Interactions Between Endosymbionts Wolbachia and Rickettsia in the Spider Mite Tetranychus turkestani: Cooperation or Antagonism?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Spider Mites
2.2. Detection of Infections Using Different Symbiotic Bacteria
2.3. Establishment of Strains Infected with Different Endosymbiotic Bacteria
2.4. Wolbachia and Rickettsia Phylogenetic Tree Construction
2.5. Detection of the Maternal Inheritance Efficiency of Wolbachia and Rickettsia
2.6. Detection of the Titers of Wolbachia and Rickettsia in T. turkestani
2.7. Effects of Different Symbiotic Bacteria Infections on the Fecundity of T. turkestani
2.8. Data Processing
3. Results
3.1. Diagram of the Life Cycle of T. turkestani and Phylogenetic Analysis of Wolbachia and Rickettsia
3.2. Analysis of the Maternal Inheritance Efficiency of Wolbachia and Rickettsia
3.3. Detection of the Titers of Wolbachia and Rickettsia in T. turkestani
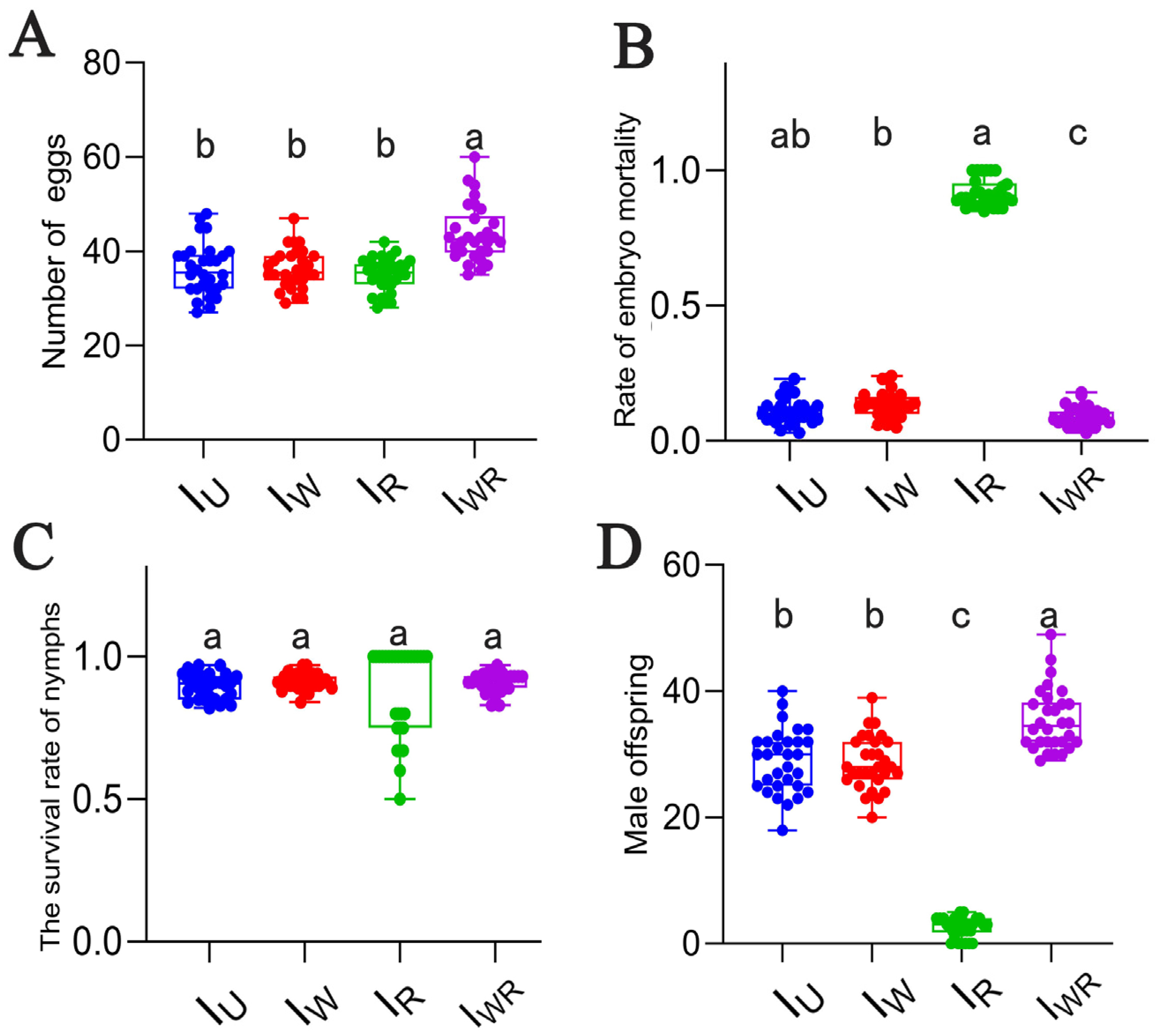
3.4. Effects of Different Endosymbiont Infections on the Parthenogenesis of T. turkestani
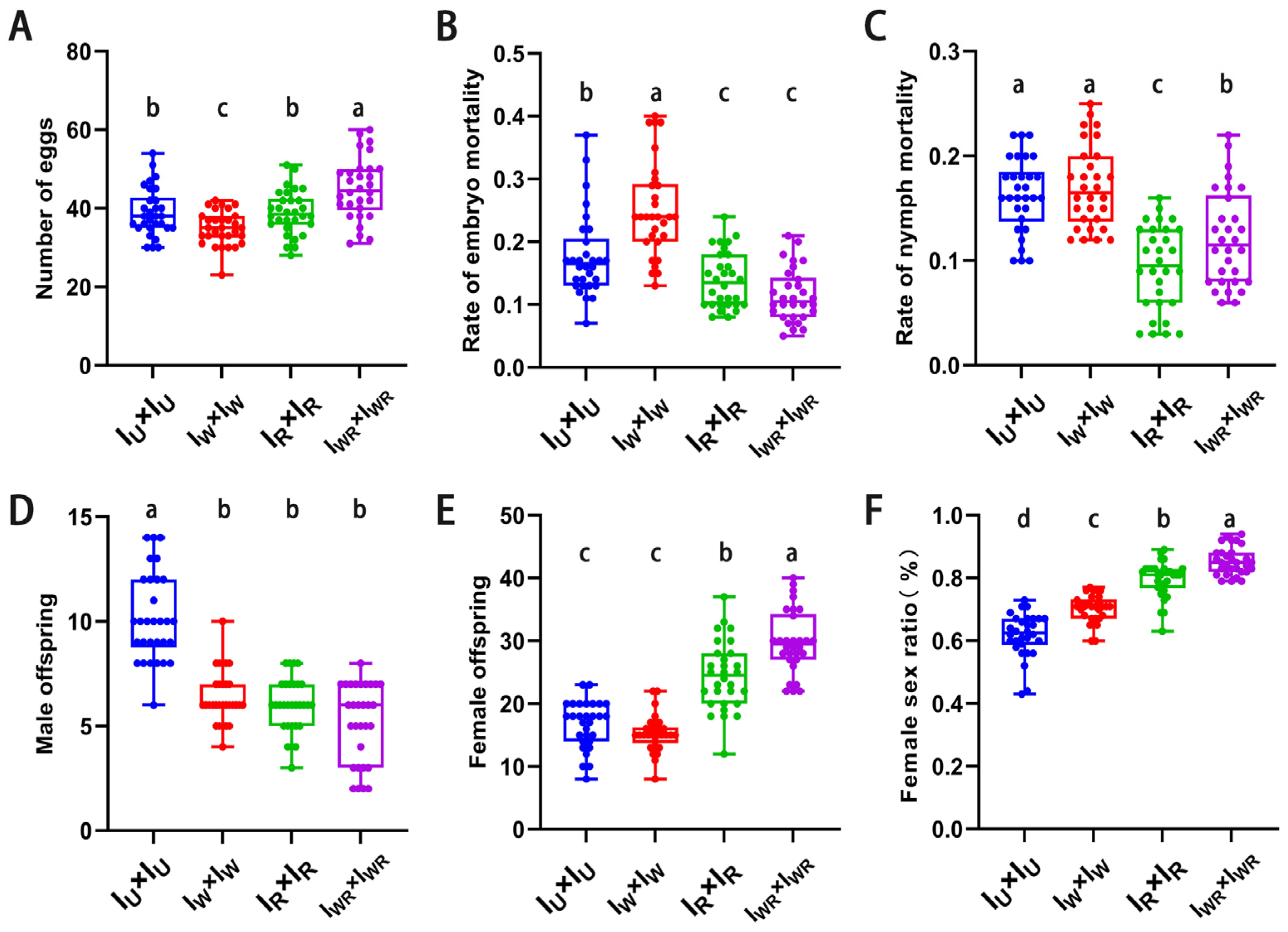
3.5. Effects of Different Endosymbiotic Bacterial Infections on the Sexual Reproduction of T. turkestani
3.6. Verification of the Male-Killing Effect of Rickettsia in T. turkestani
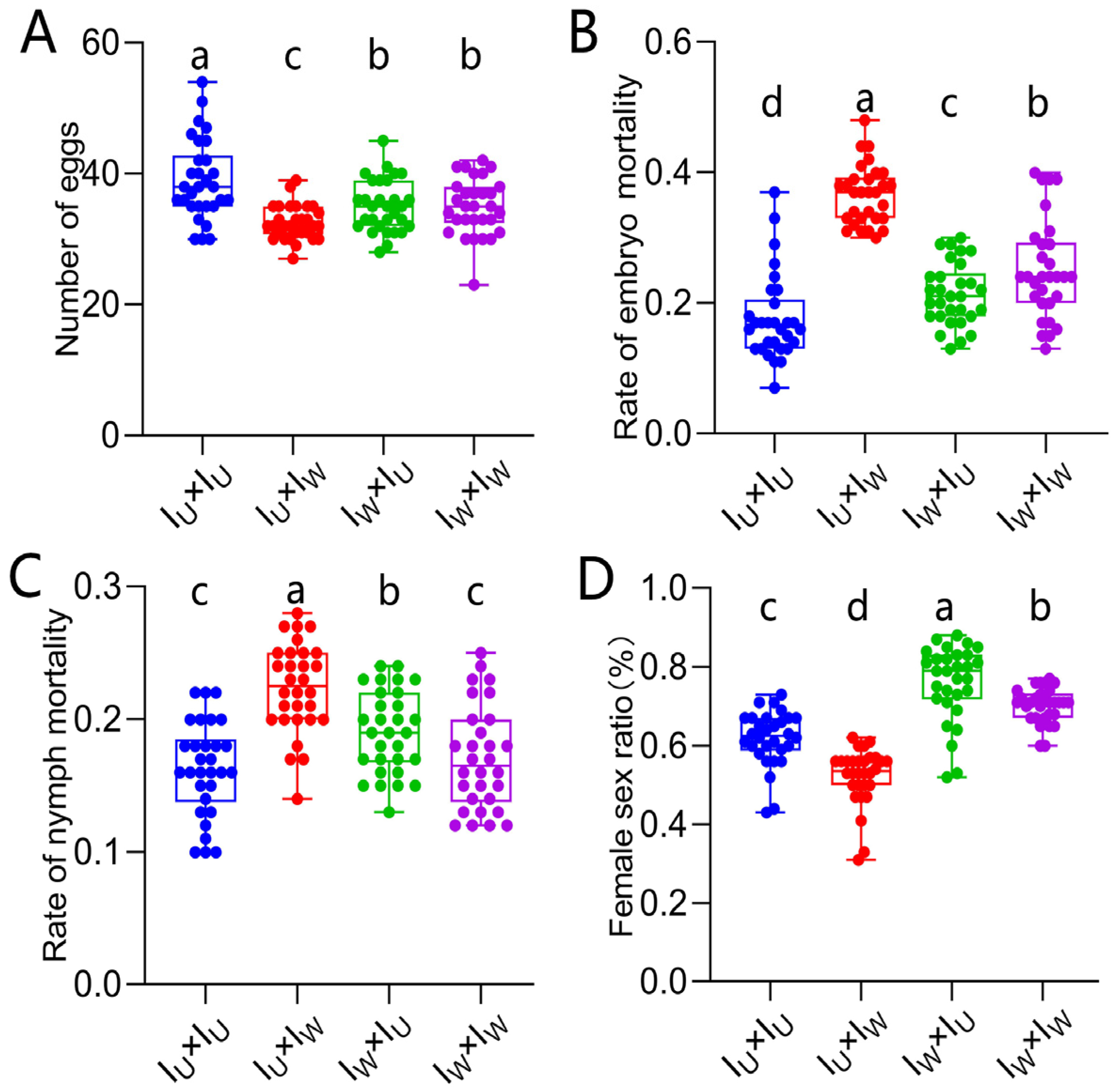
3.7. Verification of the Cytoplasmic Incompatibility (CI) Induced by Wolbachia in Singly-Infected T. turkestani
3.8. Verification of the Cytoplasmic Incompatibility (CI) Induced by Wolbachia and Rickettsia in Co-Infected T. turkestani
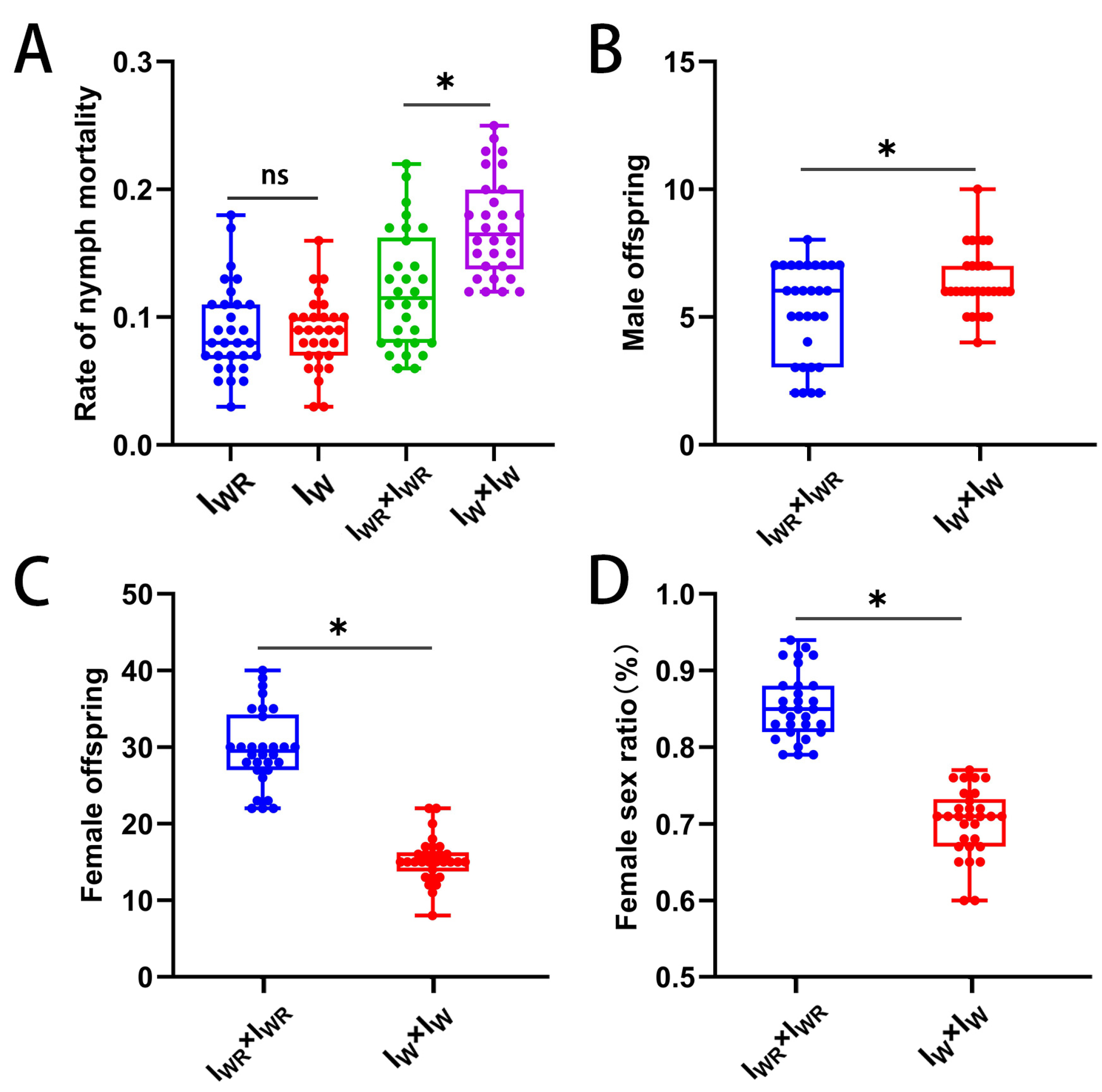
3.9. Antagonistic Effect of Rickettsia-Induced Male Killing on the Strength of Wolbachia-Induced CI
3.10. Wolbachia Does Not Have a Male-Killing Effect on T. turkestani
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How Many Species Are Infected with Wolbachia?—A Statistical Analysis of Current Data: Wolbachia Infection Rates. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The Incidence of Bacterial Endosymbionts in Terrestrial Arthropods. Proc. R. Soc. B 2015, 282, 20150249. [Google Scholar] [CrossRef]
- Brumin, M.; Levy, M.; Ghanim, M. Transovarial Transmission of Rickettsia Spp. and Organ-Specific Infection of the Whitefly Bemisia tabaci. Appl. Environ. Microbiol. 2012, 78, 5565–5574. [Google Scholar] [CrossRef]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative Bacterial Symbionts in Aphids Confer Resistance to Parasitic Wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master Manipulators of Invertebrate Biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and Virus Protection in Insects. Science 2008, 322, 702. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.J.; Hunter, M.S.; Zchori-Fein, E. The Emerging Diversity of Rickettsia. Proc. Biol. Sci. 2006, 273, 2097–2106. [Google Scholar] [CrossRef]
- Liu, B.-Q.; Bao, X.-Y.; Yan, J.-Y.; Zhang, D.; Sun, X.; Li, C.-Q.; Chen, Z.-B.; Luan, J.-B. Rickettsia Symbionts Spread via Mixed Mode Transmission, Increasing Female Fecundity and Sex Ratio Shift by Host Hormone Modulating. Proc. Natl. Acad. Sci. USA 2024, 121, e2406788121. [Google Scholar] [CrossRef]
- Dobson, S.L.; Bourtzis, K.; Braig, H.R.; Jones, B.F.; Zhou, W.; Rousset, F.; O’Neill, S.L. Wolbachia Infections Are Distributed throughout Insect Somatic and Germ Line Tissues. Insect Biochem. Mol. Biol. 1999, 29, 153–160. [Google Scholar] [CrossRef]
- Ilbeigi Khamseh Nejad, M.; Cappelli, A.; Damiani, C.; Falcinelli, M.; Catapano, P.L.; Nanfack-Minkeu, F.; Mayi, M.P.A.; Currà, C.; Ricci, I.; Favia, G. Wolbachia and Asaia Distribution among Different Mosquito Vectors Is Affected by Tissue Localization and Host Species. Microorganisms 2024, 12, 545. [Google Scholar] [CrossRef]
- Fricke, L.C.; Lindsey, A.R.I. Examining Wolbachia-Induced Parthenogenesis in Hymenoptera. Methods Mol. Biol. 2024, 2739, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Fast, E.M.; Toomey, M.E.; Panaram, K.; Desjardins, D.; Kolaczyk, E.D.; Frydman, H.M. Wolbachia Enhance Drosophila Stem Cell Proliferation and Target the Germline Stem Cell Niche. Science 2011, 334, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Dobson, S.L.; Rattanadechakul, W.; Marsland, E.J. Fitness Advantage and Cytoplasmic Incompatibility in Wolbachia Single- and Superinfected Aedes Albopictus. Heredity 2004, 93, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes Aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia Induces Reactive Oxygen Species (ROS)-Dependent Activation of the Toll Pathway to Control Dengue Virus in the Mosquito Aedes Aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar] [CrossRef]
- Hagimori, T.; Abe, Y.; Date, S.; Miura, K. The First Finding of a Rickettsia Bacterium Associated with Parthenogenesis Induction among Insects. Curr. Microbiol. 2006, 52, 97–101. [Google Scholar] [CrossRef]
- Himler, A.G.; Adachi-Hagimori, T.; Bergen, J.E.; Kozuch, A.; Kelly, S.E.; Tabashnik, B.E.; Chiel, E.; Duckworth, V.E.; Dennehy, T.J.; Zchori-Fein, E.; et al. Rapid Spread of a Bacterial Symbiont in an Invasive Whitefly Is Driven by Fitness Benefits and Female Bias. Science 2011, 332, 254–256. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Aksoy, S. Concordant Evolution of a Symbiont with Its Host Insect Species: Molecular Phylogeny of Genus Glossina and Its Bacteriome-Associated Endosymbiont, Wigglesworthia Glossinidia. J. Mol. Evol. 1999, 48, 49–58. [Google Scholar] [CrossRef]
- Fan, Z.-Y.; Liu, Y.; He, Z.-Q.; Wen, Q.; Chen, X.-Y.; Khan, M.M.; Osman, M.; Mandour, N.S.; Qiu, B.-L. Rickettsia Infection Benefits Its Whitefly Hosts by Manipulating Their Nutrition and Defense. Insects 2022, 13, 1161. [Google Scholar] [CrossRef]
- Zélé, F.; Weill, M.; Magalhães, S. Identification of Spider-Mite Species and Their Endosymbionts Using Multiplex PCR. Exp. Appl. Acarol. 2018, 74, 123–138. [Google Scholar] [CrossRef]
- Frank, S.A. Dynamics of Cytoplasmic Incompatibility with Multiple Wolbachia Infections. J. Theor. Biol. 1998, 192, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Zchori-Fein, E.; Perlman, S.J. Distribution of the Bacterial Symbiont Cardinium in Arthropods. Mol. Ecol. 2004, 13, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Dobson, S.L. Evolution of Wolbachia Cytoplasmic Incompatibility Types. Evolution 2004, 58, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Ros, V.I.D.; Breeuwer, J.A.J. The Effects of, and Interactions between, Cardinium and Wolbachia in the Doubly Infected Spider Mite Bryobia sarothamni. Heredity 2009, 102, 413–422. [Google Scholar] [CrossRef]
- Zhao, D.-X.; Zhang, X.-F.; Hong, X.-Y. Host-Symbiont Interactions in Spider Mite Tetranychus truncates Doubly Infected with Wolbachia and Cardinium. Environ. Entomol. 2013, 42, 445–452. [Google Scholar] [CrossRef]
- Ben-David, T.; Melamed, S.; Gerson, U.; Morin, S. ITS2 Sequences as Barcodes for Identifying and Analyzing Spider Mites (Acari: Tetranychidae). Exp. Appl. Acarol. 2007, 41, 169–181. [Google Scholar] [CrossRef]
- Navajas, M.; Boursot, P. Nuclear Ribosomal DNA Monophyly versus Mitochondrial DNA Polyphyly in Two Closely Related Mite Species: The Influence of Life History and Molecular Drive. Proc. Biol. Sci. 2003, 270 (Suppl. S1), S124–S127. [Google Scholar] [CrossRef]
- Mohammadi, S.; Seraj, A.A.; Rajabpour, A. Effects of Six Greenhouse Cucumber Cultivars on Reproductive Performance and Life Expectancy of Tetranychus Turkestani (Acari: Tetranychidae). Acarologia 2015, 55, 231–242. [Google Scholar] [CrossRef]
- Chu, D.; Gao, C.S.; De Barro, P.; Zhang, Y.J.; Wan, F.H.; Khan, I.A. Further Insights into the Strange Role of Bacterial Endosymbionts in Whitefly, Bemisia Tabaci: Comparison of Secondary Symbionts from Biotypes B and Q in China. Bull. Entomol. Res. 2011, 101, 477–486. [Google Scholar] [CrossRef]
- Hong, X.-Y.; Gotoh, T.; Nagata, T. Vertical Transmission of Wolbachia in Tetranychus kanzawai Kishida and Panonychus Mori Yokoyama (Acari: Tetranychidae). Heredity 2002, 88, 190–196. [Google Scholar] [CrossRef]
- Bing, X.-L.; Lu, Y.-J.; Xia, C.-B.; Xia, X.; Hong, X.-Y. Transcriptome of Tetranychus Urticae Embryos Reveals Insights into Wolbachia-Induced Cytoplasmic Incompatibility. Insect Mol. Biol. 2020, 29, 193–204. [Google Scholar] [CrossRef]
- Lv, N.; Peng, J.; Chen, X.-Y.; Guo, C.-F.; Sang, W.; Wang, X.-M.; Ahmed, M.Z.; Xu, Y.-Y.; Qiu, B.-L. Antagonistic Interaction between Male-Killing and Cytoplasmic Incompatibility Induced by Cardinium and Wolbachia in the Whitefly, Bemisia tabaci. Insect Sci. 2021, 28, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jin, Y.; He, L.; Lu, W.-C.; Li, M. Suitable Reference Gene Selection for Different Strains and Developmental Stages of the Carmine Spider Mite, Tetranychus cinnabarinus, Using Quantitative Real-Time PCR. J. Insect Sci. 2010, 10, 208. [Google Scholar] [CrossRef]
- Mouton, L.; Dedeine, F.; Henri, H.; Boulétreau, M.; Profizi, N.; Vavre, F. Virulence, Multiple Infections and Regulation of Symbiotic Population in the Wolbachia-Asobara Tabida Symbiosis. Genetics 2004, 168, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Morrow, J.L.; Spooner-Hart, R.N.; Riegler, M. Independent Cytoplasmic Incompatibility Induced by Cardinium and Wolbachia Maintains Endosymbiont Coinfections in Haplodiploid Thrips Populations. Evolution 2017, 71, 995–1008. [Google Scholar] [CrossRef]
- Sakurai, M.; Koga, R.; Tsuchida, T.; Meng, X.-Y.; Fukatsu, T. Rickettsia Symbiont in the Pea Aphid Acyrthosiphon pisum: Novel Cellular Tropism, Effect on Host Fitness, and Interaction with the Essential Symbiont Buchnera. Appl. Environ. Microbiol. 2005, 71, 4069–4075. [Google Scholar] [CrossRef]
- Ferrari, J.; Scarborough, C.L.; Godfray, H.C.J. Genetic Variation in the Effect of a Facultative Symbiont on Host-Plant Use by Pea Aphids. Oecologia 2007, 153, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kontsedalov, S.; Zchori-Fein, E.; Chiel, E.; Gottlieb, Y.; Inbar, M.; Ghanim, M. The Presence of Rickettsia Is Associated with Increased Susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to Insecticides. Pest. Manag. Sci. 2008, 64, 789–792. [Google Scholar] [CrossRef]
- Shan, H.-W.; Lu, Y.-H.; Bing, X.-L.; Liu, S.-S.; Liu, Y.-Q. Differential Responses of the Whitefly Bemisia tabaci Symbionts to Unfavorable Low and High Temperatures. Microb. Ecol. 2014, 68, 472–482. [Google Scholar] [CrossRef]
- Vavre, F.; Dedeine, F.; Quillon, M.; Fouillet, P.; Fleury, F.; Bouletreau, M. Within-Species Diversity of Wolbachia-Induced Cytoplasmic Incompatibility in Haplodiploid Insects. Evolution 2001, 55, 1710–1714. [Google Scholar] [CrossRef]
- Vala, F.; Breeuwer, J.a.J.; Sabelis, M.W. Sorting out the Effects of Wolbachia, Genotype and Inbreeding on Life-History Traits of a Spider Mite. Exp. Appl. Acarol. 2003, 29, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kriesner, P.; Conner, W.R.; Weeks, A.R.; Turelli, M.; Hoffmann, A.A. Persistence of a Wolbachia Infection Frequency Cline in Drosophila melanogaster and the Possible Role of Reproductive Dormancy. Evolution 2016, 70, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.M.; Schiffer, M.; Griffin, P.C.; Lee, S.F.; Hoffmann, A.A. Tropical Drosophila pandora Carry Wolbachia Infections Causing Cytoplasmic Incompatibility or Male Killing. Evolution 2016, 70, 1791–1802. [Google Scholar] [CrossRef] [PubMed]


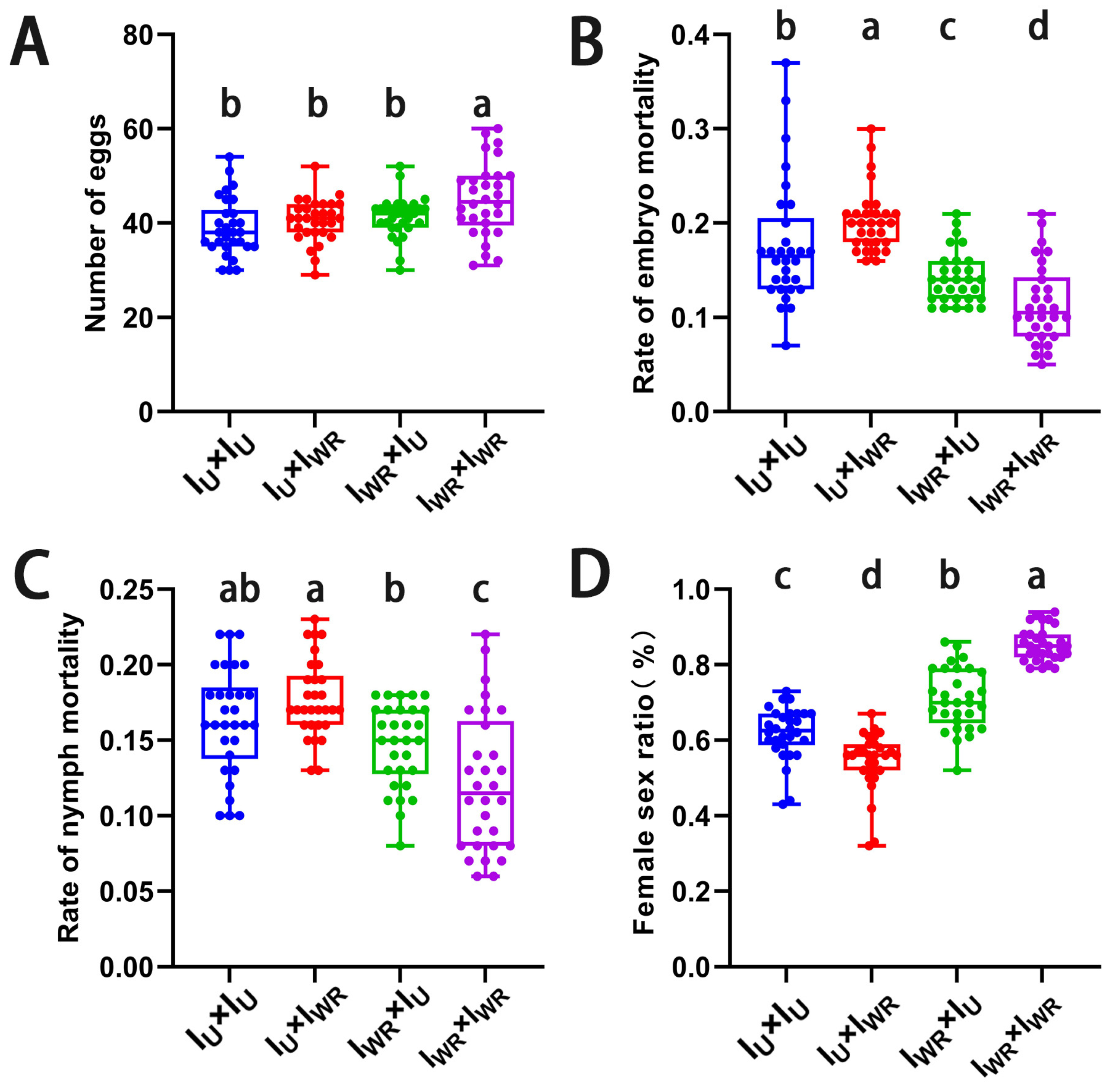

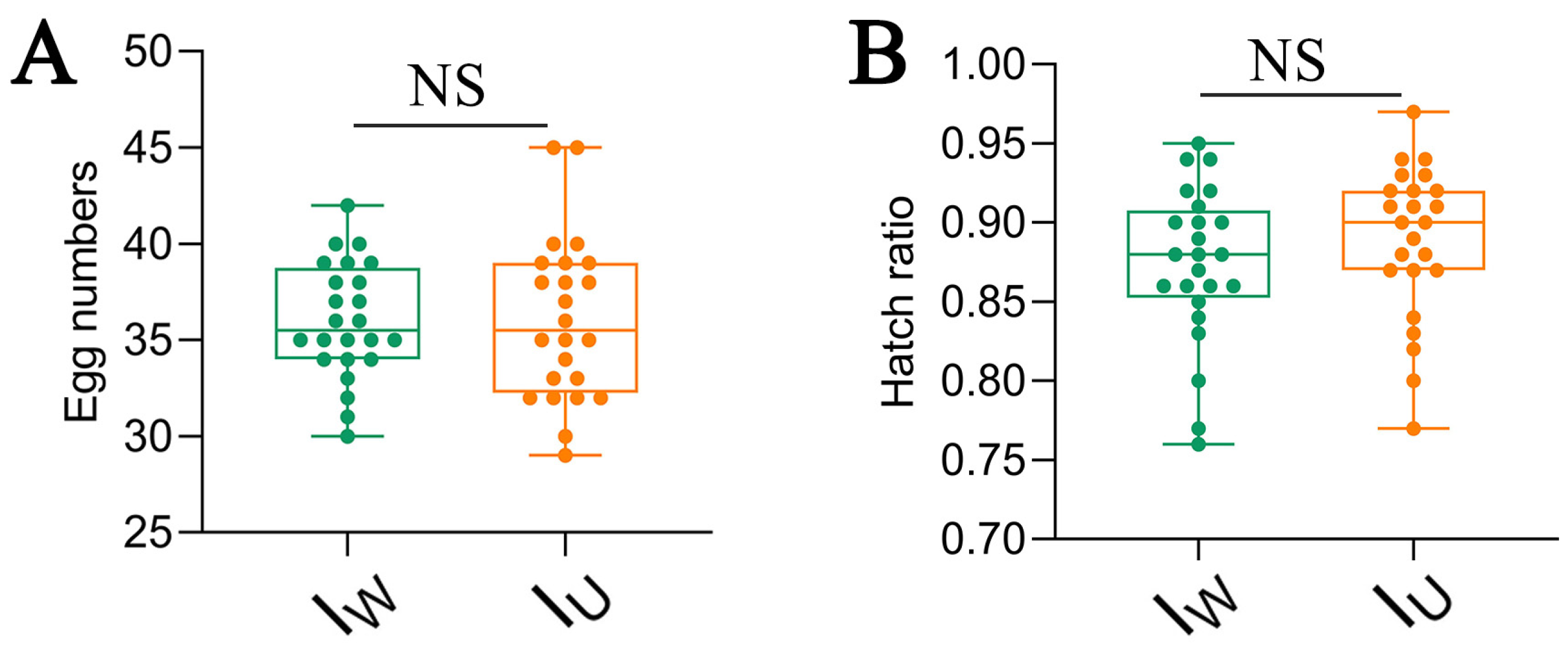
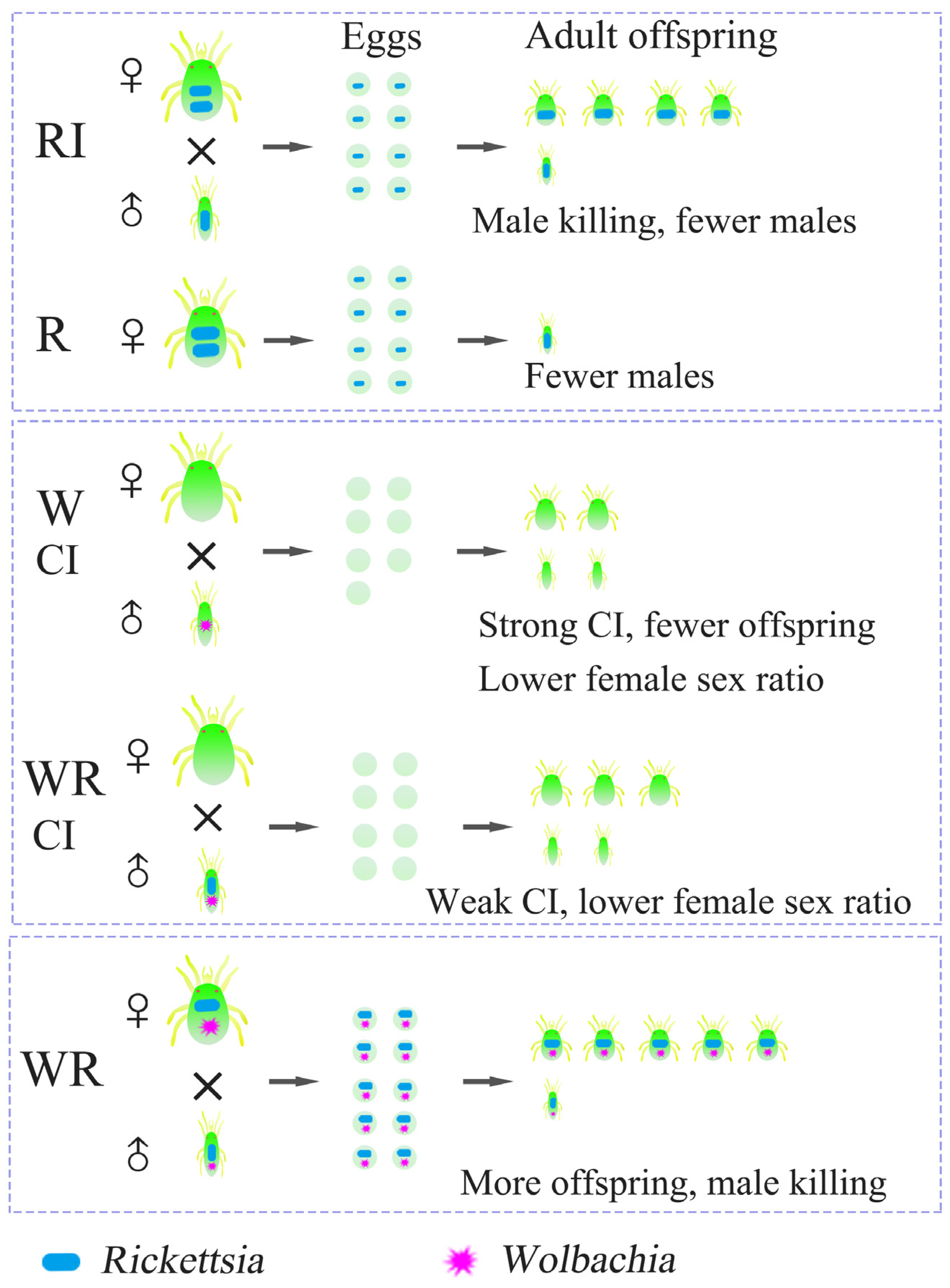
| Tetranychus turkestani Strains | Number of Adult Female Mites | Number of Offspring | Total Number of Specimens Tested | Rickettsia | Wolbachia | ||||
|---|---|---|---|---|---|---|---|---|---|
| n+ | n− | % | n+ | n− | % | ||||
| ♀IW | 10 | 10 | 100 | 0 | 100 | 0 | 100 | 0 | 100 |
| ♀IR | 50 | 2 | 100 | 100 | 0 | 100 | 0 | 100 | 0 |
| ♀IWR | 10 | 10 | 100 | 100 | 0 | 100 | 100 | 0 | 100 |
| ♀IW × ♂IU | 10 | 10 | 100 | 0 | 100 | 0 | 100 | 0 | 100 |
| ♀IR × ♂IU | 10 | 10 | 100 | 100 | 0 | 100 | 0 | 100 | 0 |
| ♀IWR × ♂IU | 10 | 10 | 100 | 100 | 0 | 100 | 100 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, X.; Basit, A.; Wei, Q.; Zhao, K.; Zhao, Y. Interactions Between Endosymbionts Wolbachia and Rickettsia in the Spider Mite Tetranychus turkestani: Cooperation or Antagonism? Microorganisms 2025, 13, 642. https://doi.org/10.3390/microorganisms13030642
Wang S, Wang X, Basit A, Wei Q, Zhao K, Zhao Y. Interactions Between Endosymbionts Wolbachia and Rickettsia in the Spider Mite Tetranychus turkestani: Cooperation or Antagonism? Microorganisms. 2025; 13(3):642. https://doi.org/10.3390/microorganisms13030642
Chicago/Turabian StyleWang, Sha, Xinlei Wang, Ali Basit, Qiancheng Wei, Kedi Zhao, and Yiying Zhao. 2025. "Interactions Between Endosymbionts Wolbachia and Rickettsia in the Spider Mite Tetranychus turkestani: Cooperation or Antagonism?" Microorganisms 13, no. 3: 642. https://doi.org/10.3390/microorganisms13030642
APA StyleWang, S., Wang, X., Basit, A., Wei, Q., Zhao, K., & Zhao, Y. (2025). Interactions Between Endosymbionts Wolbachia and Rickettsia in the Spider Mite Tetranychus turkestani: Cooperation or Antagonism? Microorganisms, 13(3), 642. https://doi.org/10.3390/microorganisms13030642







