Predisposing and Precipitating Factors in Epstein–Barr Virus-Caused Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Abstract
1. Predisposing and Precipitating Factors in Epstein–Barr Virus-Caused ME/CFS
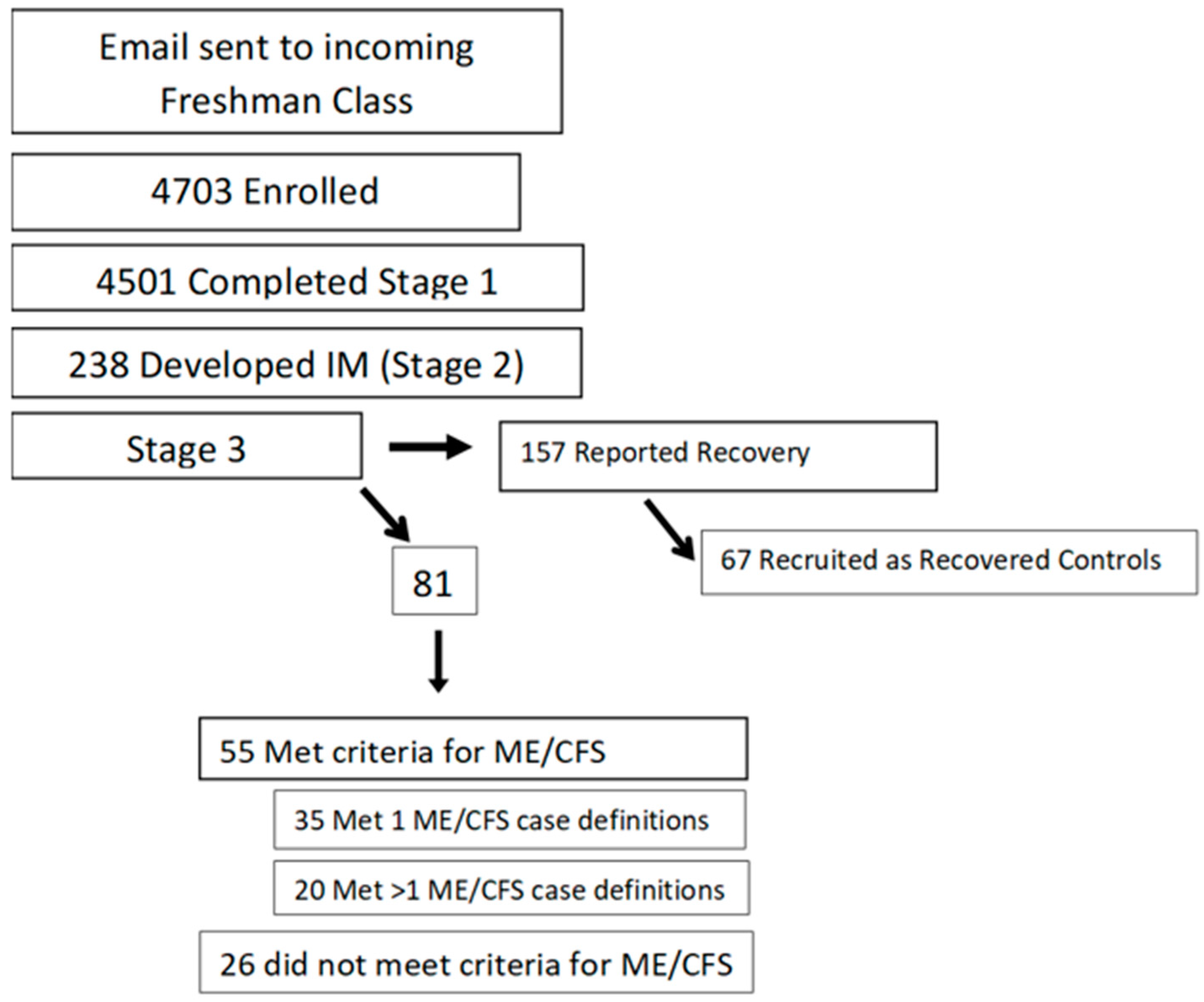
2. Prospective Study
3. Baseline Network Analysis
4. Predicting ME/CFS
5. Baseline Metabolic Pathways
6. Long COVID
7. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jason, L.A.; Williams-Harmon, Y.J. Incidence of Infectious Mononucleosis in Universities and U.S. Military Settings. J. Diagn. Tech. Biomed. Anal. 2016, 3, 2. [Google Scholar]
- White, P.D.; Thomas, J.M.; Amess, J.; Crawford, D.H.; Grover, S.A.; Clare, A.W.; Kangro, H.O. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br. J. Psychiatry 1998, 173, 475–481. [Google Scholar] [PubMed]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575–580. [Google Scholar] [CrossRef]
- Katz, B.Z.; Shiraishi, Y.; Mears, C.J.; Binns, H.J.; Taylor, R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics 2009, 124, 189–193. [Google Scholar] [PubMed]
- Brown, M.; Bell, D.S.; Jason, L.A.; Christos, C.; Bell, D.E. Understanding long-term outcomes of chronic fatigue syndrome. J. Clin. Psychol. 2012, 68, 1028–1035. [Google Scholar]
- Song, Z.; Giuriato, M. Demographic and clinical factors associated with Long COVID. Health Aff. 2023, 42, 433–442. [Google Scholar] [CrossRef]
- Natelson, B.H.; Tseng, C.-L.; Ottenweller, J.E. Spinal fluid abnormalities in patients with chronic fatigue syndrome. Clin. Diagn. Lab. Immunol. 2005, 12, 53–55. [Google Scholar]
- Broderick, G.; Fuite, J.; Kreitz, A.; Vernon, S.D.; Klimas, N.; Fletcher, M.A. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav. Immun. 2010, 24, 1209–1217. [Google Scholar]
- White, A.C.; Katz, B.Z., Jr.; Silbert, J.A. Association of Epstein-Barr virus with an angioimmunoblastic lymphadenopathy-like lymphoproliferative syndrome. Yale J. Biol. Med. 1989, 62, 263–269. [Google Scholar]
- Armstrong, C.W.; McGregor, N.R.; Butt, H.L.; Gooley, P.R. Metabolism in chronic fatigue syndrome. Adv. Clin. Chem. 2014, 66, 121–172. [Google Scholar] [PubMed]
- Germain, A.; Ruppert, D.; Levine, S.M.; Hanson, M.R. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol. Biosyst. 2017, 13, 371–379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagy-Szakal, D.; Barupal, D.K.; Lee, B.; Che, X.; Williams, B.L.; Kahn, E.J.R.; Ukaigwe, J.E.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci. Rep. 2018, 8, 10056. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E5472–E5480. [Google Scholar] [CrossRef]
- Walsh, C.M.; Zainal, N.Z.; Middleton, S.J.; Paykel, E.S. A family history study of chronic fatigue syndrome. Psych. Gen. 2001, 11, 123–128. [Google Scholar]
- Jason, L.A.; Ngonmedje, S. The influence of ME/CFS family history on patients with ME/CFS. Explor. Med. 2024, 5, 185–192. [Google Scholar] [CrossRef]
- Guo, C.; Che, X.; Briese, T.; Ranjan, A.; Allicock, O.; Yates, R.A.; Cheng, A.; March, D.; Hornig, M.; Komaroff, A.L.; et al. Deficient butyrate-producing capacity in the gut microbiome is associated with bacterial network disturbances and fatigue symptoms in ME/CFS. Cell Host Microbe 2023, 31, 288–304. [Google Scholar]
- Xiong, R.; Gunter, C.; Fleming, E.; Vernon, S.D.; Bateman, L.; Unutmaz, D.; Oh, J. Multi-omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients. Cell Host Microbe 2023, 31, 273–287. [Google Scholar]
- Jason, L.A.; Cotler, J.; Islam, M.I.; Furst, J.; Katz, B.Z. Predictors for developing severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome following Infectious Mononucleosis. J. Rehabil. Ther. 2022, 4, 1–5. [Google Scholar] [CrossRef]
- Nagy-Szakal, D.; Williams, B.L.; Mishra, N.; Che, X.; Lee, B.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; Levine, S.; Montoya, J.G.; et al. Fecal metagenomics profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2017, 5, 44. [Google Scholar] [CrossRef]
- Nunes, J.M.; Kell, D.B.; Pretorius, E. Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A role for viruses. Blood Rev. 2023, 60, 101075. [Google Scholar]
- Thapaliya, K.; Marshall-Gradisnik, S.; Barth, M.; Eaton-Fitch, N.; Barnden, L. Brainstem volume changes in myalgic encephalomyelitis/chronic fatigue syndrome and long COVID patients. Front. Neurosci. 2023, 17, 1125208. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Lapp, C. (Eds.) Understanding the Behavioral and Medical Impact of Long COVID: An Empirical Guide to Assessment and Treatment of Post-Acute Sequelae of SARS CoV-2 Infection; Routledge: New York, NY, USA, 2023. [Google Scholar]
- Yang, J.; Qiu, M. Mild Respiratory COVID-Induced Neuroinflammation Causes Neurological Deficits. Neurosci. Bull. 2023, 39, 713–715. [Google Scholar] [CrossRef]
- Zinn, M.A.; Jason, L.A. Cortical autonomic network connectivity predicts symptoms in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Int. J. Psychophysiol. 2021, 170, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Cotler, J.; Islam, M.; Sunnquist, M.; Katz, B.Z. Risks for developing ME/CFS in college students following Infectious Mononucleosis: A prospective cohort study. Clin. Infect. Dis. 2021, 73, e3740–e3746. [Google Scholar] [CrossRef]
- Jason, L.A.; Cotler, J.; Islam, M.F.; Furst, J.; Sorenson, M.; Katz, B.Z. Cytokine networks analysis uncovers further differences between those who develop Myalgic Encephalomyelitis/Chronic Fatigue Syndrome following Infectious Mononucleosis. Fatigue Biomed. Health Behav. 2021, 9, 45–57. [Google Scholar] [CrossRef]
- Sorenson, M.; Furst, J.; Mathews, H.; Jason, L.A. Dysregulation of cytokine pathways in Chronic Fatigue Syndrome and Multiple Sclerosis. Fatigue Biomed. Health Behav. 2017, 5, 145–158. [Google Scholar] [CrossRef]
- Katz, B.Z.; Reuter, C.; Lupovitch, Y.; Gleason, K.; McClellan, D.; Cotler, J.; Jason, L.A. A validated scale for assessing the severity of acute Infectious Mononucleosis. J. Pediatr. 2019, 209, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Conroy, K.E.; Furst, J.; Vasan, K.; Katz, B.Z. Pre-illness data reveals differences in multiple metabolites and metabolic pathways in those who do and do not recover. Mol. Omics 2022, 18, 662–665. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Long-Term Health Effects of COVID-19: Disability and Function Following SARS-CoV-2 Infection; The National Academies Press: Washington, DC, USA, 2024. [Google Scholar] [CrossRef]
- Jin, J.; Xie, X.; Xiao, Y.; Hu, H.; Zou, Q.; Cheng, X.; Sun, S.-C. Epigenetic regulation of the expression of Il12 and Il23 and autoimmune inflammation by the deubiquitinase Trabid. Nat. Immunol. 2016, 17, 259–268. [Google Scholar] [CrossRef]
- Loebel, M.; Strohschein, K.; Giannini, C.; Koelsch, U.; Bauer, S.; Doebis, C.; Thomas, S.; Unterwalder, N.; von Baehr, V.; Reinke, P.; et al. Deficient EBV-specific B- and T-cell response in patients with chronic fatigue syndrome. PLoS ONE 2014, 15, e85387. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2018, 50, 992–1006. [Google Scholar] [CrossRef]
- Jason, L.A.; Kalns, J.; Richarte, A.; Katz, B.Z.; Torres, C. Saliva fatigue biomarker index as a marker for severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in a community-based sample. Fatigue Biomed. Health Behav. 2021, 9, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Richman, J.A.; Rademaker, A.W.; Jordan, K.M.; Plioplys, A.V.; Taylor, R.R.; McCreedy, W.; Huang, C.; Plioplys, S. A community-based study of chronic fatigue syndrome. Arch. Intern. Med. 1999, 159, 2129–2137. [Google Scholar] [PubMed]
- Jason, L.A.; Katz, B.Z.; Sunnquist, M.; Torres, C.; Cotler, J.; Bhatia, S. The prevalence of pediatric myalgic encephalomyelitis/chronic fatigue syndrome in a community-based sample. Child. Youth Care Forum 2020, 49, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Dorri, J. Predictors of impaired functioning among Long COVID patients. WORK J. Prev. Assess. Rehabil. 2023, 74, 1215–1224. [Google Scholar]
- King, C.; Jason, L.A. Improving the diagnostic criteria and procedures for chronic fatigue syndrome. Biol. Psychol. 2005, 68, 87–106. [Google Scholar]
- Jason, L.A.; Sunnquist, M. The development of the DePaul Symptom Questionnaire: Original, expanded, brief and pediatric versions. Front. Pediatr. 2018, 6, 330. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Jason, L.A.; Unutmaz, D.; Bateman, L.; Vernon, S.D. Improvement of long COVID symptoms over one year. Front. Med. 2022, 9, 1065620. [Google Scholar] [CrossRef]
- McGarrigle, W.J.; Furst, J.; Jason, L.A. Psychometric evaluation of the DePaul Symptom Questionnaire-Short Form (DSQ-SF) among adults with Long COVID, ME/CFS, and healthy controls: A machine learning approach. J. Health Psychol. 2024, 29, 1241–1252. [Google Scholar] [CrossRef]
- Hua, C.; Schwabe, J.; Jason, L.A.; Furst, J.; Raicu, D. Predicting Myalgic Encephalomyelitis/Chronic Fatigue Syndrome from early symptoms of COVID-19 infection. Psych 2023, 5, 1101–1108. [Google Scholar] [CrossRef]
- Müller-Durovic, B.; Jäger, J.; Engelmann, C.; Schuhmachers, P.; Altermatt, S.; Schlup, Y.; Duthaler, U.; Makowiec, C.; Unterstab, G.; Roffeis, S.; et al. A metabolic dependency of EBV can be targeted to hinder B cell transformation. Science 2024, 385, eadk4898. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, N.; Ribeiro, R.A.; Camarinha-Matos, L.M. The roles of the kynurenine pathway in COVID-19 neuropathogenesis. Infection 2024, 52, 2043–2059. [Google Scholar] [CrossRef]
- Ruffieux, H.; Hanson, A.L.; Lodge, S.; Lawler, N.G.; Whiley, L.; Gray, N.; Nolan, T.H.; Bergamaschi, L.; Mescia, F.; Turner, L.; et al. A patient-centric modeling framework captures recovery from SARS-CoV-2 infection. Nat. Immunol. 2023, 24, 349–358. [Google Scholar] [CrossRef]
- Jason, L.A.; Yoo, S.; Bhatia, S. Patient perceptions of infectious illnesses preceding Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Chronic Illn. 2022, 18, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Brown, M.; Brown, A.; Evans, M.; Flores, S.; Grant-Holler, E.; Sunnquist, M. Energy Conservation/Envelope Theory interventions to help patients with chronic fatigue syndrome. Fatigue Biomed. Health Behav. 2013, 1, 27–42. [Google Scholar]
- Johnson, J.N.; Mack, K.J.; Kuntz, N.L.; Brands, C.K.; Porter, C.J.; Fischer, P.R. Postural orthostatic tachycardia syndrome: A clinical review. Pediatr. Neurol. 2010, 42, 77–85. [Google Scholar]
- Zadourian, A.; Doherty, T.A.; Swiatkiewicz, I.; Taub, P.R. Postural orthostatic tachycardia syndrome: Prevalence, pathophysiology, and management. Drugs 2018, 78, 983–994. [Google Scholar] [PubMed]
- Ruzieh, M.; Sirianni, N.; Ammari, Z.; Dasa, O.; Alhazmi, L.; Karabin, B.; Grubb, B. Ivabradine in the treatment of postural tachycardia syndrome (POTS), a single center experience. Pacing Clin. Electrophysiol. 2017, 40, 1242–1245. [Google Scholar]
- Theoharides, T.C.; Asadi, S.; Weng, Z.; Zhang, B. Serotonin-selective reuptake inhibitors and nonsteroidal anti-inflammatory drugs-important considerations of adverse interactions especially for the treatment of myalgic encephalomyelitis/ chronic fatigue syndrome. J. Clin. Psychopharmacol. 2011, 31, 403–405. [Google Scholar]
- Pajediene, E.; Bileviciute-Ljungar, I.; Friberg, D. Sleep patterns among patients with chronic fatigue: A polysomnography-based study. Clin. Respir. J. 2018, 12, 1389–1397. [Google Scholar]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The gut microbiome in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Front. Immunol. 2022, 12, 628741. [Google Scholar]
- Maes, M.; Al-Rubaye, H.T.; Almulla, A.F.; Al-Hadrawi, D.S.; Stoyanova, K.; Kubera, M.; Al-Hakeim, H.K. Lowered quality of life in long COVID is predicted by affective symptoms, chronic fatigue syndrome, inflammation and neuroimmunotoxic pathways. Int. J. Environ. Res. Public. Health 2022, 19, 10362. [Google Scholar] [CrossRef]
- Kielland, A.; Liu, J.; Jason, L.A. Do diagnostic criteria for ME matter to patient experience with services and interventions? Key results from an online RDS survey targeting fatigue patients in Norway. J. Health Psychol. 2023, 28, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, L.; Hattesohl DBRJason, L.A.; Scheibenbogen, C.; Behrends, U.; Thoma, M. Medical care situation of people with Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome in Germany. Medicina 2021, 57, 646. [Google Scholar] [CrossRef] [PubMed]
- Pipper, C.; Bliem, L.; León, L.E.; Mennickent, D.; Bodner, C.; Guzmán-Gutiérrez, E.; Stingl, M.; Untersmayr, E.; Wagner, B.; Bertinat, R.; et al. Sex and disease severity-based analysis of steroid hormones in ME/CFS. J. Endocrinol. Investig. 2024, 47, 2235–2248. [Google Scholar] [CrossRef]
- Sandler, C.X.; Wyler, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and post-infective fatigue syndrome—A review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef]


| S-CFS | Controls | |||||
|---|---|---|---|---|---|---|
| KEEG | Metabolite | M(SD) | M(SD) | U | p | |
| a | C00750 | spermine | 18.79 (0.13) | 19.54 (0.16) | 0 | 0.0000000002 |
| c | C00354…C00665 | F-1,6/2,6-DP | 14.94 (0.43) | 13.90 (0.24) | 321 | 0.0000000015 |
| a | C00315 | spermidine | 19.18 (0.66) | 17.84 (0.33) | 311 | 0.0000000822 |
| c | C00002…C00286 | ATP/dGTP | 14.57 (1.02) | 13.16 (0.54) | 301 | 0.0000012586 |
| b | C00169 | carbamoyl phosphate | 15.67 (0.45) | 16.65 (0.12) | 23 | 0.0000012586 |
| a | C00127 | glutathione disulfide | 13.63 (1.00) | 11.64 (1.11) | 300 | 0.0000015973 |
| c | C00158…C00311 | citrate/citrate(iso) | 22.90 (0.28) | 22.40 (0.30) | 290 | 0.0000135233 |
| a | C00112 | CDP | 10.84 (1.29) | 8.88 (0.85) | 297 | 0.0000169794 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jason, L.A.; Katz, B.Z. Predisposing and Precipitating Factors in Epstein–Barr Virus-Caused Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Microorganisms 2025, 13, 702. https://doi.org/10.3390/microorganisms13040702
Jason LA, Katz BZ. Predisposing and Precipitating Factors in Epstein–Barr Virus-Caused Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Microorganisms. 2025; 13(4):702. https://doi.org/10.3390/microorganisms13040702
Chicago/Turabian StyleJason, Leonard A., and Ben Z. Katz. 2025. "Predisposing and Precipitating Factors in Epstein–Barr Virus-Caused Myalgic Encephalomyelitis/Chronic Fatigue Syndrome" Microorganisms 13, no. 4: 702. https://doi.org/10.3390/microorganisms13040702
APA StyleJason, L. A., & Katz, B. Z. (2025). Predisposing and Precipitating Factors in Epstein–Barr Virus-Caused Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Microorganisms, 13(4), 702. https://doi.org/10.3390/microorganisms13040702






