food Sci. Technol. 2020, 95, 10–20. [Google Scholar] [CrossRef]
- Jonczyk-Matysiak, E.; Łodej, N.; Kula, D.; Owczarek, B.; Orwat, F.; Międzybrodzki, R.; Neuberg, J.; Bagińska, N.; Weber-Dąbrowska, B.; Górski, A. Factors determining phage stability/activity: Challenges in practical phage application. Expert Rev. Anti-Infect. Ther. 2019, 17, 583–606. [Google Scholar] [CrossRef]
- Ahmadi, H.; Radford, D.; Kropinski, A.M.; Lim, L.T.; Balamurugan, S. Thermal-stability and reconstitution ability of Listeria phages P100 and A511. Front. Microbiol. 2017, 8, 2375. [Google Scholar] [CrossRef]
- Duarte, J.; Pereira, C.; Moreirinha, C.; Salvio, R.; Lopes, A.; Wang, D.; Almeida, A. New insights on phage efficacy to control Aeromonas salmonicida in aquaculture systems: An in vitro preliminary study. Aquaculture 2018, 495, 970–982. [Google Scholar] [CrossRef]
- Ackermann, H.-W.; Tremblay, D.; Moineau, S. Long-term bacteriophage preservation. WFCC Newsl. 2004, 38, 35–40. [Google Scholar]
- Vandenheuvel, D.; Lavigne, R.; Brüssow, H. Bacteriophage therapy: Advances in formulation strategies and human clinical trials. Annu. Rev. Virol. 2015, 2, 599–618. [Google Scholar] [CrossRef]
- Mojica, K.D.A.; Brussaard, C.P.D. Factors affecting virus dynamics and microbial host-virus interactions in marine environments. FEMS Microbiol. Ecol. 2014, 89, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.A.M.; Pereira, C.; Frazão, C.; Balcão, V.; Almeida, A. Efficiency of phage φ6 for biocontrol of Pseudomonas syringae pv. syringae: An in vitro preliminary study. Microorganisms 2019, 7, 286. [Google Scholar] [CrossRef]
- Silva, Y.J.; Costa, L.; Pereira, C.; Cunha, Â.; Calado, R.; Gomes, N.C.M.; Almeida, A. Influence of environmental variables in the efficiency of phage therapy in aquaculture. Microb. Biotechnol. 2014, 7, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Tey, B.T.; Ooi, S.T.; Yong, K.C.; Ng, M.Y.T.; Ling, T.C.; Tan, W.S. Production of fusion m13 phage bearing the di-sulphide constrained peptide sequence (C-WSFFSNI-C) that interacts with hepatitis B core antigen. Afr. J. Biotechnol. 2009, 8, 268–273. [Google Scholar]
- Kowalska, J.D.; Kazimierczak, J.; Sowińska, P.M.; Wójcik, E.A.; Siwicki, A.K.; Dastych, J. Growing trend of fighting infections in aquaculture environment—Opportunities and challenges of phage therapy. Antibiotics 2020, 9, 301. [Google Scholar] [CrossRef]
- Qadir, M.I.; Chauhdary, Z. Antibacterial activity of novel strains of bacteriophages: An experimental approach. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 1–12. [Google Scholar] [CrossRef]
- Vörös, Z.; Sevcsik, G.; Csík, G.; Herényi, L.; Kellermayer, M.S. Temperature-dependent nanomechanics and topography of bacteriophage T7. Biophys. J. 2018, 114, 354A. [Google Scholar] [CrossRef]
- Broeker, N.K.; Kiele, F.; Casjens, S.R.; Gilcrease, E.B.; Thalhammer, A.; Koetz, J.; Barbirz, S. In vitro studies of lipopolysaccharide-mediated DNA release of podovirus HK620. Viruses 2018, 10, 289. [Google Scholar] [CrossRef]
- Liu, T.; Sae-Ueng, U.; Li, D.; Lander, G.C.; Zuo, X.; Jönsson, B.; Rau, D.; Shefer, I.; Evilevitch, A.; Reiss, H. Solid-to-fluid-like DNA transition in viruses facilitates infection. Proc. Natl. Acad. Sci. USA 2014, 111, 14675–14680. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Zhang, N.-H. Size dependent correlation between structure and apparent stiffness of viral DNA during temperature variation. J. Mech. Phys. Solids 2021, 154, 104501. [Google Scholar] [CrossRef]
- Taj, M.K.; Ling, J.X.; Bing, L.L.; Qi, Z.; Taj, I.; Hassani, T.M.; Samreen, Z.; Yunlin, W. Effect of dilution, temperature and pH on the lysis activity of T4 phage against E. coli BL21. J. Anim. Plant Sci. 2014, 24, 1252–1255. [Google Scholar]
- Ravi, Y.; Pooja, M.K.; Krushna Yadav, D.K. Review—Bacteriophages in food preservation. Int. J. Pure Appl. Biosci. 2017, 5, 197–205. [Google Scholar] [CrossRef]
- Leverentz, B.; Conway, W.S.; Alavidze, Z.; Janisiewicz, W.J.; Fuchs, Y.; Camp, M.J.; Chighladze, E.; Sulakvelidze, A. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: A model study. J. Food Prot. 2001, 64, 1116–1121. [Google Scholar] [CrossRef]
- Leverentz, B.; Conway, W.S.; Camp, M.J.; Janisiewicz, W.J.; Abuladze, T.; Yang, M.; Saftner, R.; Sulakvelidze, A. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 2003, 69, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and bacterial plant diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Turgeon, N.; Toulouse, M.J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of five bacteriophages as models for viral aerosol studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef]
- Vasickova, P.; Kovarcik, K. Natural persistence of food- and waterborne viruses. In Viruses in Food and Water: Risks, Surveillance and Control; Cook, N., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 179–204. [Google Scholar] [CrossRef]
- Verreault, D.; Marcoux-Voiselle, M.; Turgeon, N.; Moineau, S.; Duchaine, C. Resistance of aerosolized bacterial viruses to relative humidity and temperature. Appl. Environ. Microbiol. 2015, 81, 7305–7311. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S.; Datta, S.; Prasad, R.; Dubey, D.; Prasad, R.K.; Vairale, M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2017, 62, 17–55. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, Y.; Fealey, M.; Singh, A.; Walton, K.; Martin, C.; Harman, N.J.; Mahlie, J.; Ebner, P.D. Physiological and molecular characterization of Salmonella bacteriophages previously used in phage therapy. J. Food Prot. 2015, 78, 2143–2149. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Premaratne, A.; On, S.L.W. Inactivation of Escherichia coli O157:H7 using ultraviolet light-treated bacteriophages. Food Sci. Technol. Int. 2016, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Anany, H.; Chen, W.; Pelton, R.; Griffiths, M.W. Biocontrol of Listeria monocytogenes and Escherichia coli O157:H7 in meat by using phages immobilized on modified cellulose membranes. Appl. Environ. Microbiol. 2011, 77, 6379–6387. [Google Scholar] [CrossRef] [PubMed]
- Rode, T.M.; Axelsson, L.; Granum, P.E.; Heir, E.; Holck, A.; L’Abée-Lund, T.M. High stability of Stx2 phage in food and under food-processing conditions. Appl. Environ. Microbiol. 2011, 77, 5336–5341. [Google Scholar] [CrossRef] [PubMed]
- Carrigy, N.B.; Liang, L.; Wang, H.; Kariuki, S.; Nagel, T.E.; Connerton, I.F.; Vehring, R. Spray-dried anti-Campylobacter bacteriophage CP30A powder suitable for global distribution without cold chain infrastructure. Int. J. Pharm. 2019, 569, 118601. [Google Scholar] [CrossRef]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef]
- Zinno, P.; Devirgiliis, C.; Ercolini, D.; Ongeng, D.; Mauriello, G. Bacteriophage P22 to challenge Salmonella in foods. Int. J. Food Microbiol. 2014, 191, 69–74. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, P.; Zhang, H.; Zhou, Y.; Zhang, L.; Wang, R. Bio-control of Salmonella Enteritidis in foods using bacteriophages. Viruses 2015, 7, 4836–4853. [Google Scholar] [CrossRef]
- Thung, T.Y.; Krishanthi Jayarukshi Kumari Premarathne, J.M.; San Chang, W.; Loo, Y.Y.; Chin, Y.Z.; Kuan, C.H.; Tan, C.W.; Basri, D.F.; Jasimah Wan Mohamed Radzi, C.W.; Radu, S. Use of a lytic bacteriophage to control Salmonella Enteritidis in retail food. LWT—Food Sci. Technol. 2017, 78, 222–225. [Google Scholar] [CrossRef]
- De Siqueira, R.S.; Dodd, C.E.R.; Rees, C.E.D. Evaluation of the natural virucidal activity of teas for use in the phage amplification assay. Int. J. Food Microbiol. 2006, 111, 259–262. [Google Scholar] [CrossRef]
- García, P.; Madera, C.; Martínez, B.; Rodríguez, A.; Suárez, E. Prevalence of bacteriophages infecting Staphylococcus aureus in dairy samples and their potential as biocontrol agents. J. Dairy Sci. 2009, 92, 3019–3026. [Google Scholar] [CrossRef]
- García, P.; Rodríguez, L.; Rodríguez, A.; Martínez, B. Food biopreservation: Promising strategies using bacteriocins, bacteriophages and endolysins. Trends Food Sci. Technol. 2010, 21, 373–382. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepulveda, D.R.; Rios-Velasco, C.; Zamudio-Flores, P.B.; Acosta-Muñiz, C.H. Effect of homogenization on binding-affinity of bacteriophage A511 in bovine milk fractions. J. Food Eng. 2019, 244, 73–79. [Google Scholar] [CrossRef]
- Liu, S.; Quek, S.Y.; Huang, K. Advanced strategies to overcome the challenges of bacteriophage-based antimicrobial treatments in food and agricultural systems. Crit. Rev. Food Sci. Nutr. 2023, 64, 12574–12598. [Google Scholar] [CrossRef]
- Batalha, L.S.; Gontijo, M.T.P.; Teixeira, A.V.N.C.; Boggione, D.M.G.; Lopez, M.E.S.; Eller, M.R.; Mendonça, R.C.S. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Food Res. Int. 2021, 139, 109947. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.; Malik, D.J. Bacteriophage encapsulation in pH-responsive core-shell capsules as an animal feed additive. Viruses 2021, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef]
- Rosner, D.; Clark, J. Formulations for bacteriophage therapy and the potential uses of immobilization. Pharmaceuticals 2021, 14, 359. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Quintavalla, S.; Vicini, L. Antimicrobial food packaging in meat industry. Meat Sci. 2002, 62, 373–380. [Google Scholar] [CrossRef]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel bio-based materials and applications in antimicrobial food packaging: Recent advances and future trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Riaz, S.; Khan, M.U.; Sultan, M.; Munekata, P.E.S.; Lorenzo, J.M. Biodegradable active, intelligent, and smart packaging materials for food applications. Food Packag. Shelf Life 2022, 33, 100903. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Petka-Poniatowska, K. Antimicrobial compounds in food packaging. Int. J. Mol. Sci. 2023, 24, 2457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Ignacio, J.; Caballero, M. Biopolymers for edible films and coatings in food applications. In Biopolymers—New Materials for Sustainable Films and Coatings; Plackett, D., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 233–254. [Google Scholar] [CrossRef]
- De Dicastillo, C.L.; Settier-Ramírez, L.; Gavara, R.; Hernández-Muñoz, P.; Carballo, G.L. Development of biodegradable films loaded with phages with antilisterial properties. Polymers 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Lone, A.; Anany, H.; Hakeem, M.; Aguis, L.; Avdjian, A.C.; Bouget, M.; Atashi, A.; Brovko, L.; Rochefort, D.; Griffiths, M.W. Development of prototypes of bioactive packaging materials based on immobilized bacteriophages for control of growth of bacterial pathogens in foods. Int. J. Food Microbiol. 2016, 217, 49–58. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Bolívar-Monsalve, J.; Ramírez-Toro, C.; Bolívar, G.; Ceballos-González, C. Mechanisms of action of novel ingredients used in edible films to preserve microbial quality and oxidative stability in sausages—A review. Trends Food Sci. Technol. 2019, 89, 100–109. [Google Scholar] [CrossRef]
- Jarray, A.; Gerbaud, V.; Hemati, M. Polymer-plasticizer compatibility during coating formulation: A multi-scale investigation. Prog. Org. Coat. 2016, 101, 195–206. [Google Scholar] [CrossRef]
- Malik, G.K.; Khuntia, A.; Mitra, J. Comparative effect of different plasticizers on barrier, mechanical, optical, and sorption properties of hydroxypropyl methylcellulose (HPMC)–based edible film. J. Biosyst. Eng. 2022, 47, 93–105. [Google Scholar] [CrossRef]
- Leung, V.; Szewczyk, A.; Chau, J.; Hosseinidoust, Z.; Groves, L.; Hawsawi, H.; Anany, H.; Griffiths, M.W.; Monsur Ali, M.; Filipe, C.D.M. Long-term preservation of bacteriophage antimicrobials using sugar glasses. ACS Biomater. Sci. Eng. 2018, 4, 3802–3808. [Google Scholar] [CrossRef]
- Costa, M.J.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Bacteriophage delivery systems for food applications: Opportunities and perspectives. Viruses 2023, 15, 1271. [Google Scholar] [CrossRef] [PubMed]
- Choińska-Pulit, A.; Mituła, P.; Śliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- O’Connell, L.; Marcoux, P.R.; Roupioz, Y. Strategies for surface immobilization of whole bacteriophages: A review. ACS Biomater. Sci. Eng. 2021, 7, 1987–2014. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a phage cocktail and cinnamaldehyde on sodium alginate emulsion-based films to fight food contamination by Escherichia coli and Salmonella Enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef]
- Amarillas, L.; Lightbourn-Rojas, L.; Angulo-Gaxiola, A.K.; Basilio Heredia, J.; González-Robles, A.; León-Félix, J. The antibacterial effect of chitosan-based edible coating incorporated with a lytic bacteriophage against Escherichia coli O157:H7 on the surface of tomatoes. J. Food Saf. 2018, 38, e12571. [Google Scholar] [CrossRef]
- Choi, I.; Yoo, D.S.; Chang, Y.; Kim, S.Y.; Han, J. Polycaprolactone film functionalized with bacteriophage T4 promotes antibacterial activity of food packaging toward Escherichia coli. Food Chem. 2021, 346, 128883. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Li, C.; Ye, Y.; Chen, X.; Lin, L. Enhancing anti-E. coli O157:H7 activity of composite phage nanofiber film by D-phenylalanine for food packaging. Int. J. Food Microbiol. 2022, 376, 109762. [Google Scholar] [CrossRef]
- Korehei, R.; Kadla, J. Incorporation of T4 bacteriophage in electrospun fibres. J. Appl. Microbiol. 2013, 114, 1425–1434. [Google Scholar] [CrossRef]
- Korehei, R.; Kadla, J.F. Encapsulation of T4 bacteriophage in electrospun poly(ethylene oxide)/cellulose diacetate fibers. Carbohydr. Polym. 2014, 100, 150–157. [Google Scholar] [CrossRef]
- Liana, A.E.; Marquis, C.P.; Gunawan, C.; Gooding, J.J.; Amal, R. Antimicrobial activity of T4 bacteriophage conjugated indium tin oxide surfaces. J. Colloid Interface Sci. 2018, 514, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Greene, M.; Kimmelshue, C.; Cademartiri, R. Stabilization of T4 bacteriophage at acidic and basic pH by adsorption on paper. Colloids Surf. B Biointerfaces 2017, 160, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Tomat, D.; Soazo, M.; Verdini, R.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of an WPC edible film added with a cocktail of six lytic phages against foodborne pathogens such as enteropathogenic and Shigatoxigenic Escherichia coli. LWT—Food Sci. Technol. 2019, 113, 108316. [Google Scholar] [CrossRef]
- Vonasek, E.; Le, P.; Nitin, N. Encapsulation of bacteriophages in whey protein films for extended storage and release. Food Hydrocoll. 2014, 37, 7–13. [Google Scholar] [CrossRef]
- Vonasek, E.L.; Choi, A.H.; Sanchez, J.; Nitin, N. Incorporating phage therapy into WPI dip coatings for applications on fresh whole and cut fruit and vegetable surfaces. J. Food Sci. 2018, 83, 1871–1879. [Google Scholar] [CrossRef]
- Kamali, S.; Yavarmanesh, M.; Habibi Najafi, M.B.; Koocheki, A. Poly (lactic acid) and whey protein/pullulan composite bilayer film containing phage A511 as an anti-Listeria packaging for chicken breast at refrigerated temperatures. LWT—Food Sci. Technol. 2022, 170, 114085. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.S.; Bai, J.; Chang, Y. Antibacterial κ-carrageenan/konjac glucomannan-based edible hydrogel film containing Salmonella phage PBSE191 and its application in chicken meat. LWT—Food Sci. Technol. 2023, 180, 114707. [Google Scholar] [CrossRef]
- Radford, D.; Guild, B.; Strange, P.; Ahmed, R.; Lim, L.T.; Balamurugan, S. Characterization of antimicrobial properties of Salmonella phage Felix O1 and Listeria phage A511 embedded in xanthan coatings on poly(lactic acid) films. Food Microbiol. 2017, 66, 117–128. [Google Scholar] [CrossRef]
- Tidim, G.; Guzel, M.; Soyer, Y.; Erel-Goktepe, I. Layer-by-layer assembly of chitosan/alginate thin films containing Salmonella enterica bacteriophages for antibacterial applications. Carbohydr. Polym. 2024, 328, 121710. [Google Scholar] [CrossRef]
- Gouvêa, D.M.; Mendonça, R.C.S.; Soto, M.L.; Cruz, R.S. Acetate cellulose film with bacteriophages for potential antimicrobial use in food packaging. LWT—Food Sci. Technol. 2015, 63, 85–91. [Google Scholar] [CrossRef]
- Sezer, B.; Kubra, E.; Hakki, I. The use of bacteriophage-based edible coatings for the biocontrol of Salmonella in strawberries. Food Control 2022, 135, 108812. [Google Scholar] [CrossRef]
- Vila, M.M.D.C.; Cinto, E.C.; Pereira, A.O.; Baldo, D.Á.; Oliveira, J.M., Jr.; Balcão, V.M. An edible antibacterial coating integrating lytic bacteriophage ripened cheese. Polymers 2024, 16, 680. [Google Scholar] [CrossRef] [PubMed]
- García-Anaya, M.C.; Sepulveda, D.R.; Rios-Velasco, C.; Acosta-Muñiz, C.H. Incorporation of A511 bacteriophage in a whey protein isolate-based edible coating for the control of Listeria monocytogenes in cheese. Food Packag. Shelf Life 2023, 37, 101095. [Google Scholar] [CrossRef]
- Kamali, S.; Yavarmanesh, M.; Habibi Najafi, M.B.; Koocheki, A. Development of whey protein concentrate/pullulan composite films containing bacteriophage A511: Functional properties and anti-Listeria effects during storage. Food Packag. Shelf Life 2022, 33, 100902. [Google Scholar] [CrossRef]
- Costa, M.J.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Characterization of PHBV films loaded with FO1 bacteriophage using polyvinyl alcohol-based nanofibers and coatings: A comparative study. Innov. Food Sci. Emerg. Technol. 2021, 69, 102646–102656. [Google Scholar] [CrossRef]
- Kim, S.; Chang, Y. Anti-Salmonella polyvinyl alcohol coating containing a virulent phage PBSE191 and its application on chicken eggshell. Food Res. Int. 2022, 162, 111971. [Google Scholar] [CrossRef]
- Leung, V.; Groves, L.; Szewczyk, A.; Hosseinidoust, Z.; Filipe, C.D.M. Long-term antimicrobial activity of phage-sugar glasses is closely tied to the processing conditions. ACS Omega 2018, 3, 18295–18303. [Google Scholar] [CrossRef]
- Kalkan, S. Vibrio parahaemolyticus ATCC 17802 inactivation by using methylcellulose films containing encapsulated bacteriophages. Turk. J. Vet. Anim. Sci. 2018, 42, 480–485. [Google Scholar] [CrossRef]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ϕIBB-PF7A loaded on sodium alginate-based films to prevent microbial meat spoilage. Int. J. Food Microbiol. 2019, 291, 121–127. [Google Scholar] [CrossRef]
- Kimmelshue, C.; Goggi, A.S.; Cademartiri, R. The use of biological seed coatings based on bacteriophages and polymers against Clavibacter michiganensis subsp. nebraskensis in maize seeds. Sci. Rep. 2019, 9, 17950. [Google Scholar] [CrossRef]
- Weng, S.; López, A.; Sáez-Orviz, S.; Marcet, I.; García, P.; Rendueles, M.; Díaz, M. Effectiveness of bacteriophages incorporated in gelatine films against Staphylococcus aureus. Food Control 2021, 121, 107666. [Google Scholar] [CrossRef]

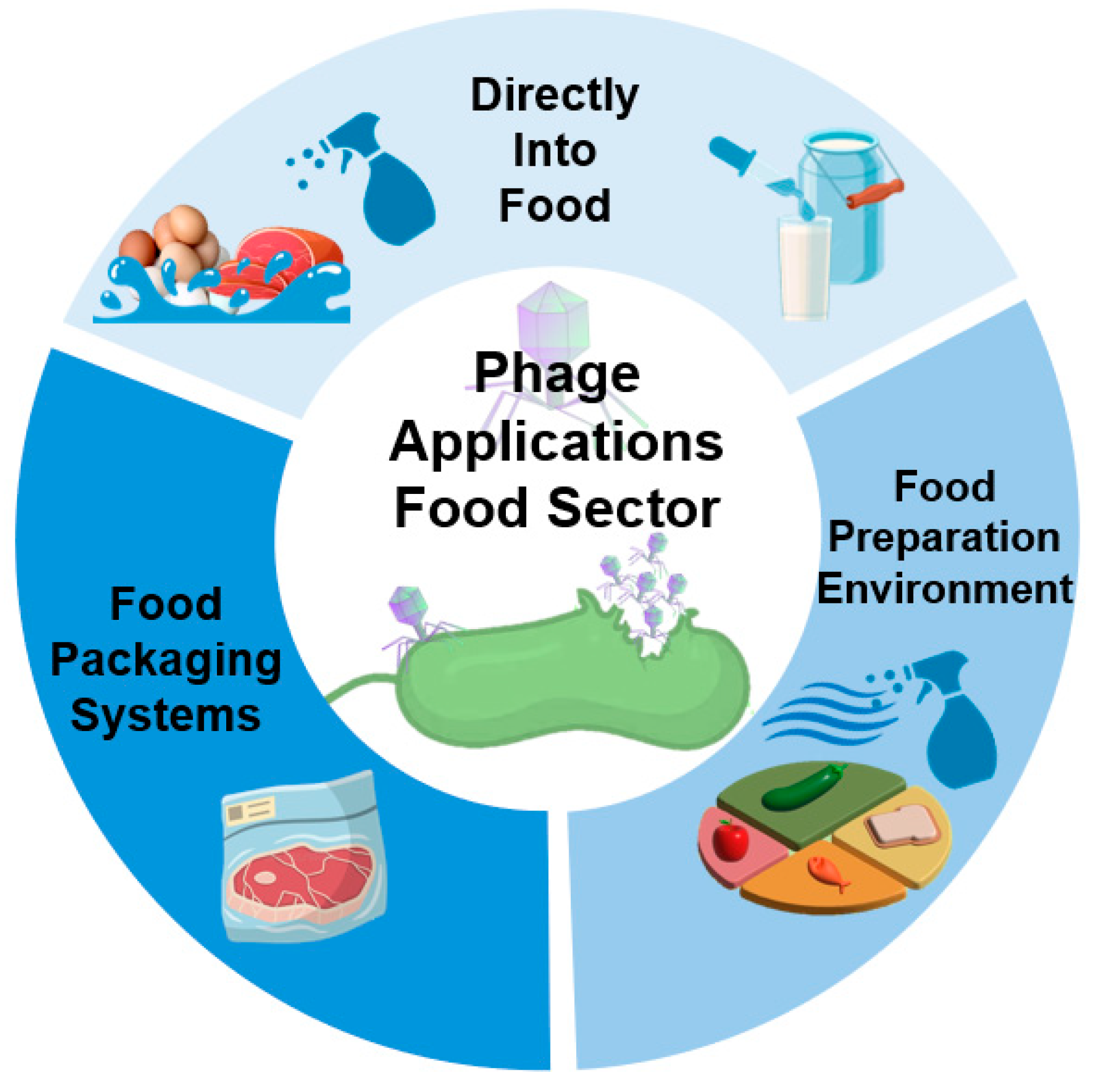
| Thermal Methods | |||
|---|---|---|---|
| Type of Decontamination Method | Advantages | Disadvantages | References |
| Conventional thermal methods | Pasteurization, sterilization, air or vacuum drying, burning charcoal, steaming, boiling, frying
|
| [23,24,25] |
| Novel thermal methods | Superheated steam
|
| [4,24] |
Radiofrequency and microwave
|
| [4,23,40] | |
Infrared
|
| [4,23,39] | |
Extrusion cooking
|
| [4,41] | |
Ohmic heating
|
| [40] | |
| Non-thermal methods | |||
| Chemical methods (natural) | Bacteriocins
|
| [42,43,44] |
Essential oils
|
| [42,45] | |
| Chemical methods (non-natural) | Chlorine-based methods
|
| [42,46] |
Ozone
|
| [3,42,47] | |
Cold Plasma
|
| [3,48] | |
| Physical methods | Pulsed electric field
|
| [3,40,46,47] |
High-pressure processing
|
| [3,40,47] | |
Ultrasound
|
| [3,40,46,47,49] | |
Ultraviolet radiation
|
| [3,23,46,50] | |
Pulsed light system
|
| [3,51] | |
Ionizing radiation
|
| [3,40,52] | |
| Biological methods | Phage treatment
|
| [9,11,53,54,55] |
| Target Bacteria (Study) | Phages | Material(s) and Type of Application | Food Products | Findings | |
| Bacteria | Phages/Films/Coatings | ||||
| Escherichia coli | |||||
| Escherichia coli O157:H7 (amp::lux) (C918) [265] | Cocktail of E. coli phages (EcoM-AG2, EcoM-AG3, and EcoM-AG10) | Modified cellulose membranes with polyvinylamine (positively charged cellulose membranes) | Raw beef | In vitro (bioluminescent assays at 25 °C): At higher phage concentrations (109, 107, and 105 PFU/mL)-similar results obtained with modified and unmodified cellulose membranes with almost complete inhibition; At lower phage concentrations (103):
| Phage-treated positively charged cellulose membranes showed higher quantities of phage plates than those with the phage-treated unmodified membranes. Drying tests:
|
| Escherichia coli ATCC 11303 [303] | Wild type T4 phage | Core (phage suspension)/shell (poly(ethylene oxide)) fibers | N/A | N/A | Better results obtained with coaxial electrospinning (relatively to simple and emulsion electrospinning)—the incorporated T4 phage totally maintained its activity after several weeks at 4 °C. |
| Escherichia coli [304] | Wild type T4 phage | Core (phage suspension)/shell electrospun fibers made from poly(ethylene oxide), cellulose diacetate, and their blends | N/A | N/A | The phage release rate was dependent on cellulose diacetate/poly(ethylene oxide) ratio and the poly(ethylene oxide) molecular weight. Increasing both parameters resulted in slower phage release. |
| Escherichia coli BL21 [308] | Coliphage T4 | Edible whey protein isolate films | Lettuce leaves (release tests) | In vitro: ~5 log difference between the bacterial control and samples containing phage and ~2 log decrease in the samples from the initial inoculation, after 24 h at 22 °C. | The phages
|
| Escherichia coli O104:H4, C1321 [289] | E. coli phage cocktail (EcoM-HG2, EcoM-HG7, and EcoM-HG8) | Paper coated with encapsulated phage in alginate beads or Paper impregnated with phage suspension | Alfalfa seed and sprout | At room temperature:
|
|
| Escherichia coli (EHEC O157:H7 CICC 21530) [301] | E. coli O157:H7 phage | Chitosan film containing liposome-encapsulated phage | Beef | Model beef suspension (room temperature with shaking): The antibacterial activity of the chitosan film containing liposome-encapsulated phages was positively associated with its concentration—better results for 400 mg/mL (around 5 log reduction after 7 days in comparison with the bacterial control) were obtained. In food (at 25 °C): The chitosan film containing liposome-encapsulated phage led to a 4.45 log reduction while the chitosan film led to a 0.29 log reduction after 15 days. |
|
| Escherichia coli O157:H7 [294] | E. coli AG10 phages | Pullulan–trehalose films | N/A | N/A | Phage infectivity was maintained for up to 1 month at ambient storage conditions (reduction of 1.90 log PFU/film). |
| Escherichia coli K12 [306] | T4, T5, and T7 phages | Filter paper with phage addition after functionalization with carboxyl methyl cellulose or chitosan | Milk | At 37 °C: All papers with the T4 phage were able to remove E. coli from milk;
| All papers extended the lifetime of the infective phage by at least a factor of four, with some papers stabilizing phages for up to one week at 37 °C. |
| Escherichia coli O157:H7 CECT 4076 [299] | vB_EcoMH2W phage | Chitosan-based edible coating | Tomato | A ~3 log bacterial reduction in the samples relative to the controls on tomato surfaces after 6 days at 20 °C. | Phage infectivity was maintained in the film that was applied on the surface of tomatoes for at least 6 days in the presence (higher titer) or absence of E. coli at 20 °C. |
| Escherichia coli BL21 [309] | T7 phage | Edible whey protein isolate-based coating | Apples Cherry tomatoes Cucumbers | After 24 h at 4 °C: A ~2 and 4 log bacterial reduction on cut apples and whole cherry tomatoes, respectively, was observed, while no significant reduction was seen for sliced cucumbers. | Films enhanced phage stability during cold storage (4 °C). |
| Escherichia coli ATCC 11303 [305] | Phage T4 (ATCC 11303-B4) | Phage-conjugated indium tin oxide systems | Food components: starch and casein | In vitro, at 37 °C, after 2 h incubation:
In vitro (in the presence of food components at pH 7): A 3–4 log bacterial reduction after 2 h was seen, similar to that observed in only medium. | All the indium tin oxide/T4 systems maintained their antimicrobial activity in the presence of model food components (starch and casein), but this activity was still affected by pH. |
| Escherichia coli (DH5α and O157:H7 STEC strains) [307] | Cocktail of T-even type phages (DT1 to DT6) | Whey protein concentrate films | Meat | In vitro (growth inhibition assay):
| Phage cocktail embedded in the films were stable within 5 weeks of storage (better results at 4 °C than at 24 °C). |
| Escherichia coli CECT 434 [298] | Salmonella phage φ135 and E. coli phage vB_EcoS-EC4 (EC4) | Phages and cinnamaldehyde incorporated on sodium alginate emulsion-based films | N/A | In vitro: Better results with a combination of both phages at a higher concentration of cinnamaldehyde (0.4%), with a reduction until reaching the detection limit of the method (~7 log) after 24 h at 20 °C. | The incorporation of phages into the film did not introduce significant changes to the film characteristics, unlike cinnamaldehyde, which increased the roughness, thickness, and swelling ability of films. |
| Escherichia coli K12 (ATCC 23724) and O157:H7 H1730 [300] | Phage T4 (ATCC 11303) | Polycaprolactone films (phage addition by physical adsorption or chemical functionalization) | Raw beef | In vitro E. coli K12 reduction with chemically functionalized film compared to films with no phages:
| Chemically functionalized film showed more antibacterial efficacy than the physically adsorbed film. |
| Escherichia coli O157:H7 (EHEC O157:H7 CICC 21530) [302] | E. coli O157 phage | Co-encapsulation of phages and D-phenylalanine into sodium alginate/polyethylene oxide nanofibers based-films | Beef Cherry tomatoes Cucumber | In vitro (37 °C):
In food (after 4 days): Beef—reduction of ~2 log of planktonic E. coli at both 4 and 25 °C; Cucumber—reduction of ~3 and 2 log of E. coli biofilm at 4 and 25 °C, respectively; Cherry tomatoes—reduction of ~2 and 1.5 log on the E. coli biofilm at 4 and 25 °C, respectively. |
|
| Salmonella spp. | |||||
| Salmonella enterica ser. Typhimurium [314] | Phage cocktail (BFSE16, BFSE18, PaDTA1, PaDTA9, PaDTA10, and PaDTA11) | Cellulose acetate films | N/A | In vitro (diffusion in liquid medium): Increase in the lag phase and slower growth of microorganisms in the sample containing incorporated phages in the films, compared to control, at 150 rpm at 35 ± 2 °C. In vitro (diffusion in solid medium): Halos of films containing phages of 1.23–1.35 cm, larger than that of the pure cellulose acetate film (1 cm), at 35 ± 2 °C for 24 h. |
|
| Salmonella sp. cocktail (S. Typhimurium DT104, 19485A96 SGI1 and ATCC 13311, S. Heidelberg ATCC 8326, S. Enteritidis ATCC 4931 and S. Newport ATCC 6962) [312] | Salmonella phage Felix O1 | Poly(lactic acid) films with a xanthan coating containing phage | Sliced turkey | In vitro (microtiter plate assay): At 37 °C, S. Typhimurium DT104 cultures showed significantly slower growth and lower final bacterial density compared to the control, while at 25 °C, S. Typhimurium was reduced in a similar way in the presence and absence of phages over a period of 20 h. In food: Better results under anaerobic packaging with reductions in Salmonella sp. cocktail in about 0.832 and 1.30 log at 4 °C and 10 °C after 30 days. | 99.9% of phage released within 30 min into meat. |
| Salmonella Newport [294] | Salmonella CG4-1 | Paper coated with pullulan–trehalose mixture containing phages or Pullulan–trehalose films incorporating phages | N/A | In vitro, a 4.59 log CFU/cm2 bacterial reduction at ambient conditions (∼22–25 °C) after 1 month was observed with paper coated with a pullulan–trehalose mixture and containing phages. | Phage infectivity was maintained for up 2 months in the pullulan–trehalose films in ambient storage conditions. |
| Salmonella Enteritidis EX2 [298] | Salmonella phage φ135 and E. coli phage vB_EcoS-EC4 (EC4) | Phages and cinnamaldehyde incorporated on sodium alginate emulsion-based films | N/A | In vitro, better results with a combination of both phages at the higher concentration of cinnamaldehyde (0.4%) were obtained, with reduction until the detection limit of the method (~8 log) was reached after 24 h at 20 °C. | The incorporation of phages into the film did not introduce significant changes in its characteristics, unlike cinnamaldehyde, which increased the roughness, thickness, and swelling ability of films. |
| Salmonella Enteritidis (H40499 SDE) [319] | Salmonella Enteritidis phage Felix O1 | Phage incorporation into polyvinyl alcohol coatings and fibers deposited by casting and electrospinning on polyhydroxybutyrate/poly-hydroxyvalerate films | N/A | N/A |
|
| Salmonella mixture [315] | Phage cocktail: S. Enteritidis F5–4, S. Typhimurium L2–1, and S. Typhimurium ICB1–1 | Phage-based edible coatings of whey protein concentrate, carboxymethyl cellulose, chitosan, or sodium alginate | Strawberries | The largest antimicrobial effect was observed with a whey protein concentrate coating, with a reduction of 3.1 log CFU/g after 5 days at 4 °C, compared to the other tested polymers. | For a period of 5 days at 4 °C:
During storage, a 0.7 log-unit (PFU/g) reduction in whey protein concentrate coating was observed compared to the phage reduction observed with other polymers (around 1.0). |
| Salmonella Enteritidis (ATCC 13076) [320] | Salmonella Enteritidis phage PBSE191 (BP-1370) | Polyvinyl alcohol film | Chicken eggshell | In vitro: Significant bacterial reduction (2 × 105 CFU/film within 2 h) in the phage-containing films compared to the bacterial control-containing film without phages, at 37 °C. On chicken eggshell surface: An about 2 log CFU reduction within 24 h at 5 °C and 50% relative humidity was observed. | Phages remained stable and were effectively released from the polyvinyl alcohol film without any substantial loss in the phage titer at 5 °C and at 50% relative humidity for 30 days. |
| Salmonella Enteritidis (ATCC 13076) [311] | Salmonella Enteritidis phage PBSE191 (BP-1370) | κ-carrageenan (KC) and konjac glucomannan (KGM) hydrogel film containing adsorbed phages | Raw chicken meat | Around 1 log CFU/mL reduction within 3 h at 25 °C and within 48 h at 5 °C. |
|
| Salmonella enterica CCCD-S004 [316] | Phage cocktail (SentS01L and SentS01T phages) | Sodium alginate edible coating | Ripened cheese | Coating containing phage cocktail was removed from a cheese matrix sample and positioned in the center of a bacterial lawn of Salmonella enterica CCCD-S004. After incubation (24 h at 37 °C), the presence of clear zones of lysis in the bacterial lawn surrounding the coating was observed. | The antibacterial coating showed adequate physicochemical characteristics and zero cytotoxicity in HaCaT and 3T3 cell lines. |
| Salmonella Enteritidis (MET S1–001) [313] | Phage MET P1-001_43 | Layer-by-layer assembly of chitosan/alginate thin films incorporating phages | Chicken meat | Wrapping a S. Enteritidis-contaminated chicken piece with aluminum foil whose surface was modified with phage-loaded chitosan/alginate multilayers reduced bacterial numbers in more than 1.0 log CFU/cm2. Similar results were obtained with free phages. |
|
| Listeria monocytogenes | |||||
| Listeria monocytogenes C391 [265] | Cocktail of Listeria phages (LinM-AG8, LmoM-AG13, and LmoM-AG20) | Modified cellulose membranes with polyvinylamine (positively charged cellulose membranes) | RTE oven-roasted turkey breast | Better results obtained at 4 °C and in vacuum conditions: Listeria reduction of 4 log CFU/g after 15 days. | Immobilized phages were able to control the growth of L. monocytogenes in meat incubated at different temperatures and under different packaging conditions. |
| Listeria monocytogenes cocktail (Li0512, Li0529, ATCC19115 and 08–5578, serotype 1/2b,1/2a, 4b and 1/2a, respectively) [289] | LISTEX™ P100 phage (RTE turkey) or L. monocytogenes phage cocktail (LinM-AG8, LmoM-AG13, and LmoM-AG20) (freshly cut cantaloupe) | Modified cellulose membranes with polyvinylamine (positively charged cellulose membranes) | RTE turkey Fresh cut cantaloupe | RTE turkey: Significant L. monocytogenes reduction at 4 °C and 10 °C, with around 1 log and 2 log CFU/cm2 reduction, respectively, after 5 days. Fresh cut cantaloupe: Reduction of ~1 log at the end of different storage conditions (4 and 12 °C for 5 days, and 25 °C for 24 h) in the samples containing the immobilized phage compared to controls;
| RTE turkey: No significant difference in antibacterial effect between free and immobilized phages. Fresh cut cantaloupe: Spray-coating free phages showed greater antibacterial effect than immobilized phages. |
| Listeria monocytogenes cocktail (FSL F6-367, ATCC 19115, 08e5578, C6-0003, LI 0512) [312] | Listeria phage A511 | Poly(lactic acid) films with a xanthan coating containing phages | Sliced turkey | In vitro (microtiter plate assay): At 37 °C, a significant decrease in final cell density in the grown cultures of L. monocytogenes ATCC 19115 in the presence of the films containing phage, relative to the controls, after 3 h at 25 °C, was observed, with a significant reduction only seen after 21 h. In food: Reductions in L. monocytogenes cocktail of 6.31 log at 4 °C and 1.52 log at 10 °C, in anaerobic packaging after 30 days, and reductions of 3.79 log at 4 °C and 2.17 log at 10 °C, after 14 days, in aerobic packaging. | 99.9% of both phages were successfully released from the film within 30 min onto the meat samples. |
| Listeria monocytogenes serotype 1/2a [294] | LISTEX P100 | Paper coated with pullulan–trehalose mixture containing phages or Pullulan–trehalose films incorporating phages | N/A | In vitro, more than 2 log CFU/cm2 reduction at ambient conditions (∼22–25 °C) after 6 weeks, with paper coated with pullulan–trehalose mixture containing phages was observed. | Phage infectivity was maintained for up to 2 months in the pullulan–trehalose films at ambient storage conditions. |
| Listeria monocytogenes serotype 1/2a [321] | LISTEX P100 | Phage incorporated into pullulan–trehalose films | N/A | N/A |
|
| Listeria monocytogenes (CECT 934, ATCC 19114) [288] | LISTEX™ P100 phage | Films of (1) sodium caseinate, (2) sodium alginate mixed with gelatine, and (3) polyvinyl alcohol | N/A |
|
|
| Listeria monocytogenes (ATCC 19113) [318] | Listeria phage A511 | Whey protein concentrate (WPC), pullulan (PULL) films, and their blends | N/A | In vitro:
|
|
| Listeria monocytogenes (ATCC 19113) [310] | Listeria phage A511 | Bilayer films of whey protein concentrate/pullulan (WP) containing phage and poly (lactic acid) (PLA) | Chicken breast | In vitro: Disk diffusion assay—The diameter of the inhibition zone in the bilayer and monolayer films were similar and did not differ significantly at 30 °C;
|
|
| Listeria monocytogenes [317] | ATCC A511 phage | Whey protein isolate (WPI)-based coating | Cheese | At the end of storage period (16 days at 4 °C), phages added to water or to WPI reduced bacterial counts in 0.39 and 0.86 log CFU/g, respectively. |
|
| Other bacteria | |||||
| Vibrio parahaemolyticus ATCC 17802 [322] | Phage isolated from raw bonito fishes (Sarda sarda) | Edible methylcellulose films coated with capsules of sodium alginate containing phages | Raw fish filets | In vitro (after 24 h at room temperature):
| Phage stability:
|
| Pseudomonas fluorescens PF7A [323] | Phage ϕIBB-PF7A | Sodium alginate-based films | Chicken breast filet | In vitro (at 4 °C): A significant decrease in P. fluorescens growth in films that incorporated phages (1.9 and 4 log reductions after 24 h) when in direct contact with P. fluorescens or when completely immersed on a bacterial suspension, respectively. In food (at 4 °C): Films incorporating phages reduced P. fluorescens counts by 2 log after 2 days and maintained a significant reduction for the next 5 days (1 log). |
|
| Clavibacter michiganensis subsp. nebraskensis (Cmn-91R) [324] | Phage CN8 | Coatings based on phages, polymers (polyvinylpyrrolidone, polyvinyl alcohol, or poly(methyl vinyl ether)), and stabilizers (whey protein isolate, skim milk, sucrose, maltodextrin, or D-mannitol) | Maize seeds | Coating of polyvinyl alcohol combined with whey protein isolate containing phage significantly reduced target bacterial cells up to 3.8 × 103 CFU/seed after four months at 10 °C, without affecting seed germination. |
|
| Staphylococcus aureus IPLA1 [325] | Phage phiIPLA-RODI | Edible gelatine films/coatings | Cottage cheese | In vitro: Reductions of 5 and 7 log for the films with the lowest and the highest phage concentrations, respectively, after 17 h at 37 °C and at 250 rpm. On cheese (at 4 °C):
| The edible films/coatings were not physically impacted by the addition of phages. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braz, M.; Pereira, C.; Freire, C.S.R.; Almeida, A. A Review on Recent Trends in Bacteriophages for Post-Harvest Food Decontamination. Microorganisms 2025, 13, 515. https://doi.org/10.3390/microorganisms13030515
Braz M, Pereira C, Freire CSR, Almeida A. A Review on Recent Trends in Bacteriophages for Post-Harvest Food Decontamination. Microorganisms. 2025; 13(3):515. https://doi.org/10.3390/microorganisms13030515
Chicago/Turabian StyleBraz, Márcia, Carla Pereira, Carmen S. R. Freire, and Adelaide Almeida. 2025. "A Review on Recent Trends in Bacteriophages for Post-Harvest Food Decontamination" Microorganisms 13, no. 3: 515. https://doi.org/10.3390/microorganisms13030515
APA StyleBraz, M., Pereira, C., Freire, C. S. R., & Almeida, A. (2025). A Review on Recent Trends in Bacteriophages for Post-Harvest Food Decontamination. Microorganisms, 13(3), 515. https://doi.org/10.3390/microorganisms13030515









