Co-Inoculation of Trichoderma harzianum and Bradyrhizobium Species Augment the Growth of Schizolobium parahyba var. parahyba (Vell.) Blake Seedlings
Abstract
:1. Introduction
2. Material and Methods
2.1. Description of the Experimental Site
2.2. Soil Characteristics: Correction and Fertilization
2.3. Seeds (Schizolobium parahyba var. parahyba): Overcoming Dormancy
2.4. Biostimulating Microorganisms: Soil Treatment
2.5. Conducting Experimental Analysis
2.6. Statistical Analysis
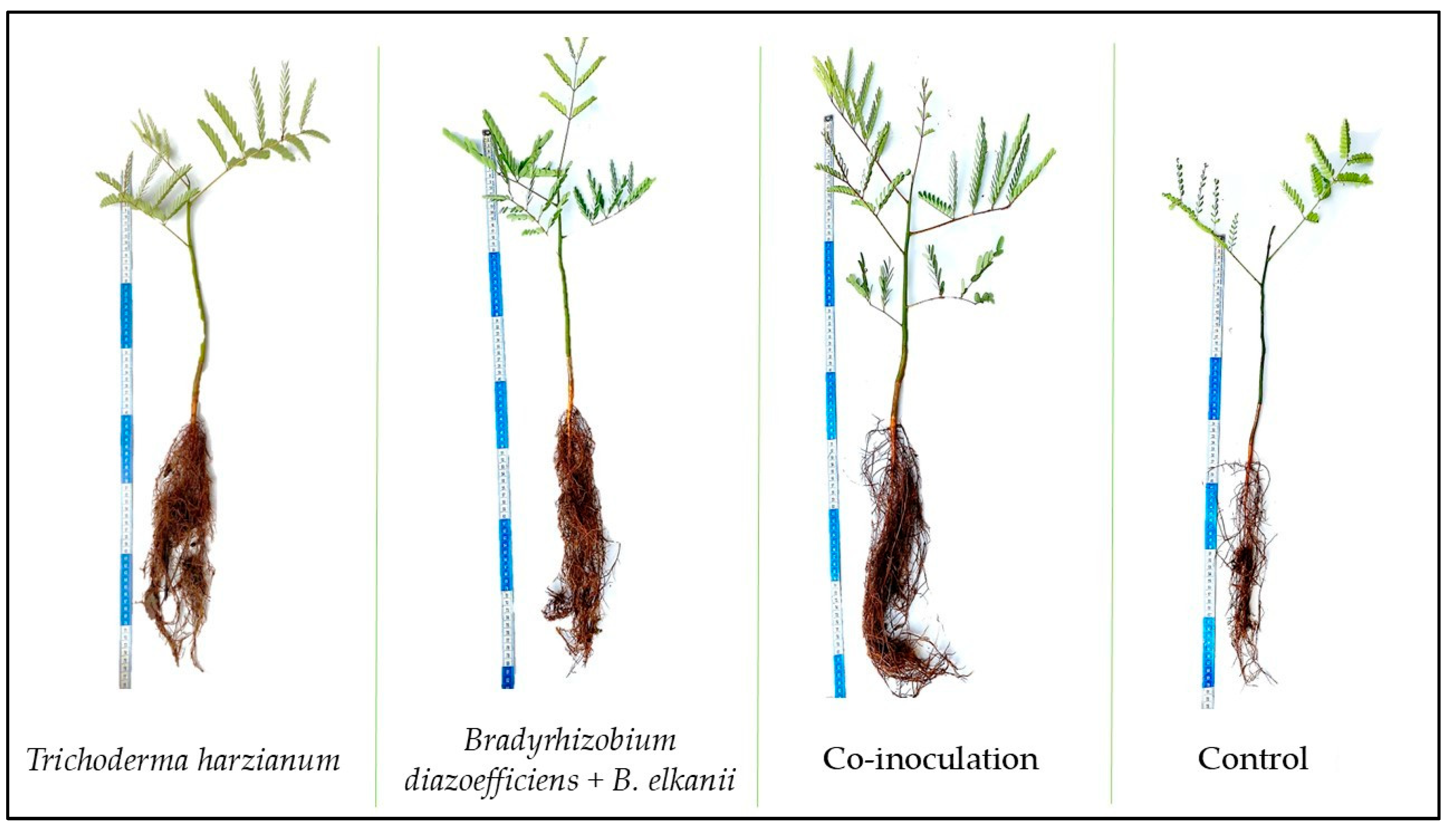
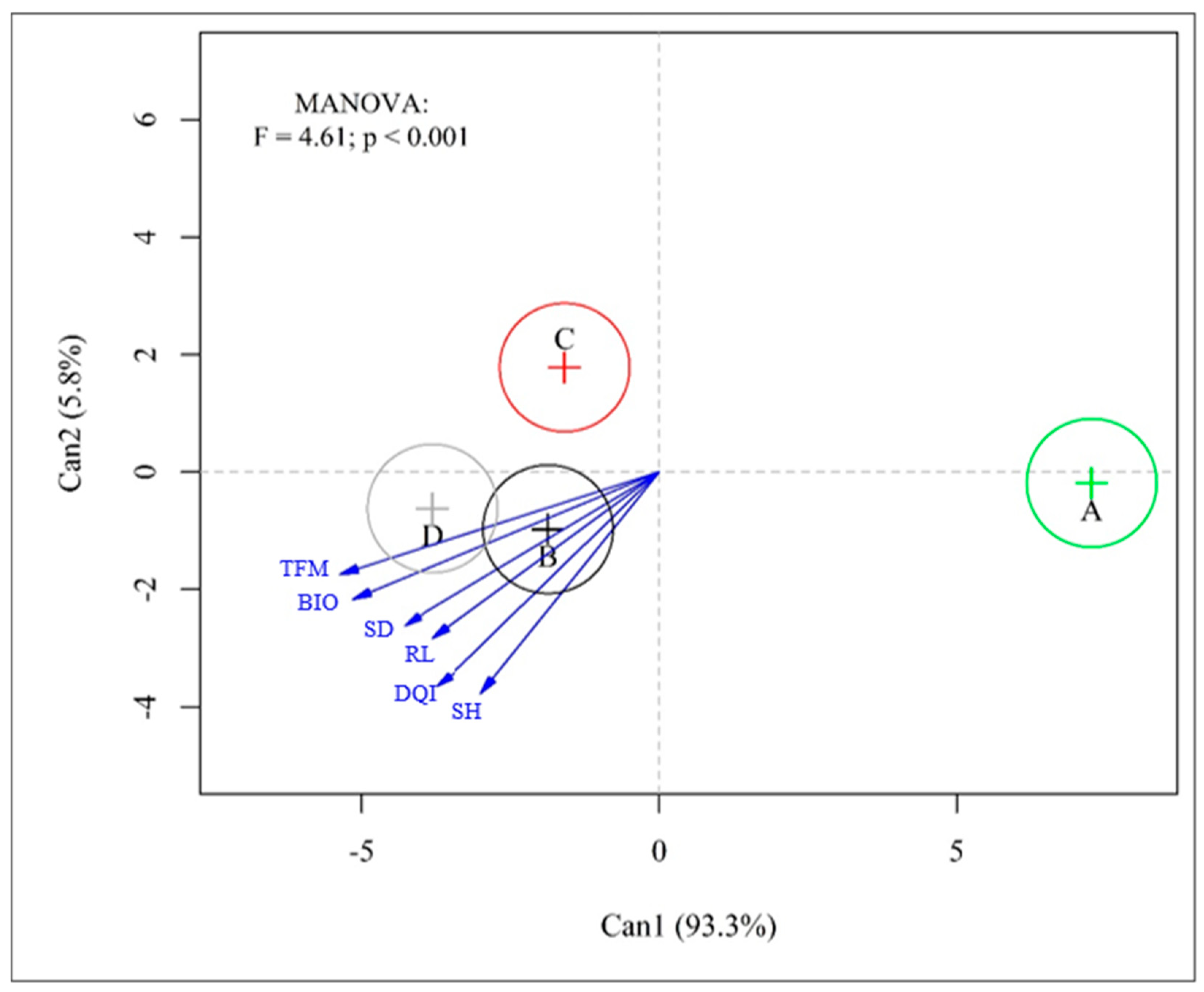
3. Results
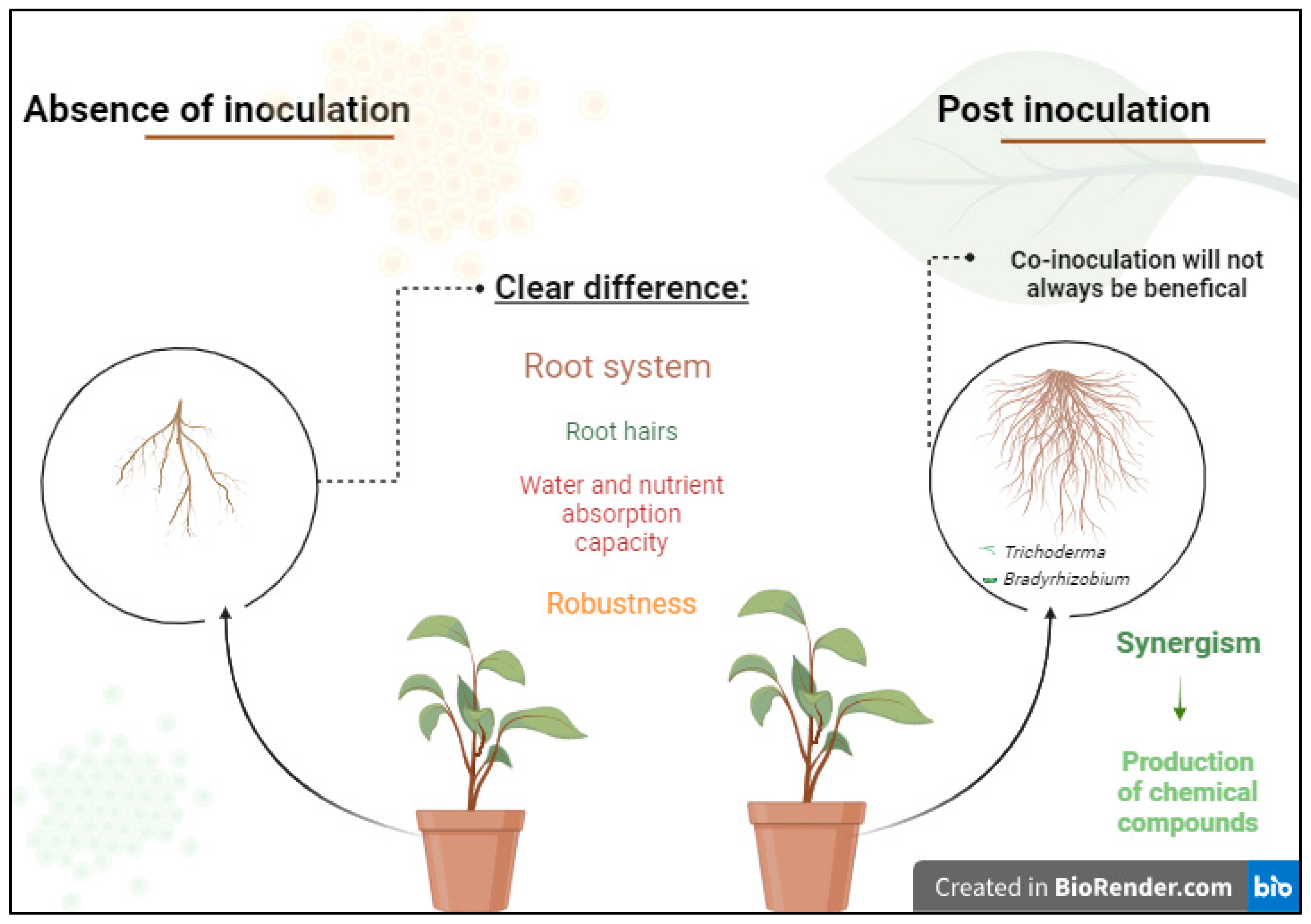
4. Discussion
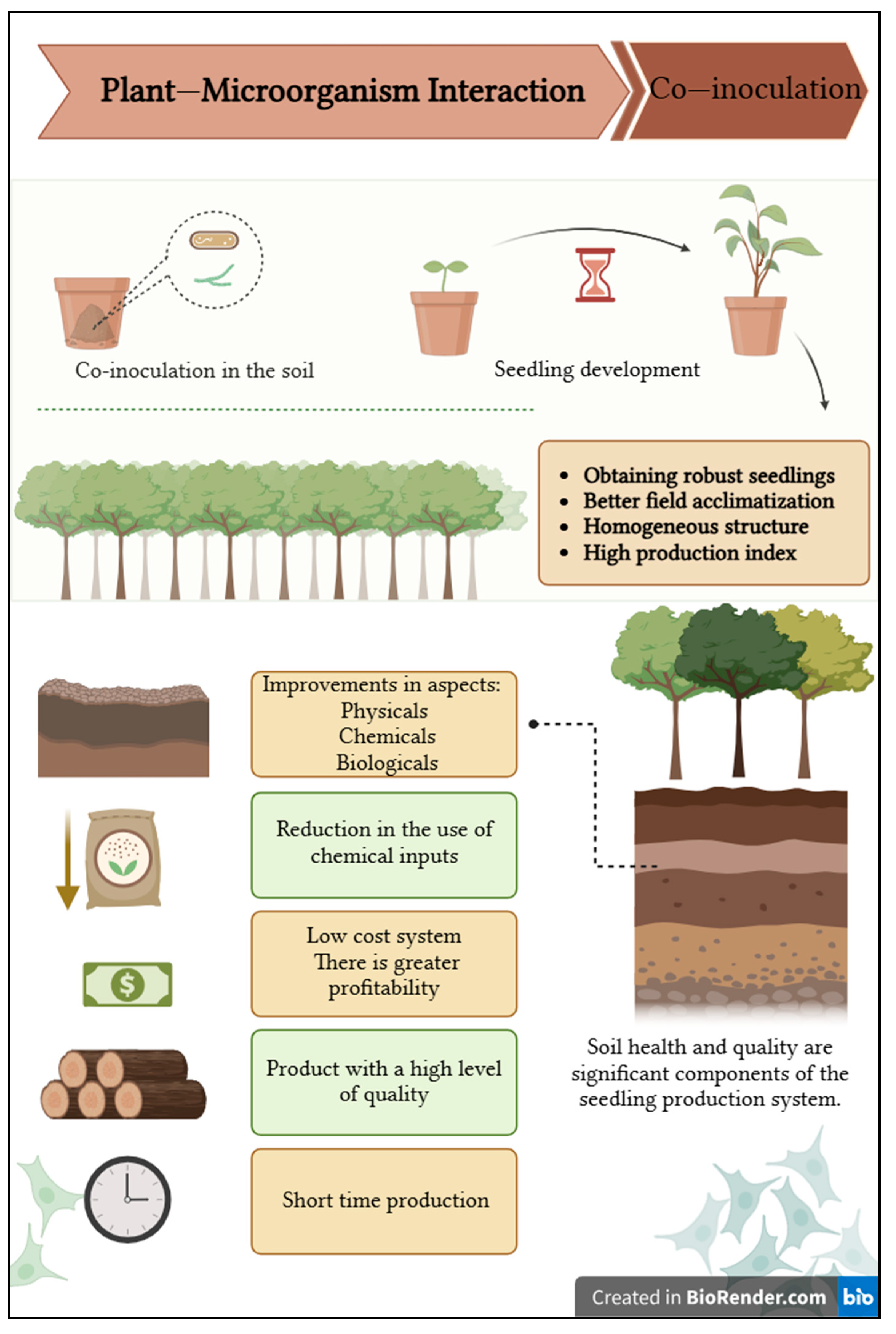
5. Considerations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castilho Silva, A.W.; Pontara, V.; Bueno, M.L.; Villa, P.M.; Walter, B.M.T.; Meira-Neto, J.A.A. The bulk of a plant hotspot: Composition, species richness and conservation status of the Cerrado herbaceous–subshrub flora. Folia Geobot. 2024, 59, 39–49. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, L.; Feng, Q.; Luo, H.; Chen, C.; Wang, S.; Li, N. Influence and role of fungi, bacteria, and mixed microbial populations on phosphorus acquisition in plants. Agriculture 2024, 14, 358. [Google Scholar] [CrossRef]
- Niu, K.; Li, M.; Lenzen, M.; Wiedmann, T.; Han, X.; Jin, S.; Gu, B. Impacts of global trade on cropland soil-phosphorus depletion and food security. Nat. Sustain. 2024, 1, 1128–1140. [Google Scholar] [CrossRef]
- Campos, E.V.; Pereira, A.; Aleksieienko, I.; Carmo, G.C.; Gohari, G.; Santaella, C.; Oliveira, H.C. Encapsulated plant growth regulators and associative microorganisms: Nature-based solutions to mitigate the effects of climate change on plants. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef] [PubMed]
- Tormes, E.C.; Barbosa, B.S.; Berle, H.; Uriarte, J.F.; Pollnow, H.E.; Pasa, M. Legal aspects of the production of seeds and seedlings of forest species. Res. Soc. Dev. 2022, 11, e37911325903. [Google Scholar]
- Abreu, G.M.; Paiva, H.N.; Megumi Kasuya, M.C.; Paula, S.D.; Guirardi, B.D.; Araújo, G.M. Soil of the parent plant and AMF mix improve Cerrado seedlings growth in forest nurseries. iForest-Biogeosciences For. 2022, 15, 197–205. [Google Scholar] [CrossRef]
- Figueiredo Guimarães Epifanio, M.L.; de Almeida Sousa, H.G.; Campos Aguiar, B.A.; Silva, R.C.; Dias, C.F.; Xavier, M.O.; Souza, P.B. Morphophysiological comparison of Schizolobium parahyba varieties seedlings cultivated under different shading levels. Aust. J. Crop Sci. 2022, 16, 408–414. [Google Scholar] [CrossRef]
- Corrêa, T.R.; Dias, D.P. Emergência e crescimento inicial de plântulas de guapuruvu (Schizolobium parahyba (Vell.) Blake) a partir de diferentes recipientes e tamanhos de sementes. Glob. Sci. Technol. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Duin, V.F.F.; Liuti, G.; Prado, N.V.; Cely, M.V.T.; Lima Andreata, M.F.; Santos, I.M.O.; Andrade, G. Effect of the fertilization and growth promoting microrganisms on Schizolobium parahyba. Semin. Cienc. Agrar. 2019, 40, 1747–1760. [Google Scholar] [CrossRef]
- Reyes, F.C.; Quiroz, D.C.; Ceja, J.E.S.; Sánchez, A.R.; Reyes, J.S. Efectos del pretratamiento con Trichoderma y Bacillus en la germinación de semillas de Agave victoriae-reginae T. Moore. Rev. Mex. Cienc. For. 2022, 13, 56–72. [Google Scholar] [CrossRef]
- Joshi, S.; Gangola, S.; Jaggi, V.; Sahgal, M. Functional characterization and molecular fingerprinting of potential phosphate solubilizing bacterial candidates from Shisham rhizosphere. Sci. Rep. 2023, 13, 7003. [Google Scholar] [CrossRef]
- Komolafe, A.F.; Kayode, C.O.; Ezekiel-Adewoyin, D.T.; Ayanfe Oluwa, O.E.; Ogunleti, D.O.; Makinde, A.I. Soil properties and performance of celosia (Celosia argentea) as affected by compost made with Trichoderma asperellum. Eurasian J. Soil Sci. 2021, 10, 199–206. [Google Scholar] [CrossRef]
- Prasad, A.; Dixit, M.; Meena, S.K.; Kumar, A. Qualitative and quantitative estimation for phosphate solubilizing ability of Trichoderma isolates: A natural soil health enhancer. Mater. Today Proc. 2021, 81, 360–366. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.J.; Anjum, M.M.; Khan, G.R.; Ali, N. Trichoderma harzianum modulates phosphate and micronutrient solubilization in the rhizosphere. Gesunde Pflanz. 2022, 74, 853–862. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystem. Microbiol. Res. 2024, 281, 127621. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Boubakri, H.; Chen, B.; Farooq, M.; Lai, Z.; Kou, H.; Fan, J. Biofertilizers as an eco-friendly approach to combat drought stress in plants. Biocatal. Agric. Biotechnol. 2025, 64, 103510. [Google Scholar] [CrossRef]
- Siregar, B.A.; Liantiqomah, D.; Gafur, A.; Tjahjono, B. Screening of endophytic Trichoderma isolates to improve the growth and health of Eucalyptus pellita seedlings. IOP Publ. 2022, 974, 012084. [Google Scholar] [CrossRef]
- Batistello, M.N.; Brito, N.F.; Sousa, W.N.; Felsemburgh, C.A.; Vieira, T.A.; Lustosa, D.C. Beneficial soil fungi and jabuticaba growth promotion. Agronomy 2022, 12, 367. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Aguirre-Noyola, J.L.; Martínez-Romero, E.; Arteaga-Garibay, R.I.; Ireta-Moreno, J.; Ruvalcaba-Gómez, J.M. A look at plant-growth-promoting bacteria. Plants 2023, 12, 1668. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A promising source of plant growth-promoting molecules and their non-legume interactions: Examining applications and mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Fernandez-Gnecco, G.; Gégu, L.; Covacevich, F.; Consolo, V.F.; Bouffaud, M.L.; Buscot, F.; Babin, D. Alone as effective as together: AMF and Trichoderma inoculation boost maize performance but differentially shape soil and rhizosphere microbiota. J. Sustain. Agric. Environ. 2024, 31, 2091. [Google Scholar] [CrossRef]
- Dwibedi, V.; Rath, S.K.; Joshi, M.; Kaur, R.; Kaur, G.; Singh, D.; Kaur, S. Microbial endophytes: Application towards sustainable agriculture and food security. Appl. Microbiol. Biotechnol. 2022, 106, 5359–5384. [Google Scholar] [CrossRef] [PubMed]
- Lima Andreata, M.F.; Afonso, L.; Niekawa, E.T.G.; Salomão, J.M.; Basso, K.R.; Andrade, G. Microbial fertilizers: A study on the current scenario of brazilian inoculants and future perspectives. Preprints 2024, 1, 2246. [Google Scholar] [CrossRef]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control 2022, 176, 105100. [Google Scholar] [CrossRef]
- Bisht, N.; Singh, T.; Ansari, M.M.; Chauhan, P.S. The hidden language of plant-beneficial microbes: Chemo-signaling dynamics in plant microenvironments. World J. Microb. Biotechnol. 2025, 41, 35. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Babalola, O.O. Fungi that promote plant growth in the rhizosphere boost crop growth. J. Fungi 2023, 9, 239. [Google Scholar] [CrossRef]
- Ratu, S.T.N.; Amelia, L.; Okazaki, S. Type III effector provides a novel symbiotic pathway in legume–rhizobia symbiosis. Biosci. Biotechnol. Biochem. 2023, 87, 28–37. [Google Scholar] [CrossRef]
- Sousa, W.N.; Brito, N.F.; Felsemburgh, C.A.; Vieira, T.A.; Lustosa, D.C. Evaluation of Trichoderma spp. isolates in cocoa seed treatment and seedling production. Plants 2021, 10, 1964. [Google Scholar] [CrossRef]
- Riseh, R.S.; Fathi, F.; Vazvani, M.G.; Tarkka, M.T. Plant Colonization by Biocontrol Bacteria and Improved Plant Health: A Review. Front. Biosci. Landmark 2025, 30, 23223. [Google Scholar] [CrossRef]
- Wahab, A.; Bibi, H.; Batool, F.; Muhammad, M.; Ullah, S.; Zaman, W.; Abdi, G. Plant growth-promoting rhizobacteria biochemical pathways and their environmental impact: A review of sustainable farming practices. Plant Growth Regul. 2024, 104, 637–662. [Google Scholar] [CrossRef]
- Ali, J.; Mukarram, M.; Ojo, J.; Dawam, N.; Riyazuddin, R.; Bayram, A. Harnessing phytohormones: Advancing plant growth and defense strategies for sustainable agriculture. Physiol. Plant. 2024, 176, 14307. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Tabassum, B.; Hashim, M.; Khan, N. Role of plant growth promoting rhizobacteria (PGPR) as a plant growth enhancer for sustainable agriculture: A review. Bacteria 2024, 3, 59–75. [Google Scholar] [CrossRef]
- Negi, R.; Sharma, B.; Parastesh, F.; Kaur, S.; Yadav, A.N. Microbial consortia mediated regulation of plant defense: A promising tool for sustaining crops protection. Phys. Mol. Plant Pat. 2024, 134, 102393. [Google Scholar] [CrossRef]
- Waqar, S.; Bhat, A.A.; Khan, A.A. Endophytic fungi: Unravelling plant-endophyte interaction and the multifaceted role of fungal endophytes in stress amelioration. Plant Phys. Biochem. 2024, 206, 108174. [Google Scholar] [CrossRef]
- Trujillo-Elisea, F.I.; Labrín-Sotomayor, N.Y.; Becerra-Lucio, P.A.; Peña-Ramírez, Y.J. Plant growth and microbiota structural effects of Rhizobacteria inoculation on mahogany (Swietenia macrophylla King [Meliaceae]) under nursery conditions. Forests 2022, 13, 1742. [Google Scholar] [CrossRef]
- Alexandre, F.S.; Della Flora, L.V.; Henrique, I.G.; Silva, D.C.; Cely, M.V. Arbuscular mycorrhizal fungi (Rhizophagus clarus) and rhizobacteria (Bacillus subtilis) can improve the clonal propagation and development of teak for commercial plantings. Front. Plant Sci. 2021, 12, 628769. [Google Scholar] [CrossRef]
- Ribeiro, A.S.N.; Junior, A.F.C.; Chagas, L.F.B.; Alves, M.V.G. Efficiency of Trichoderma and Bacillus subtilis as growth promoters in eucalyptus Corymbia citriodora. Obs. Econ. Latinoam. 2023, 21, 20380–20397. [Google Scholar] [CrossRef]
- Kumar, A.M.; Sandhya, G.M.; Karthikeyan, A. Evaluation of bio-fertiliser (bio-inoculant) consortia and their effect on plant growth performance of sandalwood (Santalum album) seedlings. J. Trop. For. Sci. 2023, 35, 311–321. [Google Scholar] [CrossRef]
- Aguirre, M.I.H.; Vega, W.O.; Peláez, J.L.D. Co-inoculation with beneficial soil microorganisms promoted growth and quality of Tabebuia rosea seedlings. For. Sci. 2022, 68, 95–103. [Google Scholar] [CrossRef]
- Chaiya, L.; Gavinlertvatana, P.; Teaumroong, N.; Lumyong, S. Enhancing teak (Tectona grandis) seedling growth by rhizosphere microbes: A sustainable way to optimize agroforestry. Microorganisms 2021, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Malgioglio, G.; Rizzo, G.F.; Nigro, S.; Lefebvre, P.V.; Herforth-Rahmé, J.; Catara, V.; Branca, F. Plant-microbe interaction in sustainable agriculture: The factors that may influence the efficacy of PGPM application. Sustainability 2022, 14, 2253. [Google Scholar] [CrossRef]
- Palma, M.; Scotti, R.; D’agostino, N.; Zaccardelli, M.; Tucci, M. Phyto-Friendly soil bacteria and fungi provide beneficial outcomes in the host plant by differently modulating its responses through (in) direct mechanisms. Plants 2022, 11, 2672. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, R.; Sarma, A.K. Phosphate solubilizing microorganisms: A review. Commun. Soil Sci. Plant Anal. 2022, 1, 1306–1315. [Google Scholar] [CrossRef]
- Gorai, P.S.; Barman, S.; Gond, S.K.; Mandal, N.C. Trichoderma. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Annapurna, K., Sankaranarayanan, A., Kumar, M.S., Kumar, K., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 1, pp. 571–591. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Valencia-Cantero, E.; Santoyo, G. Plant growth-promoting bacteria potentiate antifungal and plant-beneficial responses of Trichoderma atroviride by upregulating its effector functions. PLoS ONE 2024, 19, 190301139. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Ivetić, V. Root system development and field establishment: Effect of seedling quality. New For. 2022, 53, 1021–1067. [Google Scholar] [CrossRef]
| S | P Mehlich | K | Na | Zn | Cu | Fe | Mn | Ca | Mg | Al | H+Al |
| mg dm−3 | cmolcdm−3 | ||||||||||
| 2.00 | 0.50 | 15.00 | 3.80 | 0.50 | 0.60 | 23.40 | 8.10 | 0.20 | 0.10 | 0.00 | 2.40 |
| SOM | Organic Carbon | pH | Sand | Clay | Silt | CEC | V | Ca/CEC | Mg/CEC | K/CEC | H+Al/CEC |
| g kg−1 | CaCl2 | Texture (g kg−1) | cmolcdm−3 | % | |||||||
| 8.00 | 4.64 | 5.10 | 370.00 | 540.00 | 90.00 | 2.76 | 12.91 | 2.00 | 3.62 | 1.45 | 86.96 |
| Treatment | SH | RL | SD | SFM | RFM | TFM | SDM | RDM | BIO | DQI |
|---|---|---|---|---|---|---|---|---|---|---|
| cm | mm | g | ||||||||
| T. harzianum | 20.18 a | 43.10 a | 5.34 a | 6.18 b | 4.53 a | 10.71 b | 2.98 b | 2.00 b | 4.99 b | 0.93 b |
| B. diazoefficiens + B. elkanii | 16.10 b | 31.80 b | 4.56 b | 5.80 b | 3.20 b | 9.00 b | 2.04 c | 1.54 b | 3.58 c | 0.74 b |
| Co-inoculation | 19.10 a | 45.40 a | 5.28 a | 7.18 a | 5.83 a | 13.04 a | 3.51 a | 2.71 a | 6.22 a | 1.24 a |
| Control | 15.50 b | 24.60 c | 3.88 b | 3.01 c | 1.35 c | 4.36 c | 0.61 d | 0.45 c | 1.06 d | 0.20 c |
| CV% | 14.06 | 25.91 | 10.11 | 9.71 | 33.60 | 13.47 | 16.31 | 34.61 | 19.27 | 24.25 |
| SE | 0.64 | 1.69 | 0.17 | 0.37 | 0.27 | 0.48 | 0.25 | 0.80 | 0.49 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, N.C.d.F.; Gatto, A.; Ramos, M.L.G. Co-Inoculation of Trichoderma harzianum and Bradyrhizobium Species Augment the Growth of Schizolobium parahyba var. parahyba (Vell.) Blake Seedlings. Microorganisms 2025, 13, 630. https://doi.org/10.3390/microorganisms13030630
Ferreira NCdF, Gatto A, Ramos MLG. Co-Inoculation of Trichoderma harzianum and Bradyrhizobium Species Augment the Growth of Schizolobium parahyba var. parahyba (Vell.) Blake Seedlings. Microorganisms. 2025; 13(3):630. https://doi.org/10.3390/microorganisms13030630
Chicago/Turabian StyleFerreira, Natália Cássia de Faria, Alcides Gatto, and Maria Lucrecia Gerosa Ramos. 2025. "Co-Inoculation of Trichoderma harzianum and Bradyrhizobium Species Augment the Growth of Schizolobium parahyba var. parahyba (Vell.) Blake Seedlings" Microorganisms 13, no. 3: 630. https://doi.org/10.3390/microorganisms13030630
APA StyleFerreira, N. C. d. F., Gatto, A., & Ramos, M. L. G. (2025). Co-Inoculation of Trichoderma harzianum and Bradyrhizobium Species Augment the Growth of Schizolobium parahyba var. parahyba (Vell.) Blake Seedlings. Microorganisms, 13(3), 630. https://doi.org/10.3390/microorganisms13030630






