Effects of Fucoidan on the Inhibition of Biofilm Formation of Salmonella enterica Subsp. enterica Serovar Typhimurium on Seafoods and Its Molecular Antibiofilm Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Culture and Growth
2.2. Fucoidan and Minimum Inhibitory Concentration (MIC) Determination
2.3. Crabs and Shrimp Sample Preparation
2.4. Biofilm Formation Process
2.5. Biofilm Visualization by Field Emission Scanning Electron Microscopy (FE-SEM)
2.6. Relative Expression of Gene by Real-Time PCR (RT–PCR)
2.7. Statistical Analysis
3. Results
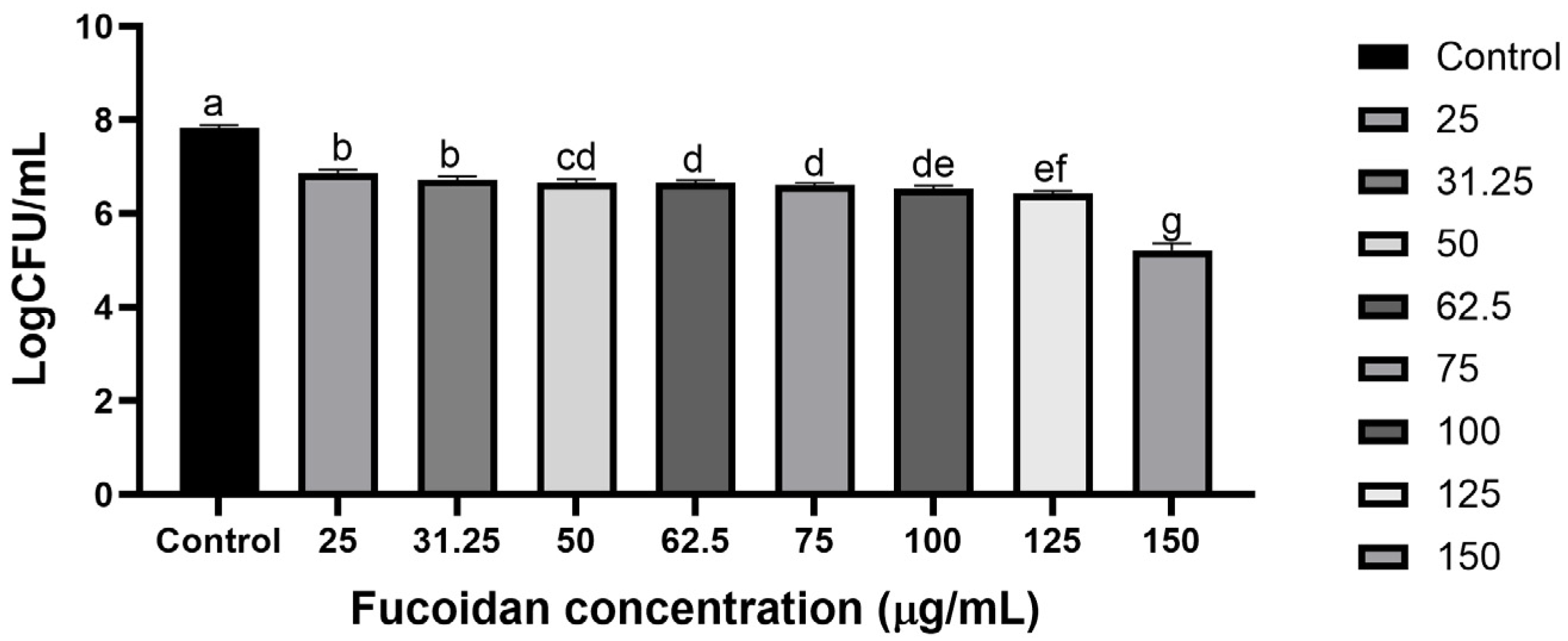
3.1. MIC Determination
3.2. Eradication Impact of Fucoidan on Crab and Shrimp Against S. Typhimurium ATCC 13311
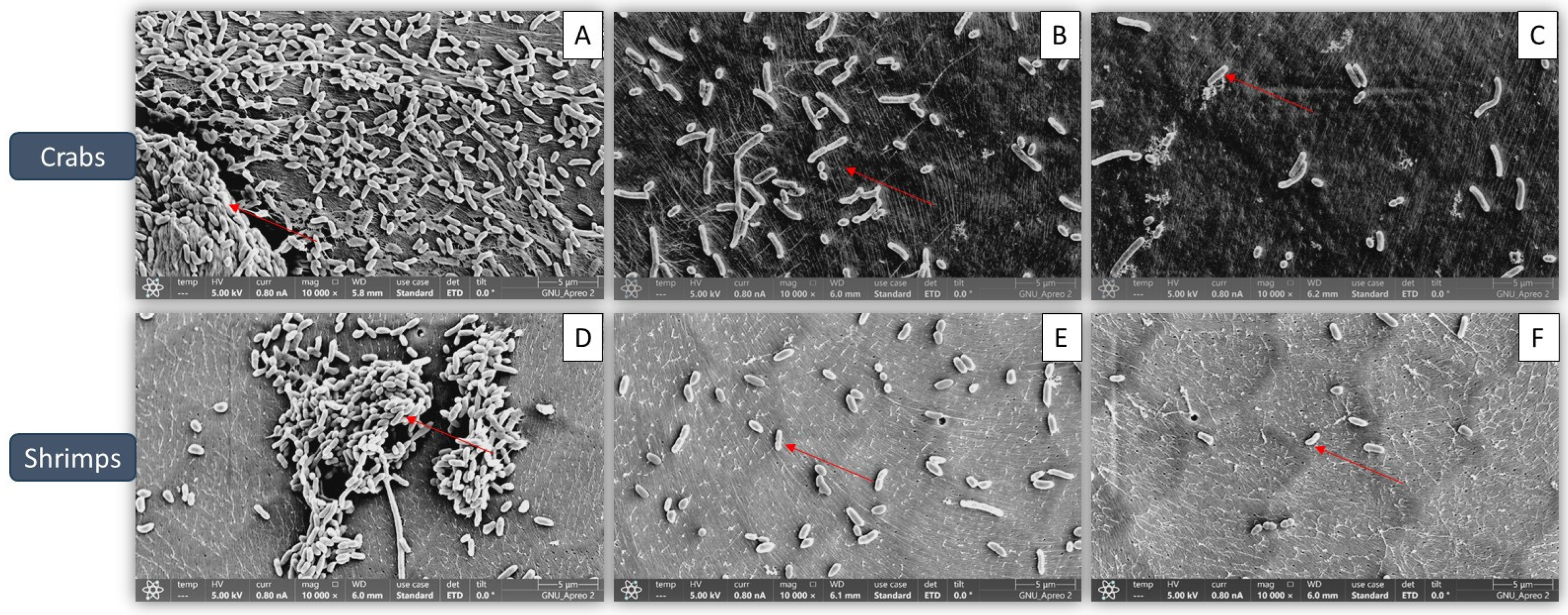
3.3. Confirmation of Biofilm Reduction Visually by Fucoidan
3.4. Relative Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, P.K.; Ha, A.J.; Nahar, S.; Hossain, M.I.; Ashrafudoulla, M.; Toushik, S.H.; Mizan, M.F.R.; Kang, I.; Ha, S.D. Inhibitory effects of vorinostat (SAHA) against food-borne pathogen Salmonella enterica serotype Kentucky mixed culture biofilm with virulence and quorum-sensing relative expression. Biofouling 2023, 39, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Dawan, J.; Zhang, S.; Ahn, J. Recent Advances in Biofilm Control Technologies for the Food Industry. Antibiotics 2025, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Kim, S.H.; Jeon, E.B.; Park, E.H.; Park, S.Y. Inhibition of Listeria monocytogenes Cocktail Culture Biofilms on Crab and Shrimp Coupons and the Expression of Biofilm-Related Genes. Antibiotics 2023, 12, 1008. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Huamán, B.R.H.; Vega-Sánchez, A.; Rolón-Verdún, P.; Gervilla-Cantero, G.; Rodríguez-Jerez, J.J.; Ripolles-Avila, C. Effect of Cinnamomum cassia essential oil combined with enzymes on the elimination and regrowth potential of Listeria monocytogenes and Salmonella enterica biofilms formed on stainless steel surfaces. Food Control 2025, 172, 111120. [Google Scholar] [CrossRef]
- Yang, P.; Huo, Y.; Yang, Q.; Zhao, F.; Li, C.; Ju, J. Synergistic anti-biofilm strategy based on essential oils and its application in the food industry. World J. Microbiol. Biotechnol. 2025, 41, 81. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Yamik, D.Y.; Vetboocha, N.; Vongkamjan, K. Effect of novel phage cocktail on Salmonella recovered from broiler sources and its anti-biofilm effect on food contact surface model. Food Control 2025, 169, 111000. [Google Scholar] [CrossRef]
- CDC. Salmonella Infection (Salmonellosis). Available online: https://www.cdc.gov/salmonella/ (accessed on 15 March 2025).
- Kim, Y.K.; Roy, P.K.; Ashrafudoulla, M.; Nahar, S.; Toushik, S.H.; Hossain, M.I.; Mizan, M.F.R.; Park, S.H.; Ha, S.-D. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression. Food Control 2022, 137, 108964. [Google Scholar] [CrossRef]
- Giaouris, E.; Habimana, O. The Battle Against Biofilms: A Focus on Novel Antimicrobial Strategies and Their Mechanisms of Action. Antibiotics 2025, 14, 111. [Google Scholar] [CrossRef]
- Rohilla, A.; Kumar, V.; Ahire, J.J. Unveiling the persistent threat: Recent insights into Listeria monocytogenes adaptation, biofilm formation, and pathogenicity in foodborne infections. J. Food Sci. Technol. 2024, 61, 1428–1438. [Google Scholar] [CrossRef]
- Alsuwat, M.A.; Shah, A.A.; Ullah, S.; Khan, R.U.; Alissa, M.; Khan, M.S. Microbial Biofilm Formation to Mitigate Foodborne Pathogens Strategies and Control Measures. Indian J. Microbiol. 2025, 1–6. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, F.; Kang, M.G.; Jo, D.M.; Cho, K.J.; Kim, Y.M. Inhibition of Polymicrobial Biofilms of Candida albicans-Staphylococcus aureus/Streptococcus mutans by Fucoidan-Gold Nanoparticles. Mar. Drugs 2023, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, L.; Shang, N.; Wu, K.; Liao, W. Recent Advances in the Structure, Extraction, and Biological Activity of Sargassum fusiforme Polysaccharides. Mar. Drugs 2025, 23, 98. [Google Scholar] [CrossRef]
- Vladkova, T.G.; Staneva, A.D.; Avramova, I.A.; Ivanova, I.A.; Gospodinova, D.N. Fucoidan-Containing, Low-Adhesive Siloxane Coatings for Medical Applications: Inhibition of Bacterial Growth and Biofilm Development. Materials 2023, 16, 3651. [Google Scholar] [CrossRef]
- Nazari, M.; Taheri, M.; Nouri, F.; Bahmanzadeh, M.; Alikhani, M.Y. The antimicrobial and antibiofilm effects of gentamicin, imipenem, and fucoidan combinations against dual-species biofilms of Staphylococcus aureus and Acinetobacter baumannii isolated from diabetic foot ulcers. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Improving potential strategies for biological activities of phlorotannins derived from seaweeds. Crit. Rev. Food Sci. Nutr. 2025, 65, 833–855. [Google Scholar] [CrossRef]
- Azeem, K.; Fatima, S.; Ali, A.; Ubaid, A.; Husain, F.M.; Abid, M. Biochemistry of Bacterial Biofilm: Insights into Antibiotic Resistance Mechanisms and Therapeutic Intervention. Life 2025, 15, 49. [Google Scholar] [CrossRef]
- Jeong, G.J.; Khan, F.; Tabassum, N.; Cho, K.J.; Kim, Y.M. Strategies for controlling polymicrobial biofilms: A focus on antibiofilm agents. Int. J. Antimicrob. Agents 2024, 64, 107243. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, M.; Munawar, A.; Nawaz, Z.; Hussain, N.; Hafeez, A.B.; Szweda, P. Antibiotic resistance and preventive strategies in foodborne pathogenic bacteria: A comprehensive review. Food Sci. Biotechnol. 2025, 1–29. [Google Scholar] [CrossRef]
- Elkhalifa, A.M.E.; Nazar, M.; Ali, S.I.; Khursheed, I.; Taifa, S.; Ahmad Mir, M.; Shah, I.H.; Malik, M.; Ramzan, Z.; Ahad, S.; et al. Novel Therapeutic Agents for Management of Diabetes Mellitus: A Hope for Drug Designing against Diabetes Mellitus. Life 2024, 14, 99. [Google Scholar] [CrossRef]
- Anestopoulos, I.; Kiousi, D.E.; Klavaris, A.; Maijo, M.; Serpico, A.; Suarez, A.; Sanchez, G.; Salek, K.; Chasapi, S.A.; Zompra, A.A.; et al. Marine-derived surface active agents: Health-promoting properties and blue biotechnology-based applications. Biomolecules 2020, 10, 885. [Google Scholar] [CrossRef]
- McGurrin, A.; Suchintita Das, R.; Soro, A.B.; Maguire, J.; Flórez Fernández, N.; Dominguez, H.; Torres, M.D.; Tiwari, B.K.; Garcia-Vaquero, M. Antimicrobial Activities of Polysaccharide-Rich Extracts from the Irish Seaweed Alaria esculenta, Generated Using Green and Conventional Extraction Technologies, Against Foodborne Pathogens. Mar. Drugs 2025, 23, 46. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Jeon, E.B.; Kim, S.H.; Park, S.Y. Antibiofilm Efficacy of Quercetin against Vibrio parahaemolyticus Biofilm on Food-Contact Surfaces in the Food Industry. Microorganisms 2022, 10, 1902. [Google Scholar] [CrossRef]
- Kim, S.H.; Roy, P.K.; Park, S.Y. Synergistic Effects of Combined Flavourzyme and Floating Electrode-Dielectric Barrier Discharge Plasma on Reduction of Escherichia coli Biofilms in Squid (Todarodes pacificus). Microorganisms 2024, 12, 1188. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Park, J.; Toushik, S.H.; Shaila, S.; Ha, A.J.W.; Rahman, M.A.; Park, S.H.; Ha, S.D. Synergistic mechanism of UV-C and postbiotic of Leuconostoc mesenteroides (J. 27) combination to eradicate Salmonella Thompson biofilm in the poultry industry. Food Control 2024, 164, 110607. [Google Scholar] [CrossRef]
- Roy, P.K.; Kim, G.; Fang, X.; Hassan, B.; Soysa, M.D.; Shin, S.T.; Cho, J.K. Optimization of post-activation systems to improve the embryonic development in porcine parthenogenesis and somatic cell nuclear transfer. J. Embryo Transf. 2017, 32, 95–104. [Google Scholar] [CrossRef]
- Roy, P.K.; Fang, X.; Hassan, B.; Shin, S.T.; Cho, J.K. Effects of roscovitine on in vitro development of porcine oocyte using brilliant cresyl blue. J. Embryo Transf. 2017, 32, 111–122. [Google Scholar] [CrossRef]
- Cao, J.; Qin, L.; Liu, M.; Yao, M.; Wang, K.; Lin, H.; Qu, C.; He, Y.; Xue, C.; Miao, J. Fucoidan from sea cucumber cooking liquid: Structural analysis, physicochemical properties, and anti-Helicobacter pylori potential. Int. J. Biol. Macromol. 2025, 306, 141593. [Google Scholar] [CrossRef]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine fucoidans: Structural, extraction, biological activities and their applications in the food industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Jun, J.Y.; Jung, M.J.; Jeong, I.H.; Yamazaki, K.; Kawai, Y.; Kim, B.M. Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Gemba, M.; Rosiak, E.; Kołożyn-Krajewska, D. Development of predictive models of biofilm formation by C. sakazakii, E. cloacae on surfaces used in the food industry and medicine. Int. J. Food Microbiol. 2025, 434, 111131. [Google Scholar] [CrossRef]
- Rajasekaran, J.; Viswanathan, P. Anti-bacterial and antibiofilm properties of seaweed polysaccharide-based nanoparticles. Aquac. Int. 2023, 31, 2799–2823. [Google Scholar] [CrossRef]
- Khan, F.; Manivasagan, P.; Lee, J.-W.; Pham, D.T.N.; Oh, J.; Kim, Y.-M. Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Mar. Drugs 2019, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Mizan, M.F.R.; Hossain, M.I.; Han, N.; Nahar, S.; Ashrafudoulla, M.; Toushik, S.H.; Shim, W.-B.; Kim, Y.-M.; Ha, S.-D. Elimination of Vibrio parahaemolyticus biofilms on crab and shrimp surfaces using ultraviolet C irradiation coupled with sodium hypochlorite and slightly acidic electrolyzed water. Food Control 2021, 128, 108179. [Google Scholar] [CrossRef]
- Han, S.; Choi, M.W.; Byun, K.H.; Kim, B.H.; Song, M.S.; Kang, I.; Ha, S.D. Characterization of Salmonella ser. Enteritidis-specific bacteriophages and biocontrol strategy to reduce S. Enteritidis on egg products using bacteriophages and essential oil compounds. Food Control 2024, 160, 110304. [Google Scholar] [CrossRef]
- Shivaprasad, D.P.; Kaushik, A.; Taneja, N.K.; Lakra, A.; Bharadwaj, D.K.; Juneja, V.K.; Taneja, P.; Chauhan, K.; Oberoi, H.S. Breaking the biofilm barrier: Vitamin C as a novel strategy against multidrug-resistant Salmonella Typhimurium. Int. J. Food Sci. Technol. 2025, 60, vvae082. [Google Scholar] [CrossRef]
- Lawal, H.; Saeed, S.I.; Gaddafi, M.S.; Kamaruzzaman, N.F. Green Nanotechnology: Naturally Sourced Nanoparticles as Antibiofilm and Antivirulence Agents Against Infectious Diseases. Int. J. Microbiol. 2025, 2025, 8746754. [Google Scholar] [CrossRef]
- Galinskaitė, A.; Gruškienė, R.; Kavleiskaja, T.; Stanevičienė, R.; Servienė, E.; Sereikaitė, J. Bioactive Fucoidan-Based Three-Component Colloidal Particles for Food Safety. Food Bioprocess Technol. 2025, 1–13. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Thirugnanasambantham, M.K.; Khan, F.; Mani, A.K. Bacteria-Inspired Synthesis of Silver-Doped Zinc Oxide Nanocomposites: A Novel Synergistic Approach in Controlling Biofilm and Quorum-Sensing-Regulated Virulence Factors in Pseudomonas aeruginosa. Antibiotics 2025, 14, 59. [Google Scholar] [CrossRef]
- Alipour, A.; Marhamatizadeh, M.H.; Mohammadi, M. Studying the shelf life of butter containing fucoidan, by evaluating sensory and chemical properties. Food Sci. Nutr. 2023, 11, 2956–2963. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Ormian, M.; Sokołowicz, Z.; Kačániová, M. The effect of the addition of hemp seeds, Amaranth, and Golden flaxseed on the nutritional value, physical, sensory characteristics, and safety of poultry Pâté. Appl. Sci. 2022, 12, 5289. [Google Scholar] [CrossRef]
- Pittia, P.; Heer, M. Space food for the future: Nutritional challenges and technological strategies for healthy and high-quality products. In In-Space Manufacturing and Resources: Earth and Planetary Exploration Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; Volume 58, pp. 251–268. [Google Scholar]



| Target Primers | Sequence (5′–3′) | Product Size (bp) |
|---|---|---|
| 16S rRNA | F: CAGAAGAAGCACCGGCTAAC | 167 |
| R: GACTCAAGCCTGCCAGTTTC | ||
| rpoS | F: GAATCTGACGAACACGCTCA | 171 |
| R: CCACGCAAGATGACGATATG | ||
| avrA | F: GAGCTGCTTTGGTCCTCAAC | 173 |
| R: AATGGAAGGCGTTGAATCTG | ||
| hilA | F: ATTAAGGCGACAGAGCTGGA | 134 |
| R: GCAGAAATGGGCGAAAGTAA | ||
| LuxS | F: CGGGTTGCAAAAACGATGA | 150 |
| R: GTTGAGGTGGTCGCGCATA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, A.; Roy, P.K.; Cho, S.R.; Park, S.Y. Effects of Fucoidan on the Inhibition of Biofilm Formation of Salmonella enterica Subsp. enterica Serovar Typhimurium on Seafoods and Its Molecular Antibiofilm Mechanisms. Microorganisms 2025, 13, 914. https://doi.org/10.3390/microorganisms13040914
Roy A, Roy PK, Cho SR, Park SY. Effects of Fucoidan on the Inhibition of Biofilm Formation of Salmonella enterica Subsp. enterica Serovar Typhimurium on Seafoods and Its Molecular Antibiofilm Mechanisms. Microorganisms. 2025; 13(4):914. https://doi.org/10.3390/microorganisms13040914
Chicago/Turabian StyleRoy, Anamika, Pantu Kumar Roy, Sung Rae Cho, and Shin Young Park. 2025. "Effects of Fucoidan on the Inhibition of Biofilm Formation of Salmonella enterica Subsp. enterica Serovar Typhimurium on Seafoods and Its Molecular Antibiofilm Mechanisms" Microorganisms 13, no. 4: 914. https://doi.org/10.3390/microorganisms13040914
APA StyleRoy, A., Roy, P. K., Cho, S. R., & Park, S. Y. (2025). Effects of Fucoidan on the Inhibition of Biofilm Formation of Salmonella enterica Subsp. enterica Serovar Typhimurium on Seafoods and Its Molecular Antibiofilm Mechanisms. Microorganisms, 13(4), 914. https://doi.org/10.3390/microorganisms13040914








