Evolution of Epstein–Barr Virus Infection Seroprevalence in a French University Hospital over 11 Years, Including the COVID-19 Pandemic, 2013–2023
Abstract
1. Introduction
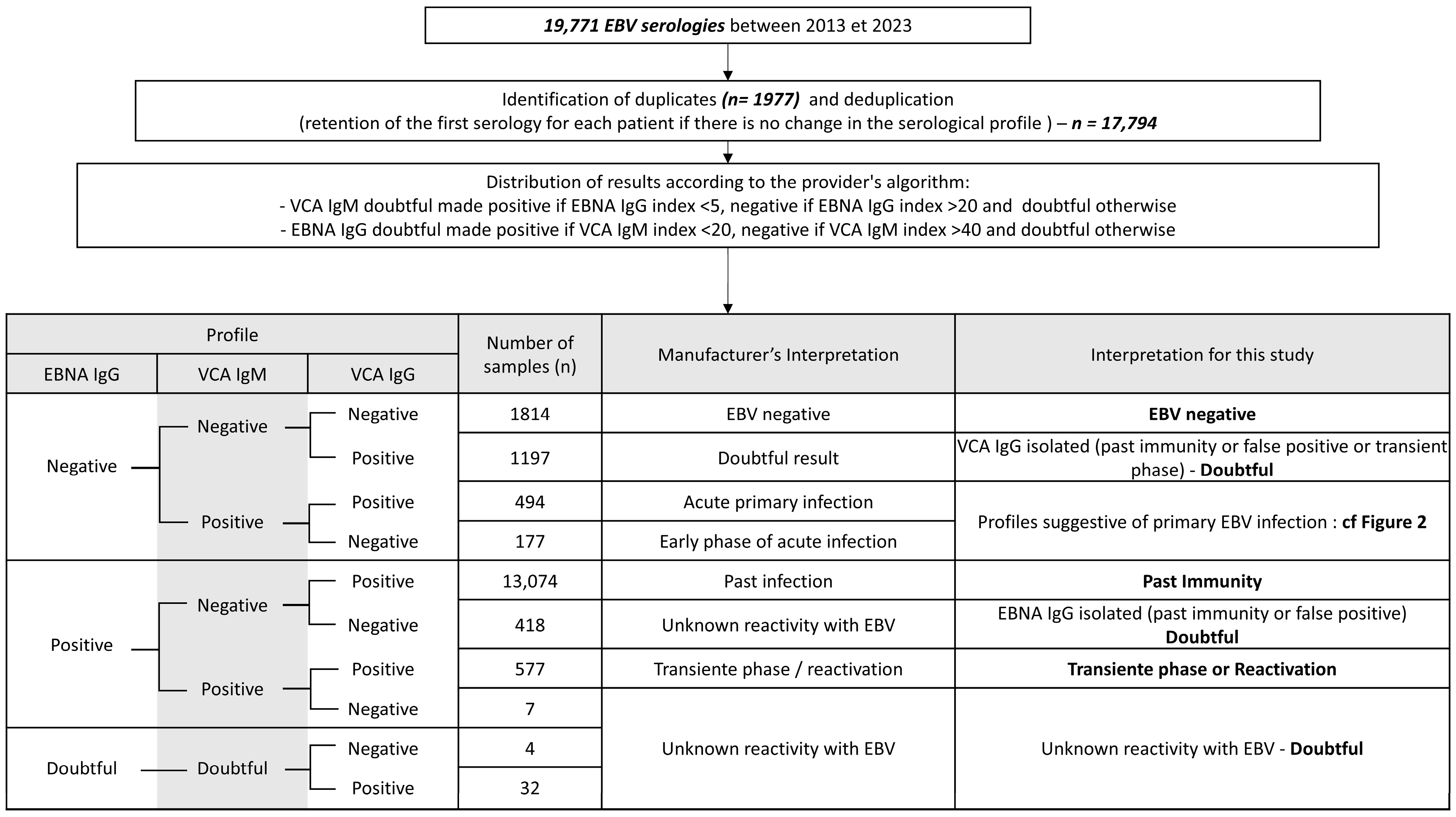
2. Materials and Methods
2.1. Data Collection
2.2. Detection of EBV Antibodies
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
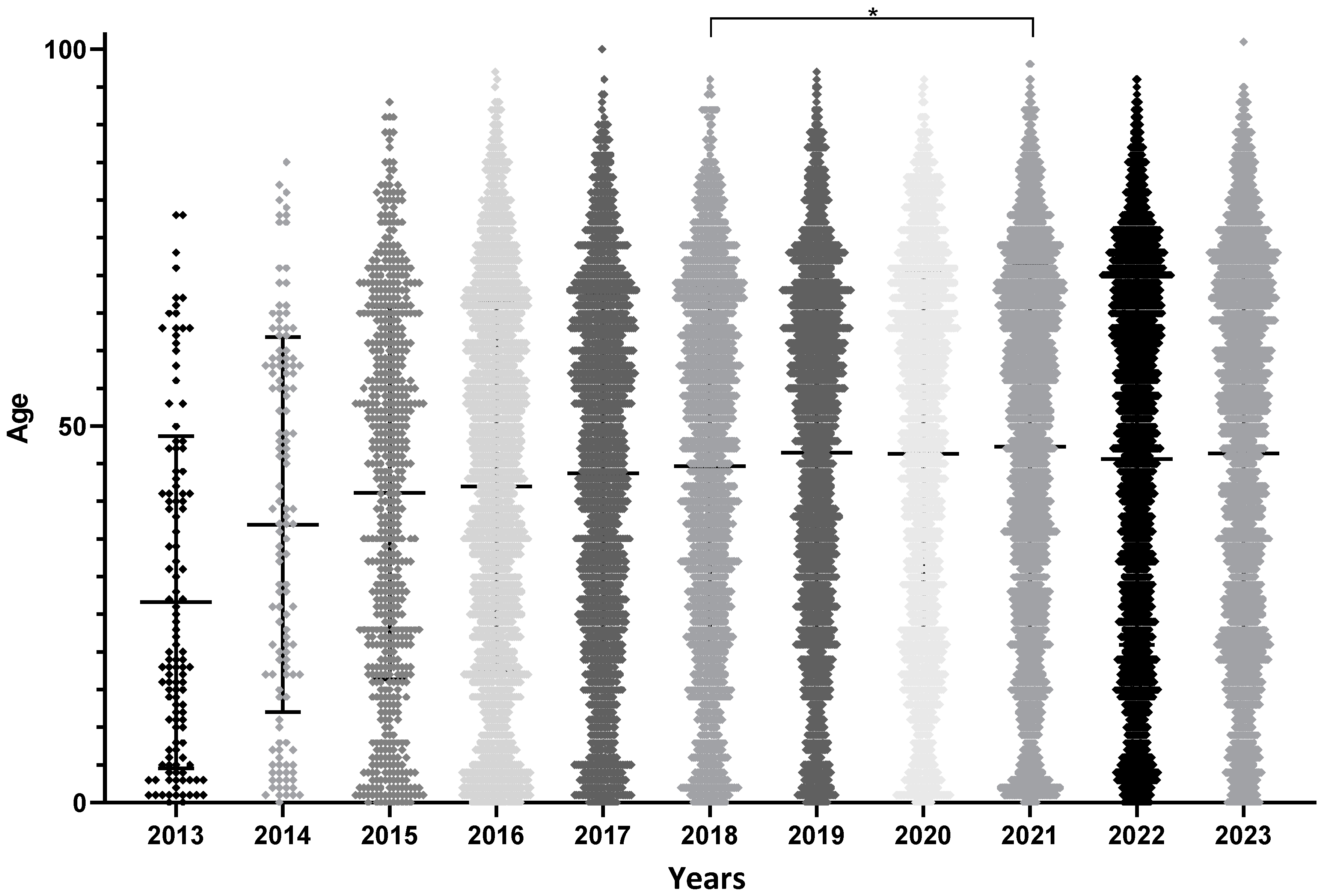
3.1. Patient Characteristics
3.2. Global EBV Seroprevalence
3.3. Primary Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H. Primary Epstein-Barr Virus Infection. J. Clin. Virol. 2018, 102, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Gloghini, A.; Dotti, G. EBV-Associated Lymphoproliferative Disorders: Classification and Treatment. Oncologist 2008, 13, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Hjalgrim, H.; Smedby, K.E.; Rostgaard, K.; Molin, D.; Hamilton-Dutoit, S.; Chang, E.T.; Ralfkiaer, E.; Sundström, C.; Adami, H.-O.; Glimelius, B.; et al. Infectious Mononucleosis, Childhood Social Environment, and Risk of Hodgkin Lymphoma. Cancer Res. 2007, 67, 2382–2388. [Google Scholar] [CrossRef] [PubMed]
- Niedobitek, G.; Meru, N.; Delecluse, H.J. Epstein-Barr Virus Infection and Human Malignancies. Int. J. Exp. Pathol. 2001, 82, 149–170. [Google Scholar] [CrossRef]
- Dowd, J.B.; Palermo, T.; Brite, J.; McDade, T.W.; Aiello, A. Seroprevalence of Epstein-Barr Virus Infection in U.S. Children Ages 6–19, 2003–2010. PLoS ONE 2013, 8, e64921. [Google Scholar] [CrossRef]
- Balfour, H.H.; Sifakis, F.; Sliman, J.A.; Knight, J.A.; Schmeling, D.O.; Thomas, W. Age-Specific Prevalence of Epstein-Barr Virus Infection among Individuals Aged 6–19 Years in the United States and Factors Affecting Its Acquisition. J. Infect. Dis. 2013, 208, 1286–1293. [Google Scholar] [CrossRef]
- Takeuchi, K.; Tanaka-Taya, K.; Kazuyama, Y.; Ito, Y.M.; Hashimoto, S.; Fukayama, M.; Mori, S. Prevalence of Epstein-Barr Virus in Japan: Trends and Future Prediction. Pathol. Int. 2006, 56, 112–116. [Google Scholar] [CrossRef]
- Fourcade, G.; Germi, R.; Guerber, F.; Lupo, J.; Baccard, M.; Seigneurin, A.; Semenova, T.; Morand, P.; Epaulard, O. Evolution of EBV Seroprevalence and Primary Infection Age in a French Hospital and a City Laboratory Network, 2000–2016. PLoS ONE 2017, 12, e0175574. [Google Scholar] [CrossRef]
- Franci, G.; Crudele, V.; Della Rocca, M.T.; Melardo, C.; Chianese, A.; Finamore, E.; Bencivenga, F.; Astorri, R.; Vitiello, M.; Galdiero, E.; et al. Epstein-Barr Virus Seroprevalence and Primary Infection at the University Hospital Luigi Vanvitelli of Naples from 2007 to 2017. Intervirology 2019, 62, 15–22. [Google Scholar] [CrossRef]
- Xiong, G.; Zhang, B.; Huang, M.; Zhou, H.; Chen, L.; Feng, Q.; Luo, X.; Lin, H.; Zeng, Y. Epstein-Barr Virus (EBV) Infection in Chinese Children: A Retrospective Study of Age-Specific Prevalence. PLoS ONE 2014, 9, e99857. [Google Scholar] [CrossRef]
- Cai, F.; Gao, H.; Ye, Q. Seroprevalence of Epstein–Barr Virus Infection in Children during the COVID-19 Pandemic in Zhejiang, China. Front. Pediatr. 2023, 11, 1064330. [Google Scholar] [CrossRef]
- Jansen, M.A.E.; van den Heuvel, D.; Bouthoorn, S.H.; Jaddoe, V.W.V.; Hooijkaas, H.; Raat, H.; Fraaij, P.L.A.; van Zelm, M.C.; Moll, H.A. Determinants of Ethnic Differences in Cytomegalovirus, Epstein-Barr Virus, and Herpes Simplex Virus Type 1 Seroprevalence in Childhood. J. Pediatr. 2016, 170, 126–134.e6. [Google Scholar] [CrossRef] [PubMed]
- Gares, V.; Panico, L.; Castagne, R.; Delpierre, C.; Kelly-Irving, M. The Role of the Early Social Environment on Epstein Barr Virus Infection: A Prospective Observational Design Using the Millennium Cohort Study. Epidemiol. Infect. 2017, 145, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Glaser, S.L. Epstein-Barr Virus-Associated Malignancies: Epidemiologic Patterns and Etiologic Implications. Crit. Rev. Oncol. Hematol. 2000, 34, 27–53. [Google Scholar] [CrossRef]
- Hjalgrim, H.; Askling, J.; Sørensen, P.; Madsen, M.; Rosdahl, N.; Storm, H.H.; Hamilton-Dutoit, S.; Eriksen, L.S.; Frisch, M.; Ekbom, A.; et al. Risk of Hodgkin’s Disease and Other Cancers after Infectious Mononucleosis. J. Natl. Cancer Inst. 2000, 92, 1522–1528. [Google Scholar] [CrossRef]
- Haahr, S.; Plesner, A.M.; Vestergaard, B.F.; Höllsberg, P. A Role of Late Epstein-Barr Virus Infection in Multiple Sclerosis. Acta Neurol. Scand. 2004, 109, 270–275. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Balfour, H.H.; Meirhaeghe, M.R.; Stancari, A.L.; Geris, J.M.; Condon, L.M.; Cederberg, L.E. Declining Epstein-Barr Virus Antibody Prevalence in College Freshmen Strengthens the Rationale for a Prophylactic EBV Vaccine. Vaccines 2022, 10, 1399. [Google Scholar] [CrossRef]
- Goscé, L.; Winter, J.R.; Taylor, G.S.; Lewis, J.E.A.; Stagg, H.R. Modelling the Dynamics of EBV Transmission to Inform a Vaccine Target Product Profile and Future Vaccination Strategy. Sci. Rep. 2019, 9, 9290. [Google Scholar] [CrossRef]
- Lupo, J.; Germi, R.; Semenova, T.; Buisson, M.; Seigneurin, J.M.; Morand, P. Performance of Two Commercially Available Automated Immunoassays for the Determination of Epstein-Barr Virus Serological Status. Clin. Vaccine Immunol. 2012, 19, 929–934. [Google Scholar] [CrossRef]
- Tortosa-Carreres, J.; Lloret-Sos, C.; Sahuquillo-Arce, J.M.; Suárez-Urquiza, P.; Prat-Fornells, J.; Molina-Moreno, J.M.; Alba-Redondo, A.; Martínez-Triguero, M.L.; Aguado-Codina, C.; Laiz-Marro, B.; et al. Evaluating the Diagnostic Performance of Liaison® Chemiluminescence Assay as Screening Tool for Detection of Acute Epstein-Barr Infection: A Comparative Study. Diagn. Microbiol. Infect. Dis. 2024, 108, 116167. [Google Scholar] [CrossRef]
- François, C.; Segard, C.; Bouvier, M.; Stefanski, M.; Pannier, C.; Zawadzki, P.; Roussel, C.; Hecquet, D.; Duverlie, G.; Brochot, E.; et al. Comparison of Abbott Architect®, Siemens Immulite®, and Diasorin Liaison® for Determination of Epstein-Barr Virus Serological Diagnosis. Diagn. Microbiol. Infect. Dis. 2018, 90, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Epstein Barr Virus Test|Immunodiagnostics|Diasorin. Available online: https://int.diasorin.com/en/immunodiagnostics/infectious-diseases/epstein-barr-virus (accessed on 14 February 2025).
- The R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files, Version 1.3.1; R Package. 2019. Available online: https://CRAN.R-project.org/package=readxl (accessed on 14 March 2025).
- Mueller, J. Writexl: Export Data Frames to Excel “xlsx” Format, Version 1.5.1; R Package. 2020. Available online: https://CRAN.R-project.org/package=writexl (accessed on 14 March 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 14 March 2025).
- Henle, G.; Henle, W. Observations on Childhood Infections with the Epstein-Barr Virus. J. Infect. Dis. 1970, 121, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Y.; Ba, L.; Liu, L.; Su, T.; Sun, Y.; Dian, Z. Epidemiological Changes in Respiratory Pathogen Transmission among Children with Acute Respiratory Infections during the COVID-19 Pandemic in Kunming, China. BMC Infect. Dis. 2024, 24, 826. [Google Scholar] [CrossRef]
- Hoagland, R.J. The Transmission of Infectious Mononucleosis. Am. J. Med. Sci. 1955, 229, 262–272. [Google Scholar] [CrossRef]




| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 119 | 130 | 640 | 1982 | 1882 | 1895 | 1968 | 1806 | 2318 | 2528 | 2526 | 17,794 |
| M/F | 57/62 | 68/62 | 334/306 | 1028/954 | 983/899 | 956/939 | 1059/909 | 957/849 | 1214/1104 | 1276/1252 | 1235/1291 | 9167/8627 |
| Past infection (%) | 38.7 | 36.2 | 59.1 | 73.6 | 75.7 | 74.0 | 76.0 | 76.9 | 74.0 | 72.0 | 75.3 | 73.5 |
| EBV negative (%) | 15.1 | 14.6 | 13.1 | 12.5 | 9.8 | 9.2 | 8.8 | 10.5 | 9.5 | 11.2 | 8.6 | 10.2 |
| Primary Infection (%) | 20.2 | 14.6 | 4.1 | 2.5 | 2.2 | 1.6 | 2.2 | 2.1 | 2.3 | 2.5 | 3.0 | 2.6 |
| Others (%) | 26.1 | 34.6 | 23.8 | 11.5 | 12.3 | 15.1 | 13.0 | 10.5 | 14.2 | 14.4 | 13.1 | 13.7 |
| Mean age (±standard deviation of the mean) | 26.6 ± 2 | 36.9 ± 2.2 | 41.1 ± 1 | 42 ± 0.5 | 43.7 ± 0.5 | 44.7 ± 0.5 | 46.5 ± 0.5 | 46.4 ± 0.6 | 47.3 ± 0.5 | 45.6 ± 0.5 | 46.4 ± 0.5 | 45.1 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubry, A.; Francois, C.; Demey, B.; Louchet-Ducoroy, M.; Pannier, C.; Segard, C.; Brochot, E.; Castelain, S. Evolution of Epstein–Barr Virus Infection Seroprevalence in a French University Hospital over 11 Years, Including the COVID-19 Pandemic, 2013–2023. Microorganisms 2025, 13, 733. https://doi.org/10.3390/microorganisms13040733
Aubry A, Francois C, Demey B, Louchet-Ducoroy M, Pannier C, Segard C, Brochot E, Castelain S. Evolution of Epstein–Barr Virus Infection Seroprevalence in a French University Hospital over 11 Years, Including the COVID-19 Pandemic, 2013–2023. Microorganisms. 2025; 13(4):733. https://doi.org/10.3390/microorganisms13040733
Chicago/Turabian StyleAubry, Aurélien, Catherine Francois, Baptiste Demey, Marie Louchet-Ducoroy, Christine Pannier, Christine Segard, Etienne Brochot, and Sandrine Castelain. 2025. "Evolution of Epstein–Barr Virus Infection Seroprevalence in a French University Hospital over 11 Years, Including the COVID-19 Pandemic, 2013–2023" Microorganisms 13, no. 4: 733. https://doi.org/10.3390/microorganisms13040733
APA StyleAubry, A., Francois, C., Demey, B., Louchet-Ducoroy, M., Pannier, C., Segard, C., Brochot, E., & Castelain, S. (2025). Evolution of Epstein–Barr Virus Infection Seroprevalence in a French University Hospital over 11 Years, Including the COVID-19 Pandemic, 2013–2023. Microorganisms, 13(4), 733. https://doi.org/10.3390/microorganisms13040733





