Comparing In Vitro Virucidal Efficacy of Commercially Available Mouthwashes Against Native High-Risk Human Papillomavirus Types 16 and 18
Abstract
1. Introduction
2. Materials and Methods
2.1. Biosafety Measures
2.2. Mouthwash and Gargling Products
2.3. Cell Culture
2.4. Growing HPV16 and HPV18 Organotypic Raft Cultures
2.5. HPV16 and HPV18 Virus Stocks Preparation
2.6. Titering HPV16 and HPV18 Virus Stocks
2.7. Disinfection Procedure
2.8. RT-qPCR Infectivity Assays in HaCaT Monolayer Cultures
3. Results
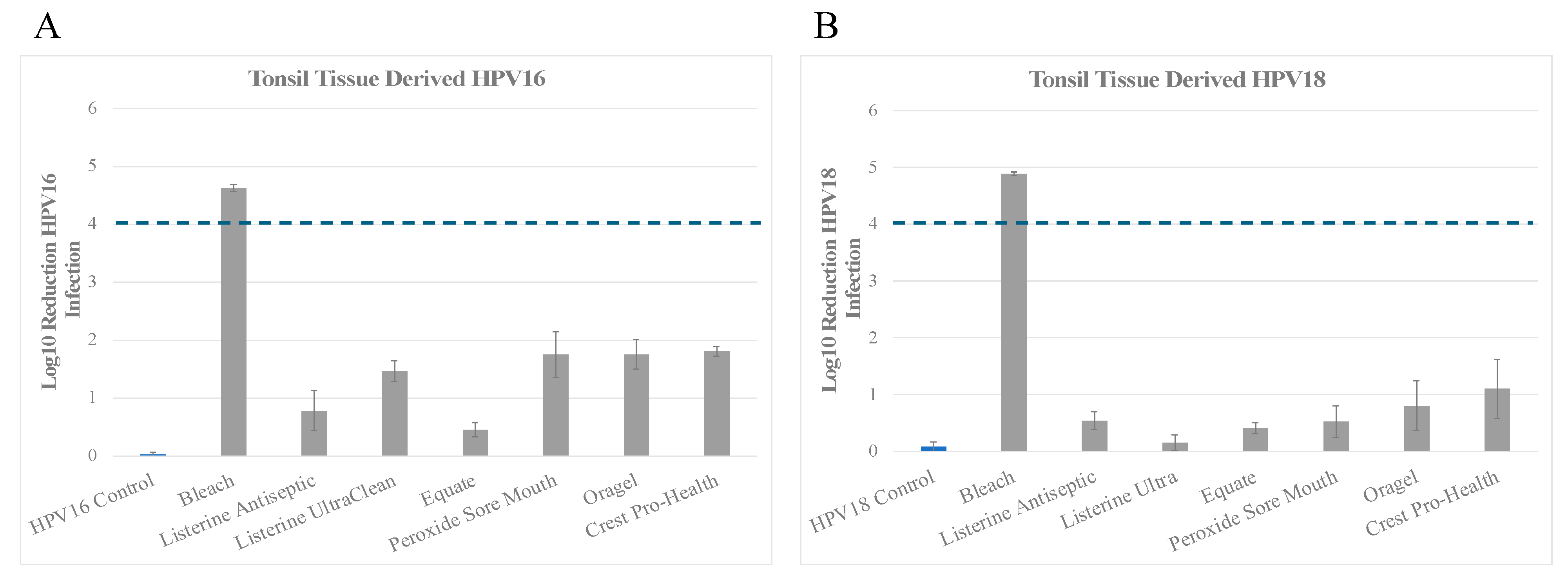
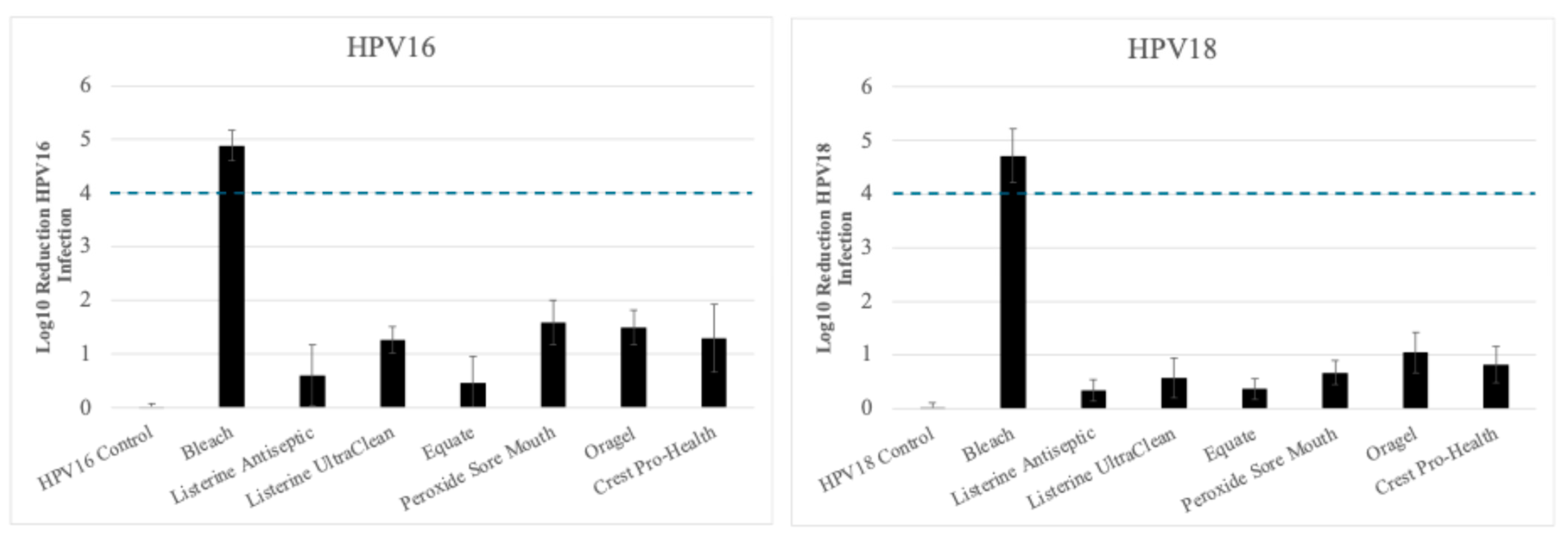
3.1. Inactivation of Tonsil Tissue Derived HPV16 (HPV16T) and HPV18 (HPV18T) Virus Stocks
Inactivation of HPV18T
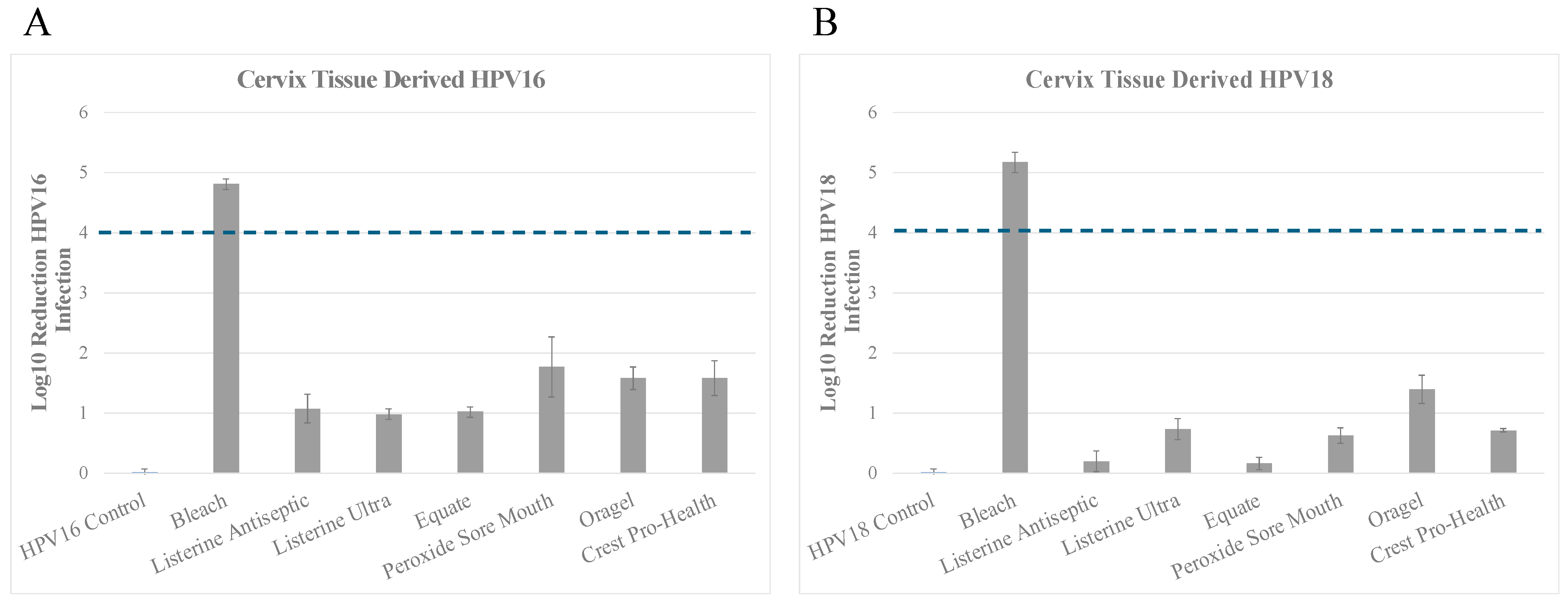
3.2. Inactivation of HPV16 (HPV16Cx) and HPV18 (HPV18Cx) Derived from Cervix Tissues
Inactivation of HPV18Cx
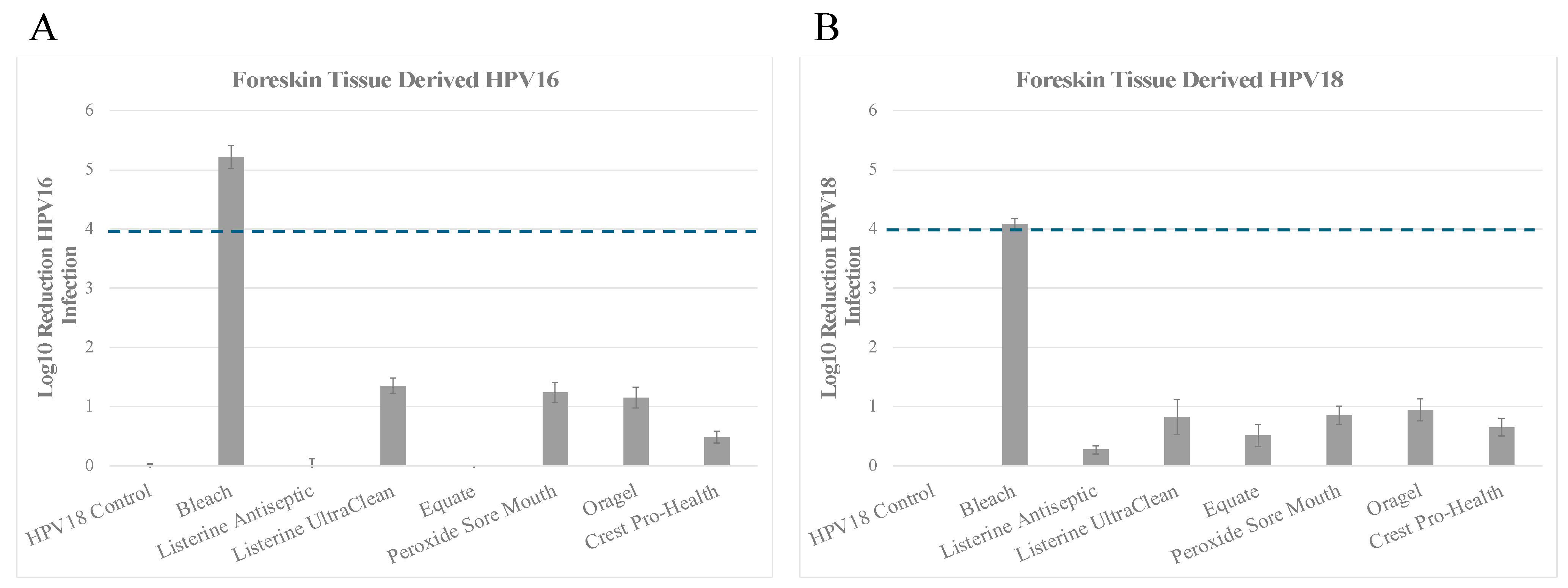
3.3. Inactivation of HPV16 (HPV16F) and HPV18 (HPV18F) Derived from Foreskin Tissues
Inactivation of HPV18F
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef]
- Chang, E.T.; Liu, Z.; Hildesheim, A.; Liu, Q.; Cai, Y.; Zhang, Z.; Chen, G.; Xie, S.H.; Cao, S.M.; Shao, J.Y.; et al. Active and Passive Smoking and Risk of Nasopharyngeal Carcinoma: A Population-Based Case-Control Study in Southern China. Am. J. Epidemiol. 2017, 185, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Wilson, L.F. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiol. 2016, 44, 203–221. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsague, X.; Laporte, L.; Bosch, F.X.; de Sanjose, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- World Health Organization. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans: Human Papillomaviruses; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.; Rosenberg, P.S.; Bray, F.; Gillison, M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013, 31, 4550–4559. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; D’Souza, G.; Gillison, M.L.; Katki, H.A. Burden of HPV-positive oropharynx cancers among ever and never smokers in the U.S. population. Oral Oncol. 2016, 60, 61–67. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Agency for Research on Cancer (IARC). Human Papillomaviruses. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Geneva, Switzerland, 2007; Volume 90, pp. 47–79. [Google Scholar]
- Syrjanen, S.; Lodi, G.; von Bultzingslowen, I.; Aliko, A.; Arduino, P.; Campisi, G.; Challacombe, S.; Ficarra, G.; Flaitz, C.; Zhou, H.M.; et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011, 17 (Suppl. S1), 58–72. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef]
- Castellsague, X.; Alemany, L.; Quer, M.; Halec, G.; Quiros, B.; Tous, S.; Clavero, O.; Alos, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef]
- Walline, H.M.; Komarck, C.; McHugh, J.B.; Byrd, S.A.; Spector, M.E.; Hauff, S.J.; Graham, M.P.; Bellile, E.; Moyer, J.S.; Prince, M.E.; et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and oral cavity cancers: Comparison of multiple methods. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1320–1327. [Google Scholar] [CrossRef]
- Van Dyne, E.A.; Henley, S.J.; Saraiya, M.; Thomas, C.C.; Markowitz, L.E.; Benard, V.B. Trends in Human Papillomavirus-Associated Cancers-United States, 1999–2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 918–924. [Google Scholar] [CrossRef]
- Preti, M.; Boldorini, R.; Gallio, N.; Cavagnetto, C.; Borella, F.; Pisapia, E.; Ribaldone, R.; Bovio, E.; Bertero, L.; Airoldi, C.; et al. Human papillomavirus genotyping in high-grade vaginal intraepithelial neoplasia: A multicentric Italian study. J. Med. Virol. 2024, 96, e29474. [Google Scholar] [CrossRef]
- Preti, M.; Rotondo, J.C.; Holzinger, D.; Micheletti, L.; Gallio, N.; McKay-Chopin, S.; Carreira, C.; Privitera, S.S.; Watanabe, R.; Ridder, R.; et al. Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect. Agent. Cancer 2020, 15, 20. [Google Scholar] [CrossRef]
- zur Hausen, H. The search for infectious causes of human cancers: Where and why (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2009, 48, 5798–5808. [Google Scholar] [CrossRef]
- Haedicke, J.; Iftner, T. Human papillomaviruses and cancer. Radiother. Oncol. 2013, 108, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Simard, E.P.; Dorell, C.; Noone, A.M.; Markowitz, L.E.; Kohler, B.; Eheman, C.; Saraiya, M.; Bandi, P.; Saslow, D.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J. Natl. Cancer Inst. 2013, 105, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Alemany, L.; Snijders, P.J.; Chaturvedi, A.; Steinberg, B.M.; Schwartz, S.; Castellsague, X. Human papillomavirus and diseases of the upper airway: Head and neck cancer and respiratory papillomatosis. Vaccine 2012, 30 (Suppl. S5), F34–F54. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef]
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef]
- Mirabello, L.; Clarke, M.A.; Nelson, C.W.; Dean, M.; Wentzensen, N.; Yeager, M.; Cullen, M.; Boland, J.F.; Workshop, N.H.; Schiffman, M.; et al. The Intersection of HPV Epidemiology, Genomics and Mechanistic Studies of HPV-Mediated Carcinogenesis. Viruses 2018, 10, 80. [Google Scholar] [CrossRef]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens--Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef]
- Stein, A.P.; Saha, S.; Kraninger, J.L.; Swick, A.D.; Yu, M.; Lambert, P.F.; Kimple, R.J. Prevalence of Human Papillomavirus in Oropharyngeal Cancer: A Systematic Review. Cancer J. 2015, 21, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.; Clifford, G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjose, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef]
- Gravitt, P.E. The known unknowns of HPV natural history. J. Clin. Investig. 2011, 121, 4593–4599. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229. [Google Scholar] [CrossRef]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef]
- Strander, B.; Ryd, W.; Wallin, K.L.; Warleby, B.; Zheng, B.; Milsom, I.; Gharizadeh, B.; Pourmand, N.; Andersson-Ellstrom, A. Does HPV-status 6-12 months after treatment of high grade dysplasia in the uterine cervix predict long term recurrence? Eur. J. Cancer 2007, 43, 1849–1855. [Google Scholar] [CrossRef]
- Rautava, J.; Willberg, J.; Louvanto, K.; Wideman, L.; Syrjanen, K.; Grenman, S.; Syrjanen, S. Prevalence, genotype distribution and persistence of human papillomavirus in oral mucosa of women: A six-year follow-up study. PLoS ONE 2012, 7, e42171. [Google Scholar] [CrossRef][Green Version]
- Kero, K.; Rautava, J.; Syrjanen, K.; Grenman, S.; Syrjanen, S. Oral mucosa as a reservoir of human papillomavirus: Point prevalence, genotype distribution, and incident infections among males in a 7-year prospective study. Eur. Urol. 2012, 62, 1063–1070. [Google Scholar] [CrossRef]
- Pickard, R.K.; Xiao, W.; Broutian, T.R.; He, X.; Gillison, M.L. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sex. Transm. Dis. 2012, 39, 559–566. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Agrawal, Y.; Halpern, J.; Bodison, S.; Gillison, M.L. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J. Infect. Dis. 2009, 199, 1263–1269. [Google Scholar] [CrossRef]
- Chung, C.H.; Bagheri, A.; D’Souza, G. Epidemiology of oral human papillomavirus infection. Oral Oncol. 2014, 50, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Gooi, Z.; Chan, J.Y.; Fakhry, C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope 2016, 126, 894–900. [Google Scholar] [CrossRef]
- D’Souza, G.; Cullen, K.; Bowie, J.; Thorpe, R.; Fakhry, C. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS ONE 2014, 9, e86023. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Felsher, M.; Waterboer, T.; Mirghani, H.; Mehanna, H.; Roberts, C.; Chen, Y.T.; Lynam, M.; Pedros, M.; Sanchez, E.; et al. Oral Human Papillomavirus Prevalence and Genotyping Among a Healthy Adult Population in the US. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 783–795. [Google Scholar] [CrossRef]
- Farsi, N.J.; El-Zein, M.; Gaied, H.; Lee, Y.C.; Hashibe, M.; Nicolau, B.; Rousseau, M.C. Sexual behaviours and head and neck cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2015, 39, 1036–1046. [Google Scholar] [CrossRef]
- Chancellor, J.A.; Ioannides, S.J.; Elwood, J.M. Oral and oropharyngeal cancer and the role of sexual behaviour: A systematic review. Community Dent. Oral Epidemiol. 2017, 45, 20–34. [Google Scholar] [CrossRef]
- Mork, J.; Lie, A.K.; Glattre, E.; Hallmans, G.; Jellum, E.; Koskela, P.; Moller, B.; Pukkala, E.; Schiller, J.T.; Youngman, L.; et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2001, 344, 1125–1131. [Google Scholar] [CrossRef]
- Gillison, M.L. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin. Oncol. 2004, 31, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, M.; San Giorgi, M.R.M.; Dikkers, F.G. Transmission and clearance of human papillomavirus infection in the oral cavity and its role in oropharyngeal carcinoma—A review. Rev. Med. Virol. 2023, 33, e2337. [Google Scholar] [CrossRef]
- Ernster, J.A.; Sciotto, C.G.; O’Brien, M.M.; Finch, J.L.; Robinson, L.J.; Willson, T.; Mathews, M. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope 2007, 117, 2115–2128. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, K.R.; Burchell, A.N.; Ramanakumar, A.V.; Rodrigues, A.; Tellier, P.P.; Hanley, J.; Coutlee, F.; Franco, E.L. Sexual transmission of oral human papillomavirus infection among men. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2959–2964. [Google Scholar] [CrossRef]
- Kellokoski, J.K.; Syrjanen, S.M.; Chang, F.; Yliskoski, M.; Syrjanen, K.J. Southern blot hybridization and PCR in detection of oral human papillomavirus (HPV) infections in women with genital HPV infections. J. Oral Pathol. Med. 1992, 21, 459–464. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Bhatia, R.K.; Messeguer, A.L.; Gonzalez, P.; Herrero, R.; Giuliano, A.R. Oral human papillomavirus in healthy individuals: A systematic review of the literature. Sex. Transm. Dis. 2010, 37, 386–391. [Google Scholar] [CrossRef]
- D’Souza, G.; Kluz, N.; Wentz, A.; Youngfellow, R.M.; Griffioen, A.; Stammer, E.; Guo, Y.; Xiao, W.; Gillison, M.L. Oral Human Papillomavirus (HPV) Infection among Unvaccinated High-Risk Young Adults. Cancers 2014, 6, 1691–1704. [Google Scholar] [CrossRef]
- Rositch, A.F.; Burke, A.E.; Viscidi, R.P.; Silver, M.I.; Chang, K.; Gravitt, P.E. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: Acquisition and reactivation in older women. Cancer Res. 2012, 72, 6183–6190. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. NHANES 2009–2012 Findings: Association of Sexual Behaviors with Higher Prevalence of Oral Oncogenic Human Papillomavirus Infections in U.S. Men. Cancer Res. 2015, 75, 2468–2477. [Google Scholar] [CrossRef]
- Laprise, C.; Madathil, S.A.; Schlecht, N.F.; Castonguay, G.; Soulieres, D.; Nguyen-Tan, P.F.; Allison, P.; Coutlee, F.; Hier, M.; Rousseau, M.C.; et al. Increased risk of oropharyngeal cancers mediated by oral human papillomavirus infection: Results from a Canadian study. Head Neck 2019, 41, 678–685. [Google Scholar] [CrossRef]
- Ragin, C.; Edwards, R.; Larkins-Pettigrew, M.; Taioli, E.; Eckstein, S.; Thurman, N.; Bloome, J.; Markovic, N. Oral HPV infection and sexuality: A cross-sectional study in women. Int. J. Mol. Sci. 2011, 12, 3928–3940. [Google Scholar] [CrossRef] [PubMed]
- Pytynia, K.B.; Dahlstrom, K.R.; Sturgis, E.M. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014, 50, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.F.; Eisenberg, M.C.; Carey, T.E.; Meza, R. Multisite HPV infections in the United States (NHANES 2003–2014): An overview and synthesis. Prev. Med. 2019, 123, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Xiao, W.; Pickard, R.K.L.; Kahle, L.; Gillison, M.L. Prevalence of Oral HPV Infection in Unvaccinated Men and Women in the United States, 2009–2016. JAMA 2019, 322, 977–979. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Klussmann, J.P.; San Giorgi, M.R.; Wuerdemann, N.; Dikkers, F.G. Oral and laryngeal HPV infection: Incidence, prevalence and risk factors, with special regard to concurrent infection in head, neck and genitals. Vaccine 2021, 39, 2344–2350. [Google Scholar] [CrossRef]
- Ali, A.; Lassi, Z.S.; Kapellas, K.; Jamieson, L.; Rumbold, A.R. A systematic review and meta-analysis of the association between periodontitis and oral high-risk human papillomavirus infection. J. Public Health 2021, 43, e610–e619. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e576. [Google Scholar] [CrossRef]
- Galvao-Moreira, L.V.; da Cruz, M.C. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016, 53, 17–19. [Google Scholar] [CrossRef]
- Martinez, A.; Kuraji, R.; Kapila, Y.L. The human oral virome: Shedding light on the dark matter. Periodontology 2000 2021, 87, 282–298. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Tezal, M.; Sullivan, M.A.; Hyland, A.; Marshall, J.R.; Stoler, D.; Reid, M.E.; Loree, T.R.; Rigual, N.R.; Merzianu, M.; Hauck, L.; et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Shigeishi, H.; Sugiyama, M.; Ohta, K. Relationship between the prevalence of oral human papillomavirus DNA and periodontal disease (Review). Biomed. Rep. 2021, 14, 40. [Google Scholar] [CrossRef]
- Tezal, M.; Wactawski-Wende, J.; Grossi, S.G.; Dmochowski, J.; Genco, R.J. Periodontal disease and the incidence of tooth loss in postmenopausal women. J. Periodontol. 2005, 76, 1123–1128. [Google Scholar] [CrossRef]
- Furquim, C.P.; Soares, G.M.; Ribeiro, L.L.; Azcarate-Peril, M.A.; Butz, N.; Roach, J.; Moss, K.; Bonfim, C.; Torres-Pereira, C.C.; Teles, F.R. The Salivary Microbiome and Oral Cancer Risk: A Pilot Study in Fanconi Anemia. J. Dent. Res. 2017, 96, 292–299. [Google Scholar] [CrossRef]
- Tezal, M. Interaction between Chronic Inflammation and Oral HPV Infection in the Etiology of Head and Neck Cancers. Int. J. Otolaryngol. 2012, 2012, 575242. [Google Scholar] [CrossRef]
- Prabhu, S.R.; Wilson, D.F. Human papillomavirus and oral disease-emerging evidence: A review. Aust. Dent. J. 2013, 58, 2–10, quiz 125. [Google Scholar] [CrossRef]
- Syrjanen, S. Oral manifestations of human papillomavirus infections. Eur. J. Oral Sci. 2018, 126 (Suppl. S1), 49–66. [Google Scholar] [CrossRef]
- Faraji, F.; Zaidi, M.; Fakhry, C.; Gaykalova, D.A. Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect. 2017, 19, 464–475. [Google Scholar] [CrossRef]
- Hormia, M.; Willberg, J.; Ruokonen, H.; Syrjanen, S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. J. Periodontol. 2005, 76, 358–363. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gorbach, P.M.; Gratzer, B.; Steinau, M.; Collins, T.; Parrish, A.; Kerndt, P.R.; Crosby, R.A.; Unger, E.R.; Markowitz, L.E.; et al. Risk Factors for Oral Human Papillomavirus Infection Among Young Men Who Have Sex With Men-2 Cities, United States, 2012–2014. Sex. Transm. Dis. 2018, 45, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Kay, P.; Meehan, K.; Williamson, A.L. The use of nested polymerase chain reaction and restriction fragment length polymorphism for the detection and typing of mucosal human papillomaviruses in samples containing low copy numbers of viral DNA. J. Virol. Methods 2002, 105, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernandez, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Pierce Campbell, C.M.; Lin, H.Y.; Fulp, W.; Papenfuss, M.R.; Abrahamsen, M.; Hildesheim, A.; Villa, L.L.; Salmeron, J.J.; Lazcano-Ponce, E.; et al. Incidence and clearance of oral human papillomavirus infection in men: The HIM cohort study. Lancet 2013, 382, 877–887. [Google Scholar] [CrossRef]
- D’Souza, G.; Fakhry, C.; Sugar, E.A.; Seaberg, E.C.; Weber, K.; Minkoff, H.L.; Anastos, K.; Palefsky, J.M.; Gillison, M.L. Six-month natural history of oral versus cervical human papillomavirus infection. Int. J. Cancer 2007, 121, 143–150. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, F.; Pan, Y.; Deng, Q.; Li, X.; He, Z.; Liu, M.; Ning, T.; Guo, C.; Liang, Y.; et al. Incidence and clearance of oral human papillomavirus infection: A population-based cohort study in rural China. Oncotarget 2017, 8, 59831–59844. [Google Scholar] [CrossRef][Green Version]
- Hang, D.; Liu, F.; Liu, M.; He, Z.; Sun, M.; Liu, Y.; Li, J.; Pan, Y.; Ning, T.; Guo, C.; et al. Oral human papillomavirus infection and its risk factors among 5,410 healthy adults in China, 2009–2011. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2101–2110. [Google Scholar] [CrossRef]
- Muller, K.; Kazimiroff, J.; Fatahzadeh, M.; Smith, R.V.; Wiltz, M.; Polanco, J.; Grossberg, R.M.; Belbin, T.J.; Strickler, H.D.; Burk, R.D.; et al. Oral Human Papillomavirus Infection and Oral Lesions in HIV-Positive and HIV-Negative Dental Patients. J. Infect. Dis. 2015, 212, 760–768. [Google Scholar] [CrossRef]
- D’Souza, G.; McNeel, T.S.; Fakhry, C. Understanding personal risk of oropharyngeal cancer: Risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann. Oncol. 2017, 28, 3065–3069. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Shiels, M.S.; Fakhry, C.; Johansson, M.; Pawlita, M.; Brennan, P.; Hildesheim, A.; Waterboer, T. Screening for human papillomavirus-driven oropharyngeal cancer: Considerations for feasibility and strategies for research. Cancer 2018, 124, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Rosenbaum, E.; Carvalho, A.L.; Koch, W.; Jiang, W.; Sidransky, D.; Califano, J. Feasibility of quantitative PCR-based saliva rinse screening of HPV for head and neck cancer. Int. J. Cancer 2005, 117, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Ryndock, E.; Robison, R.; Meyers, C. Susceptibility of HPV16 and 18 to high level disinfectants indicated for semi-critical ultrasound probes. J. Med. Virol. 2016, 88, 1076–1080. [Google Scholar] [CrossRef]
- Meyers, J.; Ryndock, E.; Conway, M.J.; Meyers, C.; Robison, R. Susceptibility of high-risk human papillomavirus type 16 to clinical disinfectants. J. Antimicrob. Chemother. 2014, 69, 1546–1550. [Google Scholar] [CrossRef]
- Alam, S.; Chatterjee, S.; Kang, S.D.; Milici, J.; Biryukov, J.; Chen, H.; Meyers, C. Anti-Retroviral Protease Inhibitors Regulate Human Papillomavirus 16 Infection of Primary Oral and Cervical Epithelium. Cancers 2020, 12, 2664. [Google Scholar] [CrossRef]
- Conway, M.J.; Alam, S.; Ryndock, E.J.; Cruz, L.; Christensen, N.D.; Roden, R.B.; Meyers, C. Tissue-spanning redox gradient-dependent assembly of native human papillomavirus type 16 virions. J. Virol. 2009, 83, 10515–10526. [Google Scholar] [CrossRef]
- Meyers, C.; Milici, J.; Robison, R. UVC radiation as an effective disinfectant method to inactivate human papillomaviruses. PLoS ONE 2017, 12, e0187377. [Google Scholar] [CrossRef]
- Meyers, C. Organotypic (raft) epithelial tissue culture system for the differentiation-dependent replication of papillomavirus. Methods Cell Sci. 1996, 18, 201–210. [Google Scholar]
- Biryukov, J.; Cruz, L.; Ryndock, E.J.; Meyers, C. Native human papillomavirus production, quantification, and infectivity analysis. Methods Mol. Biol. 2015, 1249, 317–331. [Google Scholar] [CrossRef]
- Seedorf, K.; Krammer, G.; Durst, M.; Suhai, S.; Rowekamp, W.G. Human papillomavirus type 16 DNA sequence. Virology 1985, 145, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Durst, M.; Gissmann, L.; Ikenberg, H.; zur Hausen, H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl. Acad. Sci. USA 1983, 80, 3812–3815. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.; Mayer, T.J.; Ozbun, M.A. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J. Virol. 1997, 71, 7381–7386. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, J.; Meyers, C. Superinfection Exclusion between Two High-Risk Human Papillomavirus Types during a Coinfection. J. Virol. 2018, 92, 1–20. [Google Scholar] [CrossRef]
- Robins, L.I.; Clark, A.; Gafken, P.R.; Alam, S.; Milici, J.; Hassan, R.; Wang, C.Y.; Williams, J.; Meyers, C. Hypochlorous acid as a disinfectant for high-risk HPV: Insight into the mechanism of action. J. Med. Virol. 2022, 94, 3386–3393. [Google Scholar] [CrossRef]
- Meyers, C.; Milici, J.; Robison, R. The ability of two chlorine dioxide chemistries to inactivate human papillomavirus-contaminated endocavitary ultrasound probes and nasendoscopes. J. Med. Virol. 2020, 92, 1298–1302. [Google Scholar] [CrossRef]
- Meyers, C.; Kass, R.; Goldenberg, D.; Milici, J.; Alam, S.; Robison, R. Ethanol and isopropanol inactivation of human coronavirus on hard surfaces. J. Hosp. Infect. 2021, 107, 45–49. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Levine, R.L.; Moskovitz, J.; Stadtman, E.R. Oxidation of methionine in proteins: Roles in antioxidant defense and cellular regulation. IUBMB Life 2000, 50, 301–307. [Google Scholar] [CrossRef]
- Garrido Ruiz, D.; Sandoval-Perez, A.; Rangarajan, A.V.; Gunderson, E.L.; Jacobson, M.P. Cysteine Oxidation in Proteins: Structure, Biophysics, and Simulation. Biochemistry 2022, 61, 2165–2176. [Google Scholar] [CrossRef]
- Conway, M.J.; Cruz, L.; Alam, S.; Christensen, N.D.; Meyers, C. Differentiation-dependent interpentameric disulfide bond stabilizes native human papillomavirus type 16. PLoS ONE 2011, 6, e22427. [Google Scholar] [CrossRef]
- Ryndock, E.J.; Conway, M.J.; Alam, S.; Gul, S.; Murad, S.; Christensen, N.D.; Meyers, C. Roles for human papillomavirus type 16 l1 cysteine residues 161, 229, and 379 in genome encapsidation and capsid stability. PLoS ONE 2014, 9, e99488. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.K.; Ozbun, M.A. Two highly conserved cysteine residues in HPV16 L2 form an intramolecular disulfide bond and are critical for infectivity in human keratinocytes. PLoS ONE 2009, 4, e4463. [Google Scholar] [CrossRef]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef]
- Vogt, W. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med. 1995, 18, 93–105. [Google Scholar] [CrossRef]
- Savige, W.E.; Fontana, A. Interconversion of methionine and methionine sulfoxide. Methods Enzymol. 1977, 47, 453–459. [Google Scholar] [CrossRef]
- Keremi, B.; Marta, K.; Farkas, K.; Czumbel, L.M.; Toth, B.; Szakacs, Z.; Csupor, D.; Czimmer, J.; Rumbus, Z.; Revesz, P.; et al. Effects of Chlorine Dioxide on Oral Hygiene - A Systematic Review and Meta-analysis. Curr. Pharm. Des. 2020, 26, 3015–3025. [Google Scholar] [CrossRef]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Thomas, D.; Stanton, R.; Maillard, J.Y.; Murphy, R.C.; Jones, S.A.; Humphreys, I.; Wakelam, M.J.O.; Fegan, C.; Wise, M.P.; et al. Potential Role of Oral Rinses Targeting the Viral Lipid Envelope in SARS-CoV-2 Infection. Function 2020, 1, zqaa002. [Google Scholar] [CrossRef]
- Meyers, C.; Robison, R.; Milici, J.; Alam, S.; Quillen, D.; Goldenberg, D.; Kass, R. Lowering the transmission and spread of human coronavirus. J. Med. Virol. 2021, 93, 1605–1612. [Google Scholar] [CrossRef]
- Popkin, D.L.; Zilka, S.; Dimaano, M.; Fujioka, H.; Rackley, C.; Salata, R.; Griffith, A.; Mukherjee, P.K.; Ghannoum, M.A.; Esper, F. Cetylpyridinium Chloride (CPC) Exhibits Potent, Rapid Activity Against Influenza Viruses in vitro and in vivo. Pathog. Immun. 2017, 2, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Riveira-Munoz, E.; Garcia-Vidal, E.; Bano-Polo, M.; Leon, R.; Blanc, V.; Clotet, B.; Ballana, E. Cetylpyridinium Chloride-Containing Mouthwashes Show Virucidal Activity against Herpes Simplex Virus Type 1. Viruses 2023, 15, 1433. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, C.; Al-Ahmad, A.; Hellwig, E.; Chrubasik, S. Listerine(R) Products: An Update on the Efficacy and Safety. Phytother. Res. 2016, 30, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 2017, 6, e00459. [Google Scholar] [CrossRef]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- D’Souza, G.; Zhang, H.H.; D’Souza, W.D.; Meyer, R.R.; Gillison, M.L. Moderate predictive value of demographic and behavioral characteristics for a diagnosis of HPV16-positive and HPV16-negative head and neck cancer. Oral Oncol. 2010, 46, 100–104. [Google Scholar] [CrossRef]
- Heck, J.E.; Berthiller, J.; Vaccarella, S.; Winn, D.M.; Smith, E.M.; Shan’gina, O.; Schwartz, S.M.; Purdue, M.P.; Pilarska, A.; Eluf-Neto, J.; et al. Sexual behaviours and the risk of head and neck cancers: A pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int. J. Epidemiol. 2010, 39, 166–181. [Google Scholar] [CrossRef]
- D’Souza, G.; Westra, W.H.; Wang, S.J.; van Zante, A.; Wentz, A.; Kluz, N.; Rettig, E.; Ryan, W.R.; Ha, P.K.; Kang, H.; et al. Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncol. 2017, 3, 169–177. [Google Scholar] [CrossRef]
- Damgacioglu, H.; Sonawane, K.; Zhu, Y.; Li, R.; Balasubramanian, B.A.; Lairson, D.R.; Giuliano, A.R.; Deshmukh, A.A. Oropharyngeal Cancer Incidence and Mortality Trends in All 50 States in the US, 2001–2017. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 155–165. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Cook-Wiens, G.; Yoshida, E.; Shiao, S.L.; Lee, N.Y.; Mita, A.; Jeon, C.; Goodman, M.T.; Ho, A.S. Incidence of Oropharyngeal Cancer Among Elderly Patients in the United States. JAMA Oncol. 2016, 2, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, D.R.; Egerman, M.A.; Bui, A.H.; Doucette, J.T.; Sharma, S.; Liu, J.; Gupta, V.; Miles, B.A.; Genden, E.; Westra, W.H.; et al. A new face of the HPV epidemic: Oropharyngeal cancer in the elderly. Oral Oncol. 2020, 109, 104687. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; Fakhry, C.; Khararjian, A.; Westra, W.H. Age Profile of Patients With Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef]
- Schache, A.G.; Powell, N.G.; Cuschieri, K.S.; Robinson, M.; Leary, S.; Mehanna, H.; Rapozo, D.; Long, A.; Cubie, H.; Junor, E.; et al. HPV-Related Oropharynx Cancer in the United Kingdom: An Evolution in the Understanding of Disease Etiology. Cancer Res. 2016, 76, 6598–6606. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Zumsteg, Z.S. A snapshot of the evolving epidemiology of oropharynx cancers. Cancer 2018, 124, 2893–2896. [Google Scholar] [CrossRef]
- Tota, J.E.; Best, A.F.; Zumsteg, Z.S.; Gillison, M.L.; Rosenberg, P.S.; Chaturvedi, A.K. Evolution of the Oropharynx Cancer Epidemic in the United States: Moderation of Increasing Incidence in Younger Individuals and Shift in the Burden to Older Individuals. J. Clin. Oncol. 2019, 37, 1538–1546. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Chaturvedi, A.K.; Alemany, L.; Anantharaman, D.; Bray, F.; Carrington, M.; Doorbar, J.; D’Souza, G.; Fakhry, C.; Ferris, R.L.; et al. Summary from an international cancer seminar focused on human papillomavirus (HPV)-positive oropharynx cancer, convened by scientists at IARC and NCI. Oral Oncol. 2020, 108, 104736. [Google Scholar] [CrossRef]
- D’Souza, G.; Wentz, A.; Kluz, N.; Zhang, Y.; Sugar, E.; Youngfellow, R.M.; Guo, Y.; Xiao, W.; Gillison, M.L. Sex Differences in Risk Factors and Natural History of Oral Human Papillomavirus Infection. J. Infect. Dis. 2016, 213, 1893–1896. [Google Scholar] [CrossRef]
- Sonawane, K.; Shyu, S.S.; Damgacioglu, H.; Li, R.; Nyitray, A.G.; Deshmukh, A.A. Prevalence and concordance of oral and genital HPV by sexual orientation among US men. JNCI Cancer Spectr. 2023, 7, pkac088. [Google Scholar] [CrossRef]
- Beder Ribeiro, C.M.; Ferrer, I.; Santos de Farias, A.B.; Fonseca, D.D.; Morais Silva, I.H.; Monteiro Gueiros, L.A.; Carvalho, A.T.; Porter, S.R.; Leao, J.C. Oral and genital HPV genotypic concordance between sexual partners. Clin. Oral Investig. 2014, 18, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kero, K.; Rautava, J. HPV Infections in Heterosexual Couples: Mechanisms and Covariates of Virus Transmission. Acta Cytol. 2019, 63, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.G.; Cruciani, M.; Scaggiante, R.; Boldrin, C.; Andreis, S.; Dal Bello, F.; Pagni, S.; Barelli, A.; Sattin, A.; Mengoli, C.; et al. Anal and oral human papillomavirus (HPV) infection in HIV-infected subjects in northern Italy: A longitudinal cohort study among men who have sex with men. BMC Infect. Dis. 2011, 11, 150. [Google Scholar] [CrossRef]
- Read, T.R.; Hocking, J.S.; Vodstrcil, L.A.; Tabrizi, S.N.; McCullough, M.J.; Grulich, A.E.; Garland, S.M.; Bradshaw, C.S.; Chen, M.Y.; Fairley, C.K. Oral human papillomavirus in men having sex with men: Risk-factors and sampling. PLoS ONE 2012, 7, e49324. [Google Scholar] [CrossRef]
- Chaitanya, N.C.; Allam, N.S.; Gandhi Babu, D.B.; Waghray, S.; Badam, R.K.; Lavanya, R. Systematic meta-analysis on association of human papilloma virus and oral cancer. J. Cancer Res. Ther. 2016, 12, 969–974. [Google Scholar] [CrossRef]
- Pugliese, D.B.; Bruzzesi, G.; Montaldo, C.; Porcu, L.; Landi, M.; Mastinu, A.; Torri, V.; Licitra, L.; Locati, L.D. Oral prevalence and clearance of oncogenic human papilloma virus in a rehabilitation community for substance abusers in Italy: A case of behavioral correction? J. Oral Pathol. Med. 2015, 44, 728–733. [Google Scholar] [CrossRef]
- Heard, I.; Palefsky, J.M.; Kazatchkine, M.D. The impact of HIV antiviral therapy on human papillomavirus (HPV) infections and HPV-related diseases. Antivir. Ther. 2004, 9, 13–22. [Google Scholar]
- Gillison, M.L. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck 2007, 29, 779–792. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Clooney, A.G.; Sutton, T.D.S.; Ryan, F.J.; Daly, K.M.; Nolan, J.A.; McDonnell, S.A.; Khokhlova, E.V.; Draper, L.A.; Forde, A.; et al. The Human Gut Virome Is Highly Diverse, Stable, and Individual Specific. Cell Host Microbe 2019, 26, 527–541.e525. [Google Scholar] [CrossRef]
- Vemuri, R.; Shankar, E.M.; Chieppa, M.; Eri, R.; Kavanagh, K. Beyond Just Bacteria: Functional Biomes in the Gut Ecosystem Including Virome, Mycobiome, Archaeome and Helminths. Microorganisms 2020, 8, 483. [Google Scholar] [CrossRef]
- Gao, L.; Kang, M.; Zhang, M.J.; Reza Sailani, M.; Kuraji, R.; Martinez, A.; Ye, C.; Kamarajan, P.; Le, C.; Zhan, L.; et al. Polymicrobial periodontal disease triggers a wide radius of effect and unique virome. NPJ Biofilms Microbiomes 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Shigeishi, H.; Sugiyama, M.; Ohta, K.; Yokoyama, S.; Sakuma, M.; Murozumi, H.; Kato, H.; Takechi, M. High HPV16 E6 viral load in the oral cavity is associated with an increased number of bacteria: A preliminary study. Biomed. Rep. 2018, 8, 59–64. [Google Scholar] [CrossRef]
- Chow, L.T.; Broker, T.R.; Steinberg, B.M. The natural history of human papillomavirus infections of the mucosal epithelia. Apmis 2010, 118, 422–449. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.C.; Markham, C.M.; Ross, M.W.; Mullen, P.D. Examining the association between oral health and oral HPV infection. Cancer Prev. Res. 2013, 6, 917–924. [Google Scholar] [CrossRef]
- Eliot, M.N.; Michaud, D.S.; Langevin, S.M.; McClean, M.D.; Kelsey, K.T. Periodontal disease and mouthwash use are risk factors for head and neck squamous cell carcinoma. Cancer Causes Control. 2013, 24, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Mazul, A.L.; Taylor, J.M.; Divaris, K.; Weissler, M.C.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Olshan, A.F.; Zevallos, J.P. Oral health and human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 2017, 123, 71–80. [Google Scholar] [CrossRef]
- Beachler, D.C.; Lang Kuhs, K.A.; Struijk, L.; Schussler, J.; Herrero, R.; Porras, C.; Hildesheim, A.; Cortes, B.; Sampson, J.; Quint, W.; et al. The Natural History of Oral Human Papillomavirus in Young Costa Rican Women. Sex. Transm. Dis. 2017, 44, 442–449. [Google Scholar] [CrossRef]
- Beachler, D.C.; Sugar, E.A.; Margolick, J.B.; Weber, K.M.; Strickler, H.D.; Wiley, D.J.; Cranston, R.D.; Burk, R.D.; Minkoff, H.; Reddy, S.; et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am. J. Epidemiol. 2015, 181, 40–53. [Google Scholar] [CrossRef]
- Beachler, D.C.; Waterboer, T.; Pierce Campbell Ch, M.; Ingles, D.J.; Kuhs, K.A.; Nyitray, A.G.; Hildesheim, A.; Pawlita, M.; Kreimer, A.R.; Giuliano, A.R. HPV16 E6 seropositivity among cancer-free men with oral, anal or genital HPV16 infection. Papillomavirus Res. 2016, 2, 141–144. [Google Scholar] [CrossRef]
- D’Souza, G.; Clemens, G.; Strickler, H.D.; Wiley, D.J.; Troy, T.; Struijk, L.; Gillison, M.; Fakhry, C. Long-term Persistence of Oral HPV Over 7 Years of Follow-up. JNCI Cancer Spectr. 2020, 4, pkaa047. [Google Scholar] [CrossRef]
- Boda, D.; Docea, A.O.; Calina, D.; Ilie, M.A.; Caruntu, C.; Zurac, S.; Neagu, M.; Constantin, C.; Branisteanu, D.E.; Voiculescu, V.; et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int. J. Oncol. 2018, 52, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.M.; Chan, J.Y.; Zhang, Z.; Wang, H.; Khan, Z.; Bishop, J.A.; Westra, W.; Koch, W.M.; Califano, J.A. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Kero, K.; Rautava, J.; Syrjanen, K.; Willberg, J.; Grenman, S.; Syrjanen, S. Smoking increases oral HPV persistence among men: 7-year follow-up study. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 123–133. [Google Scholar] [CrossRef] [PubMed]
| Product | Company | Active Ingredients | Inactive Ingredients |
|---|---|---|---|
| Listerine Antiseptic Cool Mint | Johnson and Johnson | Eucalyptol 0.092% Menthol 0.042% Methyl Salicylate 0.060% Thymol 0.064% | Water, Sodium Saccharin, Green 3, Alcohol (21.6%), Sodium Benzoate, Sorbitol, Flavor, Poloxamer 407, Benzoic acid |
| Listerine Antiseptic UltraClean | Johnson and Johnson | Eucalyptol 0.092% Menthol 0.042% Methyl Salicylate 0.060% Thymol 0.064% | Water, Zinc Chloride, Green 3, Alcohol (21.6%), Sodium Saccharin, Sorbitol, Sodium Benzoate, Poloxamer 407, Sucralose, Benzoic acid, Flavor |
| Equate Antiseptic Mouthrinse | Wal-Mart | Eucalyptol 0.092% Menthol 0.042% Methyl Salicylate 0.060% Thymol 0.064% | Water, Zinc Chloride, Alcohol (21.6%), Sodium Saccharin, Sorbitol, Sodium Benzoate, Poloxamer 407, Benzoic acid, FD&C Green no. 3, Flavor |
| Peroxide Sore Mouth Cleanser | CVS | Hydrogen peroxide 150 mg/10 mL | Water, Methyl salicylate, Sorbitol, Menthol, Propylene glycol, Sodium saccharin, Poloxamer 338, Blue 1, Polysorbate 20 |
| Oragel 2X Mouth Sores Rinse Medicated | Church and Dwight Co., Inc. | Hydrogen peroxide 1.5% Menthol 0.1% | Water, Alcohol (4.1% by vol.), Phosphoric acid, Sorbitol, Disodium EDTA, Poloxamer 338, FD&C Blue no.1, Polysorbate 20, Methyl salicylate, Sodium Saccharin |
| Crest Pro-Health | Proctor and Gamble | Cetylpyridinium chloride 0.1% | Water, Hydrogen peroxide, Glycerin, Sucralose Flavor, Poloxamer 407 |
| A. | ||
| Product | Tonsil Tissue Derived HPV16 (log10 reduction) contact time 30 s | % Inactivation |
| No treatment Control | 0.031 ± 0.035 | No change |
| Bleach | 4.627 ± 0.062 | Between >4 and <5 log10 (>99.99% to <99.999%) |
| Listerine Antiseptic Cool Mint | 0.787 ± 0.348 | <1 log10 (<90%) |
| Listerine Antiseptic UltraClean | 1.461 ± 0.181 | Between >1 and <2 log10 (>90% to <99%) |
| Equate Antiseptic Mouthrinse | 0.452 ± 0.121 | <1 log10 (<90%) |
| Peroxide Sore Mouth Cleanser | 1.751 ± 0.398 | Between >1 and <2 log10 (>90% to <99%) |
| Oragel 2X Mouth Sores Rinse Medicated | 1.753 ± 0.250 | Between >1 and <2 log10 (>90% to <99%) |
| Crest Pro-Health | 1.810 ± 0.082 | Between >1 and <2 log10 (>90% to <99%) |
| B. | ||
| Product | Tonsil Tissue Derived HPV18 (log10 reduction) contact time 30 s | % Inactivation |
| No treatment Control | 0.088 ± 0.083 | No change |
| Bleach | 4.89 ± 0.030 | Between >4 and <5 log10 (>99.99% to <99.999%) |
| Listerine Antiseptic Cool Mint | 0.545 ± 0.115 | <1 log10 (<90%) |
| Listerine Antiseptic UltraClean | 0.155 ± 0.141 | <1 log10 (<90%) |
| Equate Antiseptic Mouthrinse | 0.411 ± 0.097 | <1 log10 (<90%) |
| Peroxide Sore Mouth Cleanser | 0.525 ± 0.280 | <1 log10 (<90%) |
| Oragel 2X Mouth Sores Rinse Medicated | 0.806 ± 0.438 | <1 log10 (<90%) |
| Crest Pro-Health | 1.104 ± 0.516 | Between >1 and <2 log10 (>90% to <99%) |
| A. | ||
| Product | Cervix Tissue Derived HPV16 (log10 reduction) contact time 30 s | % Inactivation |
| No treatment Control | 0.022 ± 0.055 | No change |
| Bleach | 4.813 ± 0.086 | Between >4 and <5 log10 (>99.99% to <99.999%) |
| Listerine Antiseptic Cool Mint | 1.078 ± 0.241 | Between >1 and <2 log10 (>90% to <99%) |
| Listerine Antiseptic UltraClean | 0.984 ± 0.086 | <1 log10 (<90%) |
| Equate Antiseptic Mouthrinse | 1.022 ± 0.089 | Between >1 and <2 log10 (>90% to <99%) |
| Peroxide Sore Mouth Cleanser | 1.773 ± 0.496 | Between >1 and <2 log10 (>90% to <99%) |
| Oragel 2X Mouth Sores Rinse Medicated | 1.584 ± 0.183 | Between >1 and <2 log10 (>90% to <99%) |
| Crest Pro-Health | 1.586 ± 0.293 | Between >1 and <2 log10 (>90% to <99%) |
| B. | ||
| Product | Cervix Tissue Derived HPV18 (log10 reduction) contact time 30 s | % Inactivation |
| No treatment Control | 0.018 ± 0.059 | No change |
| Bleach | 5.175 ± 0.171 | Between >4 and <5 log10 (>99.99% to <99.999%) |
| Listerine Antiseptic Cool Mint | 0.203 ± 0.177 | <1 log10 (<90%) |
| Listerine Antiseptic UltraClean | 0.741 ± 0.175 | <1 log10 (<90%) |
| Equate Antiseptic Mouthrinse | 0.167 ± 0.101 | <1 log10 (<90%) |
| Peroxide Sore Mouth Cleanser | 0.633 ± 0.132 | <1 log10 (<90%) |
| Oragel 2X Mouth Sores Rinse Medicated | 1.399 ± 0.230 | Between >1 and <2 log10 (>90% to <99%) |
| Crest Pro-Health | 0.716 ± 0.029 | <1 log10 (<90%) |
| A. | ||
| Product | Foreskin Tissue Derived HPV16 (log10 reduction) contact time 30 s | % Inactivation |
| No treatment Control | −0.096 ± 0.134 | No change |
| Bleach | 5.220 ± 0.188 | Between >4 and <5 log10 (>99.99% to <99.999%) |
| Listerine Antiseptic Cool Mint | −0.074 ± 0.200 | <1 log10 (<90%) |
| Listerine Antiseptic UltraClean | 1.356 ± 0.122 | Between >1 and <2 log10 (>90% to <99%) |
| Equate Antiseptic Mouthrinse | −0.099 ± 0.104 | <1 log10 (<90%) |
| Peroxide Sore Mouth Cleanser | 1.240 ± 0.168 | Between >1 and <2 log10 (>90% to <99%) |
| Oragel 2X Mouth Sores Rinse Medicated | 1.154 ± 0.179 | Between >1 and <2 log10 (>90% to <99%) |
| Crest Pro-Health | 0.485 ± 0.097 | <1 log10 (<90%) |
| B. | ||
| Product | Foreskin Tissue Derived HPV18 (log10 reduction) contact time 30 s | % Inactivation |
| No treatment Control | −0.097 ± 0.095 | No change |
| Bleach | 4.083 ± 0.090 | Between >4 and <5 log10 (>99.99% to <99.999%) |
| Listerine Antiseptic Cool Mint | 0.273 ± 0.073 | <1 log10 (<90%) |
| Listerine Antiseptic UltraClean | 0.825 ± 0.295 | <1 log10 (<90%) |
| Equate Antiseptic Mouthrinse | 0.519 ± 0.191 | <1 log10 (<90%) |
| Peroxide Sore Mouth Cleanser | 0.858 ± 0.153 | <1 log10 (<90%) |
| Oragel 2X Mouth Sores Rinse Medicated | 0.944 ± 0.189 | <1 log10 (<90%) |
| Crest Pro-Health | 0.655 ± 0.144 | <1 log10 (<90%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, S.; Avila, J.; Barrett, W.; Meyers, C. Comparing In Vitro Virucidal Efficacy of Commercially Available Mouthwashes Against Native High-Risk Human Papillomavirus Types 16 and 18. Microorganisms 2025, 13, 734. https://doi.org/10.3390/microorganisms13040734
Alam S, Avila J, Barrett W, Meyers C. Comparing In Vitro Virucidal Efficacy of Commercially Available Mouthwashes Against Native High-Risk Human Papillomavirus Types 16 and 18. Microorganisms. 2025; 13(4):734. https://doi.org/10.3390/microorganisms13040734
Chicago/Turabian StyleAlam, Samina, Jesus Avila, William Barrett, and Craig Meyers. 2025. "Comparing In Vitro Virucidal Efficacy of Commercially Available Mouthwashes Against Native High-Risk Human Papillomavirus Types 16 and 18" Microorganisms 13, no. 4: 734. https://doi.org/10.3390/microorganisms13040734
APA StyleAlam, S., Avila, J., Barrett, W., & Meyers, C. (2025). Comparing In Vitro Virucidal Efficacy of Commercially Available Mouthwashes Against Native High-Risk Human Papillomavirus Types 16 and 18. Microorganisms, 13(4), 734. https://doi.org/10.3390/microorganisms13040734







