Photodynamic Inactivation of Bacteria in Boar Semen with Blue LED Light
Abstract
1. Introduction

2. Materials and Methods
2.1. Photosensitizer and Illumination Setup
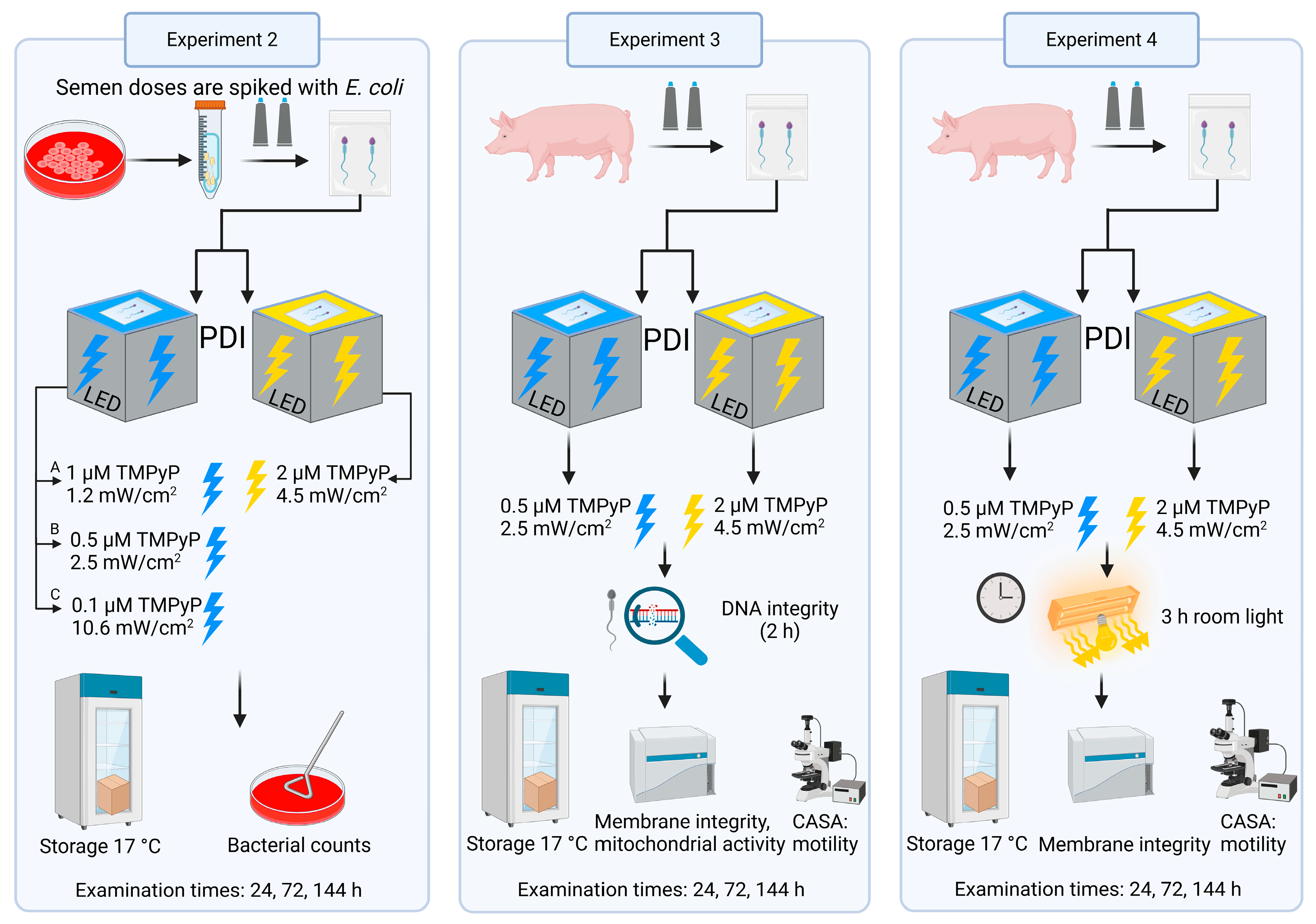
2.2. Experimental Design
2.2.1. Experiment 1: Effect of Blue LED Light on Sperm Kinematics
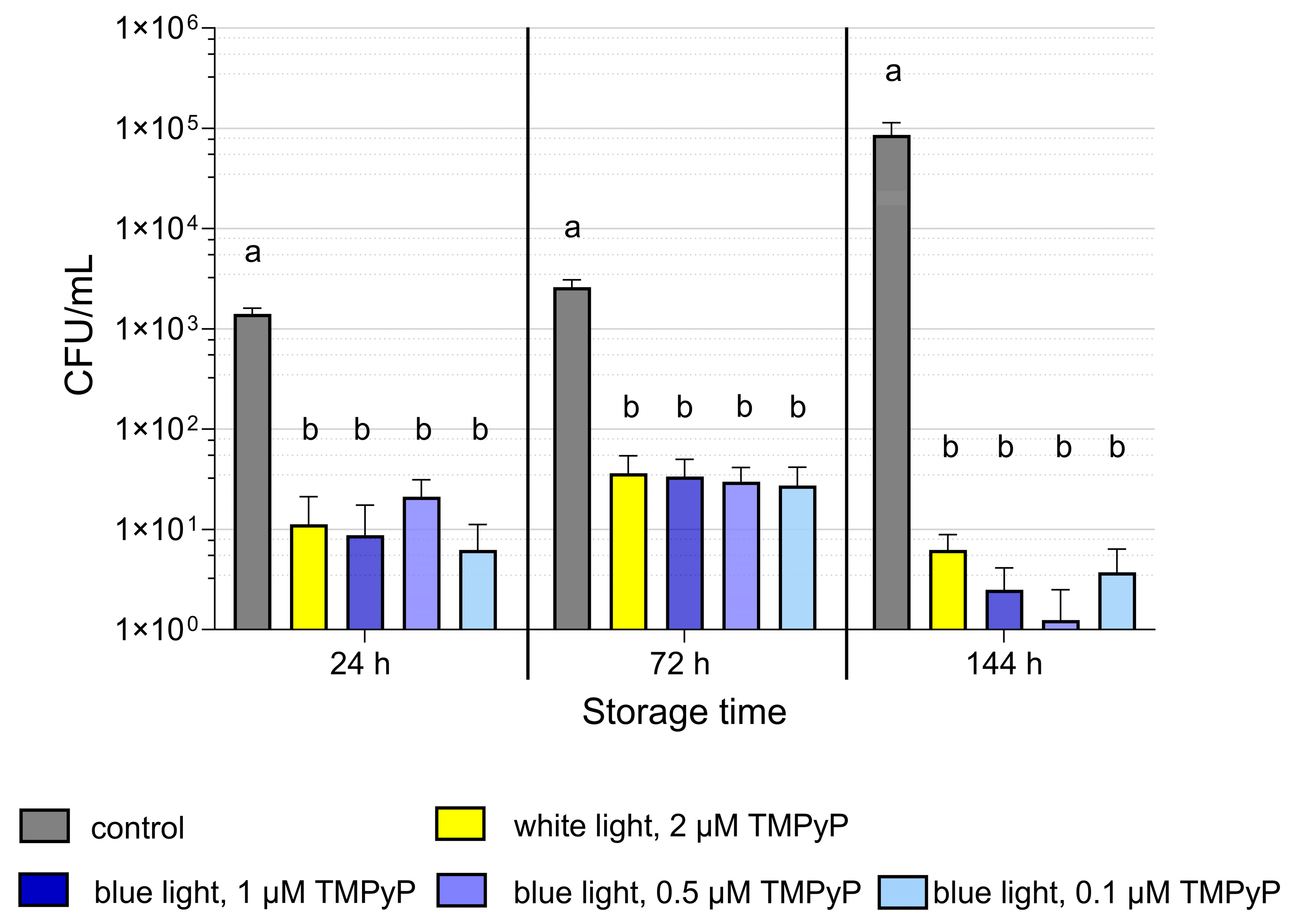
2.2.2. Experiment 2: Antimicrobial Efficiency of PDIblue Compared to PDIwhite
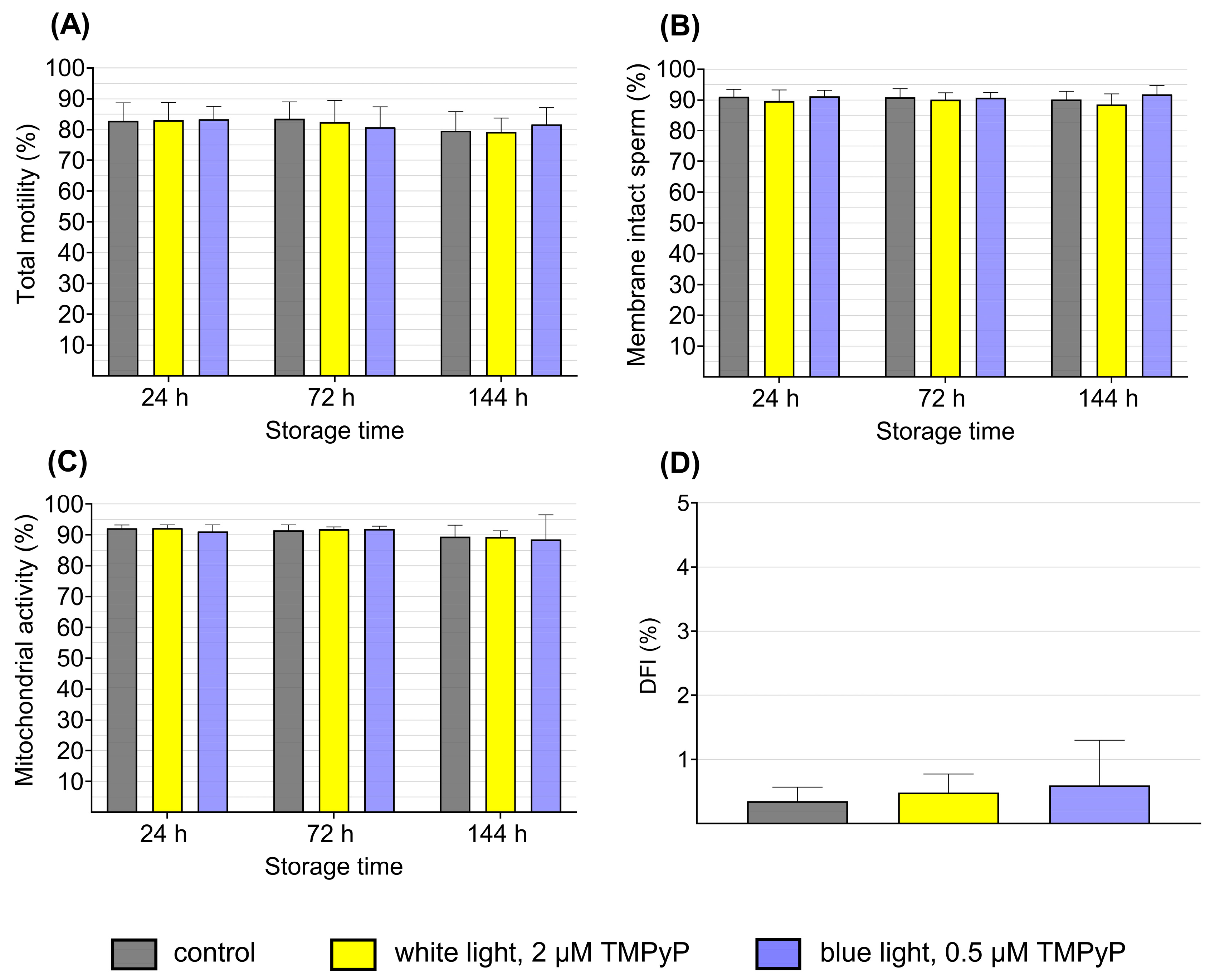
2.2.3. Experiment 3: Sperm Quality After PDIblue Compared to PDIwhite
2.2.4. Experiment 4: Effect of Ambient Light on Sperm Quality After PDIblue Compared to PDIwhite
2.3. Chemicals and Media
2.4. Semen Collection, Sample Preparation, and Storage
2.5. Microbiology
2.6. Spermatology
2.6.1. Sperm Kinematics
2.6.2. Sperm Membrane Integrity and Mitochondrial Activity
2.6.3. DNA Fragmentation
2.7. Statistical Analysis
3. Results
3.1. Experiment 1: Effect of Blue LED Light on Sperm Kinematics
3.2. Experiment 2: Antimicrobial Efficiency of PDIblue Compared to PDIwhite
3.3. Experiment 3: Sperm Quality After PDIblue Compared to PDIwhite
3.4. Experiment 4: Effect of Ambient Light on Sperm Quality After PDIblue Compared to PDIwhite
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial insemination |
| LED | Light-emitting diode |
| PDI | Photodynamic inactivation |
References
- Schulze, M.; Nitsche-Melkus, E.; Hensel, B.; Jung, M.; Jakop, U. Antibiotics and their alternatives in Artificial Breeding in livestock. Anim. Reprod. Sci. 2020, 220, 106284. [Google Scholar] [CrossRef]
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of preserved boar semen for artificial insemination: Past, present and future challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Luther, A.-M.; Varzandeh, M.; Beckermann, C.; Feyer, L.; Maaßen, I.K.; Oldenhof, H.; Hackbarth, S.; Waberski, D. Fertility after photodynamic inactivation of bacteria in extended boar semen. Front. Microbiol. 2024, 15, 1429749. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Preuß, A.; Röder, B. Photodynamic inactivation of microorganisms. Singlet Oxygen 2016, 305–318. [Google Scholar] [CrossRef]
- Frederiksen, P.K.; McIlroy, S.P.; Nielsen, C.B.; Nikolajsen, L.; Skovsen, E.; Jørgensen, M.; Mikkelsen, K.V.; Ogilby, P.R. Two-photon photosensitized production of singlet oxygen in water. J. Am. Chem. Soc. 2005, 127, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Muehler, D.; Brandl, E.; Hiller, K.A.; Cieplik, F.; Maisch, T. Membrane damage as mechanism of photodynamic inactivation using Methylene blue and TMPyP in Escherichia coli and Staphylococcus aureus. Photochem. Photobiol. Sci. 2022, 21, 209–220. [Google Scholar] [CrossRef]
- Maisch, T.; Spannberger, F.; Regensburger, J.; Felgenträger, A.; Bäumler, W. Fast and effective: Intense pulse light photodynamic inactivation of bacteria. J. Ind. Microbiol. Biotechnol. 2012, 39, 1013–1021. [Google Scholar] [CrossRef]
- Preuss, A.; Zeugner, L.; Hackbarth, S.; Faustino, M.A.; Neves, M.G.; Cavaleiro, J.A.; Roeder, B. Photoinactivation of Escherichia coli (SURE2) without intracellular uptake of the photosensitizer. J. Appl. Microbiol. 2013, 114, 36–43. [Google Scholar] [CrossRef]
- Diaz Suarez, E.; Lima, F.; Dias, P.M.; Constantino, V.R.L.; Petrilli, H.M. Theoretical UV-Vis spectra of tetracationic porphyrin: Effects of environment on electronic spectral properties. J. Mol. Model. 2019, 25, 264. [Google Scholar] [CrossRef]
- Serrage, H.; Heiskanen, V.; Palin, W.M.; Cooper, P.R.; Milward, M.R.; Hadis, M.; Hamblin, M.R. Under the spotlight: Mechanisms of photobiomodulation concentrating on blue and green light. Photochem. Photobiol. Sci. 2019, 18, 1877–1909. [Google Scholar] [CrossRef] [PubMed]
- Lavi, R.; Shainberg, A.; Shneyvays, V.; Hochauser, E.; Isaac, A.; Zinman, T.; Friedmann, H.; Lubart, R. Detailed analysis of reactive oxygen species induced by visible light in various cell types. Lasers Surg. Med. 2010, 42, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Shahar, S.; Hillman, P.; Lubart, R.; Ickowicz, D.; Breitbart, H. Activation of sperm EGFR by light irradiation is mediated by reactive oxygen species. Photochem. Photobiol. 2014, 90, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Ramírez, M.; Espinoza, J.H.; Ruiz-Suárez, J.; Mercado-Uribe, H. The effect of green light on the motility of mouse sperm at two different temperatures. Photochem. Photobiol. Sci. 2019, 18, 2893–2900. [Google Scholar] [CrossRef]
- Yeste, M.; Castillo-Martín, M.; Bonet, S.; Rodríguez-Gil, J.E. Impact of light irradiation on preservation and function of mammalian spermatozoa. Anim. Reprod. Sci. 2018, 194, 19–32. [Google Scholar] [CrossRef]
- Bonnans, M.; Fouque, L.; Pelletier, M.; Chabert, R.; Pinacolo, S.; Restellini, L.; Cucumel, K. Blue light: Friend or foe? J. Photochem. Photobiol. B Biol. 2020, 212, 112026. [Google Scholar] [CrossRef]
- Moradi, A.; Ghaffari Novin, M.; Bayat, M. A Comprehensive Systematic Review of the Effects of Photobiomodulation Therapy in Different Light Wavelength Ranges (Blue, Green, Red, and Near-Infrared) on Sperm Cell Characteristics In Vitro and In Vivo. Reprod. Sci. 2024, 31, 3275–3302. [Google Scholar] [CrossRef]
- Schulz, S.; Ziganshyna, S.; Lippmann, N.; Glass, S.; Eulenburg, V.; Habermann, N.; Schwarz, U.T.; Voigt, A.; Heilmann, C.; Rüffer, T. The meta-substituted isomer of TMPyP enables more effective photodynamic bacterial inactivation than para-TMPyP in vitro. Microorganisms 2022, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Aalbers, J.; Grooten, H.G. Artificial Insemination of Swine: Fecundity of Boar Semen Stored in Beltsville TS (BTS), Modified Modena (MM), or MR-A and Inseminated on One, Three and Four Days After Collection 1 2. Reprod. Domest. Anim. 1988, 23, 49–55. [Google Scholar] [CrossRef]
- Höfner, L.; Luther, A.M.; Palladini, A.; Fröhlich, T.; Waberski, D. Tolerance of Stored Boar Spermatozoa to Autologous Seminal Plasma: A Proteomic and Lipidomic Approach. Int. J. Mol. Sci. 2020, 21, 6474. [Google Scholar] [CrossRef]
- Boyers, S.P. Fertilization and implantation. Curr. Opin. Obstet. Gynecol. 1989, 1, 45–54. [Google Scholar] [PubMed]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm chromatin structure assay: Its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Luther, A.M.; Grünther, B.; Jäkel, H.; Henning, H.; Vogel, C.; Peralta, W.; Weitze, K.F. Sperm function in vitro and fertility after antibiotic-free, hypothermic storage of liquid preserved boar semen. Sci. Rep. 2019, 9, 14748. [Google Scholar] [CrossRef] [PubMed]
- Lacalle, E.; Nunez, A.; Fernandez-Alegre, E.; Crespo-Felez, I.; Dominguez, J.C.; Alonso, M.E.; Gonzalez-Urdiales, R.; Martinez-Pastor, F. Cold-Shock Test Is a Practical Method for Selecting Boar Ejaculates Yielding Appropriate Seminal Plasma for Post-Thawing Supplementation. Animals 2021, 11, 871. [Google Scholar] [CrossRef]
- Eckl, D.B.; Dengler, L.; Nemmert, M.; Eichner, A.; Bäumler, W.; Huber, H. A Closer Look at Dark Toxicity of the Photosensitizer TMPyP in Bacteria. Photochem. Photobiol. 2018, 94, 165–172. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 1–7. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Guthrie, H.; Welch, G. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef]
- Zan-Bar, T.; Bartoov, B.; Segal, R.; Yehuda, R.; Lavi, R.; Lubart, R.; Avtalion, R. Influence of visible light and ultraviolet irradiation on motility and fertility of mammalian and fish sperm. Photomed. Laser Surg. 2005, 23, 549–555. [Google Scholar] [CrossRef]
- Mohammed, S.E.; Al-ameri, L.M. Laser Biostimulation Effect on Human Sperm Motility. Iraqi J. Laser 2021, 20, 39–42. [Google Scholar]
- Ban Frangez, H.; Frangez, I.; Verdenik, I.; Jansa, V.; Virant Klun, I. Photobiomodulation with light-emitting diodes improves sperm motility in men with asthenozoospermia. Lasers Med. Sci. 2015, 30, 235–240. [Google Scholar] [CrossRef]
- Yeste, M.; Codony, F.; Estrada, E.; Lleonart, M.; Balasch, S.; Peña, A.; Bonet, S.; Rodríguez-Gil, J.E. Specific LED-based red light photo-stimulation procedures improve overall sperm function and reproductive performance of boar ejaculates. Sci. Rep. 2016, 6, 22569. [Google Scholar] [CrossRef]
- Amaral, A.L.; Aoki, A.; Andrade, S.A. Could light be a broad-spectrum antimicrobial? Evid.-Based Dent. 2024, 25, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Sabino, C.P.; Wainwright, M.; Dos Anjos, C.; Sellera, F.P.; Baptista, M.S.; Lincopan, N.; Ribeiro, M.S. Inactivation kinetics and lethal dose analysis of antimicrobial blue light and photodynamic therapy. Photodiagnosis Photodyn. Ther. 2019, 28, 186–191. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Umesh Hebbar, H. Efficacy of blue LED in microbial inactivation: Effect of photosensitization and process parameters. Int. J. Food Microbiol. 2019, 290, 296–304. [Google Scholar] [CrossRef]
- Munir, Z.; Banche, G.; Cavallo, L.; Mandras, N.; Roana, J.; Pertusio, R.; Ficiarà, E.; Cavalli, R.; Guiot, C. Exploitation of the Antibacterial Properties of Photoactivated Curcumin as ‘Green’ Tool for Food Preservation. Int. J. Mol. Sci. 2022, 23, 2600. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Pummer, A.; Leibl, C.; Regensburger, J.; Schmalz, G.; Buchalla, W.; Hiller, K.A.; Maisch, T. Photodynamic Inactivation of Root Canal Bacteria by Light Activation through Human Dental Hard and Simulated Surrounding Tissue. Front. Microbiol. 2016, 7, 929. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Can light-based approaches overcome antimicrobial resistance? Drug Dev. Res. 2019, 80, 48–67. [Google Scholar] [CrossRef]
- Althouse, G.C.; Kuster, C.E.; Clark, S.G.; Weisiger, R.M. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology 2000, 53, 1167–1176. [Google Scholar] [CrossRef]
- Luther, A.M.; Beckermann, C.; Nguyen, T.Q.; Verspohl, J.; Waberski, D. Growth Dynamic and Threshold Values for Spermicidal Effects of Multidrug-Resistant Bacteria in Extended Boar Semen. Microorganisms 2023, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Maroto Martín, L.O.; Muñoz, E.C.; De Cupere, F.; Van Driessche, E.; Echemendia-Blanco, D.; Rodríguez, J.M.; Beeckmans, S. Bacterial contamination of boar semen affects the litter size. Anim. Reprod. Sci. 2010, 120, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Pinart, E.; Domènech, E.; Bussalleu, E.; Yeste, M.; Bonet, S. A comparative study of the effects of Escherichia coli and Clostridium perfringens upon boar semen preserved in liquid storage. Anim. Reprod. Sci. 2017, 177, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, L.; Bussalleu, E.; Yeste, M.; Bonet, S. Effects of different concentrations of Pseudomonas aeruginosa on boar sperm quality. Anim. Reprod. Sci. 2014, 150, 96–106. [Google Scholar] [CrossRef]
- Ubeda, J.L.; Ausejo, R.; Dahmani, Y.; Falceto, M.V.; Usan, A.; Malo, C.; Perez-Martinez, F.C. Adverse effects of members of the Enterobacteriaceae family on boar sperm quality. Theriogenology 2013, 80, 565–570. [Google Scholar] [CrossRef]


| Illumination Regime | Parameter | |||
|---|---|---|---|---|
| Progressive Motility (%) | Velocity Curvilinear Line VCL (µm/s) | Beat Cross Frequency BCF (Hz) | Amplitude of Lateral Head Displacement ALH (µm) | |
| Dark control | 77.2 ± 7.8 | 159 ± 20 | 28.7 ± 2.3 | 1.3 ± 0.1 |
| 2.9 mW/cm2, 60 s | 77.0 ± 7.9 | 156 ± 17 | 29.7 ± 1.2 | 1.3 ± 0.1 |
| 5.8 mW/cm2, 90 s | 75.3 ± 7.3 | 154 ± 16 | 29.9 ± 1.5 | 1.2 ± 0.1 |
| 11.2 mW/cm2, 90 s | 77.6 ± 7.9 | 158 ± 17 | 29.4 ± 1.7 | 1.3 ± 0.1 |
| 11.2 mW/cm2, 3 × 180 s (6 min break) | 77.2 ± 8.3 | 155 ± 18 | 28.8 ± 2.2 | 1.2 ± 0.1 |
| 17.8 mW/cm2, 60 s | 73.9 ± 6.6 | 154 ± 17 | 29.0 ± 1.5 | 1.2 ± 0.1 |
| 25.6 mW/cm2, 60 s | 74.8 ± 5.2 | 157 ± 16 | 29.4 ± 1.8 | 1.2 ± 0.1 |
| 32.7 mW/cm2, 60 s | 74.8 ± 5.2 | 155 ± 17 | 29.6 ± 2.2 | 1.2 ± 0.1 |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Bacterial Count (CFU/mL) | 1.53 × 104 | 1.34 × 103 | 1.1 × 103 | 4.6 × 104 |
| Bacterial species | ||||
| Gram-negative | Pseudomonas aeruginosa; Providencia stuartii; anhemolytic Escherichia coli; Klebsiella pneuomoniae; Citrobacter koseri; Pantoea agglomerans; non-fermenter | |||
| Gram-positive | Staphylococcus (coagulase-negative); Coryneform bacteria; Enterococcus faecalis; Streptococcus (ß-hemolytic); Bacillus species | |||
| Parameter | Treatment | 24 h | 72 h | 144 h |
|---|---|---|---|---|
| Progressive motility (%) | dark control | 76.1 ± 6.8 | 74.7 ± 9.2 | 73.6 ± 6.7 |
| white LED, 2 µM TMPyP | 79.4 ± 7.1 | 77.8 ± 9.0 | 72.9 ± 5.9 | |
| blue LED, 0.5 µM TMPyP | 79.7 ± 5.6 | 76.4 ± 8.2 | 75.7 ± 7.0 | |
| Velocity curvilinear line VCL (µm/s) | dark control | 120 ± 18 | 132 ± 18 | 174 ± 19 |
| white LED, 2 µM TMPyP | 151 ± 22 | 155 ± 18 | 173 ± 20 | |
| blue LED, 0.5 µM TMPyP | 154 ± 23 | 155 ± 25 | 172 ± 21 | |
| Beat cross frequency BCF (Hz) | dark control | 32.6 ± 2.0 | 30.7 ± 1.6 | 26.6 ± 2.0 |
| white LED, 2 µM TMPyP | 30.6 ± 2.6 | 30.2 ± 1.7 | 27.0 ± 1.9 | |
| blue LED, 0.5 µM TMPyP | 30.8 ± 2.5 | 30.3 ± 2.7 | 28.5 ± 2.2 | |
| Amplitude of lateral head displacement ALH (µm) | dark control | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.2 |
| white LED, 2 µM TMPyP | 1.2 ± 0.2 | 1.3 ± 0.1 | 1.4 ± 0.2 | |
| blue LED, 0.5 µM TMPyP | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.4 ± 0.2 |
| Parameter | Treatment | 24 h | 72 h | 144 h |
|---|---|---|---|---|
| Progressive motility (%) | dark control | 78.3 ± 9.9 a | 74.4 ± 11.8 a | 74.3 ± 8.8 a |
| white LED, 2 µM TMPyP | 77.5 ± 9.3 a | 72.6 ± 9.4 a | 65.7 ± 9.0 b | |
| white LED, 2 µM TMPyP, room light | 72.5 ± 9.5 b | 58.0 ± 10.5 b | 30.5 ± 20.0 c | |
| blue LED, 0.5 µM TMPyP | 78.3 ± 9.0 a | 73.4 ± 8.6 a | 72.3 ± 9.6 ad | |
| blue LED, 0.5 µM TMPyP, room light | 76.3 ± 9.6 a | 71.2 ± 10.2 a | 69.7 ± 10.2 bd | |
| Velocity curvilinear line VCL (µm/s) | dark control | 172 ± 25 a | 155 ± 20 a | 165 ± 24 ab |
| white LED, 2 µM TMPyP, | 151 ± 19 a | 151 ± 22 a | 162 ± 27 ab | |
| white LED, 2 µM TMPyP, room light | 130 ± 19 b | 147 ± 25 a | 132 ± 45 a | |
| blue LED, 0.5 µM TMPyP | 157 ± 28 a | 155 ± 17 a | 165 ± 24 ab | |
| blue LED, 0.5 µM TMPyP, room light | 148 ± 19 ab | 150 ± 24 a | 168 ± 28 b | |
| Beat cross frequency BCF (Hz) | dark control | 29.8 ± 2.4 a | 28.1 ± 2.4 a | 25.5 ± 2.4 a |
| white LED, 2 µM TMPyP | 29.9 ± 1.7 a | 27.3 ± 2.6 a | 24.7 ± 3.1 a | |
| white LED, 2 µM TMPyP, room light | 28.4 ± 3.2 a | 24.2 ± 3.9 b | 20.1 ± 2.5 b | |
| blue LED, 0.5 µM TMPyP | 29.1 ± 2.2 a | 27.3 ± 2.7 a | 25.1 ± 2.6 a | |
| blue LED, 0.5 µM TMPyP, room light | 29.5 ± 2.2 a | 27.7 ± 3.0 a | 24.6 ± 2.5 a | |
| Amplitude of lateral head displacement ALH (µm) | dark control | 1.3 ± 0.2 a | 1.2 ± 0.1 a | 1.3 ± 0.2 a |
| white LED, 2 µM TMPyP, | 1.2 ± 0.1 a | 1.2 ± 0.2 a | 1.3 ± 0.2 a | |
| white LED, 2 µM TMPyP, room light | 1.0 ± 0.1 b | 1.2 ± 0.2 a | 1.2 ± 0.3 a | |
| blue LED, 0.5 µM TMPyP | 1.2 ± 0.2 a | 1.2 ± 0.1 a | 1.3 ± 0.2 a | |
| blue LED, 0.5 µM TMPyP, room light | 1.2 ± 0.1 ab | 1.2 ± 0.2 a | 1.3 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maaßen, I.K.; Luther, A.-M.; Varzandeh, M.; Hackbarth, S.; Waberski, D. Photodynamic Inactivation of Bacteria in Boar Semen with Blue LED Light. Microorganisms 2025, 13, 643. https://doi.org/10.3390/microorganisms13030643
Maaßen IK, Luther A-M, Varzandeh M, Hackbarth S, Waberski D. Photodynamic Inactivation of Bacteria in Boar Semen with Blue LED Light. Microorganisms. 2025; 13(3):643. https://doi.org/10.3390/microorganisms13030643
Chicago/Turabian StyleMaaßen, Isabel Katharina, Anne-Marie Luther, Mohammad Varzandeh, Steffen Hackbarth, and Dagmar Waberski. 2025. "Photodynamic Inactivation of Bacteria in Boar Semen with Blue LED Light" Microorganisms 13, no. 3: 643. https://doi.org/10.3390/microorganisms13030643
APA StyleMaaßen, I. K., Luther, A.-M., Varzandeh, M., Hackbarth, S., & Waberski, D. (2025). Photodynamic Inactivation of Bacteria in Boar Semen with Blue LED Light. Microorganisms, 13(3), 643. https://doi.org/10.3390/microorganisms13030643






