The Role of Anode Potential in Electromicrobiology
Abstract
:1. Introduction
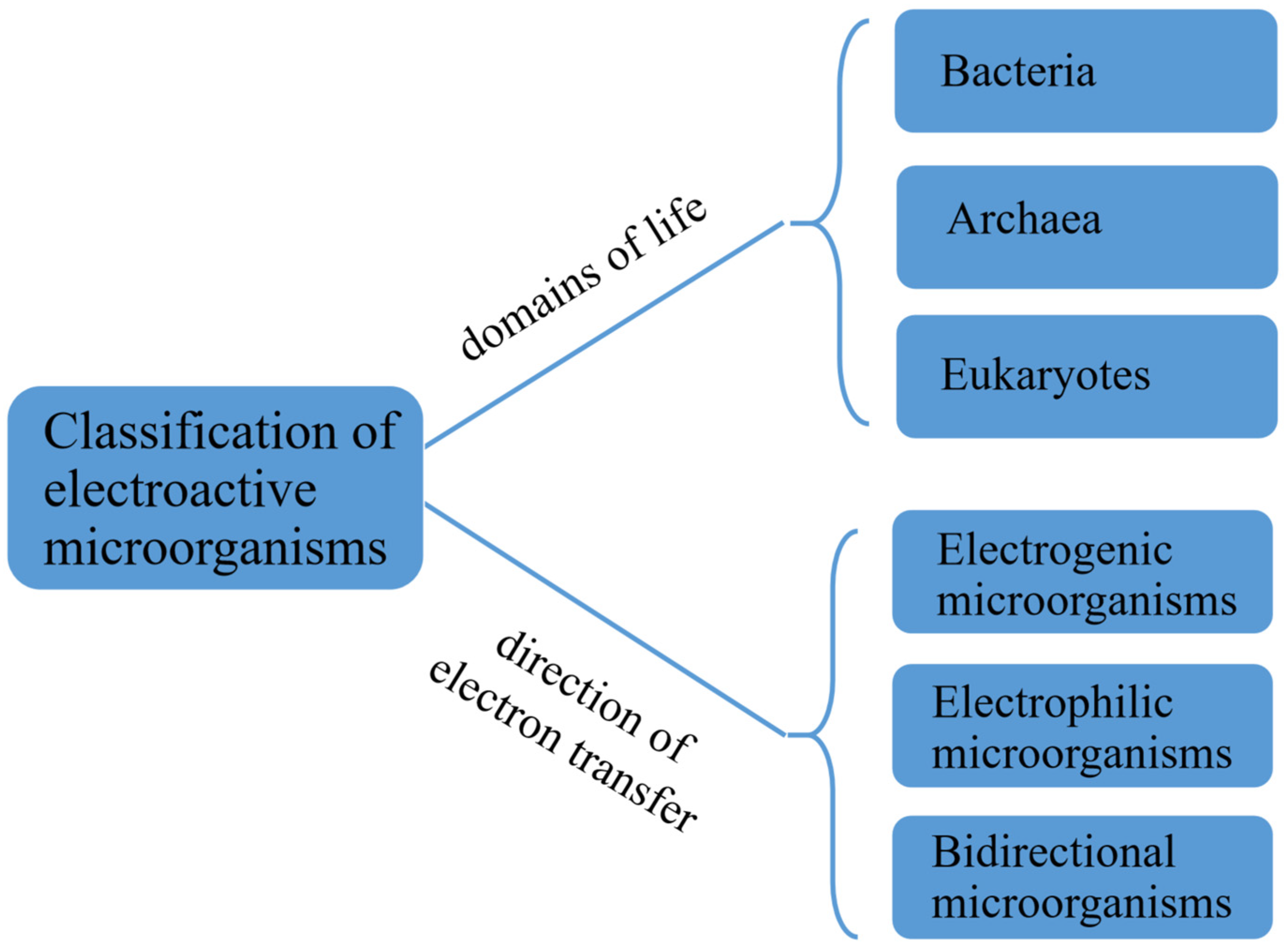
2. Classification and Characteristics of Electroactive Microorganisms
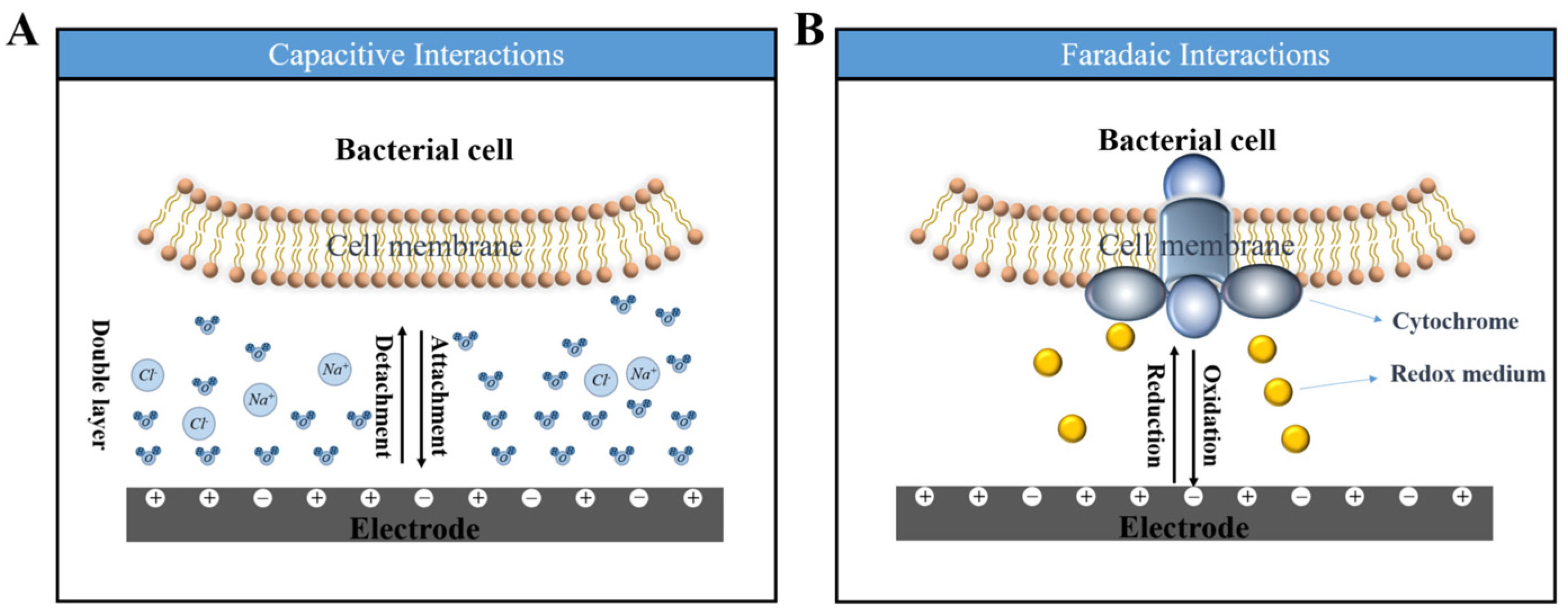
3. Electron Transfer Mechanism
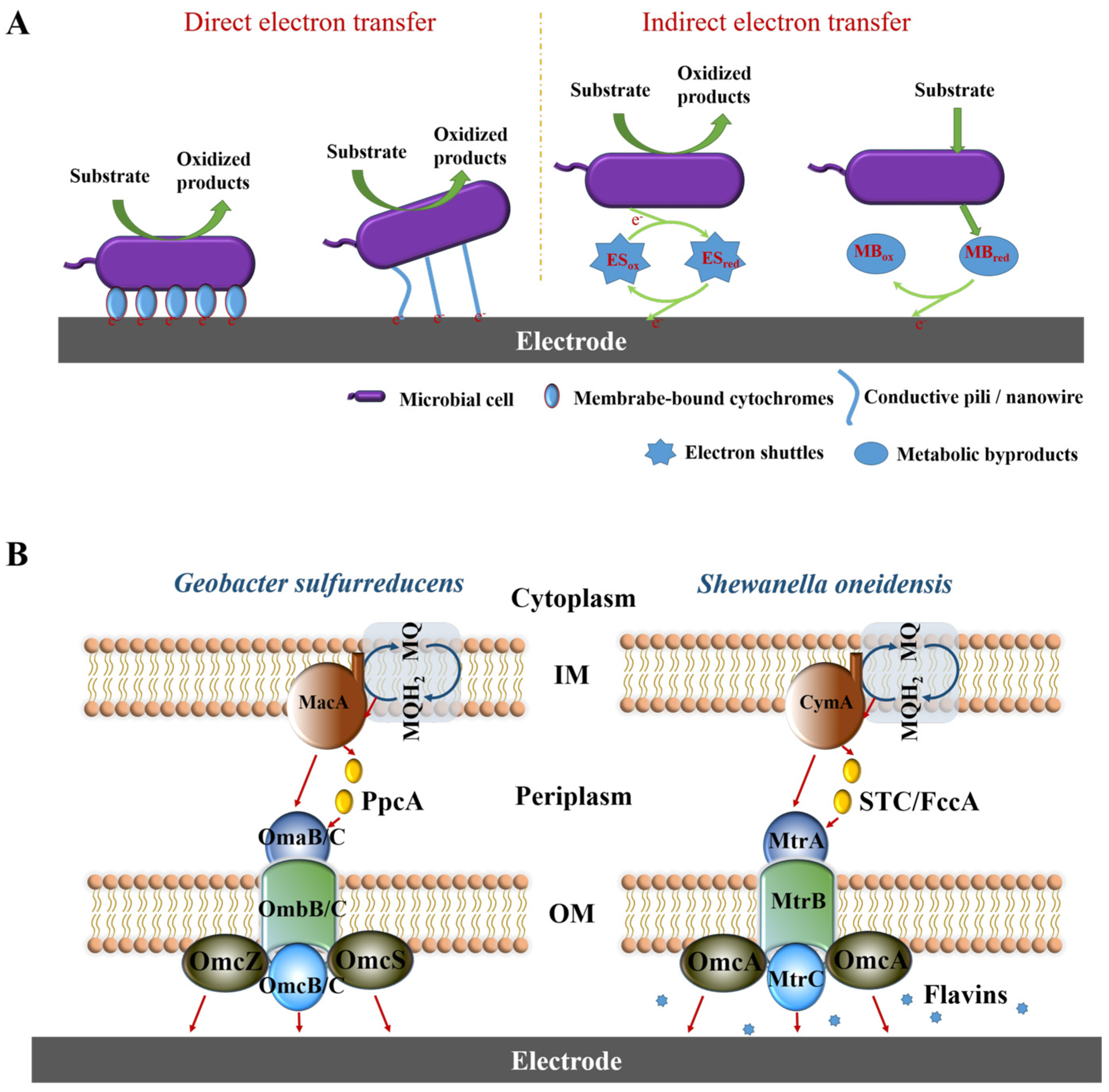
4. Effect of Anode Potential on Electricity Generation Behavior
4.1. Effect of Anode Potential on Start-Up Time and Electric Current/Power Density
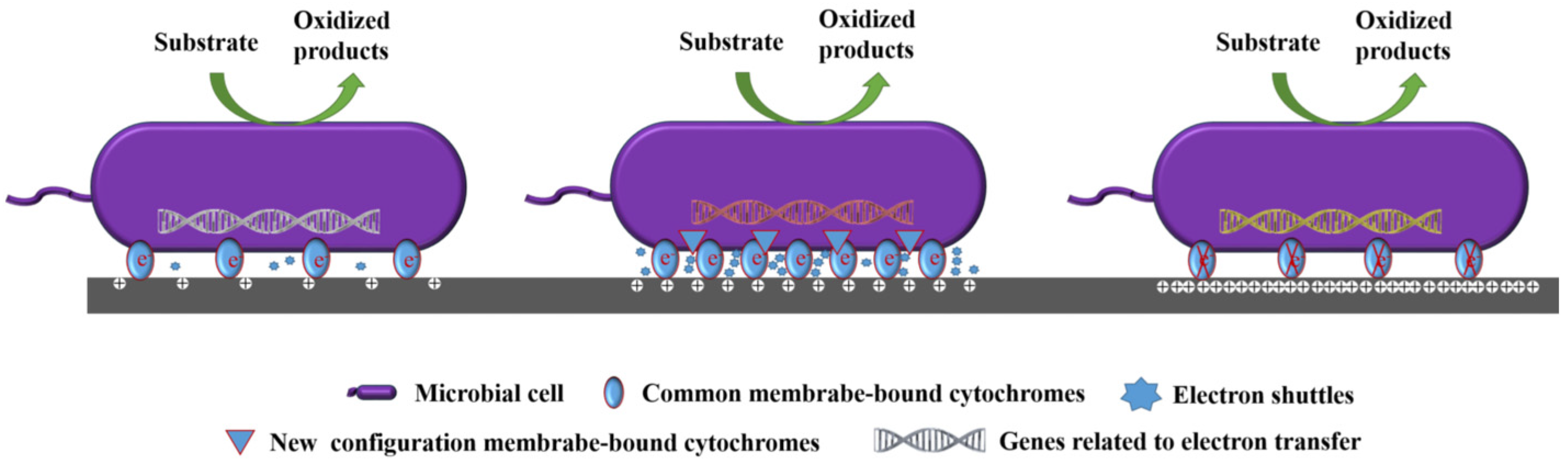
4.2. Effect on the Electron Transfer Pathways of Microorganisms
4.3. Effect on Microbial Community
4.4. Summary of Anode Potential Effects on Electricity Generation Behavior
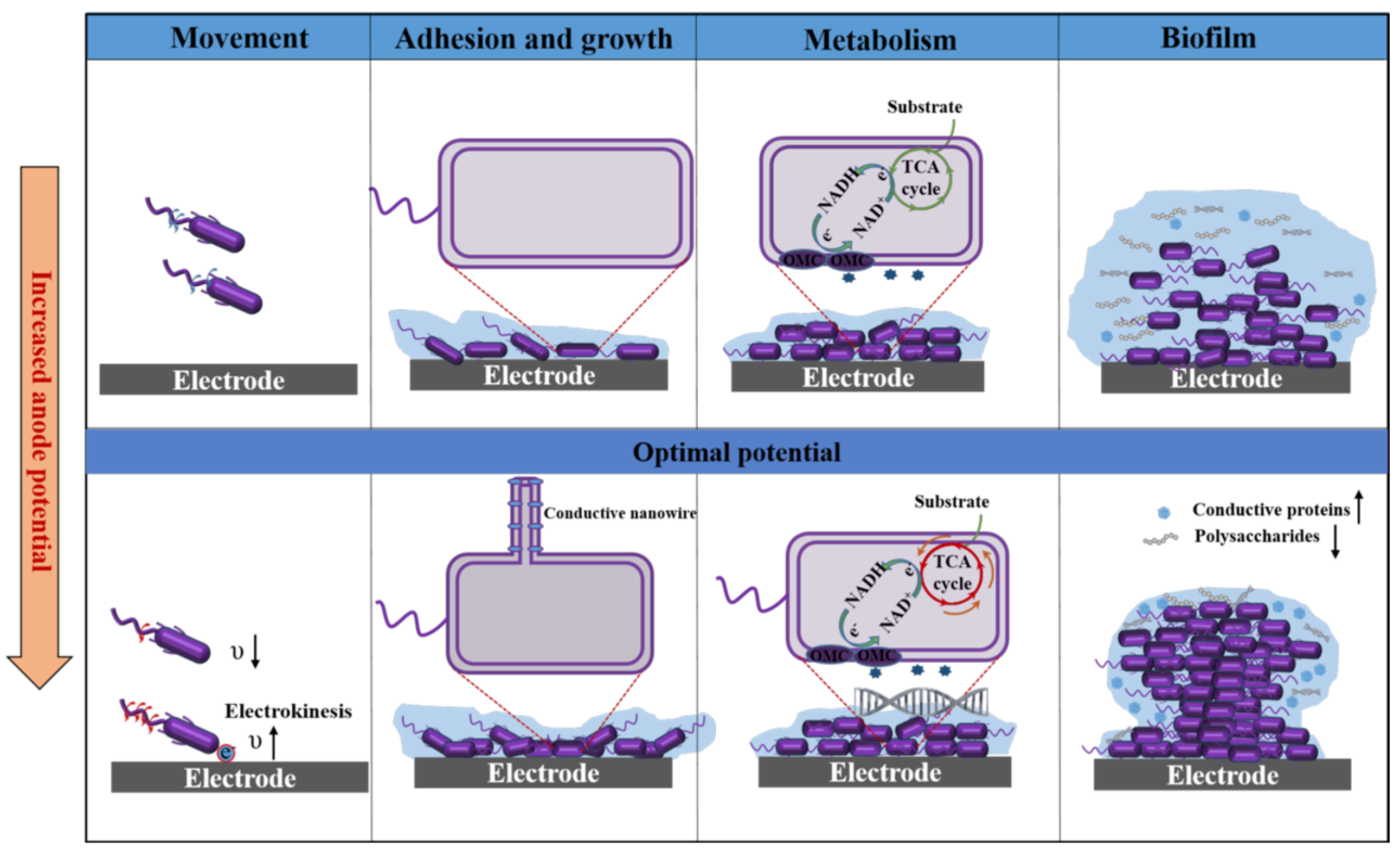
5. Effect of Anode Potential on Physiology
5.1. Effect on the Mobility of Microorganisms
5.2. Effect on the Adhesion and Growth of Microorganisms
5.3. Effect on the Metabolism of Microorganisms
5.4. Effect on the Formation of Electroactive Biofilms
5.5. Summary of Anode Potential Effects on Microbial Physiology
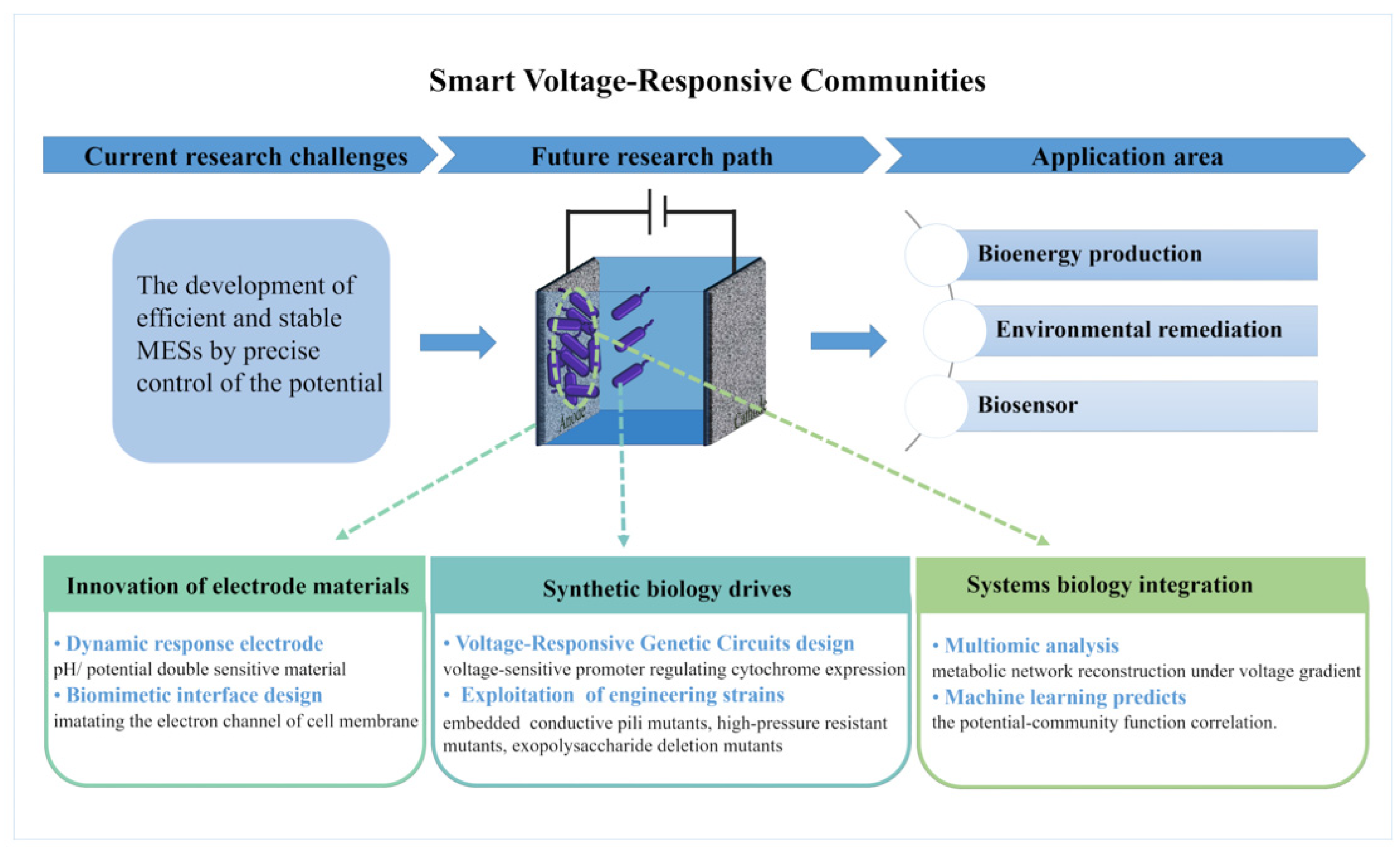
6. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schröder, U.; Harnisch, F.; Angenent, L.T. Microbial electrochemistry and technology: Terminology and classification. Energy Environ. Sci. 2015, 8, 513–519. [Google Scholar] [CrossRef]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhao, Z.; Peng, L.; Shiu, H.-Y.; Ding, M.; Song, F.; Guan, X.; Lee, C.K.; Huang, J.; Zhu, D. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells. Science 2021, 373, 1336–1340. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Lee, Y.M.; Nam, W. Fuel production from seawater and fuel cells using seawater. ChemSusChem 2017, 10, 4264–4276. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Li, H.; Opgenorth, P.H.; Wernick, D.G.; Rogers, S.; Wu, T.-Y.; Higashide, W.; Malati, P.; Huo, Y.-X.; Cho, K.M.; Liao, J.C. Integrated electromicrobial conversion of CO2 to higher alcohols. Science 2012, 335, 1596. [Google Scholar] [CrossRef]
- Fan, G.; Graham, A.J.; Kolli, J.; Lynd, N.A.; Keitz, B.K. Aerobic radical polymerization mediated by microbial metabolism. Nat. Chem. 2020, 12, 638–646. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.Y. Biosynthesis of inorganic nanomaterials using microbial cells and bacteriophages. Nat. Rev. Chem. 2020, 4, 638–656. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Lu, L.; Guest, J.S.; Peters, C.A.; Zhu, X.; Rau, G.H.; Ren, Z.J. Wastewater treatment for carbon capture and utilization. Nat. Sustain. 2018, 1, 750–758. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, M.; Liu, M.; Yang, W.; Gu, T. Microbial fuel cells for biosensor applications. Biotechnol Lett. 2015, 37, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.P.; TerAvest, M.A.; Doud, D.F.R.; Chakravorty, A.; Holmes, E.C.; Radens, C.M.; Sureka, S.; Gralnick, J.A.; Angenent, L.T. An arsenic-specific biosensor with genetically engineered Shewanella oneidensis in a bioelectrochemical system. Biosens. Bioelectron. 2014, 62, 320–324. [Google Scholar] [CrossRef]
- Kumar, A.; Katuri, K.; Lens, P.; Leech, D. Does bioelectrochemical cell configuration and anode potential affect biofilm response? Biochem. Soc. T. 2012, 40, 1308–1314. [Google Scholar] [CrossRef]
- Wagner, R.C.; Call, D.F.; Logan, B.E. Optimal set anode potentials vary in bioelectrochemical systems. Environ. Sci. Technol. 2010, 44, 6036–6041. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Liang, D.; Liu, X.; Woodard, T.L.; Holmes, D.E.; Smith, J.A.; Nevin, K.P.; Feng, Y.; Lovley, D.R. Extracellular electron exchange capabilities of Desulfovibrio ferrophilus and Desulfopila corrodens. Environ. Sci. Technol. 2021, 55, 16195–16203. [Google Scholar] [CrossRef]
- Tian, L.; Yan, X.; Wang, D.; Du, Q.; Wan, Y.; Zhou, L.; Li, T.; Liao, C.; Li, N.; Wang, X. Two key Geobacter species of wastewater-enriched electroactive biofilm respond differently to electric field. Water Res. 2022, 213, 118185. [Google Scholar] [CrossRef]
- Choi, S. Electrogenic bacteria promise new opportunities for powering, sensing, and synthesizing. Small 2022, 18, 2107902. [Google Scholar] [CrossRef]
- Mayer, F.; Enzmann, F.; Lopez, A.M.; Holtmann, D. Performance of different methanogenic species for the microbial electrosynthesis of methane from carbon dioxide. Bioresour. Technol. 2019, 289, 121706. [Google Scholar] [CrossRef]
- Yang, Y.K.; Chen, S.; Yang, D.S.; Zhang, W.; Wang, H.J.; Zeng, R.J. Anaerobic reductive bio-dissolution of jarosites by Acidithiobacillus ferrooxidans using hydrogen as electron donor. Sci. Total Environ. 2019, 686, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Um, Y.; Sang, B.I. Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol. Bioeng. 2012, 109, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Achtnich, C.; Bak, F.; Conrad, R. Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil. Biol. Fertil. Soils 1995, 19, 65–72. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Q.; Su, J.; Dong, G.; Cao, M.; Wang, Y. Metabolic regulation of Shewanella oneidensis for microbial electrosynthesis: From extracellular to intracellular. Metab. Eng. 2023, 80, 1–11. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Lloyd, J.R.; Scott, K.; Premier, G.C.; Eileen, H.Y.; Curtis, T.; Head, I.M. A critical review of integration analysis of microbial electrosynthesis (MES) systems with waste biorefineries for the production of biofuel and chemical from reuse of CO2. Renew. Sustain. Energy Rev. 2016, 56, 116–132. [Google Scholar] [CrossRef]
- Ross, D.E.; Flynn, J.M.; Baron, D.B.; Gralnick, J.A.; Bond, D.R. Towards electrosynthesis in Shewanella: Energetics of reversing the Mtr pathway for reductive metabolism. PLoS ONE 2011, 6, e16649. [Google Scholar] [CrossRef] [PubMed]
- Strycharz, S.M.; Glaven, R.H.; Coppi, M.V.; Gannon, S.M.; Perpetua, L.A.; Liu, A.; Nevin, K.P.; Lovley, D.R. Gene expression and deletion analysis of mechanisms for electron transfer from electrodes to Geobacter sulfurreducens. Bioelectrochemistry 2011, 80, 142–150. [Google Scholar] [CrossRef]
- Guang, L.; Koomson, D.A.; Jingyu, H.; Ewusi-Mensah, D.; Miwornunyuie, N. Performance of exoelectrogenic bacteria used in microbial desalination cell technology. Int. J. Environ. Res. Public Health 2020, 17, 1121. [Google Scholar] [CrossRef]
- Yan, X.; Lee, H.-S.; Li, N.; Wang, X. The micro-niche of exoelectrogens influences bioelectricity generation in bioelectrochemical systems. Renew. Sustain. Energy Rev. 2020, 134, 110184. [Google Scholar] [CrossRef]
- Yang, F.-A.; Hou, Y.-N.; Cao, C.; Ren, N.; Wang, A.-J.; Guo, J.; Liu, Z.; Huang, C. Mechanistic insights into the response of electroactive biofilms to Cd2+ shock: Bacterial viability and electron transfer behavior at the cellular and community levels. J. Hazard. Mater. 2023, 459, 132183. [Google Scholar] [CrossRef]
- Li, X.; Zheng, R.; Zhang, X.; Liu, Z.; Zhu, R.; Zhang, X.; Gao, D. A novel exoelectrogen from microbial fuel cell: Bioremediation of marine petroleum hydrocarbon pollutants. J. Environ. Manag. 2019, 235, 70–76. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Harnisch, F.; Kuhlicke, U.; Neu, T.R.; Schröder, U. Electron transfer and biofilm formation of Shewanella putrefaciens as function of anode potential. Bioelectrochemistry 2013, 93, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Korth, B.; Rosa, L.F.; Harnisch, F.; Picioreanu, C. A framework for modeling electroactive microbial biofilms performing direct electron transfer. Bioelectrochemistry 2015, 106, 194–206. [Google Scholar] [CrossRef]
- Sydow, A.; Krieg, T.; Mayer, F.; Schrader, J.; Holtmann, D. Electroactive bacteria—Molecular mechanisms and genetic tools. Appl. Microbiol. Biotechnol. 2014, 98, 8481–8495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, F.; Cao, Y.; Zhang, X.; Chen, T.; Song, H.; Wang, Z. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol. Adv. 2021, 53, 107682. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Girguis, P.; Nielsen, L.K. Metabolic and practical considerations on microbial electrosynthesis. Curr. Opin. Biotechnol. 2011, 22, 371–377. [Google Scholar] [CrossRef]
- Firer-Sherwood, M.; Pulcu, G.S.; Elliott, S.J. Electrochemical interrogations of the Mtr cytochromes from Shewanella: Opening a potential window. J. Biol. Inorg. Chem. 2008, 13, 849–854. [Google Scholar] [CrossRef]
- Zhuo, S.; Yang, G.; Zhuang, L. The electrically conductive pili of Geobacter soli. bioRxiv 2020. [Google Scholar] [CrossRef]
- Pirbadian, S.; Barchinger, S.E.; Leung, K.M.; Byun, H.S.; Jangir, Y.; Bouhenni, R.A.; Reed, S.B.; Romine, M.F.; Saffarini, D.A.; Shi, L.; et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl. Acad. Sci. USA 2014, 111, 12883–12888. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Wahid, Z.A.; Din, M.F.M. Exoelectrogens in microbial fuel cells toward bioelectricity generation: A review. Int. J. Energ. Res. 2015, 39, 1048–1067. [Google Scholar] [CrossRef]
- Richter, H.; Nevin, K.P.; Jia, H.; Lowy, D.A.; Lovley, D.R.; Tender, L.M. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2009, 2, 506–516. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Y.; O’Brien, J.P.; Sophia, M.Y.; Yalcin, S.E.; Srikanth, V.; Shen, C.; Vu, D.; Ing, N.L.; Hochbaum, A.I. Structure of microbial nanowires reveals stacked hemes that transport electrons over micrometers. Cell 2019, 177, 361–369.e10. [Google Scholar] [CrossRef]
- Yalcin, S.E.; O’Brien, J.P.; Gu, Y.; Reiss, K.; Yi, S.M.; Jain, R.; Srikanth, V.; Dahl, P.J.; Huynh, W.; Vu, D. Electric field stimulates production of highly conductive microbial OmcZ nanowires. Nat. Chem. Biol. 2020, 16, 1136–1142. [Google Scholar] [CrossRef]
- Xiong, J.; Chan, D.; Guo, X.; Chang, F.; Chen, M.; Wang, Q.; Song, X.; Wu, C. Hydrogen production driven by formate oxidation in Shewanella oneidensis MR-1. Appl. Microbiol. Biotechnol. 2020, 104, 5579–5591. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. P. Natl. A. Sci. 2008, 105, 3968–3973. [Google Scholar] [CrossRef]
- Bosire, E.M.; Rosenbaum, M.A. Electrochemical potential influences phenazine production, electron transfer and consequently electric current generation by Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 260405. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; Masuda, M.; Tsujimura, S.; Kano, K. Lactococcus lactis catalyses electricity generation at microbial fuel cell anodes via excretion of a soluble quinone. Bioelectrochemistry 2009, 76, 14–18. [Google Scholar] [CrossRef]
- Schröder, U. A basic introduction into microbial fuel cells and microbial electrocatalysis. ChemTexts 2018, 4, 19. [Google Scholar] [CrossRef]
- Aiyer, K.S. How does electron transfer occur in microbial fuel cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.; Hu, Y.; Cao, B.; Rice, S.A.; Kjelleberg, S.; Song, H. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS Synth. Biol. 2015, 4, 815–823. [Google Scholar] [CrossRef]
- Yong, Y.C.; Yu, Y.Y.; Li, C.M.; Zhong, J.J.; Song, H. Bioelectricity enhancement via overexpression of quorum sensing system in Pseudomonas aeruginosa-inoculated microbial fuel cells. Biosens. Bioelectron. 2011, 30, 87–92. [Google Scholar] [CrossRef]
- Patil, S.A.; Hägerhäll, C.; Gorton, L. Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems. Bioanal. Rev. 2012, 4, 159–192. [Google Scholar] [CrossRef]
- Pereira, J.; Mediayati, Y.; van Veelen, H.P.J.; Temmink, H.; Sleutels, T.; Hamelers, B.; Heijne, A.T. The effect of intermittent anode potential regimes on the morphology and extracellular matrix composition of electro-active bacteria. Biofilm 2022, 4, 100064. [Google Scholar] [CrossRef] [PubMed]
- Rittmann, B.E. Ironies in microbial electrochemistry. J. Environ. Eng. 2017, 143, 03117001. [Google Scholar] [CrossRef]
- Tran, H.V.; Kim, E.; Jung, S.P. Anode biofilm maturation time, stable cell performance time, and time-course electrochemistry in a single-chamber microbial fuel cell with a brush-anode. J. Ind. Eng. Chem. 2022, 106, 269–278. [Google Scholar] [CrossRef]
- Jung, S.P.; Pandit, S. Important factors influencing microbial fuel cell performance. In Microbial Electrochemical Technology, 1st ed.; Venkata Mohan, S.V.S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 377–406. [Google Scholar]
- Wang, X.; Feng, Y.; Ren, N.; Wang, H.; Lee, H.; Li, N.; Zhao, Q. Accelerated start-up of two-chambered microbial fuel cells: Effect of anodic positive poised potential. Electrochim. Acta 2009, 54, 1109–1114. [Google Scholar] [CrossRef]
- Torres, C.I.; Krajmalnik-Brown, R.; Parameswaran, P.; Marcus, A.K.; Wanger, G.; Gorby, Y.A.; Rittmann, B.E. Selecting anode-respiring bacteria based on anode potential: Phylogenetic, electrochemical, and microscopic characterization. Environ. Sci. Technol. 2009, 43, 9519–9524. [Google Scholar] [CrossRef]
- Buitrón, G.; López-Prieto, I.; Zúñiga, I.T.; Vargas, A. Reduction of start-up time in a microbial fuel cell through the variation of external resistance. Energy Procedia 2017, 142, 694–699. [Google Scholar] [CrossRef]
- Busalmen, J.P.; Esteve-Nuñez, A.; Feliu, J.M. Whole cell electrochemistry of electricity-producing microorganisms evidence an adaptation for optimal exocellular electron transport. Environ. Sci. Technol. 2008, 42, 2445–2450. [Google Scholar] [CrossRef]
- Yang, G.; Huang, L.; Yu, Z.; Liu, X.; Chen, S.; Zeng, J.; Zhou, S.; Zhuang, L. Anode potentials regulate Geobacter biofilms: New insights from the composition and spatial structure of extracellular polymeric substances. Water Res. 2019, 159, 294–301. [Google Scholar] [CrossRef]
- Dumas, C.; Basseguy, R.; Bergel, A. Electrochemical activity of Geobacter sulfurreducens biofilms on stainless steel anodes. Electrochim. Acta 2008, 53, 5235–5241. [Google Scholar] [CrossRef]
- Wei JinCheng, W.J.; Liang Peng, L.P.; Cao XiaoXin, C.X.; Huang Xia, H.X. A new insight into potential regulation on growth and power generation of Geobacter sulfurreducens in microbial fuel cells based on energy viewpoint. Environ. Sci. Technol. 2010, 44, 3187–3191. [Google Scholar]
- Korth, B.; Harnisch, F. Spotlight on the energy harvest of electroactive microorganisms: The impact of the applied anode potential. Front. Microbiol. 2019, 10, 1352. [Google Scholar]
- Zhu, X.; Yates, M.D.; Logan, B.E. Set potential regulation reveals additional oxidation peaks of Geobacter sulfurreducens anodic biofilms. Electrochem. Commun. 2012, 22, 116–119. [Google Scholar] [CrossRef]
- Grobbler, C.; Virdis, B.; Nouwens, A.; Harnisch, F.; Rabaey, K.; Bond, P.L. Effect of the anode potential on the physiology and proteome of Shewanella oneidensis MR-1. Bioelectrochemistry 2018, 119, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.W.; El-Naggar, M.Y.; Bretschger, O.; Ward, M.J.; Romine, M.F.; Obraztsova, A.Y.; Nealson, K.H. Electrokinesis is a microbial behavior that requires extracellular electron transport. Proc. Natl. Acad. Sci. USA 2010, 107, 326–331. [Google Scholar] [CrossRef] [PubMed]
- TerAvest, M.A.; Angenent, L.T. Oxidizing electrode potentials decrease current production and coulombic efficiency through cytochrome c inactivation in Shewanella oneidensis MR-1. ChemElectroChem 2014, 1, 2000–2006. [Google Scholar] [CrossRef]
- Kitayama, M.; Koga, R.; Kasai, T.; Kouzuma, A.; Watanabe, K. Structures, compositions, and activities of live Shewanella biofilms formed on graphite electrodes in electrochemical flow cells. Appl. Environ. Microbiol. 2017, 83, e00903-17. [Google Scholar] [CrossRef]
- Grobbler, C.; Virdis, B.; Nouwens, A.; Harnisch, F.; Rabaey, K.; Bond, P.L. Use of SWATH mass spectrometry for quantitative proteomic investigation of Shewanella oneidensis MR-1 biofilms grown on graphite cloth electrodes. Syst. Appl. Microbiol. 2015, 38, 135–139. [Google Scholar] [CrossRef]
- Matsuda, S.; Liu, H.; Kouzuma, A.; Watanabe, K.; Hashimoto, K.; Nakanishi, S. Electrochemical gating of tricarboxylic acid cycle in electricity-producing bacterial cells of Shewanella. PLoS ONE 2013, 8, e72901. [Google Scholar] [CrossRef]
- Hirose, A.; Kasai, T.; Aoki, M.; Umemura, T.; Watanabe, K.; Kouzuma, A. Electrochemically active bacteria sense electrode potentials for regulating catabolic pathways. Nat. Commun. 2018, 9, 1083. [Google Scholar] [CrossRef]
- Nakagawa, G.; Kouzuma, A.; Hirose, A.; Kasai, T.; Yoshida, G.; Watanabe, K. Metabolic characteristics of a glucose-utilizing Shewanella oneidensis strain grown under electrode-respiring conditions. PLoS ONE 2015, 10, e0138813. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Ellington, A.D. Optimization of the biological component of a bioelectrochemical cell. Bioelectrochemistry 2007, 70, 165–172. [Google Scholar] [CrossRef]
- Zhu, X.; Tokash, J.C.; Hong, Y.; Logan, B.E. Controlling the occurrence of power overshoot by adapting microbial fuel cells to high anode potentials. Bioelectrochemistry 2013, 90, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yates, M.D.; Hatzell, M.C.; Ananda Rao, H.; Saikaly, P.E.; Logan, B.E. Microbial community composition is unaffected by anode potential. Environ. Sci. Technol. 2014, 48, 1352–1358. [Google Scholar] [CrossRef]
- Guo, J.; Yang, G.; Zhuang, Z.; Mai, Q.; Zhuang, L. Redox potential-induced regulation of extracellular polymeric substances in an electroactive mixed community biofilm. Sci. Total Environ. 2021, 797, 149207. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, D.A.; Tender, L.M.; Zeikus, J.G. Effect of electrode potential on electrode-reducing microbiota. Environ. Sci. Technol. 2006, 40, 6990–6995. [Google Scholar] [CrossRef]
- Inoue, K.; Qian, X.; Morgado, L.; Kim, B.C.; Mester, T.; Izallalen, M.; Salgueiro, C.A.; Lovley, D.R. Purification and characterization of OmcZ, an outer-surface, octaheme c-type cytochrome essential for optimal current production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 2010, 76, 3999–4007. [Google Scholar] [CrossRef]
- Ueki, T. Cytochromes in Extracellular Electron Transfer in Geobacter. Appl. Environ. Microbiol. 2021, 87, e03109-20. [Google Scholar] [CrossRef]
- Methe, B.; Nelson, K.E.; Eisen, J.; Paulsen, I.; Nelson, W.; Heidelberg, J.; Wu, D.; Wu, M.; Ward, N.; Beanan, M. Genome of Geobacter sulfurreducens: Metal reduction in subsurface environments. Science 2003, 302, 1967–1969. [Google Scholar] [CrossRef]
- Levar, C.E.; Chan, C.H.; Mehta-Kolte, M.G.; Bond, D.R. An inner membrane cytochrome required only for reduction of high redox potential extracellular electron acceptors. mBio 2014, 5, e02034-14. [Google Scholar] [CrossRef] [PubMed]
- Zacharoff, L.; Chan, C.H.; Bond, D.R. Reduction of low potential electron acceptors requires the CbcL inner membrane cytochrome of Geobacter sulfurreducens. Bioelectrochemistry 2016, 107, 7–13. [Google Scholar] [CrossRef]
- Katuri, K.P.; Kavanagh, P.; Rengaraj, S.; Leech, D. Geobacter sulfurreducens biofilms developed under different growth conditions on glassy carbon electrodes: Insights using cyclic voltammetry. Chem. Commun. 2010, 46, 4758–4760. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Leang, C.; Hodges Myerson, A.L.; Coppi, M.V.; Cuifo, S.; Methe, B.; Sandler, S.J.; Lovley, D.R. Biochemical and genetic characterization of PpcA, a periplasmic c-type cytochrome in Geobacter sulfurreducens. Biochem. J. 2003, 369, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; You, S.-J.; Wang, J.-Y. Electrode potential regulates cytochrome accumulation on Shewanella oneidensis cell surface and the consequence to bioelectrocatalytic current generation. Biosens. Bioelectron. 2010, 25, 2530–2533. [Google Scholar] [CrossRef]
- Zhao, F.; Heidrich, E.S.; Curtis, T.P.; Dolfing, J. The effect of anode potential on current production from complex substrates in bioelectrochemical systems: A case study with glucose. Appl. Microbiol. Biotechnol. 2020, 104, 5133–5143. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Chen, Y.-C.; Yu, C.-P. Microbial community dynamics in electroactive biofilms across time under different applied anode potentials. Sustain. Environ. Res. 2022, 32, 19. [Google Scholar] [CrossRef]
- Kokko, M.E.; Mäkinen, A.E.; Sulonen, M.L.; Puhakka, J.A. Effects of anode potentials on bioelectrogenic conversion of xylose and microbial community compositions. Biochem. Eng. J. 2015, 101, 248–252. [Google Scholar] [CrossRef]
- Ishii, S.i.; Suzuki, S.; Norden-Krichmar, T.M.; Phan, T.; Wanger, G.; Nealson, K.H.; Sekiguchi, Y.; Gorby, Y.A.; Bretschger, O. Microbial population and functional dynamics associated with surface potential and carbon metabolism. ISME J. 2014, 8, 963–978. [Google Scholar] [CrossRef]
- Commault, A.S.; Lear, G.; Packer, M.A.; Weld, R.J. Influence of anode potentials on selection of Geobacter strains in microbial electrolysis cells. Bioresour. Technol. 2013, 139, 226–234. [Google Scholar] [CrossRef]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Tenney, A.; Chain, P.S.; Scholz, M.B.; Nealson, K.H.; Bretschger, O. A novel metatranscriptomic approach to identify gene expression dynamics during extracellular electron transfer. Nat. Commun. 2013, 4, 1601. [Google Scholar] [CrossRef]
- Lesnik, K.L.; Cai, W.; Liu, H. Microbial community predicts functional stability of microbial fuel cells. Environ. Sci. Technol. 2019, 54, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, R.; Neethirajan, S. Harnessing electrical energy for anti-biofilm therapies: Effects of current on cell morphology and motility. J. Exp. Nanosci. 2017, 12, 197–207. [Google Scholar] [CrossRef]
- Bao, M.-M.; Igwe, I.E.; Chen, K.; Zhang, T.-H. Modulated collective motions and condensation of bacteria. Chin. Phys. Lett. 2022, 39, 108702. [Google Scholar] [CrossRef]
- Wheeler, J.H.; Foster, K.R.; Durham, W.M. Individual bacterial cells can use spatial sensing of chemical gradients to direct chemotaxis on surfaces. Nat. Microbiol. 2024, 9, 2308–2322. [Google Scholar] [CrossRef] [PubMed]
- Busalmen, J.P.; de Sánchez, S.R. Electrochemical polarization-induced changes in the growth of individual cells and biofilms of Pseudomonas fluorescens (ATCC 17552). Appl. Environ. Microbiol. 2005, 71, 6235–6240. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sasaki, D.; Sasaki, K.; Kato, S.; Kondo, A.; Hashimoto, K.; Nakanishi, S. Comprehensive metabolomic analyses of anode-respiring Geobacter sulfurreducens cells: The impact of anode-respiration activity on intracellular metabolite levels. Process Biochem. 2016, 51, 34–38. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond risk: Bacterial biofilms and their regulating approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Masi, E.; Ciszak, M.; Santopolo, L.; Frascella, A.; Giovannetti, L.; Marchi, E.; Viti, C.; Mancuso, S. Electrical spiking in bacterial biofilms. J. R. Soc. Interface 2015, 12, 20141036. [Google Scholar] [CrossRef]
- Czerwińska-Główka, D.; Krukiewicz, K. A journey in the complex interactions between electrochemistry and bacteriology: From electroactivity to electromodulation of bacterial biofilms. Bioelectrochemistry 2020, 131, 107401. [Google Scholar] [CrossRef]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, B.; Chang, I.S. Tracking of Shewanella oneidensis MR-1 biofilm formation of a microbial electrochemical system via differential pulse voltammetry. Bioresour. Technol. 2018, 254, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Prévoteau, A.; Louro, R.O.; Paquete, C.M.; Rabaey, K. Periodic polarization of electroactive biofilms increases current density and charge carriers concentration while modifying biofilm structure. Biosens. Bioelectron. 2018, 121, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, X.; Li, J.; Liao, Q.; Ye, D. Biofilm formation and electricity generation of a microbial fuel cell started up under different external resistances. J. Power Sources 2011, 196, 6029–6035. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, F. Electrochemical roles of extracellular polymeric substances in biofilms. Curr. Opin. Electrochem. 2017, 4, 206–211. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.; Ulstrup, J.; Zhao, F. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 2017, 3, e1700623. [Google Scholar] [CrossRef]
- Cao, B.; Shi, L.; Brown, R.N.; Xiong, Y.; Fredrickson, J.K.; Romine, M.F.; Marshall, M.J.; Lipton, M.S.; Beyenal, H. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ. Microbiol. 2011, 13, 1018–1031. [Google Scholar] [CrossRef]
- Li, S.-W.; Sheng, G.-P.; Cheng, Y.-Y.; Yu, H.-Q. Redox properties of extracellular polymeric substances (EPS) from electroactive bacteria. Sci. Rep. 2016, 6, 39098. [Google Scholar] [CrossRef]
| Inoculum | Applied Potential (V) | Working Electrode | Reference Electrode | Optimal Potential (V) | Ref. |
|---|---|---|---|---|---|
| G. sulfurreducens | 0.1, 0.4, 0.6 vs. Ag/AgCl | Graphite felt | Ag/AgCl | 0.6 | [60] |
| G. soli | −0.4, −0.2, 0, 0.2, 0.4, 0.6 vs. SCE | Graphite plate | SCE | 0, 0.2 | [61] |
| G. sulfurreducens | −0.2, −0.1, 0, 0.2 vs. Ag/AgCl | Stainless steel | Ag/AgCl | 0.2 | [62] |
| G. sulfurreducens | −0.16, 0, 0.4 vs. SHE | Carbon paper | SCE | 0, 0.4 | [63] |
| G. sulfurreducens | −0.2, −0.1, 0, 0.2, 0.4, 0.6 vs. SHE | —— | —— | ≥0.2 | [64] |
| G. sulfurreducens | −0.46, −0.3, 0, 0.3, 0.6 vs. Ag/AgCl | Graphite plate | Ag/AgCl | 0, 0.3, 0.6 | [65] |
| S. oneidensis | −0.19, 0.21, 0.71 vs. SHE | Carbon cloth | Ag/AgCl | 0.71 | [66] |
| S. oneidensis | −0.6, 0, 0.3, 0.6 vs. graphite | Graphite fiber | graphite | 0.6 | [67] |
| S. oneidensis | −0.003, 0.197, 0.397, 0.597, 0.797 vs. SHE | Graphite paper | Ag/AgCl | 0.397 | [68] |
| S. putrefaciens | −0.1, 0, 0.1, 0.2, 0.3, 0.4 vs. Ag/AgCl | Polycrystalline carbon rod | Ag/AgCl | 0.4 | [32] |
| S. oneidensis | 0, 0.4 vs. SHE | Graphite plate | Ag/AgCl | 0.4 | [69] |
| S. oneidensis | −0.19, 0.21, 0.71 vs. SHE | Carbon cloth | Ag/AgCl | 0.71 | [70] |
| S. loihica | 0, 0.4 vs. SHE | ITO-coated glass | Ag/AgCl | 0 | [71] |
| S. oneidensis | 0, 0.2, 0.5 vs. SHE | Graphite felt | Ag/AgCl | 0.5 | [72] |
| Engineered S. oneidensis | 0, 0.4 vs. Ag/AgCl | Graphite felt | Ag/AgCl | 0.4 | [73] |
| S. oneidensis | 0, 0.2, 0.35, 0.5 vs. Ag/AgCl | Graphite plate | Ag/AgCl | 0.5 | [74] |
| Primary clarifier effluent | −0.46, −0.24, 0, 0.5 vs. Ag/AgCl | Carbon fiber brush | Ag/AgCl | 0.5 | [75] |
|
Primary clarifier effluent | −0.25, −0.09, 0.21, 0.51, 0.81 vs. SHE | Graphite plate | Ag/AgCl | 0.21 | [76] |
| artificial brewery wastewater | −0.3, 0, 0.3, 0.6 vs. SCE | Graphite plates | SCE | 0 | [77] |
| ARB communities | −0.15, −0.09, 0.02, 0.37 vs. SHE | Graphite rod | Ag/AgCl | −0.15 | [58] |
| Domestic wastewater | 0, 0.2 vs. Ag/AgCl | Graphite | Ag/AgCl | 0.2 | [57] |
| Marine sediment | −0.058, 0.103, 0.618 vs. Ag/AgCl | Graphite | Ag/AgCl | 0.618 | [78] |
| Electricity Generation Aspect | Key Findings | Ref. |
|---|---|---|
| Start-up time and electric current/power density | A more positive anodic potential can increase current density and reduce start-up time. | [60,66] |
| Electron transfer pathways | Electrode potential induces structural transformations of cytochrome to form specific electron transfer pathways, thereby enhancing electron transfer. | [60,65] |
| The anode potential could regulate the accumulation of c-type cytochromes at the bacteria–electrode interface, leading to a sudden increase in current followed by a gradual decrease over time. | [86] | |
| As the voltage increased, there was a significant upregulation in the expression of key EET proteins and elongation factor involved in protein synthesis | [66] | |
| Under the more positive potential, the reduction of coulomb efficiency is attributed to the direct damaged electron transfer proteins. | [68] | |
| Microbial community | Elevated anode potentials can enhance the abundance of microbial communities in the anode biofilm | [87] |
| Electrode potential can screen microorganisms with electrochemical activity. | [92] |
| Physiological Aspect | Key Findings | Ref. |
|---|---|---|
| Mobility | Applied electric potentials decrease the swimming speed of bacteria but enhance the directionality of movement. | [66,94] |
| Electrokinesis enhances the transient swimming speed of bacteria due to near-electrode electron flow. | [67] | |
| Adhesion and Growth | Lower potentials favor microorganisms dominated by DET, while higher anode potentials favor microorganisms by IET or non-electroactive microorganisms. | [58,60]. |
| Higher anode potentials reduce cell length and increase doubling times. | [97] | |
| Applied electric fields induce bacteria to produce conductive appendages. | [43] | |
| Metabolism | High potentials activate NADH-dependent pathways, induce branching in TCA cycle, and upregulate the expression of metabolism-related genes. | [70,72,73,98] |
| TCA cycle was deactivated at excessive high potential. | [71] | |
| Biofilm | Applied anode potentials promote accumulation of biomass. | [68,76] |
| The applied mode of the anode potential and the magnitude of the external resistance also influence the flatness and compactness of biofilms. | [104,105] | |
| Anode potential affects the proportion of conductive substances and non-conductive polysaccharides in bacterial extracellular polymers. | [61,69,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zong, Y.; Feng, C.; Zhao, K. The Role of Anode Potential in Electromicrobiology. Microorganisms 2025, 13, 631. https://doi.org/10.3390/microorganisms13030631
Li Y, Zong Y, Feng C, Zhao K. The Role of Anode Potential in Electromicrobiology. Microorganisms. 2025; 13(3):631. https://doi.org/10.3390/microorganisms13030631
Chicago/Turabian StyleLi, Yanran, Yiwu Zong, Chunying Feng, and Kun Zhao. 2025. "The Role of Anode Potential in Electromicrobiology" Microorganisms 13, no. 3: 631. https://doi.org/10.3390/microorganisms13030631
APA StyleLi, Y., Zong, Y., Feng, C., & Zhao, K. (2025). The Role of Anode Potential in Electromicrobiology. Microorganisms, 13(3), 631. https://doi.org/10.3390/microorganisms13030631






