Process Technologies for Disinfection of Food-Contact Surfaces in the Dry Food Industry: A Review
Abstract
:1. Introduction
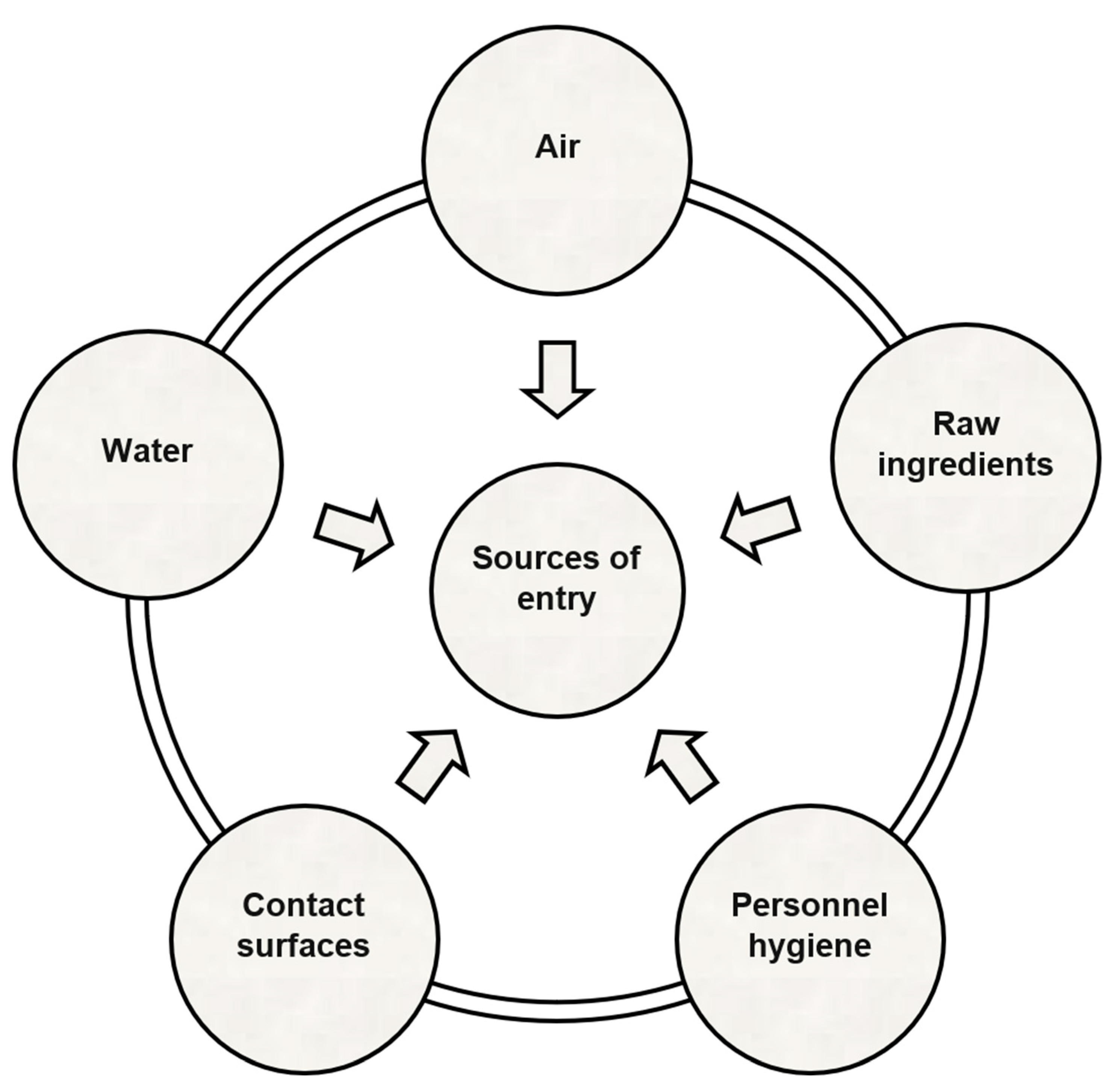
2. Factors Influencing the Disinfection Process in Low-Water-Activity Food Processing Environments
2.1. Biotransfer Potential
2.2. Presence of Persistent and Non-Persistent Strains
2.3. Presence of Organic Matter
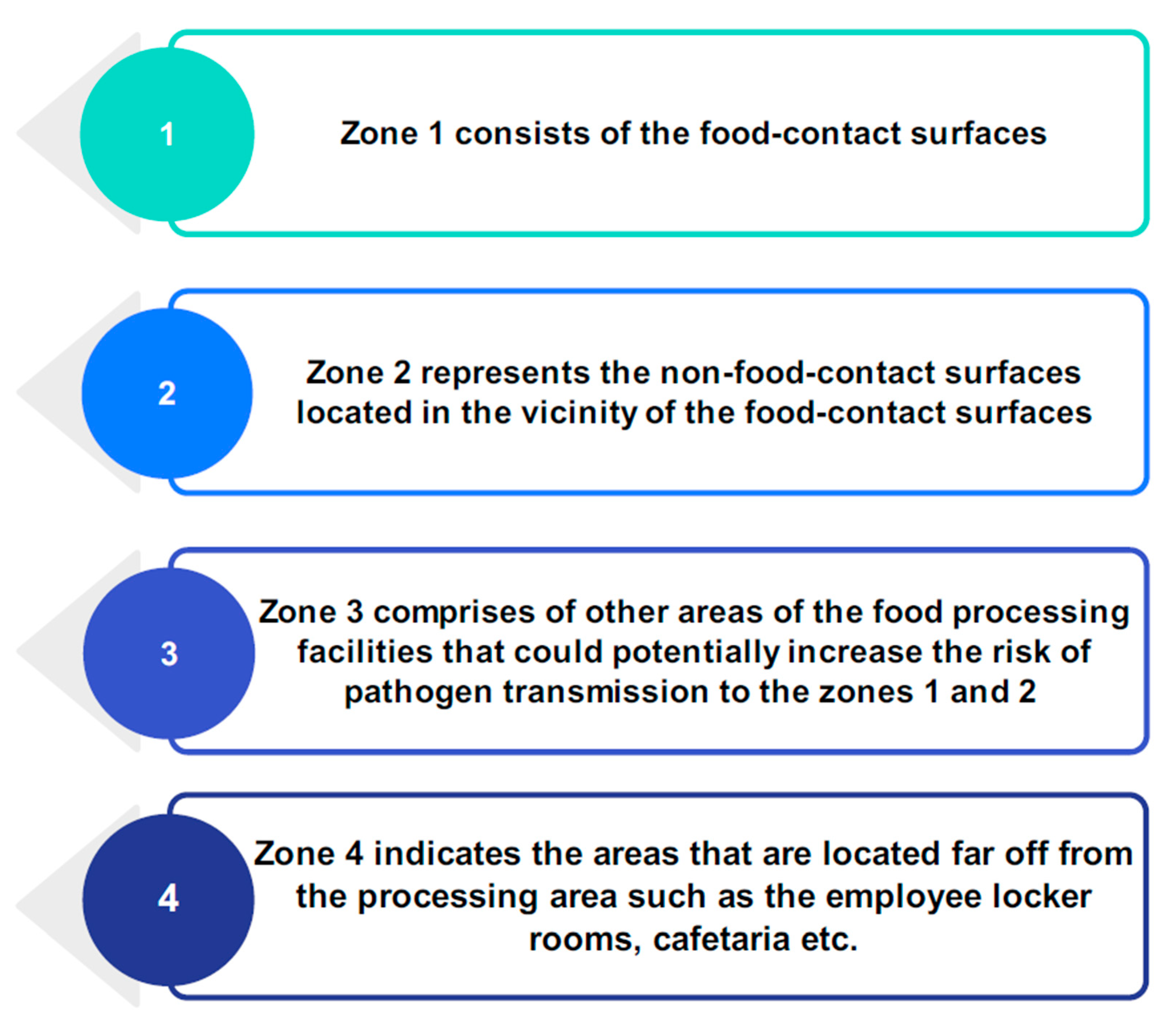
2.4. Types of Food-Contact Surfaces
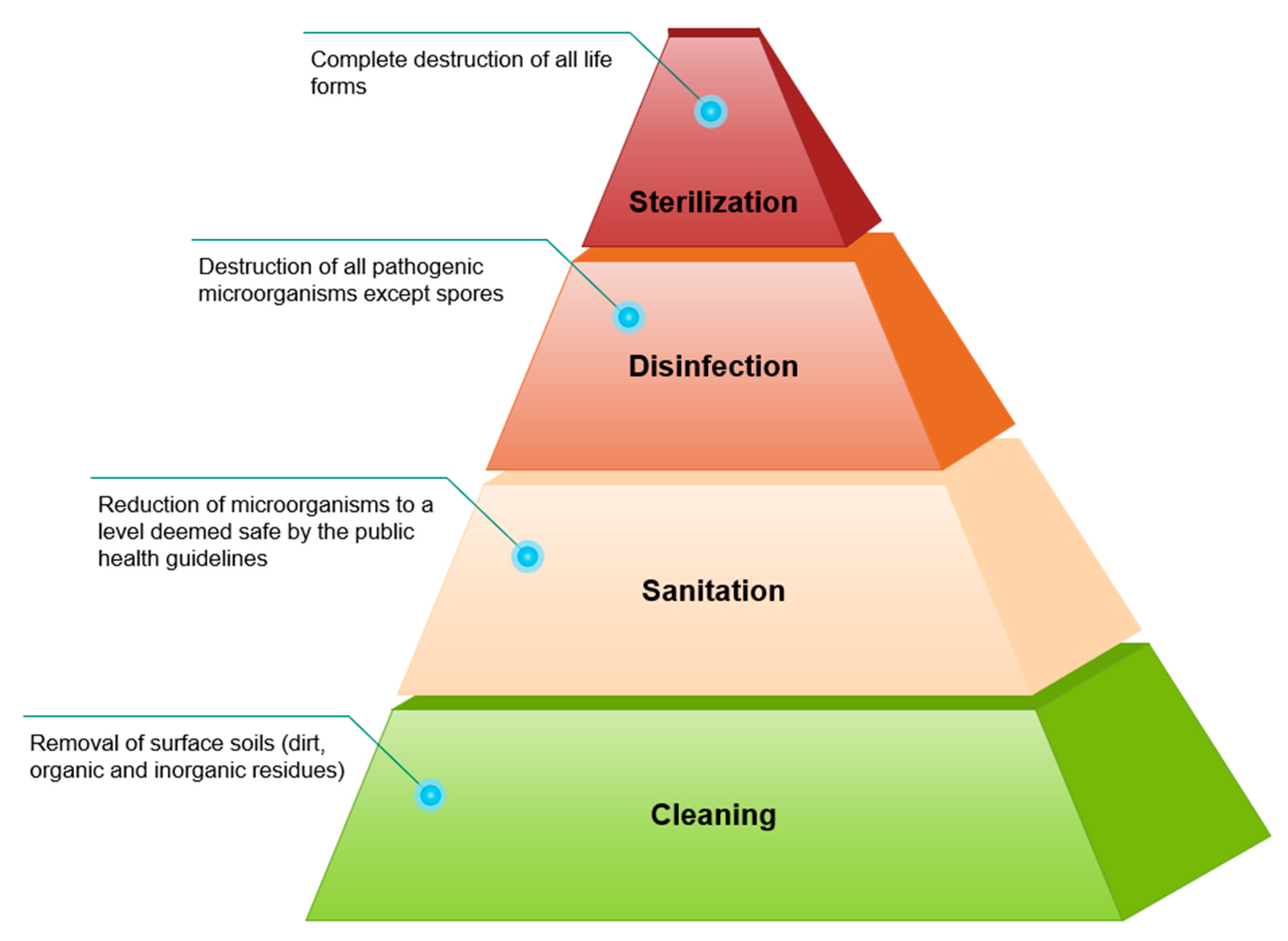
3. Surface Disinfection Challenges Faced by the Dry Food Industry
4. Dry Disinfection Methods for Microbial Inactivation in Dry Food Processing Facilities
4.1. Conventional Dry Disinfection Methods
4.1.1. Isopropyl Alcohol-Quaternary Ammonium-Based Disinfectants
4.1.2. Fumigation with Gaseous Antimicrobials
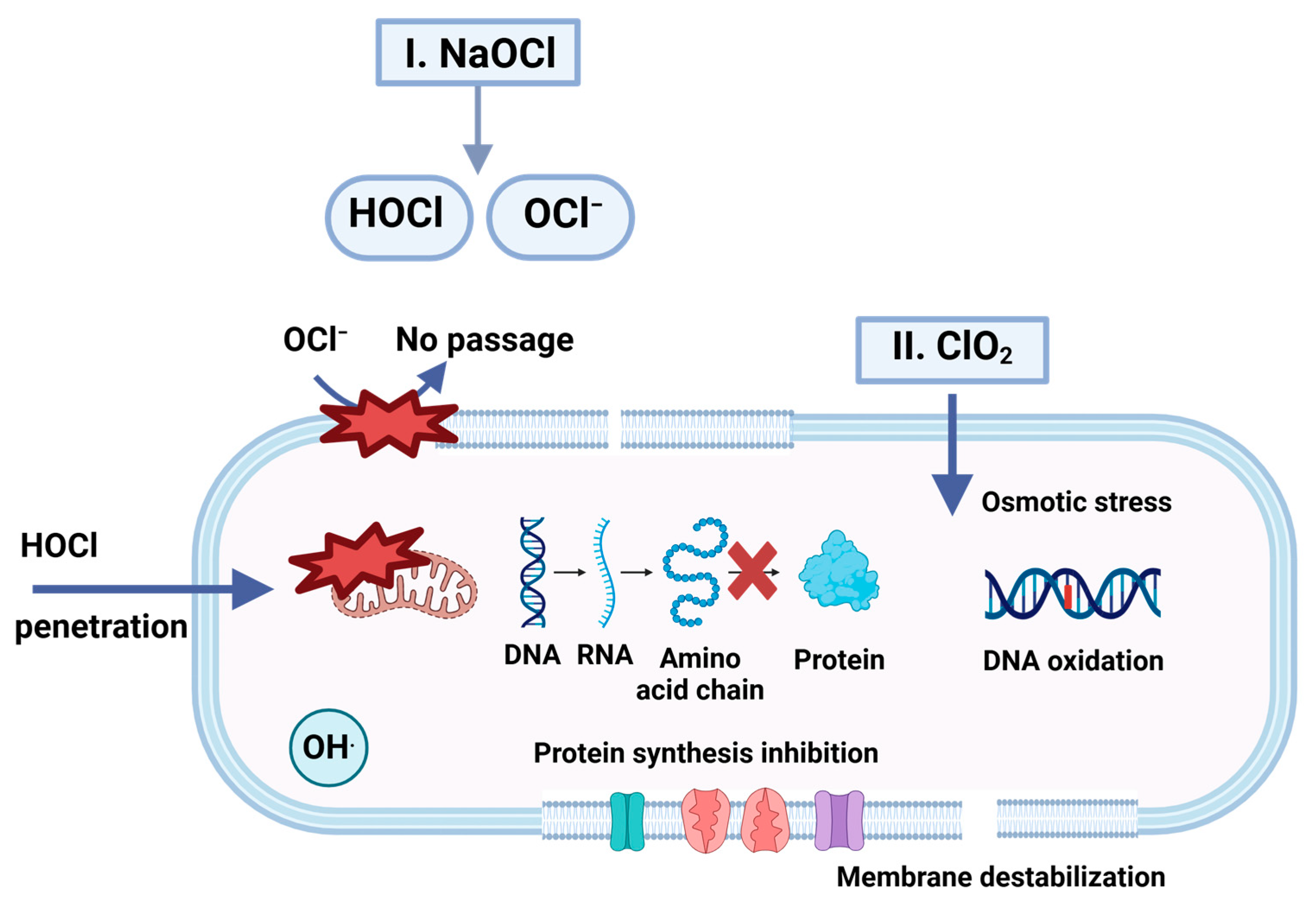
4.1.3. Chlorine Dioxide Gas Fumigation
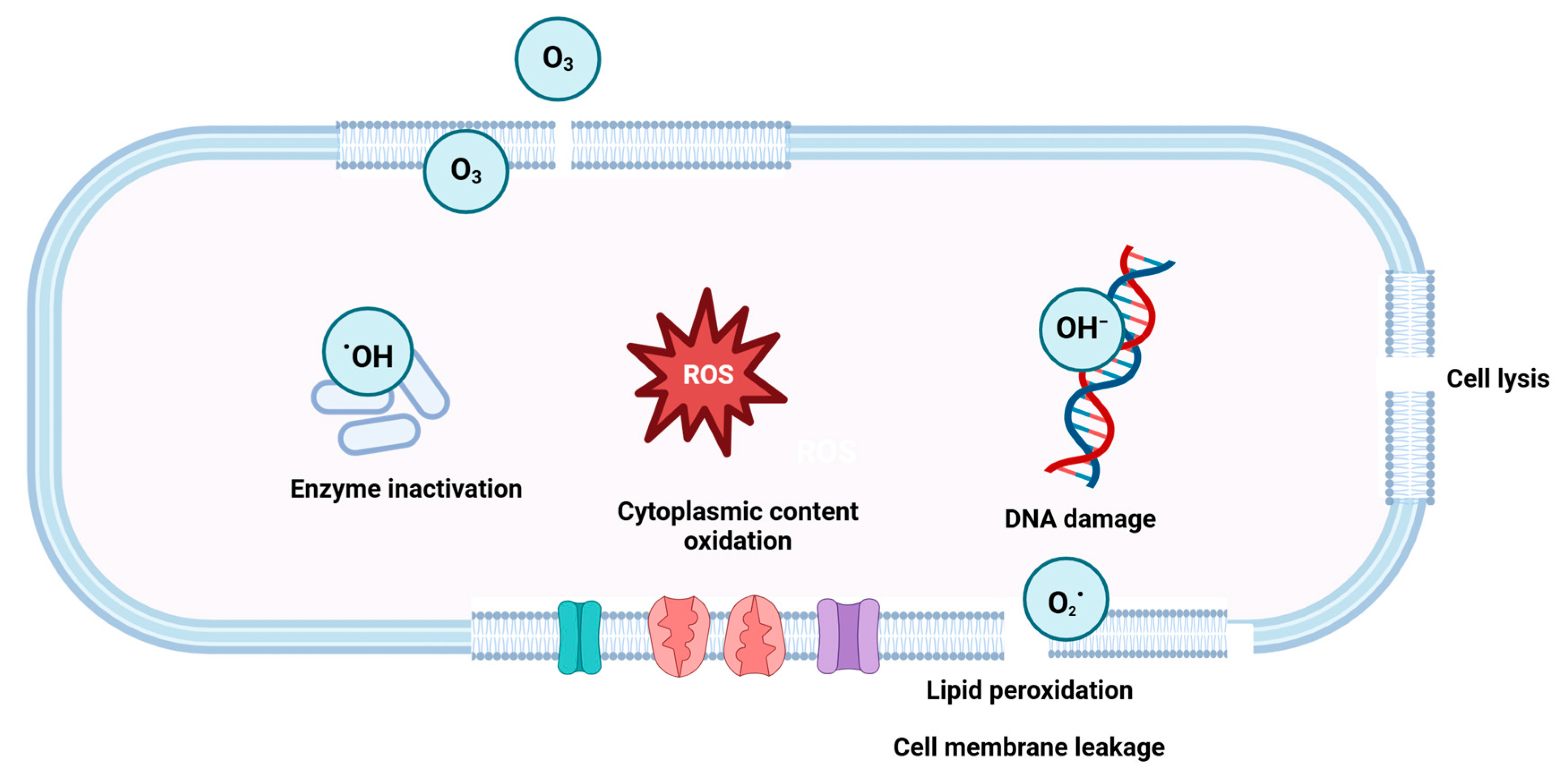
4.1.4. Ozone Gas Fumigation
4.2. Novel Dry Disinfection Technologies
4.2.1. Superheated Steam
4.2.2. UV Light Disinfection
- (a)
- Fluorescence, wherein the molecule returns to its ground state by emitting a photon.
- (b)
- Phosphorescence, which indicates that the molecule will maintain its excited state.
- (c)
- Internal conversion, in which heat is lost as the medium returns to its initial state.
- (d)
- Photochemical reaction, involving chemical conversion by altering the chemical structure of the molecules such as DNA/RNA.
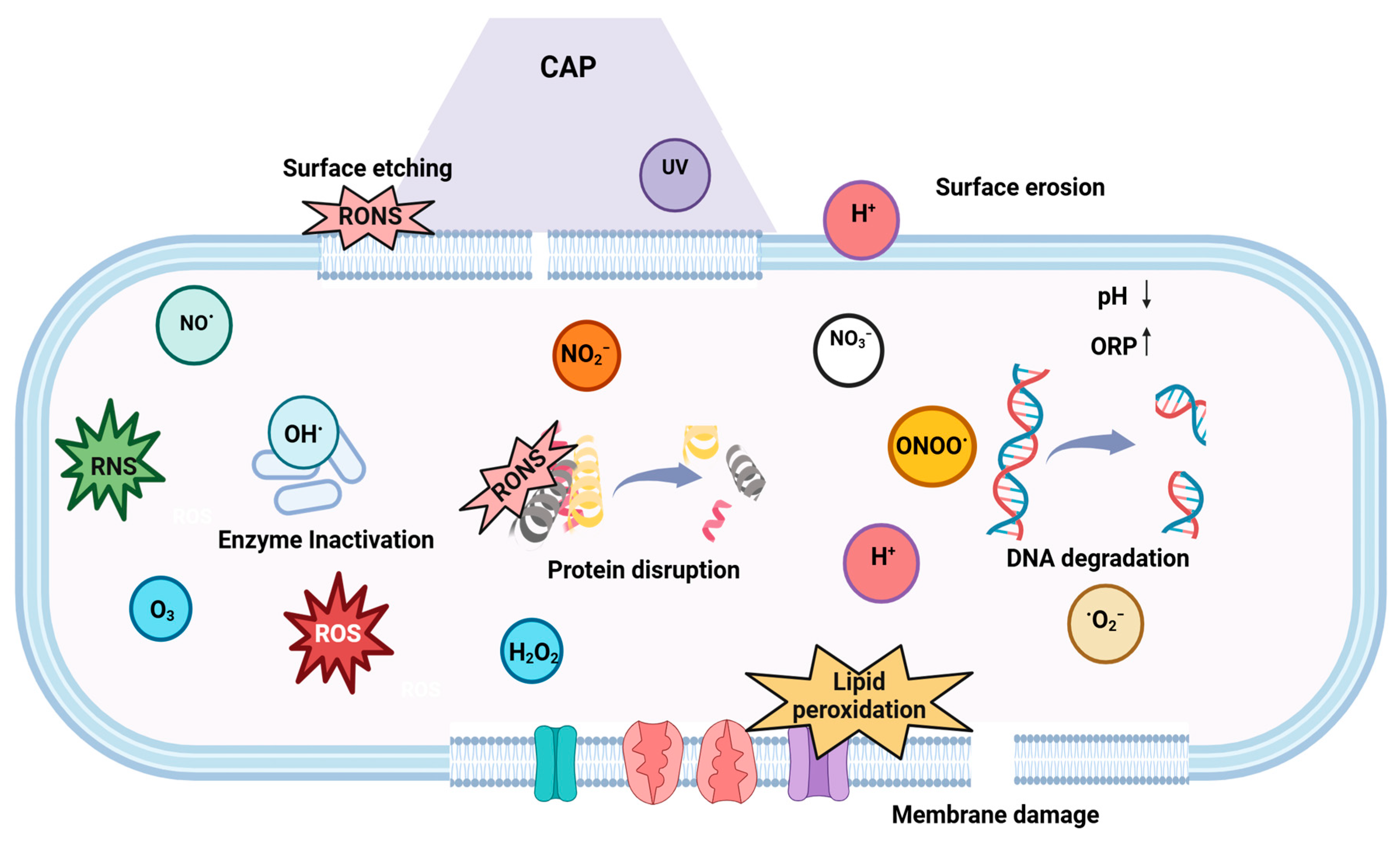
4.2.3. Cold Plasma
5. Wet Disinfection Methods for Microbial Inactivation in Dry Food Processing Facilities
5.1. Conventional Wet Disinfection Methods
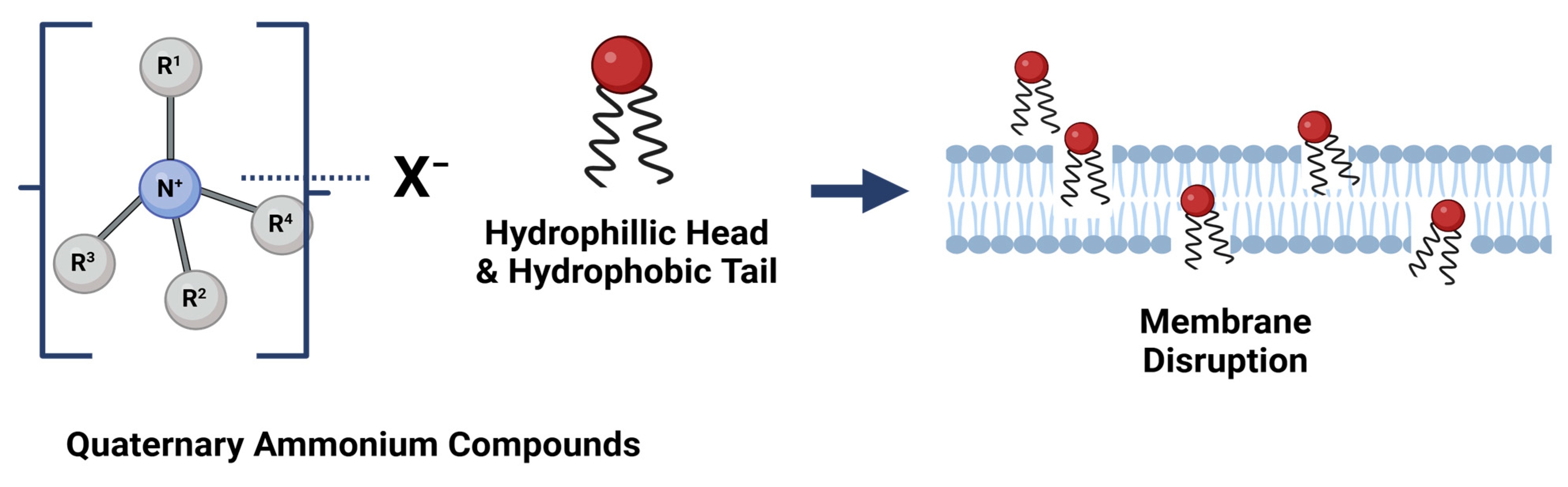
5.1.1. Quaternary Ammonium Compounds
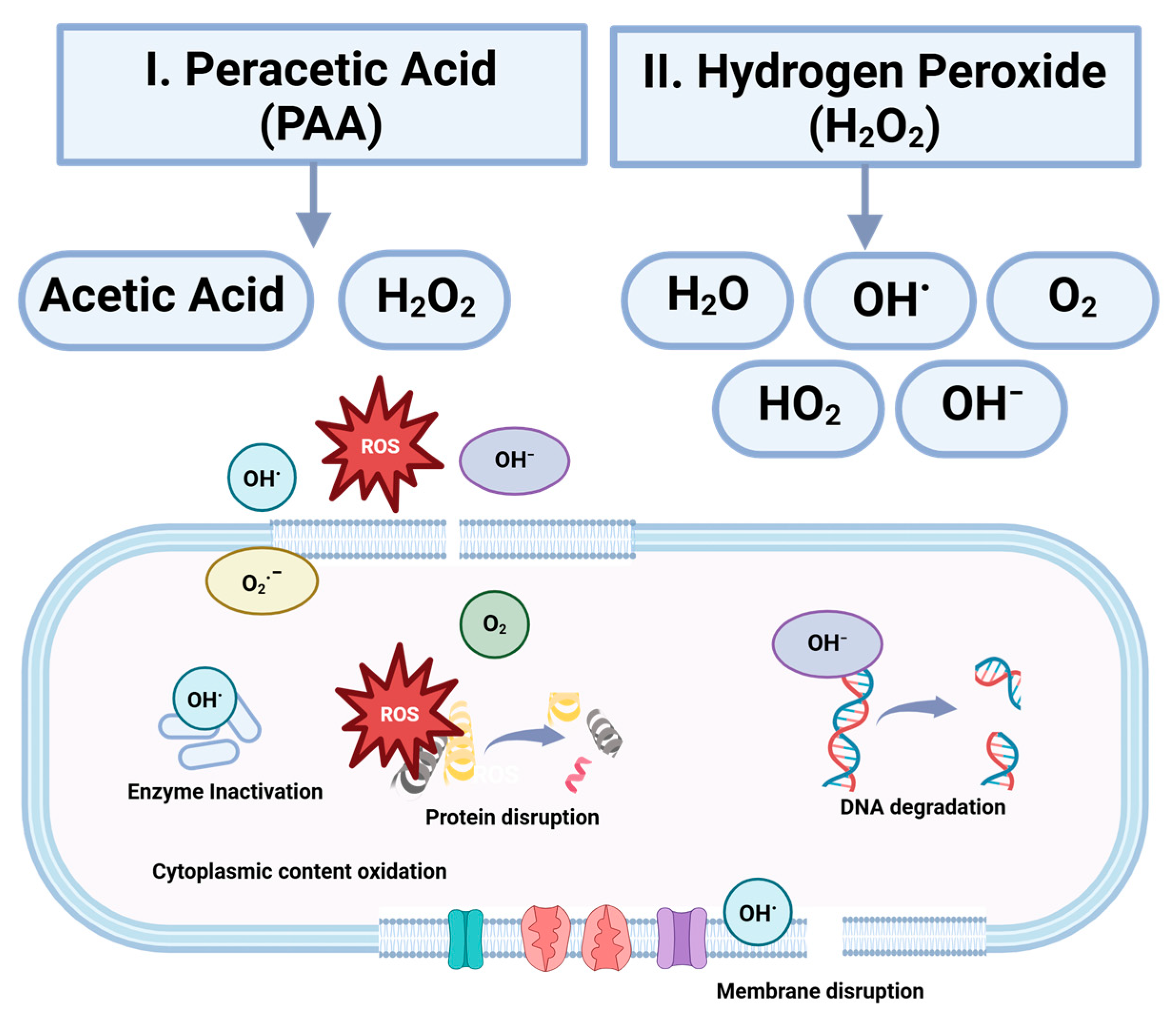
5.1.2. Peracetic Acid
5.1.3. Hydrogen Peroxide
5.1.4. Sodium Hypochlorite
5.2. Novel Wet Disinfection Methods
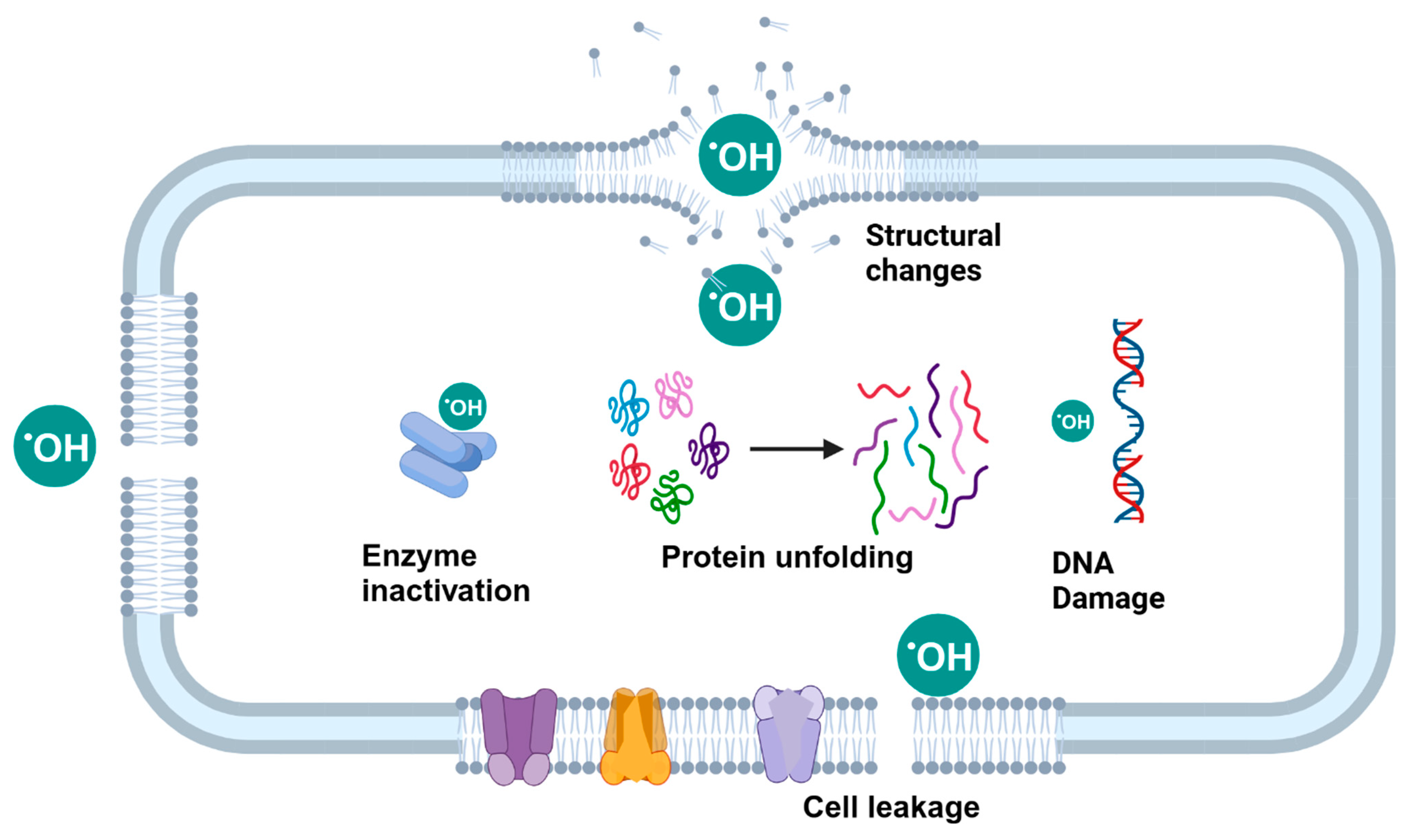
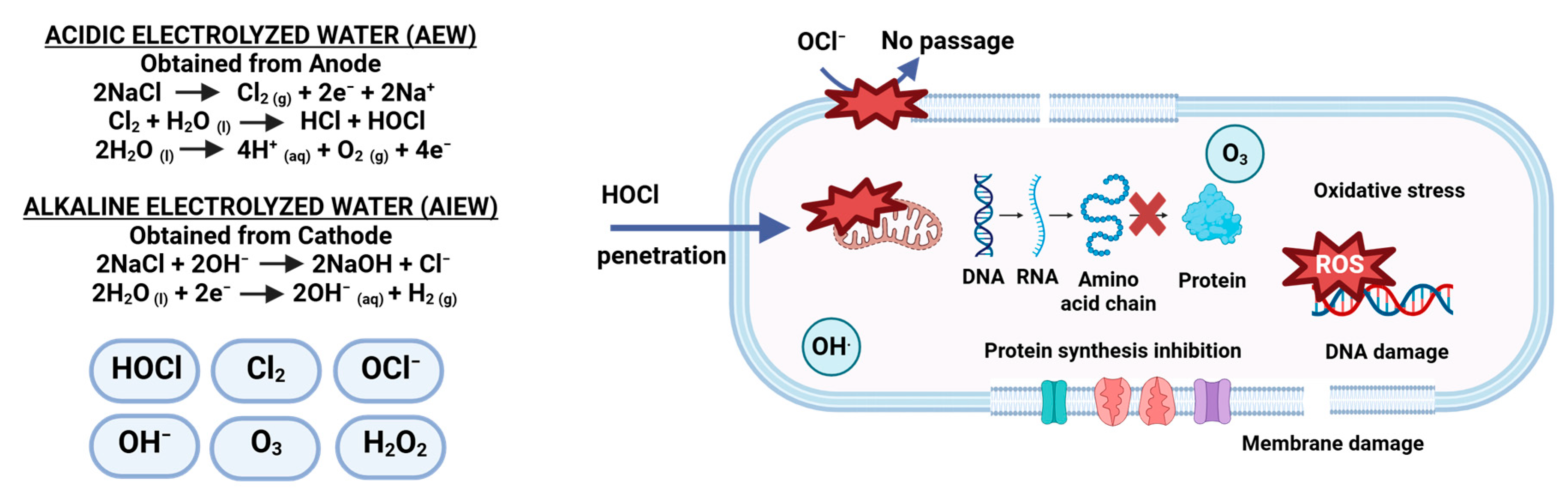
5.2.1. Electrolyzed Water (EW)
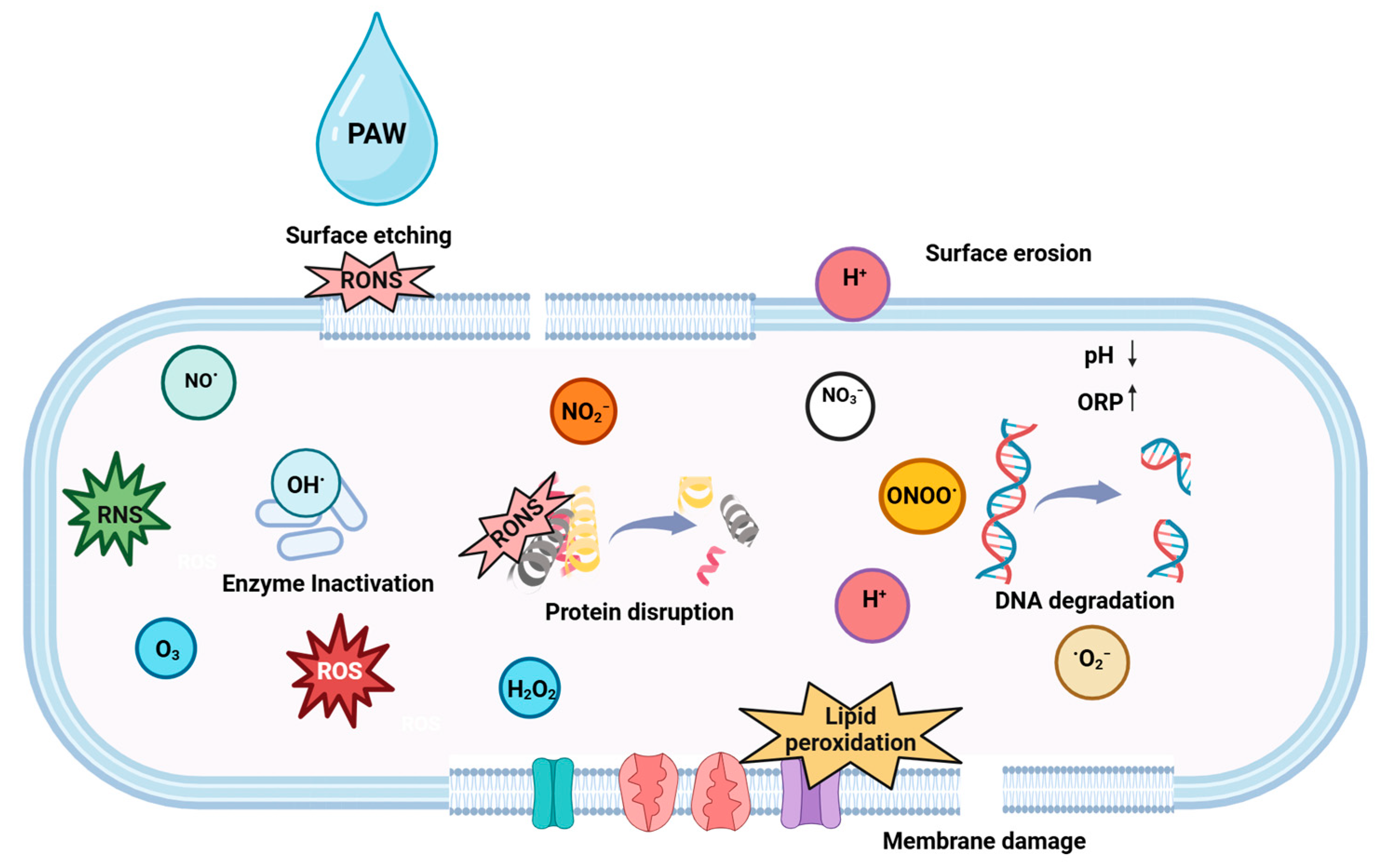
5.2.2. Plasma Activated Water
6. Commercial Status and Future Perspectives
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beuchat, L.R.; Komitopoulou, E.; Beckers, H.; Betts, R.P.; Bourdichon, F.; Fanning, S.; Joosten, H.M.; Kuile, B.H.T. Low-Water Activity Foods: Increased Concern as Vehicles of Foodborne Pathogens. J. Food Prot. 2013, 76, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Blessington, T.; Mitcham, E.J.; Harris, L.J. Survival of Salmonella enterica, Escherichia coli O157:H7, and Listeria monocytogenes on Inoculated Walnut Kernels during Storage. J. Food Prot. 2012, 75, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, N.; Pinto, R.; Sela, S. Effect of Desiccation on Tolerance of Salmonella enterica to Multiple Stresses. Appl. Environ. Microbiol. 2011, 77, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Salmonella Outbreak Linked to Flour. Available online: https://www.cdc.gov/salmonella/infantis-03-23/index.html (accessed on 10 August 2023).
- Centers for Disease Control and Prevention (CDC). Salmonella Outbreak Linked to Peanut Butter. Available online: https://www.cdc.gov/salmonella/senftenberg-05-22/index.html (accessed on 10 August 2023).
- Centers for Disease Control and Prevention (CDC). Outbreak of Salmonella Infections Linked to Karawan Brand Tahini. Available online: https://www.cdc.gov/salmonella/concord-05-19/index.html (accessed on 10 August 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella typhimurium Infections Linked to Dried Coconut (Final Update). Available online: https://www.cdc.gov/salmonella/typhimurium-03-18/index.html (accessed on 5 January 2023).
- Public Health Agency of Canada. Public Health Notice—Outbreak of Salmonella Infections Related to Sprouted Chia Seed Powder. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2014/public-health-notice-outbreak-salmonella-infections-related-sprouted-chia-seed-powder.html (accessed on 7 October 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Listeriosis Linked to Soft Cheeses Distributed by Karoun Dairies, Inc. (Final Update). Available online: https://www-cdc-gov.login.ezproxy.library.ualberta.ca/listeria/outbreaks/soft-cheeses-09-15/index.html (accessed on 18 January 2023).
- Jernberg, C.; Hjertqvist, M.; Sundborger, C.; Castro, E.; Löfdahl, M.; Pääjärvi, A.; Sundqvist, L.; Löf, E. Outbreak of Salmonella Enteritidis Phage Type 13a Infection in Sweden Linked to Imported Dried-Vegetable Spice Mixes, December 2014 to July 2015. Eurosurveillance 2015, 20, 21194. [Google Scholar] [CrossRef]
- Ladd-Wilson, S.G.; Morey, K.; Koske, S.E.; Burkhalter, B.; Bottichio, L.; Brandenburg, J.; Fontana, J.; Tenney, K.; Kutumbaka, K.K.; Samadpour, M.; et al. Notes from the Field: Multistate Outbreak of Salmonella Agbeni Associated with Consumption of Raw Cake Mix—Five States 2018. Morb. Mortal. Wkly. Rep. 2019, 68, 751. [Google Scholar] [CrossRef]
- Podolak, R.; Enache, E.; Stone, W.; Black, D.G.; Elliott, P.H. Sources and Risk Factors for Contamination, Survival, Persistence, and Heat Resistance of Salmonella in Low-Moisture Foods. J. Food Prot. 2010, 73, 1919–1936. [Google Scholar] [CrossRef]
- Frank, J.F. Microbial Attachment to Food and Food Contact Surfaces. Adv. Food Nutr. Res. 2001, 43, 319–370. [Google Scholar] [CrossRef]
- Norwood, D.E.; Gilmour, A. Adherence of Listeria monocytogenes Strains to Stainless Steel Coupons. J. Appl. Microbiol. 1999, 86, 576–582. [Google Scholar] [CrossRef]
- Miettinen, M.K.; Björkroth, K.J.; Korkeala, H.J. Characterization of Listeria monocytogenes from an Ice Cream Plant by Serotyping and Pulsed-Field Gel Electrophoresis. Int. J. Food Microbiol. 1999, 46, 187–192. [Google Scholar] [CrossRef]
- Collins, R.N.; Treger, M.D.; Goldsby, J.B.; Boring, J.R.; Coohon, D.B.; Barr, R.N. Interstate Outbreak of Salmonella newbrunswick Infection Traced to Powdered Milk. JAMA 1968, 203, 838–844. [Google Scholar] [CrossRef]
- Craven, P.C.; Baine, W.B.; Mackel, D.C.; Barker, W.H.; Gangarosa, E.J.; Goldfield, M.; Rosenfeld, H.; Altman, R.; Lachapelle, G.; Davies, J.W.; et al. International Outbreak of Salmonella eastbourne Infection Traced to Contaminated Chocolate. Lancet 1975, 1, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Agona Infections Linked to Rice and Wheat Puff Cereal (Final Update). Available online: https://www.cdc.gov/salmonella/2008/rice-wheat-puff-cereal-5-13-2008.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Wandsworth Infections Linked to Veggie Booty (Final Update). Available online: https://www.cdc.gov/salmonella/2007/veggie-booty-7-18-2007.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Schwarzengrund Infections Linked to Dry Pet Food (Final Update). Available online: https://www.cdc.gov/salmonella/2007/pet-food-9-4-2007.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Tennessee Infections Linked to Peanut Butter (Final Update). Available online: https://www.cdc.gov/salmonella/2007/peanut-butter-3-7-2007.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Infections Linked to Pistachio Nuts (Final Update). Available online: https://www.cdc.gov/salmonella/2009/pistachio-nuts-4-14-2009.html (accessed on 5 January 2023).
- Binter, C.; Straver, J.M.; Häggblom, P.; Bruggeman, G.; Lindqvist, P.A.; Zentek, J.; Andersson, M.G. Transmission and Control of Salmonella in the Pig Feed Chain: A Conceptual Model. Int. J. Food Microbiol. 2011, 145, S7–S17. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of E. coli O157:H7 Infections Associated with in-Shell Hazelnuts (Final Update). Available online: https://www.cdc.gov/ecoli/2011/hazelnuts-4-7-11.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Bredeney Infections Linked to Peanut Butter Manufactured by Sunland, Inc. (Final Update). Available online: https://www.cdc.gov/salmonella/bredeney-09-12/index.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Montevideo and Salmonella Mbandaka Infections Linked to Tahini Sesame Paste (Final Update). Available online: https://www.cdc.gov/salmonella/montevideo-tahini-05-13/index.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Braenderup Infections Linked to Nut Butter Manufactured by NSpired Natural Foods, Inc. (Final Update). Available online: https://www.cdc.gov/salmonella/braenderup-08-14/index.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Montevideo and Salmonella Senftenberg Infections Linked to Wonderful Pistachios (Final Update). Available online: https://www.cdc.gov/salmonella/montevideo-03-16/index.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). 2015 Salmonella Outbreak Linked to JEM Raw Brand Sprouted Nut Butter Spreads—Consumer Advice. Available online: https://archive.cdc.gov/www_cdc_gov/salmonella/paratyphi-b-12-15/advice.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Shiga Toxin-Producing Escherichia coli Infections Linked to Flour (Final Update). Available online: https://www.cdc.gov/ecoli/2016/o121-06-16/index.html (accessed on 5 January 2023).
- Centers for Disease Control and Prevention (CDC). Multistate Outbreak of Salmonella Infections Linked to Coconut Tree Brand Frozen Shredded Coconut (Final Update). Available online: https://www.cdc.gov/salmonella/coconut-01-18/index.html (accessed on 5 January 2023).
- Gupta, P.; Adhikari, A. Novel Approaches to Environmental Monitoring and Control of Listeria monocytogenes in Food Production Facilities. Foods 2022, 11, 1760. [Google Scholar] [CrossRef]
- Verran, J.; Airey, P.; Packer, A.; Whitehead, K.A. Chapter 8 Microbial Retention on Open Food Contact Surfaces and Implications for Food Contamination. Adv. Appl. Microbiol. 2008, 64, 223–246. [Google Scholar] [CrossRef]
- Lisle, J.T.; Broadaway, S.C.; Prescott, A.M.; Pyle, B.H.; Fricker, C.; Feters, G.A.M.C.C. Effects of Starvation on Physiological Activity and Chlorine Disinfection Resistance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 1998, 64, 4658–4662. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.C.; Schaffner, D.W. Longer Contact Times Increase Cross-Contamination of Enterobacter Aerogenes from Surfaces to Food. Appl. Environ. Microbiol. 2016, 82, 6490–6496. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in Food Industry Equipment and Premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Moerman, F.; Mager, K. Cleaning and Disinfection in Dry Food Processing Facilities. In Handbook of Hygiene Control in the Food Industry: Second Edition; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 521–554. ISBN 9780081001974. [Google Scholar]
- Block, S.S. Disinfection, Sterilization, and Preservation, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Food Safety Preventive Controls Alliance (FSPCA). FSPCA Preventive Controls for Human Food Training Curriculum, 1st ed.; FSPCA: Bedford Park, IL, USA, 2016. [Google Scholar]
- Marriott, N.G.; Schilling, M.W.; Gravani, R.B. Principles of Food Sanitation; Springer: New York, NY, USA, 2005; ISBN 9783319671642. [Google Scholar]
- Jones, F.T.; Richardson, K.E. Salmonella in Commercially Manufactured Feeds. Poult. Sci. 2004, 83, 384–391. [Google Scholar] [CrossRef]
- Carpentier, B.; Chassaing, D. Interactions in Biofilms between Listeria monocytogenes and Resident Microorganisms from Food Industry Premises. Int. J. Food Microbiol. 2004, 97, 111–122. [Google Scholar] [CrossRef]
- Sasahara, K.C.; Zottola, E.A. Biofilm Formation by Listeria monocytogenes Utilizes a Primary Colonizing Microorganism in Flowing Systems. J. Food Prot. 1993, 56, 1022–1028. [Google Scholar] [CrossRef]
- Bremer, P.J.; Monk, I.; Osborne, C.M. Survival of Listeria monocytogenes Attached to Stainless Steel Surfaces in the Presence or Absence of Flavobacterium spp. J. Food Prot. 2001, 64, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.S.; Koohmaraie, M. Cell Surface Charge Characteristics and Their Relationship to Bacterial Attachment to Meat Surfaces. Appl. Environ. Microbiol. 1989, 55, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Leriche, V.; Carpentier, B. Limitation of Adhesion and Growth of Listeria monocytogenes on Stainless Steel Surfaces by Staphylococcus sciuri Biofilms. J. Appl. Microbiol. 2000, 88, 594–605. [Google Scholar] [CrossRef]
- Hansen, L.T.; Vogel, B.F. Desiccation of Adhering and Biofilm Listeria monocytogenes on Stainless Steel: Survival and Transfer to Salmon Products. Int. J. Food Microbiol. 2011, 146, 88–93. [Google Scholar] [CrossRef]
- Kastbjerg, V.G.; Gram, L. Model Systems Allowing Quantification of Sensitivity to Disinfectants and Comparison of Disinfectant Susceptibility of Persistent and Presumed Nonpersistent Listeria Monocytogenes. J. Appl. Microbiol. 2009, 106, 1667–1681. [Google Scholar] [CrossRef]
- Vogel, B.F.; Hansen, L.T.; Mordhorst, H.; Gram, L. The Survival of Listeria monocytogenes during Long Term Desiccation Is Facilitated by Sodium Chloride and Organic Material. Int. J. Food Microbiol. 2010, 140, 192–200. [Google Scholar] [CrossRef]
- Posada-Izquierdo, G.D.; Pérez-Rodríguez, F.; Zurera, G. Mathematical Quantification of Microbial Inactivation of Escherichia coli O157: H7 and Salmonella spp. on Stainless Steel Surfaces Soiled with Different Vegetable Juice Substrates. Food Res. Int. 2013, 54, 1688–1698. [Google Scholar] [CrossRef]
- Takahashi, H.; Kuramoto, S.; Miya, S.; Kimura, B. Desiccation Survival of Listeria monocytogenes and Other Potential Foodborne Pathogens on Stainless Steel Surfaces Is Affected by Different Food Soils. Food Control 2011, 22, 633–637. [Google Scholar] [CrossRef]
- Lim, S.M.; Lim, E.S.; Kim, J.S.; Paik, H.D.; Koo, O.K. Survival of Foodborne Pathogens on Stainless Steel Soiled with Different Food Residues. Food Sci. Biotechnol. 2020, 29, 729–737. [Google Scholar] [CrossRef]
- Kuda, T.; Shibata, G.; Takahashi, H.; Kimura, B. Effect of Quantity of Food Residues on Resistance to Desiccation of Food-Related Pathogens Adhered to a Stainless Steel Surface. Food Microbiol. 2015, 46, 234–238. [Google Scholar] [CrossRef]
- Takahashi, H.; Ohuchi, A.; Miya, S.; Izawa, Y.; Kimura, B. Effect of Food Residues on Norovirus Survival on Stainless Steel Surfaces. PLoS ONE 2011, 6, e21951. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bang, J.; Beuchat, L.R.; Ryu, J.H. Fate of Enterobacter sakazakii Attached to or in Biofilms on Stainless Steel upon Exposure to Various Temperatures or Relative Humidities. J. Food Prot. 2008, 71, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, R.; Matsumoto, M.; Sakae, K.; Miyazaki, Y. Ability of Shiga Toxin-Producing Escherichia coli and Salmonella spp. To Survive in a Desiccation Model System and in Dry Foods. Appl. Environ. Microbiol. 2005, 71, 6657–6663. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Kuda, T.; Yano, T.; Kuda, M.T. Resistances to Benzalkonium Chloride of Bacteria Dried with Food Elements on Stainless Steel Surface. LWT—Food Sci. Technol. 2008, 41, 988–993. [Google Scholar] [CrossRef]
- Kuda, T.; Koyanagi, T.; Shibata, G.; Takahashi, H.; Kimura, B. Effect of Carrot Residue on the Desiccation and Disinfectant Resistances of Food Related Pathogens Adhered to a Stainless Steel Surfaces. LWT—Food Sci. Technol. 2016, 74, 251–254. [Google Scholar] [CrossRef]
- Kuda, T.; Iwase, T.; Yuphakhun, C.; Takahashi, H.; Koyanagi, T.; Kimura, B. Surfactant-Disinfectant Resistance of Salmonella and Staphylococcus Adhered and Dried on Surfaces with Egg Compounds. Food Microbiol. 2011, 28, 920–925. [Google Scholar] [CrossRef]
- Li, R.; Kuda, T.; Yano, T. Effect of Food Residues on Efficiency of Surfactant Disinfectants against Food Related Pathogens Adhered on Polystyrene and Ceramic Surfaces. LWT—Food Sci. Technol. 2014, 57, 200–206. [Google Scholar] [CrossRef]
- Medilanski, E.; Kaufmann, K.; Wick, L.Y.; Wanner, O.; Harms, H. Influence of the Surface Topography of Stainless Steel on Bacterial Adhesion. Biofouling 2002, 18, 193–203. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Chia, T.W.R.; Turner, M.S.; Fegan, N.; Dykes, G.A. Quantification of Acid-Base Interactions Based on Contact Angle Measurement Allows XDLVO Predictions to Attachment of Campylobacter jejuni but Not Salmonella. J. Microbiol. Methods 2011, 86, 89–96. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, C.; Liu, Y.; Wang, S. Bacterial Adhesion on the Metal-Polymer Composite Coatings. Int. J. Adhes. Adhes. 2007, 27, 85–91. [Google Scholar] [CrossRef]
- Meylheuc, T.; Methivier, C.; Renault, M.; Herry, J.M.; Pradier, C.M.; Bellon-Fontaine, M.N. Adsorption on Stainless Steel Surfaces of Biosurfactants Produced by Gram-Negative and Gram-Positive Bacteria: Consequence on the Bioadhesive Behavior of Listeria monocytogenes. Colloids Surf. B Biointerfaces 2006, 52, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Simões, L.C.; Simões, M.; Oliveira, R.; Vieira, M.J. Potential of the Adhesion of Bacteria Isolated from Drinking Water to Materials. J. Basic. Microbiol. 2007, 47, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Logan, B.E. Bacterial Adhesion to Glass and Metal-Oxide Surfaces. Colloids Surf. B Biointerfaces 2004, 36, 81–90. [Google Scholar] [CrossRef]
- Bayoudh, S.; Othmane, A.; Bettaieb, F.; Bakhrouf, A.; Ben Ouada, H.; Ponsonnet, L. Quantification of the Adhesion Free Energy between Bacteria and Hydrophobic and Hydrophilic Substrata. Mater. Sci. Eng. C 2006, 26, 300–305. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Valero, A.; Carrasco, E.; García, R.M.; Zurera, G. Understanding and Modelling Bacterial Transfer to Foods: A Review. Trends Food Sci. Technol. 2008, 19, 131–144. [Google Scholar] [CrossRef]
- Kusumaningrum, H.D.; Riboldi, G.; Hazeleger, W.C.; Beumer, R.R. Survival of Foodborne Pathogens on Stainless Steel Surfaces and Cross-Contamination to Foods. Int. J. Food Microbiol. 2003, 85, 227–236. [Google Scholar] [CrossRef]
- Chen, L.; Snyder, A.B. Surface Inoculation Method Impacts Microbial Reduction and Transfer of Salmonella Enteritidis PT 30 and Potential Surrogates during Dry Sanitation. Int. J. Food Microbiol. 2023, 406, 110405. [Google Scholar] [CrossRef]
- Larsen, M.H.; Dalmasso, M.; Ingmer, H.; Langsrud, S.; Malakauskas, M.; Mader, A.; Møretrø, T.; Smole Možina, S.; Rychli, K.; Wagner, M.; et al. Persistence of Foodborne Pathogens and Their Control in Primary and Secondary Food Production Chains. Food Control 2014, 44, 92–109. [Google Scholar] [CrossRef]
- Jourdan, N.; Le Hello, S.; Delmas, G.; Clouzeau, J.; Manteau, C.; Désaubliaux, B.; Chagnon, V.; Thierry-Bled, F.; Demare, N.; Weill, F.X.; et al. Nationwide Outbreak of Salmonella enterica Serotype Give Infection in Infants in France, Linked to Infant Milk Formula, September 2008. Eurosurveillance 2008, 13, 38–39. [Google Scholar] [CrossRef]
- Jones, G.; de la Gandara, M.P.; Herrera-Leon, L.; Herrera-Leon, S.; Martinez, C.V.; Hureaux-Roy, R.; Abdallah, Y.; Nisavanh, A.; Fabre, L.; Renaudat, C.; et al. Outbreak of Salmonella enterica Serotype Poona in Infants Linked to Persistent Salmonella Contamination in an Infant Formula Manufacturing Facility, France, August 2018 to February 2019. Eurosurveillance 2019, 24, 1900161. [Google Scholar] [CrossRef]
- Wulff, G.; Gram, L.; Ahrens, P.; Vogel, B.F. One Group of Genetically Similar Listeria monocytogenes Strains Frequently Dominates and Persists in Several Fish Slaughter- and Smokehouses. Appl. Environ. Microbiol. 2006, 72, 4313–4322. [Google Scholar] [CrossRef] [PubMed]
- Chaitiemwong, N.; Hazeleger, W.C.; Beumer, R.R. Survival of Listeria monocytogenes on a Conveyor Belt Material with or without Antimicrobial Additives. Int. J. Food Microbiol. 2010, 142, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Lambert, P.A.; Stedman, Y.; Hilton, A.C. Combined Effect of Temperature and Relative Humidity on the Survival of Salmonella Isolates on Stainless Steel Coupons. Int. J. Environ. Res. Public Health 2022, 19, 909. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, N.; Pinto, R.; Sela Saldinger, S. Persistence of Salmonella enterica during Dehydration and Subsequent Cold Storage. Food Microbiol. 2012, 32, 415–422. [Google Scholar] [CrossRef]
- Navarro Llorens, J.M.; Tormo, A.; Martínez-García, E. Stationary Phase in Gram-Negative Bacteria. FEMS Microbiol. Rev. 2010, 34, 476–495. [Google Scholar] [CrossRef]
- Angela, M.; Gibson, N.; Bratchell, T.A.R. Predicting Microbial Growth: Growth Responses of Salmonellae in a Laboratory Medium as Affected by PH, Sodium Chloride and Storage Temperature. Int. J. Food Microbiol. 1988, 6, 155–178. [Google Scholar] [CrossRef]
- Lunden, J.M.; Miettinen, M.K.; Autio, T.J.; Korkeala, H.J. Persistent Listeria monocytogenes Strains Show Enhanced Adherence to Food Contact Surface after Short Contact Times. J. Food Prot. 2000, 63, 1204–1207. [Google Scholar] [CrossRef]
- Martínez-Suárez, J.V.; Ortiz, S.; López-Alonso, V. Potential Impact of the Resistance to Quaternary Ammonium Disinfectants on the Persistence of Listeria monocytogenes in Food Processing Environments. Front. Microbiol. 2016, 7, 638. [Google Scholar] [CrossRef]
- Giaouris, E.D.; Nychas, G.J.E. The Adherence of Salmonella Enteritidis PT4 to Stainless Steel: The Importance of the Air-Liquid Interface and Nutrient Availability. Food Microbiol. 2006, 23, 747–752. [Google Scholar] [CrossRef]
- Kuda, T.; Iwase, T.; Chaturongkasumrit, Y.; Takahashi, H.; Koyanagi, T.; Kimura, B. Resistances to UV-C Irradiation of Salmonella typhimurium and Staphylococcus aureus in Wet and Dried Suspensions on Surface with Egg Residues. Food Control 2012, 23, 485–490. [Google Scholar] [CrossRef]
- Park, H.W.; Balasubramaniam, V.M.; Snyder, A.B.; Sekhar, J.A. Influence of Superheated Steam Temperature and Moisture Exchange on the Inactivation of Geobacillus Stearothermophilus Spores in Wheat Flour-Coated Surfaces. Food Bioprocess Technol. 2022, 15, 1550–1562. [Google Scholar] [CrossRef]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and Sucrose Protect Both Membranes and Proteins in Intact Bacteria during Drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Hingston, P.A.; Stea, E.C.; Knøchel, S.; Hansen, T. Role of Initial Contamination Levels, Biofilm Maturity and Presence of Salt and Fat on Desiccation Survival of Listeria monocytogenes on Stainless Steel Surfaces. Food Microbiol. 2013, 36, 46–56. [Google Scholar] [CrossRef]
- Abban, S.; Jakobsen, M.; Jespersen, L. Attachment Behaviour of Escherichia coli K12 and Salmonella typhimurium P6 on Food Contact Surfaces for Food Transportation. Food Microbiol. 2012, 31, 139–147. [Google Scholar] [CrossRef]
- Djebbi-Simmons, D.; Xu, W.; Janes, M.; King, J. Survival and Inactivation of Salmonella enterica Serovar Typhimurium on Food Contact Surfaces during Log, Stationary and Long-Term Stationary Phases. Food Microbiol. 2019, 84, 103272. [Google Scholar] [CrossRef]
- Boulangé-Petermann, L.; Rault, J.; Bellon-Fontaine, M.N. Adhesion of Streptococcus thermophilus to Stainless Steel with Different Surface Topography and Roughness. Biofouling 1997, 11, 201–216. [Google Scholar] [CrossRef]
- Characklis, W.G.; Engineering, C.; State, M. Bioengineering Report: Fouling Biofilm Development: A Process Analysis. Biotechnol. Bioeng. 1981, 23, 1923–1960. [Google Scholar] [CrossRef]
- Bower, C.K.; McGuire, J.; Daeschel, M.A. The Adhesion and Detachment of Bacteria and Spores on Food-Contact Surfaces. Trends Food Sci. Technol. 1996, 7, 152–157. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, D.H. Influence of Surface Properties of Produce and Food Contact Surfaces on the Efficacy of Chlorine Dioxide Gas for the Inactivation of Foodborne Pathogens. Food Control 2017, 81, 88–95. [Google Scholar] [CrossRef]
- Kim, D.K.; Kang, D.H. Effect of Surface Characteristics on the Bactericidal Efficacy of UVC LEDs. Food Control 2020, 108, 106869. [Google Scholar] [CrossRef]
- Faille, C.; Membre, J.M.; Tissier, J.P.; Bellon-Fontaine, M.N.; Carpentier, B.; Laroche, M.A.; Benezech, T. Influence of Physicochemical Properties on the Hygienic Status of Stainless Steel with Various Finishes. Biofouling 2000, 15, 261–274. [Google Scholar] [CrossRef]
- Warriner, K. Low Moisture Foods Cause of Numerous Outbreaks—Global Food Safety Resource. Available online: https://globalfoodsafetyresource.com/low-moisture-foods-implicated-numerous-outbreaks-part-1/ (accessed on 5 January 2023).
- Prestes, F.S.; Yotsuyanagi, S.E.; Alonso, V.P.; Nascimento, M.S. Dry Sanitization in the Food Industry: A Review. Curr. Opin. Food Sci. 2024, 57. [Google Scholar] [CrossRef]
- Cordier, J.-L. Methodological and Sampling Challenges to Testing Spices and Low-Water Activity Food for the Presence of Foodborne Pathogens. In The Microbiological Safety of Low Water Activity Foods and Spices; Springer: New York, NY, USA, 2014; pp. 367–386. ISBN 9781493920617. [Google Scholar]
- Harada, A.M.M.; Nascimento, M.S. Efficacy of Dry Sanitizing Methods on Listeria monocytogenes Biofilms. Food Control 2021, 124, 107897. [Google Scholar] [CrossRef]
- Harada, A.M.M.; Nascimento, M.S. Effect of Dry Sanitizing Methods on Bacillus cereus Biofilm. Braz. J. Microbiol. 2021, 52, 919–926. [Google Scholar] [CrossRef]
- Lin, L.C.; Beuchat, L.R. Survival of Enterobacter Sakazakii in Infant Cereal as Affected by Composition, Water Activity, and Temperature. Food Microbiol. 2007, 24, 767–777. [Google Scholar] [CrossRef]
- Stanga, M. Sanitation: Cleaning and Disinfection in the Food Industry; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 9780470074756. [Google Scholar]
- Kane, D.M.; Getty, K.J.K.; Mayer, B.; Mazzotta, A. Sanitizing in Dry-Processing Environments Using Isopropyl Alcohol Quaternary Ammonium Formula. J. Food Prot. 2016, 79, 112–116. [Google Scholar] [CrossRef]
- Grasso, E.M.; Grove, S.F.; Halik, L.A.; Arritt, F.; Keller, S.E. Cleaning and Sanitation of Salmonella-Contaminated Peanut Butter Processing Equipment. Food Microbiol. 2015, 46, 100–106. [Google Scholar] [CrossRef]
- Matatiele, P.; Southon, B.; Dabula, B.; Marageni, T.; Poongavanum, P.; Kgarebe, B. Assessment of Quality of Alcohol-Based Hand Sanitizers Used in Johannesburg Area during the COVID-19 Pandemic. Sci. Rep. 2022, 12, 4231. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (Qacs) and Ionic Liquids (Ils) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Pereira, B.M.P.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar]
- Kane, D.M. Evaluation of a Sanitizing System Using Isopropyl Alcohol Quaternary Ammonium Formula and Carbon Dioxide for Dry-Processing Environments. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2012. [Google Scholar]
- Du, W.; Danyluk, M.D.; Harris, L.J. Evaluation of Cleaning Treatments for Almond-Contact Surfaces in Hulling and Shelling Facilities. Food Prot. Trends 2007, 27, 678–683. [Google Scholar]
- Zhang, Y.; Meng, D.; Wang, Z.; Guo, H.; Wang, Y. Oxidative Stress Response in Two Representative Bacteria Exposed to Atrazine. FEMS Microbiol. Lett. 2012, 334, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Mohapatra, P.; Pakeeraiah, K.; Bandaru, R.K.; Ahmad, I.; Mal, S.; Dandela, R.; Sahoo, S.K.; Patel, H.; Paidesetty, S.K. In-Vitro Anticancer Evaluation of Newly Designed and Characterized Tri/Tetra-Substituted Imidazole Congeners- Maternal Embryonic Leucine Zipper Kinase Inhibitors: Molecular Docking and MD Simulation Approaches. Int. J. Biol. Macromol. 2023, 249, 126084. [Google Scholar] [CrossRef]
- Yeung, Y.W.S.; Ma, Y.; Liu, S.Y.; Pun, W.H.; Chua, S.L. Prevalence of Alcohol-Tolerant and Antibiotic-Resistant Bacterial Pathogens on Public Hand Sanitizer Dispensers. J. Hosp. Infect. 2022, 127, 26–33. [Google Scholar] [CrossRef]
- Lorcheim, K. Plant Contamination Control Close-up-New Food Safety Requirements and Improved Microbial Sampling Methodologies Demand Better Sanitation Tools and Tactics in Food Facilities. Food Technol. 2012, 66, 46–53. [Google Scholar]
- Ducom, P. Methyl Bromide Alternatives. Pest Manag. Focus. 1999, 5, 19–20. [Google Scholar]
- Msayleb, N.; Kanwar, R.; Van Leeuwen, J.; Robertson, A.; Tylka, G. Soil Disinfection with Ozone (O3) as an Alternative to Methyl Bromide—A Sustainable Practice in Agriculture. Proceedings of the 2013 ASABE Annual International Meeting Volume 4, 2735–2746. [CrossRef]
- Penkett, S.A.; Jones, B.M.R.; Rycroft, M.J.; Simmons, D.A. An Interhemispheric Comparison of the Concentrations of Bromine Compounds in the Atmosphere. Nature 1985, 318, 550–553. [Google Scholar] [CrossRef]
- Griffith, T.; Warren, M. Propylene Oxide, a Registered Fumigant, a Proven Insecticide. In Proceedings of the International Conference of Controlled Atmosphers and Fumigation in Stored Products, Fresno, CA, USA, 29 October–3 November 2000; p. 763. [Google Scholar]
- Danyluk, M.D.; Uesugi, A.R.; Harris, L.J. Survival of Salmonella Enteritidis PT 30 on Inoculated Almonds after Commercial Fumigation with Propylene Oxide. J. Food Prot. 2005, 68, 1613–1622. [Google Scholar] [CrossRef]
- Zettler, J.L.; Hartsell, P.L.; Allred, D.B.; Muhareb, J.S.; Hurley, J.M.; Gill, R.F. Sorption and Insect Toxicity of Propylene Oxide in Dried Fruits and Nuts. In Proceedings of the 8th International Working Conference on Stored Product Protection, York, UK, 22–26 July 2002; CABI Publishing: Wallingford, UK, 2003; pp. 921–924. [Google Scholar]
- Jimenez, L.R.; Hall, W.A.; Rodriquez, M.S.; Cooper, W.J.; Muhareb, J.; Jones, T.; Walse, S.S. Quantifying Residues from Postharvest Propylene Oxide Fumigation of Almonds and Walnuts. J. AOAC Int. 2015, 98, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Meylan, W.; Papa, L.; de Rosa, C.T.; Stara, J.F. Chemical of Current Interest Propylene Oxide: Health and Environmental Effects Profile. Toxicol. Ind. Health 1986, 2, 219–260. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Isikber, A.A.; Finkelman, S.; Rindner, M.; Azrieli, A.; Dias, R. Effectiveness of Short Exposures of Propylene Oxide Alone and in Combination with Low Pressure or Carbon Dioxide against Tribolium Castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2004, 40, 197–205. [Google Scholar] [CrossRef]
- United States Food and Drug Administration (US FDA). Direct Food Substances Affirmed as Generally Recognized as Safe. In Code Federal Regulations Title 21 1977 (Chapter 1), Section 184; National Archives and Records Administration: Washington, DC, USA, 2018; Volume 184. [Google Scholar]
- Chen, L.; Wei, X.; Chaves, B.D.; Jones, D.; Ponder, M.A.; Subbiah, J. Inactivation of Salmonella enterica and Enterococcus faecium NRRL B2354 on Cumin Seeds Using Gaseous Ethylene Oxide. Food Microbiol. 2021, 94, 103656. [Google Scholar] [CrossRef]
- Wei, X.; Chen, L.; Chaves, B.D.; Ponder, M.A.; Subbiah, J. Modeling the Effect of Temperature and Relative Humidity on the Ethylene Oxide Fumigation of Salmonella and Enterococcus faecium in Whole Black Peppercorn. LWT 2021, 140, 110742. [Google Scholar] [CrossRef]
- Dudkiewicz, A.; Dutta, P.; Kołożyn-Krajewska, D. Ethylene Oxide in Foods: Current Approach to the Risk Assessment and Practical Considerations Based on the European Food Business Operator Perspective. Eur. Food Res. Technol. 2022, 248, 1951–1958. [Google Scholar] [CrossRef]
- European Union. No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. Publ. Off. Eur. Union 2008, 353, 1–1355. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Ethylene Dichloride Decision Guidance Document Ethylene Dichloride; FAO: Rome, Italy, 2001. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Manual of for Insect Control. Available online: https://www.fao.org/4/x5042e/x5042e00.htm (accessed on 5 January 2023).
- Food and Agriculture Organisation of the United Nations; United Nations Environment Programme. Decision Guide Document. In Ethylene Oxide; Interim Secretariat for the Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade: Rome, Italy, 2017; Available online: https://www.pic.int/Portals/5/ConventionText/UNEP-FAO-RC-CONVTEXT-2017.English.pdf (accessed on 5 January 2023).
- Park, S.H.; Kang, D.H. Effect of Temperature on Chlorine Dioxide Inactivation of Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes on Spinach, Tomatoes, Stainless Steel, and Glass Surfaces. Int. J. Food Microbiol. 2018, 275, 39–45. [Google Scholar] [CrossRef]
- Morino, H.; Fukuda, T.; Miura, T.; Shibata, T. Effect of Low-Concentration Chlorine Dioxide Gas against Bacteria and Viruses on a Glass Surface in Wet Environments. Lett. Appl. Microbiol. 2011, 53, 628–634. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.; Bai, J. Applications of Gaseous Chlorine Dioxide on Postharvest Handling and Storage of Fruits and Vegetables—A Review. Food Control 2019, 95, 18–26. [Google Scholar] [CrossRef]
- Yeap, J.W.; Kaur, S.; Lou, F.; DiCaprio, E.; Morgan, M.; Linton, R.; Li, J. Inactivation Kinetics and Mechanism of a Human Norovirus Surrogate on Stainless Steel Coupons via Chlorine Dioxide Gas. Appl. Environ. Microbiol. 2016, 82, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Hong, A.; Kim, H.; Beuchat, L.R.; Rhee, M.S.; Kim, Y.; Ryu, J.H. Inactivation of Escherichia coli O157: H7 in Biofilm on Food-Contact Surfaces by Sequential Treatments of Aqueous Chlorine Dioxide and Drying. Int. J. Food Microbiol. 2014, 191, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; Vaid, R.; Xu, Q.; Linton, R.; Morgan, M. Inactivation of Listeria monocytogenes on Ready-to-Eat Food Processing Equipment by Chlorine Dioxide Gas. Food Control 2012, 26, 357–362. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhu, N.; Jia, H.Q.; Wu, J.H.; Yi, Y.; Qi, J.C. Decontamination of Bacillus subtilis Var. Niger. Spores on Selected Surfaces by Chlorine Dioxide Gas. J. Zhejiang Univ. Sci. B 2012, 13, 254–260. [Google Scholar] [CrossRef]
- Thorn, R.M.S.; Robinson, G.M.; Reynolds, D.M. Comparative Antimicrobial Activities of Aerosolized Sodium Hypochlorite, Chlorine Dioxide, and Electrochemically Activated Solutions Evaluated Using a Novel Standardized Assay. Antimicrob. Agents Chemother. 2013, 57, 2216–2225. [Google Scholar] [CrossRef]
- Han, Y.; Applegate, B.; Linton, R.H.; Nelson, P.E. Decontamination of Bacillus thuringiensis Spores on Selected Surfaces by Chlorine Dioxide Gas. J. Environ. Health 2003, 66, 16–20. [Google Scholar]
- Montazeri, N.; Manuel, C.; Moorman, E.; Khatiwada, J.R.; Williams, L.L.; Jaykus, L.A. Virucidal Activity of Fogged Chlorine Dioxide- and Hydrogen Peroxide-Based Disinfectants against Human Norovirus and Its Surrogate, Feline Calicivirus, on Hard-to-Reach Surfaces. Front. Microbiol. 2017, 8, 1031. [Google Scholar] [CrossRef]
- Nam, H.; Seo, H.S.; Bang, J.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Efficacy of Gaseous Chlorine Dioxide in Inactivating Bacillus cereus Spores Attached to and in a Biofilm on Stainless Steel. Int. J. Food Microbiol. 2014, 188, 122–127. [Google Scholar] [CrossRef]
- Taylor, J.B.; Wohlers, D.W.; Amata, R. Toxicological Profile for Chlorine Dioxide and Chlorite; U.S. Department of Health and Human Services: Washington, DC, USA, 2004.
- Yogendra Patel, D.W. Toxicological Review of Chlorine Dioxide and Chlorite. In Support of Summary Information on the Integrated Risk Information System; CAS Nos. 10049-04-4 and 7758-19-2; U.S. Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- Clark, E.H.; East, J.M.; Lee, A.G. The Role of Tryptophan Residues in an Integral Membrane Protein: Diacylglycerol Kinase. Biochemistry 2003, 42, 11065–11073. [Google Scholar] [CrossRef]
- Ge, Y.; Lei, Y.; Lei, X.; Gan, W.; Shu, L.; Yang, X. Exploration of Reaction Rates of Chlorine Dioxide with Tryptophan Residue in Oligopeptides and Proteins. J. Environ. Sci. 2020, 93, 129–136. [Google Scholar] [CrossRef]
- Byun, K.H.; Han, S.H.; Yoon, J.W.; Park, S.H.; Ha, S. Do Efficacy of Chlorine-Based Disinfectants (Sodium Hypochlorite and Chlorine Dioxide) on Salmonella Enteritidis Planktonic Cells, Biofilms on Food Contact Surfaces and Chicken Skin. Food Control 2021, 123, 107838. [Google Scholar] [CrossRef]
- Morino, H.; Fukuda, T.; Miura, T.; Lee, C.; Shibata, T.; Sanekata, T. Inactivation of Feline Calicivirus, a Norovirus Surrogate, by Chlorine Dioxide Gas. Biocontrol Sci. 2009, 14, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kály-Kullai, K.; Wittmann, M.; Noszticzius, Z.; Rosivall, L. Can Chlorine Dioxide Prevent the Spreading of Coronavirus or Other Viral Infections? Medical Hypotheses. Physiol. Int. 2020, 107, 1–11. [Google Scholar] [CrossRef]
- Fu, M.R.; Zhang, X.M.; Jin, T.; Li, B.Q.; Zhang, Z.Q.; Tian, S.P. Inhibitory of Grey Mold on Green Pepper and Winter Jujube by Chlorine Dioxide (ClO2) Fumigation and Its Mechanisms. LWT 2019, 100, 335–340. [Google Scholar] [CrossRef]
- Moore, G.; Griffith, C.; Peters, A. Bactericidal Properties of Ozone and Its Potential Application as a Terminal Disinfectant. J. Food Prot. 2000, 63, 1100–1106. [Google Scholar] [CrossRef]
- Torres-Mata, L.B.; García-Pérez, O.; Rodríguez-Esparragón, F.; Blanco, A.; Villar, J.; Ruiz-Apodaca, F.; Martín-Barrasa, J.L.; González-Martín, J.M.; Serrano-Aguilar, P.; Piñero, J.E.; et al. Ozone Eliminates SARS-CoV-2 from Difficult-to-Clean Office Supplies and Clinical Equipment. Int. J. Environ. Res. Public Health 2022, 19, 8672. [Google Scholar] [CrossRef]
- Oliveira, M.; Tiwari, B.K.; Duffy, G. Emerging Technologies for Aerial Decontamination of Food Storage Environments to Eliminate Microbial Cross-Contamination. Foods 2020, 9, 1779. [Google Scholar] [CrossRef]
- Canut, A.; Pascual, A. Pollution Prevention in Food Industries through Both Cleaning of Closed Equipment with Ozonated Water and Cleaning in Place (CIP) Systems. WIT Trans. Ecol. Environ. 2008, 111, 615–625. [Google Scholar] [CrossRef]
- Sujayasree, O.J.; Chaitanya, A.K.; Bhoite, R.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Mousavi Khaneghah, A. Ozone: An Advanced Oxidation Technology to Enhance Sustainable Food Consumption through Mycotoxin Degradation. Ozone Sci. Eng. 2022, 44, 17–37. [Google Scholar] [CrossRef]
- Cullen, P.J.; Norton, T. Ozone Sanitisation in the Food Industry. In Ozone in Food Processing; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 163–176. [Google Scholar]
- Volkoff, S.J.; Carlson, T.J.; Leik, K.; Smith, J.J.; Graves, D.; Dennis, P.; Aris, T.; Cuthbertson, D.; Holmes, A.; Craig, K.; et al. Demonstrated SARS-CoV-2 Surface Disinfection Using Ozone. Ozone Sci. Eng. 2021, 43, 296–305. [Google Scholar] [CrossRef]
- de Candia, S.; Morea, M.; Baruzzi, F. Eradication of High Viable Loads of Listeria monocytogenes Contaminating Food-Contact Surfaces. Front. Microbiol. 2015, 6, 733. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.; Li, C. Inactivation of Surface Viruses by Gaseous Ozone. J. Environ. Health 2008, 70, 56–62. [Google Scholar] [PubMed]
- Mascarenhas, L.A.B.; Oliveira, F.O.; Da Silva, E.S.; Dos Santos, L.M.C.; Rodrigues, L.D.A.P.; Neves, P.R.F.; Santos, A.Á.B.; Moreira, G.A.F.; Lobato, G.M.; Nascimento, C.; et al. Technological Advances in Ozone and Ozonized Water Spray Disinfection Devices. Appl. Sci. 2021, 11, 3081. [Google Scholar] [CrossRef]
- OSHA. OSHA Occupational Chemical Database—Ozone. Available online: https://www.osha.gov/chemicaldata/9 (accessed on 27 March 2023).
- Jian, J.; Hashemi, H.; Wu, H.; Jasper, A.W.; Glarborg, P. A Reaction Mechanism for Ozone Dissociation and Reaction with Hydrogen at Elevated Temperature. Fuel 2022, 322, 124138. [Google Scholar] [CrossRef]
- Xue, W.; Macleod, J.; Blaxland, J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods 2023, 12, 814. [Google Scholar] [CrossRef]
- Kim, J.G.; Yousef, A.E. Inactivation Kinetics of Foodborne Spoilage and Pathogenic Bacteria by Ozone. J. Food Sci. 2000, 65, 521–528. [Google Scholar] [CrossRef]
- Alwi, N.A.; Ali, A. Reduction of Escherichia coli O157, Listeria monocytogenes and Salmonella enterica Sv. Typhimurium Populations on Fresh-Cut Bell Pepper Using Gaseous Ozone. Food Control 2014, 46, 304–311. [Google Scholar] [CrossRef]
- Gibson, K.E.; Almeida, G.; Jones, S.L.; Wright, K.; Lee, J.A. Inactivation of Bacteria on Fresh Produce by Batch Wash Ozone Sanitation. Food Control 2019, 106, 106747. [Google Scholar] [CrossRef]
- Yesil, M.; Kasler, D.R.; Huang, E.; Yousef, A.E. Efficacy of Gaseous Ozone Application during Vacuum Cooling against Escherichia coli O157:H7 on Spinach Leaves as Influenced by Bacterium Population Size. J. Food Prot. 2017, 80, 1066–1071. [Google Scholar] [CrossRef]
- Bailey, R.; Fielding, L.; Young, A.; Griffith, C. Effect of Ozone and Open Air Factor against Aerosolized Micrococcus luteus. J. Food Prot. 2007, 70, 2769–2773. [Google Scholar] [CrossRef]
- Dubuis, M.E.; Dumont-Leblond, N.; Laliberté, C.; Veillette, M.; Turgeon, N.; Jean, J.; Duchaine, C. Ozone Efficacy for the Control of Airborne Viruses: Bacteriophage and Norovirus Models. PLoS ONE 2020, 15, e0231164. [Google Scholar] [CrossRef] [PubMed]
- O’Donell, C.; Tiwari, B.K.; Cullen, P.J.; Rice, R.G. Status and Trends of Ozone in Food Processing. In Ozone in Food Processing; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kim, S.-H.; Park, S.-H.; Kim, S.-S.; Kang, D.-H. Inactivation of Staphylococcus aureus Biofilms on Food Contact Surfaces by Superheated Steam Treatment. J. Food Prot. 2019, 82, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-S.; Kim, S.-H.; Park, S.-H.; Kang, D.-H. Inactivation of Bacillus cereus Spores on Stainless Steel by Combined Superheated Steam and UV-C Irradiation Treatment. J. Food Prot. 2020, 83, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Ban, G.H.; Kang, D.H. Effectiveness of Superheated Steam for Inactivation of Escherichia coli O157: H7, Salmonella typhimurium, Salmonella Enteritidis Phage Type 30, and Listeria monocytogenes on Almonds and Pistachios. Int. J. Food Microbiol. 2016, 220, 19–25. [Google Scholar] [CrossRef]
- Fang, J.; Liu, C.; Law, C.-L.; Mujumdar, A.S.; Xiao, H.-W.; Zhang, C. Superheated Steam Processing: An Emerging Technology to Improve Food Quality and Safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 8720–8736. [Google Scholar] [CrossRef]
- Park, H.W.; Xu, J.; Balasubramaniam, V.M.; Snyder, A.B. The Effect of Water Activity and Temperature on the Inactivation of Enterococcus faecium in Peanut Butter during Superheated Steam Sanitation Treatment. Food Control 2021, 125, 107942. [Google Scholar] [CrossRef]
- Ban, G.H.; Yoon, H.; Kang, D.H. A Comparison of Saturated Steam and Superheated Steam for Inactivation of Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes Biofilms on Polyvinyl Chloride and Stainless Steel. Food Control 2014, 40, 344–350. [Google Scholar] [CrossRef]
- Maleki, F. Bacterial Heat Shock Protein Activity. J. Clin. Diagn. Res. 2016, 10, BE01–BE03. [Google Scholar] [CrossRef]
- Yura, T.; Nakahigashi, K. Regulation of the Heat-Shock Response. Curr. Opin. Microbiol. 1999, 2, 153–158. [Google Scholar] [CrossRef]
- Jonas, K. To Divide or Not to Divide: Control of the Bacterial Cell Cycle by Environmental Cues. Curr. Opin. Microbiol. 2014, 18, 54–60. [Google Scholar] [CrossRef]
- Ban, G.H.; Kang, D.H.; Yoon, H. Transcriptional Response of Selected Genes of Salmonella enterica Serovar Typhimurium Biofilm Cells during Inactivation by Superheated Steam. Int. J. Food Microbiol. 2015, 192, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Haughton, P.N.; Lyng, J.G.; Cronin, D.A.; Morgan, D.J.; Fanning, S.; Whyte, P. Efficacy of UV Light Treatment for the Microbiological Decontamination of Chicken, Associated Packaging, and Contact Surfaces. J. Food Prot. 2011, 74, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Harrison, M.A. Effectiveness of UV Light as a Means to Reduce Salmonella Contamination on Tomatoes and Food Contact Surfaces. Food Control 2016, 66, 166–173. [Google Scholar] [CrossRef]
- Sommers, C.H.; Sites, J.E.; Musgrove, M. Ultraviolet Light (254 Nm) Inactivation of Pathogens on Foods and Stainless Steel Surfaces. J. Food Saf. 2010, 30, 470–479. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, A.N.; Lee, K.H.; Ha, S. Do Ultraviolet-C Efficacy against a Norovirus Surrogate and Hepatitis A Virus on a Stainless Steel Surface. Int. J. Food Microbiol. 2015, 211, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.; Fernandez, M.; Montoya, B.; Schmidt, M.; Thompson, J. UV-C LED Irradiation Reduces Salmonella on Chicken and Food Contact Surfaces. Foods 2021, 10, 1459. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-Induced DNA Damage and Repair: A Review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and Repair of Oxidatively Generated Damage in Cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, T. Molecular Mechanisms Involved in Adaptive Responses to Radiation, UV Light, and Heat. J. Radiat. Res. 2009, 50, 385–393. [Google Scholar] [CrossRef]
- Yoon, J.H.; Hyun, J.E.; Song, H.; Kim, J.Y.; Kim, J.H.; Lee, S.Y. Food Residuals on the Food-Contacting Surfaces of Stainless Steel and Polypropylene Influence the Efficacy of Ultraviolet Light in Killing Foodborne Pathogens. J. Food Saf. 2018, 38, e12506. [Google Scholar] [CrossRef]
- Gabriel, A.A.; Ballesteros, M.L.P.; Rosario, L.M.D.; Tumlos, R.B.; Ramos, H.J. Elimination of Salmonella enterica on Common Stainless Steel Food Contact Surfaces Using UV-C and Atmospheric Pressure Plasma Jet. Food Control 2018, 86, 90–100. [Google Scholar] [CrossRef]
- Kim, T.; Silva, J.L.; Chen, T.C. Effects of UV Irradiation on Selected Pathogens in Peptone Water and on Stainless Steel and Chicken Meat. J. Food Prot. 2002, 65, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Morey, A.; McKee, S.R.; Dickson, J.S.; Singh, M. Efficacy of Ultraviolet Light Exposure against Survival of Listeria monocytogenes on Conveyor Belts. Foodborne Pathog. Dis. 2010, 7, 737–740. [Google Scholar] [CrossRef]
- Bae, Y.M.; Lee, S.Y. Inhibitory Effects of UV Treatment and a Combination of UV and Dry Heat against Pathogens on Stainless Steel and Polypropylene Surfaces. J. Food Sci. 2012, 77, 61–64. [Google Scholar] [CrossRef]
- Katsigiannis, A.S.; Bayliss, D.L.; Walsh, J.L. Cold Plasma Decontamination of Stainless Steel Food Processing Surfaces Assessed Using an Industrial Disinfection Protocol. Food Control 2021, 121, 107543. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S. Do Assessment of Cold Oxygen Plasma Technology for the Inactivation of Major Foodborne Viruses on Stainless Steel. J. Food Eng. 2018, 223, 42–45. [Google Scholar] [CrossRef]
- Butscher, D.; Zimmermann, D.; Schuppler, M.; Rudolf von Rohr, P. Plasma Inactivation of Bacterial Endospores on Wheat Grains and Polymeric Model Substrates in a Dielectric Barrier Discharge. Food Control 2016, 60, 636–645. [Google Scholar] [CrossRef]
- Katsigiannis, A.S.; Bayliss, D.L.; Walsh, J.L. Cold Plasma for the Disinfection of Industrial Food-Contact Surfaces: An Overview of Current Status and Opportunities. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1086–1124. [Google Scholar] [CrossRef]
- Dasan, B.G.; Onal-Ulusoy, B.; Pawlat, J.; Diatczyk, J.; Sen, Y.; Mutlu, M. A New and Simple Approach for Decontamination of Food Contact Surfaces with Gliding Arc Discharge Atmospheric Non-Thermal Plasma. Food Bioprocess Technol. 2017, 10, 650–661. [Google Scholar] [CrossRef]
- Sen, Y.; Mutlu, M. Sterilization of Food Contacting Surfaces via Non-Thermal Plasma Treatment: A Model Study with Escherichia coli-Contaminated Stainless Steel and Polyethylene Surfaces. Food Bioprocess Technol. 2013, 6, 3295–3304. [Google Scholar] [CrossRef]
- Leipold, F.; Kusano, Y.; Hansen, F.; Jacobsen, T. Decontamination of a Rotating Cutting Tool during Operation by Means of Atmospheric Pressure Plasmas. Food Control 2010, 21, 1194–1198. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-Phase Chemistry and Bactericidal Effects from an Air Discharge Plasma in Contact with Water: Evidence for the Formation of Peroxynitrite through a Pseudo-Second-Order Post-Discharge Reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, C.R.; Hindle, B.J.; Saad, S.; Stratakos, A.C. Inactivation of Listeria monocytogenes and Salmonella on Stainless Steel by a Piezoelectric Cold Atmospheric Plasma Generator. Appl. Sci. 2021, 11, 3567. [Google Scholar] [CrossRef]
- Timmons, C.; Pai, K.; Jacob, J.; Zhang, G.; Ma, L.M. Inactivation of Salmonella enterica, Shiga Toxin-Producing Escherichia coli, and Listeria monocytogenes by a Novel Surface Discharge Cold Plasma Design. Food Control 2018, 84, 455–462. [Google Scholar] [CrossRef]
- Miao, H.; Yun, G. The Sterilization of Escherichia coli by Dielectric-Barrier Discharge Plasma at Atmospheric Pressure. Appl. Surf. Sci. 2011, 257, 7065–7070. [Google Scholar] [CrossRef]
- Lis, K.A.; Kehrenberg, C.; Boulaaba, A.; von Köckritz-Blickwede, M.; Binder, S.; Li, Y.; Zimmermann, J.L.; Pfeifer, Y.; Ahlfeld, B. Inactivation of Multidrug-Resistant Pathogens and Yersinia enterocolitica with Cold Atmospheric-Pressure Plasma on Stainless-Steel Surfaces. Int. J. Antimicrob. Agents 2018, 52, 811–818. [Google Scholar] [CrossRef]
- Laroussi, M.; Mendis, D.A.; Rosenberg, M. Plasma Interaction with Microbes. New J. Phys. 2003, 5, 41. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Nisar, M.; Nayak, G.; Nagaraja, K.V.; Collins, J.; Bruggeman, P.J.; Goyal, S.M. Bactericidal Efficacy of a Two-Dimensional Array of Integrated, Coaxial, Microhollow, Dielectric Barrier Discharge Plasma against Salmonella enterica Serovar Heidelberg. Foodborne Pathog. Dis. 2020, 17, 157–165. [Google Scholar] [CrossRef]
- Naítali, M.; Kamgang-Youbi, G.; Herry, J.M.; Bellon-Fontaine, M.N.; Brisset, J.L. Combined Effects of Long-Living Chemical Species during Microbialinactivation Using Atmospheric Plasma-Treated Water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef]
- Cahill, O.J.; Claro, T.; O’Connor, N.; Cafolla, A.A.; Stevens, N.T.; Daniels, S.; Humphreys, H. Cold Air Plasma to Decontaminate Inanimate Surfaces of the Hospital Environment. Appl. Environ. Microbiol. 2014, 80, 2004–2010. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, M.G.; Jeon, E.B.; Kim, J.S.; Lee, J.S.; Choi, E.H.; Lim, J.S.; Choi, J.S.; Park, S.Y. Antibacterial Effects of Non-Thermal Dielectric Barrier Discharge Plasma against Escherichia coli and Vibrio Parahaemolyticus on the Surface of Wooden Chopping Board. Innov. Food Sci. Emerg. Technol. 2021, 73, 102784. [Google Scholar] [CrossRef]
- Margas, E.; Meneses, N.; Conde-Petit, B.; Dodd, C.E.R.; Holah, J. Survival and Death Kinetics of Salmonella Strains at Low Relative Humidity, Attached to Stainless Steel Surfaces. Int. J. Food Microbiol. 2014, 187, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Skåra, T.; Rosnes, J.T. Emerging Methods and Principles in Food Contact Surface Decontamination/Prevention. In Innovation and Future Trends in Food Manufacturing and Supply Chain Technologies; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 151–172. ISBN 9781782424703. [Google Scholar]
- Al-Qadiri, H.M.; Ovissipour, M.; Al-Alami, N.; Govindan, B.N.; Shiroodi, S.G.; Rasco, B. Efficacy of Neutral Electrolyzed Water, Quaternary Ammonium and Lactic Acid-based Solutions in Controlling Microbial Contamination of Food Cutting Boards Using a Manual Spraying Technique. J. Food Sci. 2016, 81, M1177–M1183. [Google Scholar] [CrossRef] [PubMed]
- Loyawattananan, S. Characterization and Application of Alternative Anitmicrobial Surfactants on Food Contact Surface. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 2020. [Google Scholar]
- Bragg, R.; Jansen, A.; Coetzee, M.; van der Westhuizen, W.; Boucher, C. Bacterial Resistance to Quaternary Ammonium Compounds (QAC) Disinfectants BT —Infectious Diseases and Nanomedicine II; Adhikari, R., Thapa, S., Eds.; Springer: New Delhi, India, 2014; pp. 1–13. [Google Scholar]
- United States Food and Drug Administration (US FDA). CFR—Code of Federal Regulations Title 21. Available online: https://www.ecfr.gov/current/title-21 (accessed on 7 March 2023).
- Hui, Y.H. Plant Sanitation for Food Processing and Food Service; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429101533. [Google Scholar]
- Marriott, N.G.; Robertson, G. Essentials of Food Sanitation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997; ISBN 9780412080111. [Google Scholar]
- Mustpha, A.; Liewen, M.B. Destruction of Listeria monocytogenes by Sodium Hypochlorite and Quaternary Ammonium Sanitizers. J. Food Prot. 1989, 52, 306–311. [Google Scholar] [CrossRef]
- Ríos-Castillo, A.G.; Umaña, F.F.; Rodríguez-Jerez, J.J. Long-Term Antibacterial Efficacy of Disinfectants Based on Benzalkonium Chloride and Sodium Hypochlorite Tested on Surfaces against Resistant Gram-Positive Bacteria. Food Control 2018, 93, 219–225. [Google Scholar] [CrossRef]
- Kim, H.; Ryu, J.H.; Beuchat, L.R. Effectiveness of Disinfectants in Killing Enterobacter Sakazakii in Suspension, Dried on the Surface of Stainless Steel, and in a Biofilm. Appl. Environ. Microbiol. 2007, 73, 1256–1265. [Google Scholar] [CrossRef]
- Crismaru, M.; Asri, L.A.T.W.; Loontjens, T.J.A.; Krom, B.P.; De Vries, J.; Van Der Mei, H.C.; Busscher, H.J. Survival of Adhering Staphylococci during Exposure to a Quaternary Ammonium Compound Evaluated by Using Atomic Force Microscopy Imaging. Antimicrob. Agents Chemother. 2011, 55, 5010–5017. [Google Scholar] [CrossRef]
- André, S.; Hédin, S.; Remize, F.; Zuber, F. Evaluation of Peracetic Acid Sanitizers Efficiency against Spores Isolated from Spoiled Cans in Suspension and on Stainless Steel Surfaces. J. Food Prot. 2012, 75, 371–375. [Google Scholar] [CrossRef]
- Choi, E.S.; Han, S.; Son, J.W.; Song, G.B.; Ha, S.-D. Inactivation Methods for Human Coronavirus 229E on Various Food-Contact Surfaces and Foods. Food Control 2022, 142, 109271. [Google Scholar] [CrossRef]
- Kreske, A.C.; Ryu, J.H.; Beuchat, L.R. Evaluation of Chlorine, Chlorine Dioxide, and a Peroxyacetic Acid-Based Sanitizer for Effectiveness in Killing Bacillus cereus and Bacillus thuringiensis Spores in Suspensions, on the Surface of Stainless Steel, and on Apples. J. Food Prot. 2006, 69, 1892–1903. [Google Scholar] [CrossRef]
- Horn, H.; Niemeyer, B. Corrosion Inhibition of Peracetic Acid-Based Disinfectants. Chem. Eng. Technol. 2022, 45, 129–134. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Makarova, K.; Golovina, E.A.; Van As, H.; Virkutyte, J. Free Radical Reaction Pathway, Thermochemistry of Peracetic Acid Homolysis, and Its Application for Phenol Degradation: Spectroscopic Study and Quantum Chemistry Calculations. Environ. Sci. Technol. 2010, 44, 6815–6821. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzza, N.; Mutti, P.; Cigarini, M.; Berni, E. Effect of Peracetic Acid on Ascospore-Forming Molds and Test Microorganisms Used for Bio-Validations of Sanitizing Processes in Food Plants. Int. J. Food Microbiol. 2020, 332, 108772. [Google Scholar] [CrossRef]
- Magulski, T.; Paulmann, D.; Bischoff, B.; Becker, B.; Steinmann, E.; Steinmann, J.; Goroncy-Bermes, P.; Steinman, J. Inactivation of Murine Norovirus by Chemical Biocides on Stainless Steel. BMC Infect. Dis. 2009, 9, 107. [Google Scholar] [CrossRef]
- Moon, Y.; Han, S.; Son, J.W.; Park, S.H.; Ha, S.-D. Impact of Ultraviolet-C and Peroxyacetic Acid against Murine Norovirus on Stainless Steel and Lettuce. Food Control 2021, 130, 108378. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Kaiser, K.; Jahn, R. Review: Removal of Organic Matter from Soils Using Hydrogen Peroxide, Sodium Hypochlorite and Disodium Peroxodisulfate Reexamined. Soil Sci. Soc. Am. J. 2005, 69, 120–135. [Google Scholar] [CrossRef]
- Song, M.; Hossain, M.I.; Jung, S.; Yeo, D.; Wang, Z.; Min, A.; Zhao, Z.; Park, S.; Choi, C. Comparison of Virucidal Efficacy of Sodium Hypochlorite, Chlorine Dioxide, Peracetic Acid, and Ethanol against Hepatitis A Virus by Carrier and Suspension Tests. Int. J. Food Microbiol. 2022, 363, 109506. [Google Scholar] [CrossRef]
- Møretrø, T.; Fanebust, H.; Fagerlund, A.; Langsrud, S. Whole Room Disinfection with Hydrogen Peroxide Mist to Control Listeria monocytogenes in Food Industry Related Environments. Int. J. Food Microbiol. 2019, 292, 118–125. [Google Scholar] [CrossRef]
- Goyal, S.M.; Chander, Y.; Yezli, S.; Otter, J.A. Evaluating the Virucidal Efficacy of Hydrogen Peroxide Vapour. J. Hosp. Infect. 2014, 86, 255–259. [Google Scholar] [CrossRef]
- Johnston, M.D.; Lawson, S.; Otter, J.A. Evaluation of Hydrogen Peroxide Vapour as a Method for the Decontamination of Surfaces Contaminated with Clostridium Botulinum Spores. J. Microbiol. Methods 2005, 60, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Heir, E.; Nesse, L.L.; Vestby, L.K.; Langsrud, S. Control of Salmonella in Food Related Environments by Chemical Disinfection. Food Res. Int. 2012, 45, 532–544. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Use of Hydrogen Peroxide as a Biocide: New Consideration of Its Mechanisms of Biocidal Action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Kure, C.F.; Langsrud, S.; Møretrø, T. Efficient Reduction of Food Related Mould Spores on Surfaces by Hydrogen Peroxide Mist. Foods 2021, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Gulati, B.R.; Allwood, P.B.; Hedberg, C.W.; Goyal, S.M. Efficacy of Commonly Used Disinfectants for the Inactivation of Calicivirus on Strawberry, Lettuce, and a Food-Contact Surface. J. Food Prot. 2001, 64, 1430–1434. [Google Scholar] [CrossRef]
- Doll, M.; Morgan, D.J.; Anderson, D.; Bearman, G. Touchless Technologies for Decontamination in the Hospital: A Review of Hydrogen Peroxide and UV Devices. Curr. Infect. Dis. Rep. 2015, 17, 44. [Google Scholar] [CrossRef]
- Choi, N.Y.; Baek, S.Y.; Yoon, J.H.; Choi, M.R.; Kang, D.H.; Lee, S.Y. Efficacy of Aerosolized Hydrogen Peroxide-Based Sanitizer on the Reduction of Pathogenic Bacteria on a Stainless Steel Surface. Food Control 2012, 27, 57–63. [Google Scholar] [CrossRef]
- Neighbor, N.K.; Newberry, L.A.; Bayyari, G.R.; Skeeles, J.K.; Beasley, J.N.; McNew, R.W. The Effect of Microaerosolized Hydrogen Peroxide on Bacterial and Viral Poultry Pathogens. Poult. Sci. 1994, 73, 1511–1516. [Google Scholar] [CrossRef]
- Dhaliwal, H.K.; Sonkar, S.; Gänzle, M.; Roopesh, M.S. Efficacy of Oxidative Disinfectants, Quaternary Ammonium Compounds and Dry Heat on the Inactivation of Salmonella Enteritidis in Different Cellular States. Food Microbiol. 2025, 128, 104713. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of Actions of Sodium Hypochlorite in Cleaning and Disinfection Processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Dye, M.; Mead, G.C. The Effect of Chlorine on the Viability of Clostridial Spores. Int. J. Food Sci. Technol. 1972, 7, 173–181. [Google Scholar] [CrossRef]
- Bridges, D.F.; Lacombe, A.; Wu, V.C.H. Fundamental Differences in Inactivation Mechanisms of Escherichia coli O157:H7 Between Chlorine Dioxide and Sodium Hypochloritei. Front. Microbiol. 2022, 13, 923964. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, Z.G.; Dinc, O.; Cinar, B.; Gedik, S.T.; Dimoglo, A. Comparative Evaluation of Disinfection Mechanism of Sodium Hypochlorite, Chlorine Dioxide and Electroactivated Water on Enterococcus Faecalis. LWT 2019, 102, 205–213. [Google Scholar] [CrossRef]
- Sapers, G.M. Disinfection of Contaminated Produce with Conventional Washing and Sanitizing Technology; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780124046115. [Google Scholar]
- United States Food and Drug Administration (US FDA). US Public Health Service FDA Food Code; FDA: Washington, DC, USA, 1999.
- Friedrich, L.M.; Goodrich-Schneider, R.; Parish, M.E.; Danyluk, M.D. Mitigation of Alicyclobacillus spp. Spores on Food Contact Surfaces with Aqueous Chlorine Dioxide and Hypochlorite. Food Microbiol. 2009, 26, 936–941. [Google Scholar] [CrossRef]
- Deza, M.A.; Araujo, M.; Garrido, M.J. Efficacy of Neutral Electrolyzed Water to Inactivate Escherichia coli, Listeria Monocytogenes, Pseudomonas Aeruginosa, and Staphylococcus Aureus on Plastic and Wooden Kitchen Cutting Boards. J. Food Prot. 2007, 70, 102–108. [Google Scholar] [CrossRef]
- Maryline, G.; Solange, N.; Kirsten, M.; Julie, J. Attachment of Noroviruses to Stainless Steel and Their Inactivation, Using Household Disinfectants. J. Food Prot. 2010, 73, 400–404. [Google Scholar] [CrossRef]
- Kim, S.W.; Baek, S.B.; Ha, J.H.; Lee, M.H.; Choi, C.; Ha, S. Do Chlorine Treatment to Inactivate Norovirus on Food Contact Surfaces. J. Food Prot. 2012, 75, 184–188. [Google Scholar] [CrossRef]
- Whitehead, K.; McCue, K.A. Virucidal Efficacy of Disinfectant Actives against Feline Calicivirus, a Surrogate for Norovirus, in a Short Contact Time. Am. J. Infect. Control 2010, 38, 26–30. [Google Scholar] [CrossRef]
- Chiu, S.; Skura, B.; Petric, M.; McIntyre, L.; Gamage, B.; Isaac-Renton, J. Efficacy of Common Disinfectant/Cleaning Agents in Inactivating Murine Norovirus and Feline Calicivirus as Surrogate Viruses for Human Norovirus. Am. J. Infect. Control 2015, 43, 1208–1212. [Google Scholar] [CrossRef]
- Meyer, B. Does Microbial Resistance to Biocides Create a Hazard to Food Hygiene? Int. J. Food Microbiol. 2006, 112, 275–279. [Google Scholar] [CrossRef]
- Yan, P.; Daliri, E.B.M.; Oh, D.H. New Clinical Applications of Electrolyzed Water: A Review. Microorganisms 2021, 9, 136. [Google Scholar] [CrossRef]
- Phuvasate, S.; Su, Y.C. Effects of Electrolyzed Oxidizing Water and Ice Treatments on Reducing Histamine-Producing Bacteria on Fish Skin and Food Contact Surface. Food Control 2010, 21, 286–291. [Google Scholar] [CrossRef]
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The Generation and Inactivation Mechanism of Oxidation-Reduction Potential of Electrolyzed Oxidizing Water. J. Food Eng. 2007, 78, 1326–1332. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, I.; Oh, D.H. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Possas, A.; Pérez-Rodríguez, F.; Tarlak, F.; García-Gimeno, R.M. Quantifying and Modelling the Inactivation of Listeria monocytogenes by Electrolyzed Water on Food Contact Surfaces. J. Food Eng. 2021, 290, 110287. [Google Scholar] [CrossRef]
- Adal, S.; Delikanlı Kıyak, B.; Çalışkan Koç, G.; Süfer, Ö.; Özkan Karabacak, A.; İnan Çınkır, N.; Çelebi, Y.; Jeevarathinam, G.; Rustagi, S.; Pandiselvam, R. Applications of Electrolyzed Water in the Food Industry: A Comprehensive Review of Its Effects on Food Texture. Future Foods 2024, 9, 100369. [Google Scholar] [CrossRef]
- Han, Q.; Song, X.; Zhang, Z.; Fu, J.; Wang, X.; Malakar, P.K.; Liu, H.; Pan, Y.; Zhao, Y. Removal of Foodborne Pathogen Biofilms by Acidic Electrolyzed Water. Front. Microbiol. 2017, 8, 988. [Google Scholar] [CrossRef]
- Ni, L.; Zheng, W.; Zhang, Q.; Cao, W.; Li, B. Application of Slightly Acidic Electrolyzed Water for Decontamination of Stainless Steel Surfaces in Animal Transport Vehicles. Prev. Vet. Med. 2016, 133, 42–51. [Google Scholar] [CrossRef]
- Jee, D.Y.; Ha, J.W. Synergistic Interaction of Tap Water-Based Neutral Electrolyzed Water Combined with UVA Irradiation to Enhance Microbial Inactivation on Stainless Steel. Food Res. Int. 2021, 150, 110773. [Google Scholar] [CrossRef]
- Deza, M.A.; Araujo, M.; Garrido, M.J. Inactivation of Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa and Staphylococcus aureus on Stainless Steel and Glass Surfaces by Neutral Electrolysed Water. Lett. Appl. Microbiol. 2005, 40, 341–346. [Google Scholar] [CrossRef]
- Smet, C.; Govaert, M.; Kyrylenko, A.; Easdani, M.; Walsh, J.L.; Van Impe, J.F. Inactivation of Single Strains of Listeria monocytogenes and Salmonella typhimurium Planktonic Cells Biofilms with Plasma Activated Liquids. Front. Microbiol. 2019, 10, 1539. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Appl. Environ. Microbiol. 2018, 84, e00726-18. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Idris Muhammad, A.; Hu, Y.; Koseki, S.; Liao, X.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Inactivation Kinetics of Bacillus cereus Spores by Plasma-Activated Water (PAW). Food Res. Int. 2020, 131, 109041. [Google Scholar] [CrossRef] [PubMed]
- Kamgang-Youbi, G.; Herry, J.; Meylheuc, T.; Laminsi, S.; Naïtali, M. Microbial Decontamination of Stainless Steel and Polyethylene Surfaces Using GlidArc Plasma Activated Water without Chemical Additives. J. Chem. Technol. Biotechnol. 2018, 93, 2544–2551. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of Plasma-Activated Water with Biofilms: Inactivation, Dispersal Effects and Mechanisms of Action. NPJ Biofilm. Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential Alternative Disinfection Methods for Organic Fresh-Cut Industry for Minimizing Water Consumption and Environmental Impact. LWT—Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Patange, A.; Sun, D.W.; Tiwari, B. Plasma-Activated Water: Physicochemical Properties, Microbial Inactivation Mechanisms, Factors Influencing Antimicrobial Effectiveness, and Applications in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3951–3979. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.K.; Bazaka, K. Plasma-Activated Water: Generation, Origin of Reactive Species and Biological Applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Z.; Cheng, C.; Wei, J.; Lan, Y.; Ni, G.; Sun, Q.; Qian, S.; Zhang, H.; Xia, W.; et al. Bactericidal Effects of Plasma Induced Reactive Species in Dielectric Barrier Gas–Liquid Discharge. Plasma Chem. Plasma Process. 2017, 37, 415–431. [Google Scholar] [CrossRef]
- Herianto, S.; Hou, C.Y.; Lin, C.M.; Chen, H.L. Nonthermal Plasma-Activated Water: A Comprehensive Review of This New Tool for Enhanced Food Safety and Quality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 583–626. [Google Scholar] [CrossRef]
- Baek, K.H.; Kim, H.J.; Kang, T.; Lee, Y.E.; Kim, D.K.; Kang, D.H.; Jo, C. Blue Light Promotes Bactericidal Action of Plasma-Activated Water against Staphylococcus aureus on Stainless Steel Surfaces. Innov. Food Sci. Emerg. Technol. 2021, 69, 102663. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Ultraviolet Radiation for the Processing and Treatment of Food; FDA: Washington, DC, USA, 2000.
- Rana, Y.S.; Chen, L.; Balasubramaniam, V.M.; Snyder, A.B. Superheated Steam Effectively Inactivates Diverse Microbial Targets despite Mediating Effects from Food Matrices in Bench-Scale Assessments. Int. J. Food Microbiol. 2022, 378, 109838. [Google Scholar] [CrossRef] [PubMed]


| Pathogens | Year | Remarks | References |
|---|---|---|---|
| Salmonella Newbrunswick | 1965–1966 | Inadequate hygiene standards in the spray dryer resulted in the isolation of Salmonella from the air filter | [16] |
| Salmonella Eastbourne | 1975 | Dust-induced airborne contamination of chocolate | [17] |
| Salmonella Agona | 1998 and in 2008 | Long-term persistence of Salmonella in the dry environments of the cereal manufacturing plant | [18] |
| Salmonella Wandsworth and Salmonella Typhimurium | 2007 | The initial examination revealed that the puffed rice snack was contaminated with Salmonella. The recall was expanded to include other items containing the same components or processed with the same equipment | [19] |
| Salmonella Schwarzengrund | 2007 | Two prominent brands of dry dog food related to Salmonella contamination manufactured in the same facility | [20] |
| Salmonella Tennessee | 2007 | Outbreak related to peanut butter of 2 different brands (Peter Pan and Great Value brand) manufactured in the same facility | [21] |
| Salmonella serotypes Montevideo, Newport, and Senftenberg | 2009 | Contamination of pistachio nuts and pistachio-nut-containing products produced in the same facility | [22] |
| Salmonella | 2011 | Presence of Salmonella in the air, broom, floor, and processing equipment of the feed mills | [23] |
| E. coli O157:H7 | 2011 | E. coli contamination of hazelnuts and hazelnut-containing products, sourced from the same distributor | [24] |
| Salmonella Bredeney | 2012 | Outbreak related to peanut butter of 2 different brands manufactured in the same facility | [25] |
| Salmonella Montevideo and Salmonella Mbandaka | 2013 | Salmonella infection of tahini sesame paste. To avoid the potential risk of Salmonella, subsequent batches produced on the same production line were also recalled | [26] |
| Salmonella Braenderup | 2014 | Contamination of almond and peanut butter manufactured in the same plant | [27] |
| Salmonella Montevideo and Salmonella Senftenberg | 2016 | Pistachios contaminated by the same farms’ production | [28] |
| Salmonella Paratyphi B | 2016 | Nut butter, sprouted nut butter, and all other items made on the manufacturing line were recalled owing to potential contamination | [29] |
| E. coli | 2016 | Recalled various varieties of flour manufactured at the same plant | [30] |
| Salmonella Typhimurium | 2018 | Multiple products of dried coconut contamination | [7] |
| Salmonella Newport | 2018 | The outbreak was linked to two distinct brands of dry shredded coconut manufactured in the same plant | [31] |
| Pathogens | Contact Surface | Drying Conditions | Food Sediment | Log Reduction | Reference |
|---|---|---|---|---|---|
| L. monocytogenes N53-1 | Stainless steel | Storage at 15 °C at 43% RH for 91 days | Smoked salmon juice with 5% salt | ~4.5 | [49] |
| S. enterica | Stainless steel | Storage at 6.5 °C at 60–70% RH for 168 h | Chard (66.8 g/100 mL) | 6.26 | [50] |
| Romaine lettuce (66.8 g/100 mL) | 7.68 | ||||
| L. monocytogenes | Stainless steel | Air-dried, storage for 30 days at 25 °C | Minced tuna (100 g/100 mL) | ~5 | [51] |
| No food residue | >7 | ||||
| L. monocytogenes | Stainless steel | Biosafety drying for 120 h | Soy milk (50%) | 0.48 | [52] |
| No food residue | 3.08 | ||||
| S. Enteritidis | Stainless steel | Biosafety drying for 120 h | Soy milk (50%) | 1.83 | |
| No food residue | 4.4 | ||||
| S. aureus | Stainless steel | Biosafety drying for 120 min | Carrot juice (50%) | <1 | [53] |
| Distilled water (no residue) | ~2 | ||||
| Murine norovirus-1 (MNV-1) | Stainless steel | Storage for 30 days | Cabbage (100 g/100 mL) | 1.4 | [54] |
| No food residues | 6.2 | ||||
| Enterobacter sakazakii | Stainless steel | Biosafety drying for 2 h, and storage at 43% RH at 4 °C for 60 days | Infant formula | 1.07–1.21 | [55] |
| No food residues | 1.73–2.02 | ||||
| L. monocytogenes | Stainless steel | Drying at 43% RH at 15 °C for 23 days | 0.5% NaCl | 2.46 | [47] |
| 5% NaCl | 0.88 | ||||
| Salmonella spp. | Paper discs | Drying for 25 h at 35 °C | No food residues | 2.43–3.51 | [56] |
| Storage of the dried cells at 4 °C for 22–24 months | <1 | ||||
| S. Typhimurium “DS” | Stainless steel | Drying for 80 min at 30 °C, storage at 33% RH at 25 °C for 30 days | No food residues | 4.3 | [57] |
| S. Typhimurium DT104 | 1.3 |
| Pathogens | Contact Surface | Drying Conditions | Disinfection Technique | Treatment Conditions | Food Sediment | Log Reduction | Reference |
|---|---|---|---|---|---|---|---|
| E. coli O26 | Stainless steel | Biosafety drying for 90 min | Benzalkonium chloride | 2 mg/L, 10 min | Milk | 0.39 | [58] |
| S. Typhimurium | Stainless steel | Biosafety drying for 120 min | Sodium hypochlorous acid | 0.01% w/v, 10 min | Carrot | <1 | [59] |
| S. Typhimurium | Glass | Biosafety drying for 180 min | Benzalkonium chloride | 2 mg/mL, 10 min | Whole egg solutions | <1 | [60] |
| S. aureus | Polystyrene | Biosafety drying for 90 min | Benzalkonium chloride | 0.5 mg/mL, 10 min | Bovine serum albumin (BSA) | N. D. | [61] |
| S. aureus | Polystyrene | Biosafety drying for 90 min | Benzalkonium chloride | 2.0 mg/mL, 10 min | Milk | 1.85 | [61] |
| E. coli | Stainless steel | Biosafety drying for 120 h | Benzalkonium chloride | 500 mg/L, 10 min | Soy milk (25%) | 1.5 | [52] |
| S. Typhimurium | Glass | Biosafety drying for 180 min | UV-C (254 nm) | 1 min | Egg yolk (15%) | ~3 | [62] |
| Materials | Contact Angle | Surface Energy Parameters | References | ||
|---|---|---|---|---|---|
| θw (°) | γLW (mJ/m2) | γ+ (mJ/m2) | γ− (mJ/m2) | ||
| Stainless steel (type 304, P80 finish) | 51.8 ± 9.8 | ND | ND | ND | [62] |
| Stainless steel (type 304, diamond-polished) | 76.1 ± 10.6 | ND | ND | ND | [62] |
| Stainless steel (type 304, electropolished) | 58.9 ± 4.4 | ND | ND | ND | [62] |
| Stainless steel (type 304, #4 finish) | 32.0 ± 3.6 | 37.9 | 0.5 | 1.8 | [63] |
| Stainless steel 304 | 65.8 | 39.62 | 0.0 | 18.43 | [64] |
| Stainless steel 316 L | 48.8 | 39.0 | 0.02 | 36.39 | [64] |
| Stainless steel (type 304) | 86 ± 2 | 35.5 | 0.0 | 3.8 | [65] |
| Titanium | 42.0 | 41.32 | 0.04 | 41.14 | [64] |
| Glass | 73.5 ± 3.1 | 29.6 | 0.0 | 20 | [66] |
| Glass with metal oxide finish (TiO2) | 59 ± 2 | ND | ND | ND | [67] |
| Glass with metal oxide finish (Fe2O3) | 68 ± 5 | ND | ND | ND | [67] |
| Glass | 12 ± 3 | 39.9 | 1.5 | 51.8 | [68] |
| Silicone | 122 ± 1.8 | 12.4 | 0.0 | 0.9 | [66] |
| Polyethylene | 102 ± 2.4 | 36.4 | 0.0 | 0.6 | [66] |
| Polypropylene | 107 ± 3 | 28.4 | 0.0 | 1.7 | [66] |
| Polyurethane | 80.4 | 36.34 | 0.00 | 7.85 | [64] |
| Polyvinyl chloride | 95.4 ± 2.9 | 33.9 | 0.0 | 5.8 | [66] |
| Pathogens | Contact Surface | Drying Conditions | ClO2 Gas Parameters | Log Reduction | References |
|---|---|---|---|---|---|
| S. Typhimurium | Stainless steel | Biosafety drying for 1 h | 20 ppmv, at 15 °C for 30 min | <1 | [131] |
| 20 ppmv, at 25 °C for 30 min | 1.5–2.0 | ||||
| L. monocytogenes | Stainless steel | Biosafety drying for 2 h | 2 mg/L for 10 min | 3.8 | [136] |
| E. coli O157:H7 | Polyvinyl chloride | Biosafety drying for 1 h | 20 ppmv for 15 min | 3.0 | [131] |
| Bacillus subtilis | Glass | Biosafety drying for 12 h | 0.080% for 3 h | >6.5 | [137] |
| Stainless steel | <5 | ||||
| Bacillus thuringiensis | Wood | Biosafety drying for 3 h | 5 mg/L under 85–92% RH for 12 h | 3.6 | [139] |
| Pathogens | Contact Surface | Drying Conditions | UV Exposure Conditions | Log Reduction | References |
|---|---|---|---|---|---|
| S. enterica | Stainless steel | Biosafety drying for 90 min | UV-C light (254 nm) at 656 µW/cm2 for 5 s (3.3 mJ/cm2) | 2.75 | [181] |
| High-density polyethylene | 2.93 | ||||
| Waxed cardboard | 1.39 | ||||
| Polyvinyl chloride | 1.91 | ||||
| S. enterica | Stainless steel 304 hairline | Biosafety drying for 4 h | UV-C (254 nm) at 15 W for 0–180 s | >4 | [189] |
| S. Typhimurium | Stainless steel | Air-drying for 30 min | UV-C (254 nm) at 250 µW/cm2 for 3 min | 4.35 | [190] |
| E. coli O157:H7 | 5.2 | ||||
| Salmonella spp. | Electroplated stainless steel | Biosafety drying for 30 min | UV-C (254 nm) at a dose of 0.20 J/cm2 | 3.34 | [182] |
| L. monocytogenes | 2.89 | ||||
| S. aureus | 2.58 | ||||
| L. monocytogenes | Polyurethane | Biosafety drying for 30 min | UV light (254 nm) at 5.53 mW/cm2 for 3 s | 4.97 | [191] |
| S. Typhimurium DT104 | Stainless steel | Biosafety drying for 30 min | UV (253.7 nm) at 0.236 ± 0.013 mW/cm2 for 30 min | 0.82 | [192] |
| Polypropylene | 1.62 |
| Pathogens | Contact Surface | Drying Conditions | Plasma Type | Plasma Exposure Conditions | Log Reduction | References |
|---|---|---|---|---|---|---|
| E. coli | Stainless steel | Biosafety drying for 30 min | Surface micro-discharge plasma | Air (90% rH, 5 SLM), for 20 min | 4.13 | [204] |
| S. aureus | 3.38 | |||||
| S. enterica | Stainless steel | Biosafety drying for 4 h | Atmospheric pressure plasma jet system | Air (5 SLM), for 14 s | ≥6 | [189] |
| E. coli | Stainless steel | - | Atmospheric pressure plasma jet system | Air (12 SLM) for 90 s | 3.40 | [208] |
| Polypropylene | 3.40 | |||||
| S. Typhimurium | Stainless steel | Biosafety drying for 1 h | Piezoelectric cold atmospheric plasma | 15 V, 50 kHz, Air for 300 s at 10 mm distance | 3.5 | [201] |
| S. enterica | Glass | Biosafety drying for 1 h | Surface dielectricbarrier discharge | 7 kV, 13.5 V, Air, 1 cm distance, 4 min | 3.0 | [202] |
| S. epidermidis | Stainless steel | 1–2 h drying at 35 °C | Gliding arc discharge | Nitrogen (0.5 m3/h) for 5 min | 3.94 | [197] |
| E. coli | 3.65 | |||||
| E. coli | Wood chopping board | Biosafety drying for 30 min | Atmospheric dielectric barrier discharge plasma | Nitrogen (1.5 lpm) for 60 min | 1.6 | [209] |
| Pathogens | Contact Surface | Drying Conditions | PAA Concentration | Log Reduction | References |
|---|---|---|---|---|---|
| A. brasiliensis | Aluminium | Biosafety drying for 1 h | 1000 mg/L at 40 °C | ~6 | [227] |
| Geobacillus stearothermophilus spores | Stainless steel | Biosafety drying | 200 ppm for 5 min | <1.5 | [228] |
| Murine norovirus | Stainless steel | Drying for 18–24 h, soiled with bovine serum albumin | 200 ppm for 3 min | N. C. (no cytopathic effect) | [229] |
| Feline calicivirus | Stainless steel | Biosafety drying for 30 min | 15% PAA and 11% H2O2 at 1:500 dilution | 3.00 | [230] |
| Hepatitis A virus | Stainless steel | Biosafety drying for 1 h | 200 ppm for 10 min | 4.43 | [231] |
| Pathogens | Contact Surface | Drying Conditions | H2O2 Concentration | Log Reduction | References |
|---|---|---|---|---|---|
| Feline calicivirus | Stainless steel | Air-drying for 45 min in the biosafety cabinet | H2O2 (7.5%) for 5 min | 4.3 | [140] |
| E. coli | Glass | Biosafety drying for 30 min | H2O2 (5%) micro aerosol mist for 30 min | 5.31 | [241] |
| S. Typhimurium | Glass | Biosafety drying for 22 h at 25 °C and 40% RH | H2O2 (2%) for 5 min | 4.3 | [3] |
| S. Enteritidis | Stainless steel | 1 h of biosafety drying of the stationary-phase cells | H2O2 (3.4%) for 10 min | 5.26 | [242] |
| Pathogens | Contact Surface | Drying Conditions | NaOCl Concentration | Log Reduction | References |
|---|---|---|---|---|---|
| S. Typhimurium | Plastic cutting board | Biosafety drying for 24 h (using high microbial load) | 0.0095% for 2 min | 1.75 | [89] |
| S. Enteritidis | Glass | Biosafety drying for 22 h at 25 °C and 40% RH | 100 ppm for 5 min | 5.8 | [249] |
| L. monocytogenes | Stainless steel | Biosafety drying for 24 h | 200 ppm chlorine for 5 min | >3 | [218] |
| S. aureus | Wood | Biosafety drying for 20 min | 62.3 mg/L chlorine for 1 min | 5.54 | [250] |
| Polypropylene | 6 | ||||
| Human norovirus | Stainless steel | Biosafety drying for 40 min | 3% for 5 min | <2 | [251] |
| Feline calicivirus | Stainless steel | Biosafety drying for 30 min | 5.25% for 10 min | 1.1 | [238] |
| Feline calicivirus | Stainless steel | Drying for 1 h | 12% (5000 ppm) for 5 min | 5.20 | [252] |
| Feline calicivirus | Polystyrene | Drying for 30 to 60 min | 5.7% (100 ppm available chlorine) for 1 min | <2.27 | [253] |
| Hepatitis A virus | Stainless steel | Biosafety drying for 1 h, in the presence of 5% soil | PAA 500 ppm for 10 min, | 3.76 | [231] |
| Murine norovirus | Stainless steel | Drying for 30 min | 500 ppm for 5 min | <4 | [54] |
| Murine norovirus | Stainless steel | Biosafety drying for 60 to 90 min | 1350 ppm for 5 min | 5.5 | [254] |
| Alicyclobacillus spp. spores | Stainless steel | - | 2000 ppm for 30 min | 1.0 | [249] |
| Disinfection Method | Advantages | Disadvantages | Cost | Reference |
|---|---|---|---|---|
| Dry disinfection methods | ||||
| Brushing and scraping | Easily available | Laborious, not appropriate for all low-aw foods, small spectrum | Low | [104] |
| Isopropyl alcohol-quaternary ammonium-based disinfectants | Less concentration needed compared to QUAT, easily available | Harmful byproduct residue, potential for microbial resistance in certain strains | Comparable | [109,110] |
| Ethylene oxide gas fumigation | Higher coverage area, more penetrative depth | Shadowing effect, vapor or fog may not travel a greater distance, carcinogenic properties | Comparable/high | [114,115,116] |
| Methyl bromide gas fumigation | Broad antimicrobial spectrum, high efficiency | Contributes to ozone layer’s depletion, potent carcinogen | Low | [114,116] |
| Propylene oxide gas fumigation | Non-hazardous byproducts | Used with CO2 combination | Comparable | [118,119,121] |
| Chlorine dioxide gas fumigation | Greater penetration depth, fast action, on-site production, broad pH range | Unstable at higher concentrations, difficult to handle and transport | High | [133,134,143] |
| Ozone gas fumigation | Less environmental effect, broad antimicrobial spectrum, good coverage area | Unstable, harmful in gaseous state, poses greater health risk when exposed for longer time | Low/Comparable | [151,153,160,163] |
| Superheated steam | Non-polluting, fairly good antimicrobial spectrum, no toxic byproducts | Pre-cleaning required, efficiency depends on surface characteristics | High | [85,171] |
| UV-light disinfection | Non-thermal, less energy required, no odor | Shadowing effect, low penetrability | Low | [180,181,183] |
| Cold plasma | Non thermal, broad antimicrobial spectrum | Higher energy required for production, skilled labor required | Comparable/High | [57,185,193,196] |
| Wet disinfection methods | ||||
| Quaternary ammonium compounds | Convenient, broader pH range, non-corrosive | Can promote antibiotic resistance, formation of bacteriostatic film, incompatible with detergent | Low | [212,217,219] |
| Peracetic acid | Broad antimicrobial spectrum, environmentally friendly | Thermodynamically unstable, health concern at higher concentration, effectiveness varies with microorganism | Low | [38,223,224] |
| Hydrogen peroxide | Fast-acting, broad antimicrobial spectrum, no toxic byproducts, greater dispersion | Highly corrosive, highly sensitive to the presence of heavy metals | Low | [102,232,237] |
| Sodium hypochlorite | Convenient, broad antimicrobial spectrum | Requirement for handling precautions | Low | [155,243] |
| Electrolyzed water | Broad antimicrobial spectrum, lesser environmental impacts, no effect on sensory properties | Equipment corrosion, skin irritation, high initial cost, additional cleaning requirement | Comparable | [259,265] |
| Plasma activated water | Broad range of application, no hazardous byproducts, easy to use | Shorter lifespan of reactive species, cannot store for a longer time | Comparable/high | [269,270,272,273] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaliwal, H.K.; Sonkar, S.; V, P.; Puente, L.; Roopesh, M.S. Process Technologies for Disinfection of Food-Contact Surfaces in the Dry Food Industry: A Review. Microorganisms 2025, 13, 648. https://doi.org/10.3390/microorganisms13030648
Dhaliwal HK, Sonkar S, V P, Puente L, Roopesh MS. Process Technologies for Disinfection of Food-Contact Surfaces in the Dry Food Industry: A Review. Microorganisms. 2025; 13(3):648. https://doi.org/10.3390/microorganisms13030648
Chicago/Turabian StyleDhaliwal, Harleen Kaur, Shivani Sonkar, Prithviraj V, Luis Puente, and M. S. Roopesh. 2025. "Process Technologies for Disinfection of Food-Contact Surfaces in the Dry Food Industry: A Review" Microorganisms 13, no. 3: 648. https://doi.org/10.3390/microorganisms13030648
APA StyleDhaliwal, H. K., Sonkar, S., V, P., Puente, L., & Roopesh, M. S. (2025). Process Technologies for Disinfection of Food-Contact Surfaces in the Dry Food Industry: A Review. Microorganisms, 13(3), 648. https://doi.org/10.3390/microorganisms13030648







