Gut–X Axis and Its Role in Poultry Bone Health: A Review
Abstract
1. Introduction
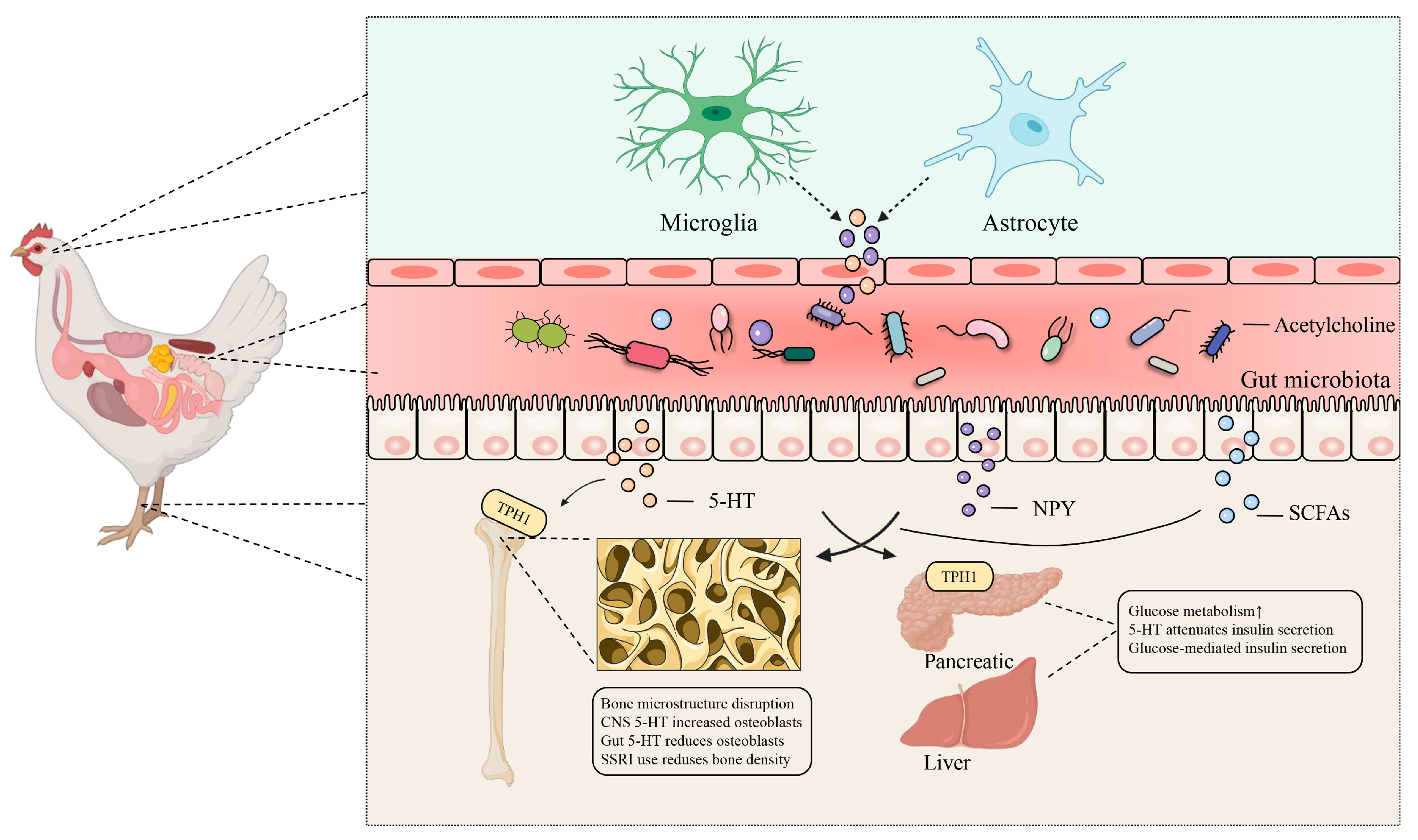
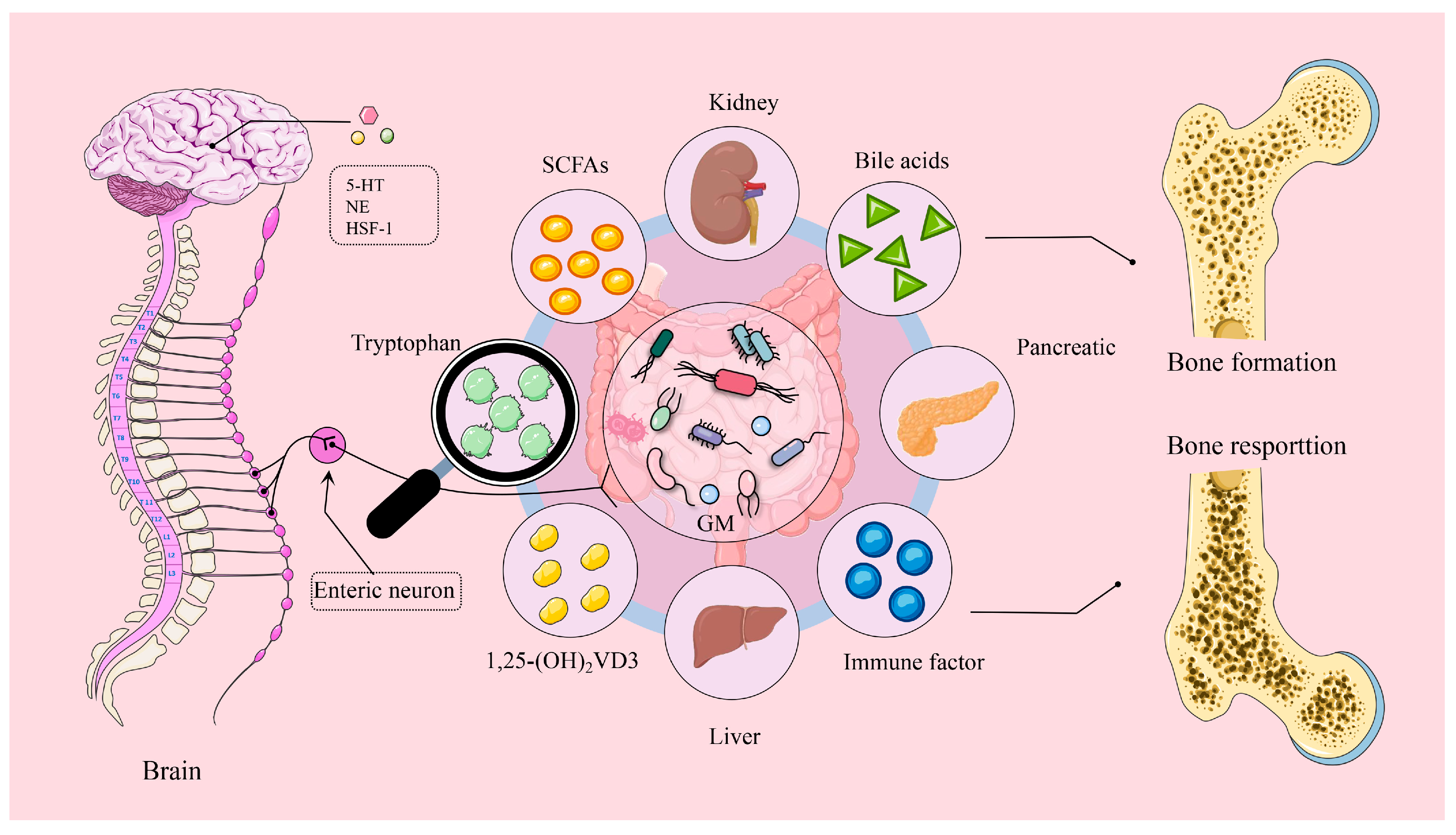
2. Brain–Gut–Bone Axis: Transmitting Sensory Signals That Drive Gut Microbiota
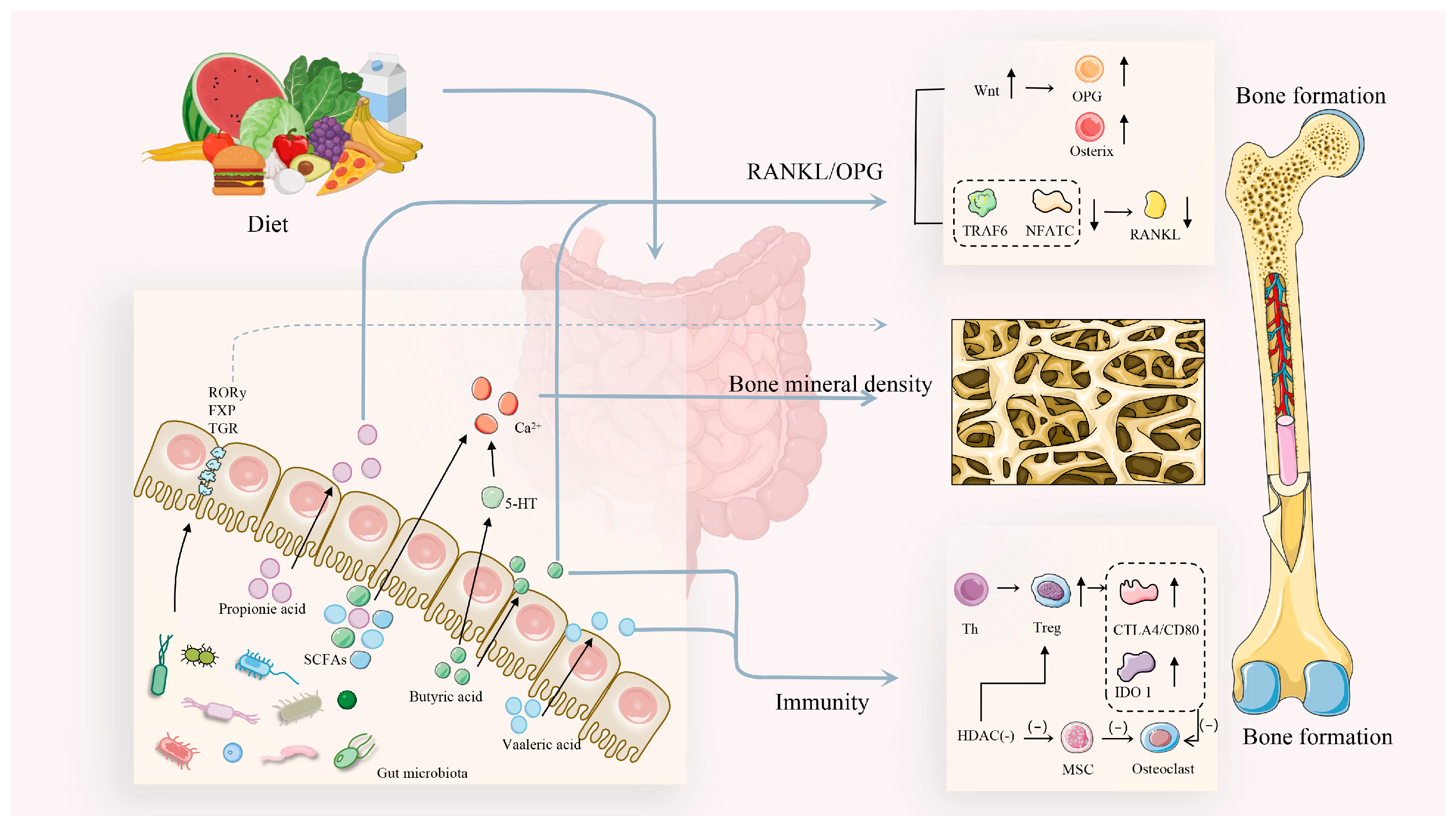
3. Gut–Bone Axis: Bridging Bone Health Through Metabolites
3.1. Short-Chain Fatty Acids and Bone Health
3.2. Bile Acids and Bone Health
3.3. Tryptophan Metabolites and Bone Health
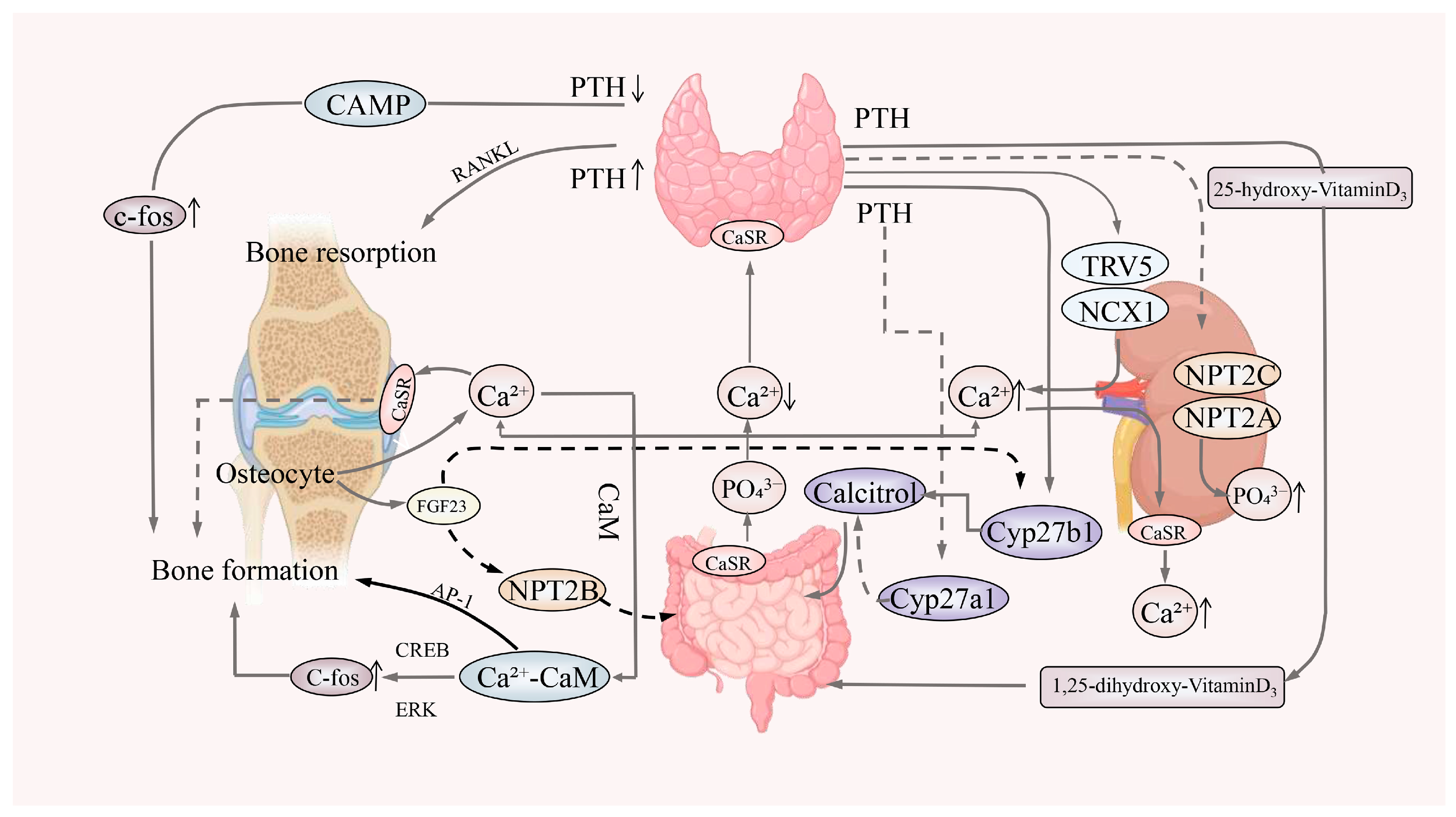
3.4. 1,25-Dihydroxyvitamin D3 and Bone Health
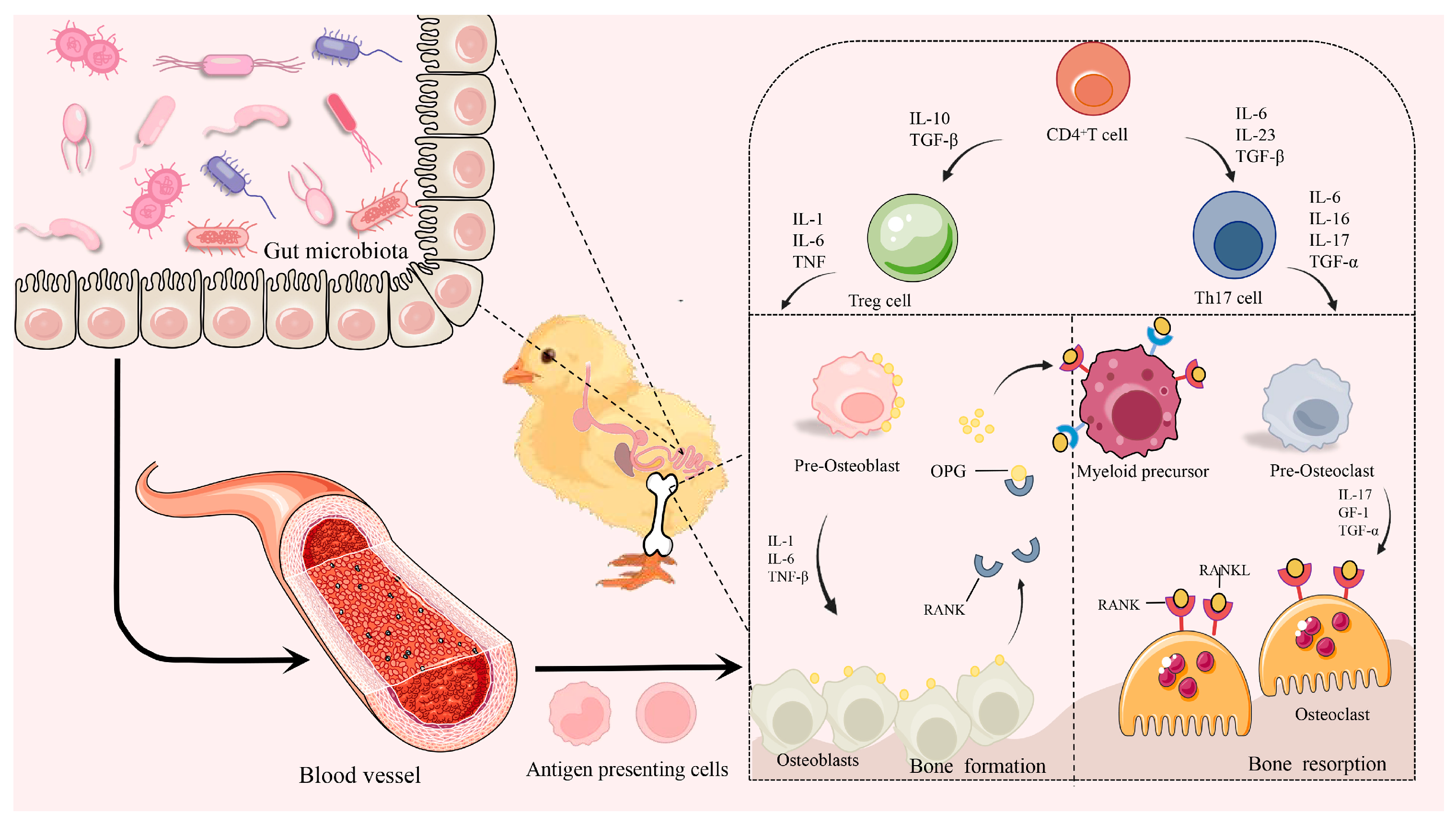
3.5. Inflammatory Cytokines and Bone Health
4. Gut–Organ Axis: Driving the Distal Organs for Bone Health
4.1. Gut–Kidney Axis and Bone Health
4.2. Gut–Liver Axis and Bone Health
4.3. Gut–Pancreatic Axis and Bone Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, M.N.; Widowski, T.M.; Kiarie, E.G.; Guerin, M.T.; Edwards, A.M.; Torrey, S. In pursuit of a better broiler: Tibial morphology, breaking strength, and ash content in conventional and slower-growing strains of broiler chickens. Poult. Sci. 2022, 101, 101755. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.Q.; Huo, W.R.; Li, Y.; Zhang, Y.; Zhang, Y.; Chen, G.H.; Xu, Q. Fiber characteristics and meat quality of different muscular tissues from slow-and fast-growing broilers. Poult. Sci. 2022, 101, 101537. [Google Scholar] [CrossRef]
- Liu, K.L.; He, Y.F.; Xu, B.W.; Lin, L.X.; Chen, P.; Iqbal, M.K.; Mehmood, K.; Huang, S.C. Leg disorders in broiler chickens: A review of current knowledge. Anim. Biotechnol. 2023, 34, 5124–5138. [Google Scholar] [CrossRef]
- Huang, S.C.; Rehman, M.U.; Lan, Y.F.; Qiu, G.; Zhang, H.; Iqbal, M.K.; Luo, H.Q.; Mehmood, K.; Zhang, L.H.; Li, J.K. Tibial dyschondroplasia is highly associated with suppression of tibial angiogenesis through regulating the HIF-1α/VEGF/VEGFR signaling pathway in chickens. Sci. Rep. 2017, 7, 9089. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Zhang, L.H.; Zhang, J.L.; Rehman, M.U.; Tong, X.L.; Qiu, G.; Jiang, X.; Iqbal, M.; Shahzad, M.; Shen, Y.Q. Role and regulation of growth plate vascularization during coupling with osteogenesis in tibial dyschondroplasia of chickens. Sci. Rep. 2018, 8, 3680. [Google Scholar] [CrossRef]
- Komori, T. Cell death in chondrocytes, osteoblasts, and osteocytes. Int. J. Mol. Sci. 2016, 17, 2045. [Google Scholar] [CrossRef]
- Ye, J.; Chi, X.; Wang, J.; Shen, Z.; Li, S.; Xu, S. High fat induces activation of the tryptophan-ERK-CREB pathway and promotes bone absorption in cage layers. Poult. Sci. 2021, 100, 101149. [Google Scholar] [CrossRef]
- Tang, H.; Guo, Y.; Zhang, Z.; Li, Z.; Zhang, Y.; Li, Y.; Kang, X.; Han, R. Integrative analysis of long non-coding RNA and mRNA in broilers with valgus-varus deformity. PLoS ONE 2020, 15, e0239450. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Cao, Q.Q.; Cao, Y.B.; Yang, Y.r.; Xu, T.t.; Yue, K.; Liu, F.; Tong, Z.x.; Wang, X.b. Morinda officinalis polysaccharides improve meat quality by reducing oxidative damage in chickens suffering from tibial dyschondroplasia. Food Chem. 2021, 344, 128688. [Google Scholar] [CrossRef]
- Villa, C.R.; Ward, W.E.; Comelli, E.M. Gut microbiota-bone axis. Crit. Rev. Food Sci. Nutr. 2017, 57, 1664–1672. [Google Scholar] [CrossRef]
- Chukwudi, P.; Umeugokwe, P.I.; Ikeh, N.E.; Amaefule, B.C. The effects of organic acids on broiler chicken nutrition: A review. Anim. Res. One Health 2025, 3, 43–53. [Google Scholar] [CrossRef]
- de Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of Dietary Supplements and Probiotics in Modulating Microbiota and Bone Health: The Gut-Bone Axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Shanmugasundaram, R.; Adams, D.; Selvaraj, R.K. Gastrointestinal Microbiota and Their Manipulation for Improved Growth and Performance in Chickens. Foods 2022, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Wu, X. Review of traditional Chinese medicines in ameliorating neuropsychiatric diseases by improving the levels of monoamine neurotransmitters via gut microbiota regulation. Zhongguo Zhong Yao Za Zhi 2023, 48, 853–860. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, J.; Zhang, H.; Erasmus, M.A.; Johnson, T.A.; Cheng, H.W. The Impact of Early-Life Cecal Microbiota Transplantation on Social Stress and Injurious Behaviors in Egg-Laying Chickens. Microorganisms 2024, 12, 471. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Liu, L.; Zhang, G.; Peng, X. Reprogrammed intestinal functions in Astragalus polysaccharide-alleviated osteoporosis: Combined analysis of transcriptomics and DNA methylomics demonstrates the significance of the gut-bone axis in treating osteoporosis. Food Funct. 2021, 12, 4458–4470. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.H.; Keum, M.C.; Han, E.; An, B.K.; Chang, H.H.; Choi, Y.H.; Moon, B.H.; Lee, K.W. Effects of fumonisin B1 and mycotoxin binders on growth performance, tibia characteristics, gut physiology, and stress indicators in broiler chickens raised in different stocking densities. Poult. Sci. 2018, 97, 845–854. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Dai, G.C.; Lu, P.P.; Zhang, M.; Bai, L.Y.; Chen, X.X.; Zhang, C.; Shi, L. The regulative effect and repercussion of probiotics and prebiotics on osteoporosis: Involvement of brain-gut-bone axis. Crit. Rev. Food Sci. Nutr. 2023, 63, 7510–7528. [Google Scholar] [CrossRef]
- Yan, F.; Wang, W.; Cheng, H. Bacillus subtilis based probiotic improved bone mass and altered brain serotoninergic and dopaminergic systems in broiler chickens. J. Funct. Foods 2018, 49, 501–509. [Google Scholar] [CrossRef]
- Fu, Y.C.; Hu, J.Y.; Cheng, H.W. Research Note: Probiotic, Bacillus subtilis, alleviates neuroinflammation in the hippocampus via the gut microbiota-brain axis in heat-stressed chickens. Poult. Sci. 2023, 102, 102635. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Li, Y.J.; Lu, P.P.; Dai, G.C.; Chen, X.X.; Rui, Y.F. The modulatory effect and implication of gut microbiota on osteoporosis: From the perspective of “brain–gut–bone” axis. Food Funct. 2021, 12, 5703–5718. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Chowdhury, V.S.; Cline, M.A.; Gilbert, E.R. The microbiota-gut-brain axis during heat stress in chickens: A review. Front. Physiol. 2021, 12, 752265. [Google Scholar] [CrossRef]
- Beldowska, A.; Barszcz, M.; Dunislawska, A. State of the art in research on the gut-liver and gut-brain axis in poultry. J. Anim. Sci. Biotechnol. 2023, 14, 37. [Google Scholar] [CrossRef]
- Johnson, A.M.; Clark, A.; Anderson, M.G.; Corbin, E.; Arguelles-Ramos, M.; Ali, A.B. The Influence of Dietary Synbiotic on Agonistic Behavior, Stress, and Brain Monoamines via Modulation of the Microbiota–Gut–Brain Axis in Laying Hens. Poultry 2024, 3, 129–146. [Google Scholar] [CrossRef]
- Villageliũ, D.N.; Lyte, M. Microbial endocrinology: Why the intersection of microbiology and neurobiology matters to poultry health. Poult. Sci. 2017, 96, 2501–2508. [Google Scholar] [CrossRef]
- Bai, H.; Geng, D.D.; Xue, F.G.; Li, X.F.; Wang, C.X.; Wang, C.Y.; Guo, Q.X.; Jiang, Y.; Wang, Z.X.; Bi, Y.L. Gut–brain bidirectional determination in regulating the residual feed intake of small-sized meat ducks. Poult. Sci. 2024, 103, 103778. [Google Scholar] [CrossRef]
- Wang, Z.X.; Shao, D.; Wu, S.; Song, Z.G.; Shi, S.R. Heat stress-induced intestinal barrier damage and dimethylglycine alleviates via improving the metabolism function of microbiota gut brain axis. Ecotoxicol. Environ. Saf. 2022, 244, 114053. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, F.F.; Hu, J.Y.; Mohammed, A.; Cheng, H.W. Bacillus subtilis-based probiotic improves skeletal health and immunity in broiler chickens exposed to heat stress. Animals 2021, 11, 1494. [Google Scholar] [CrossRef]
- Pawlak, D.; Domaniewski, T.; Znorko, B.; Oksztulska-Kolanek, E.; Lipowicz, P.; Doroszko, M.; Karbowska, M.; Pawlak, K. The impact of peripheral serotonin on leptin-brain serotonin axis, bone metabolism and strength in growing rats with experimental chronic kidney disease. Bone 2017, 105, 1–10. [Google Scholar] [CrossRef]
- Arneaud, S.L.B.; McClendon, J.; Tatge, L.; Watterson, A.; Zuurbier, K.R.; Madhu, B.; Gumienny, T.L.; Douglas, P.M. Reduced bone morphogenic protein signaling along the gut-neuron axis by heat shock factor promotes longevity. Aging Cell 2022, 21, e13693. [Google Scholar] [CrossRef]
- Barna, J.; Princz, A.; Kosztelnik, M.; Hargitai, B.; Takács-Vellai, K.; Vellai, T. Heat shock factor-1 intertwines insulin/IGF-1, TGF-β and cGMP signaling to control development and aging. BMC Dev. Biol. 2012, 12, 32. [Google Scholar] [CrossRef]
- Chen, P.; Xu, T.; Zhang, C.; Tong, X.; Shaukat, A.; He, Y.; Liu, K.; Huang, S. Effects of probiotics and gut microbiota on bone metabolism in chickens: A review. Metabolites 2022, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G., Jr.; Arreguin-Nava, M.; Maguey, J.; Michel, M.; Latorre, J.; Merino-Guzman, R.; Hernandez-Velasco, X.; Moore, P., Jr.; Hargis, B.; Tellez-Isaias, G. Effect of Bacillus–direct-fed microbial on leaky gut, serum peptide YY concentration, bone mineralization, and ammonia excretion in neonatal female turkey poults fed with a rye-based diet. Poult. Sci. 2020, 99, 4514–4520. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.T.; Yu, Y.; Wang, H.X.; Yang, H.J.; Tao, F.; Yang, S.L.; Liu, J.S.; Li, Z.M.; Yang, C.M. Dietary Clostridium butyricum and 25-Hydroxyvitamin D3 modulate bone metabolism of broilers through the gut–brain axis. Poult. Sci. 2024, 103, 103966. [Google Scholar] [CrossRef]
- Zou, X.Y.; Jiang, S.; Zhang, M.; Hu, H.Q.; Wu, X.L.; Liu, J.Z.; Jin, M.L.; Cheng, H.W. Effects of Bacillus subtilis on production performance, bone physiological property, and hematology indexes in laying hens. Animals 2021, 11, 2041. [Google Scholar] [CrossRef]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. Effects of long-term Bacillus subtilis CGMCC 1.921 supplementation on performance, egg quality, and fecal and cecal microbiota of laying hens. Poult. Sci. 2017, 96, 1280–1289. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Zhou, D.; Fan, J.G. Microbial metabolites in non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 2019–2028. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.A.; Montag, D. Exploring the relationship between gut microbiota and exercise: Short-chain fatty acids and their role in metabolism. BMJ Open Sport Exerc. Med. 2021, 7, e000930. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- De Bruyn, F.; Bonnet, N.; Baruchet, M.; Sabatier, M.; Breton, I.; Bourqui, B.; Jankovic, I.; Horcajada, M.-N.; Prioult, G. Galacto-oligosaccharide preconditioning improves metabolic activity and engraftment of Limosilactobacillus reuteri and stimulates osteoblastogenesis ex vivo. Sci. Rep. 2024, 14, 4329. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.M.; Mo, Q.; Kulyar, M.F.-e.-A.; He, Y.Y.; Yao, W.Y.; Quan, C.X.; Gong, S.S.; Li, F.R.; Fu, Y.H. Sodium butyrate ameliorates thiram-induced tibial dyschondroplasia and gut microbial dysbiosis in broiler chickens. Ecotoxicol. Environ. Saf. 2022, 245, 114134. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Z.; Zhang, X.S.; Hao, G.J.; Lin, H.; Sun, S.H. Clostridium butyricum Can Promote Bone Development by Regulating Lymphocyte Function in Layer Pullets. Int. J. Mol. Sci. 2023, 24, 1457. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Qin, S.M.; Zhu, Y.; Zhang, X.L.; Du, P.F.; Huang, Y.Q.; Michiels, J.; Zeng, Q.F.; Chen, W. Dietary resistant starch from potato regulates bone mass by modulating gut microbiota and concomitant short-chain fatty acids production in meat ducks. Front. Nutr. 2022, 9, 860086. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Qin, S.M.; Zhang, X.L.; Du, P.F.; Zhu, Y.; Huang, Y.Q.; Michiels, J.; Zeng, Q.F.; Chen, W. Dietary resistant starch alleviates Escherichia coli-induced bone loss in meat ducks by promoting short-chain fatty acid production and inhibiting Malt1/NF-κB inflammasome activation. J. Anim. Sci. Biotechnol. 2022, 13, 92. [Google Scholar] [CrossRef]
- Nguyen, H.; Morgan, N.; Roberts, J.; Wu, S.-B.; Swick, R.; Toghyani, M. Zinc hydroxychloride supplementation improves tibia bone development and intestinal health of broiler chickens. Poult. Sci. 2021, 100, 101254. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; He, Y.F.; Chen, P.; Liu, K.L.; Shaukat, A. Gut microbiota as a target in the bone health of livestock and poultry: Roles of short-chain fatty acids. Anim. Dis. 2023, 3, 23. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhou, Z.; Ju, L.; Shao, Q.; Xu, Y.; Song, Y.; Gao, W.; Lei, L.; Liu, G.; Du, X.; et al. Free fatty acids induce bile acids overproduction and oxidative damage of bovine hepatocytes via inhibiting FXR/SHP signaling. J. Steroid Biochem. Mol. Biol. 2024, 244, 106589. [Google Scholar] [CrossRef]
- Du, X.; Liu, M.; Trevisi, E.; Ju, L.; Yang, Y.; Gao, W.; Song, Y.; Lei, L.; Zolzaya, M.; Li, X.; et al. Expression of hepatic genes involved in bile acid metabolism in dairy cows with fatty liver. J. Dairy Sci. 2024, 107, 8629–8641. [Google Scholar] [CrossRef]
- Long, S.L.; Gahan, C.G.; Joyce, S.A. Interactions between gut bacteria and bile in health and disease. Mol. Asp. Med. 2017, 56, 54–65. [Google Scholar] [CrossRef]
- de Boer, J.F.; Verkade, E.; Mulder, N.L.; de Vries, H.D.; Huijkman, N.; Koehorst, M.; Boer, T.; Wolters, J.C.; Bloks, V.W.; van de Sluis, B. A human-like bile acid pool induced by deletion of hepatic Cyp2c70 modulates effects of FXR activation in mice. J. Lipid Res. 2020, 61, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Distrutti, E.; Carino, A.; Zampella, A.; Biagioli, M. Bile acids and their receptors in metabolic disorders. Prog. Lipid Res. 2021, 82, 101094. [Google Scholar] [CrossRef]
- Chen, L.; Wen, T.; Cao, A.; Wang, J.; Pan, H.; Zhao, R. Bile Acids Promote Hepatic Biotransformation and Excretion of Aflatoxin B1 in Broiler Chickens. Toxins 2023, 15, 694. [Google Scholar] [CrossRef]
- Geng, S.; Zhang, Y.; Cao, A.; Liu, Y.; Di, Y.; Li, J.; Lou, Q.; Zhang, L. Effects of Fat Type and Exogenous Bile Acids on Growth Performance, Nutrient Digestibility, Lipid Metabolism and Breast Muscle Fatty Acid Composition in Broiler Chickens. Animals 2022, 12, 1258. [Google Scholar] [CrossRef]
- Xiang, T.; Deng, Z.; Yang, C.; Tan, J.; Dou, C.; Luo, F.; Chen, Y. Bile acid metabolism regulatory network orchestrates bone homeostasis. Pharmacol. Res. 2023, 196, 106943. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wei, W.; Li, Y.; Ge, S.; Shen, J.; Guo, J.; Zhang, Y.; Huang, X.; Sun, X.; Cheng, D.; et al. Cholestyramine alleviates bone and muscle loss in irritable bowel syndrome via regulating bile acid metabolism. Cell Prolif. 2024, 57, e13638. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Song, Y.W.; Zhang, L.; Zheng, F.J.; Wang, X.M.; Zhuang, X.H.; Wu, F.; Liu, J. Association between bile acid metabolism and bone mineral density in postmenopausal women. Clinics 2020, 75, e1486. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Chen, Y.X.; Luo, Q. The association of serum total bile acids with bone mineral density in chinese adults aged 20–59: A retrospective cross-Sectional study. Front. Endocrinol. 2022, 13, 817437. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, J.; Wang, F.; Li, W.; Wu, X.; Zhao, C.; Zhao, J.; Wei, H.; Wu, Z.; Qian, M.; et al. Dual Targeting of Bile Acid Receptor-1 (TGR5) and Farnesoid X Receptor (FXR) Prevents Estrogen-Dependent Bone Loss in Mice. J. Bone Miner. Res. 2019, 34, 765–776. [Google Scholar] [CrossRef]
- Liu, X.F.; Zhang, X.; Shao, J.H.; Li, C. Intestinal flora affecting metabolites and bone metabolism: Research progress. Chin. J. Microecol. 2023, 35, 1228–1232, 1236. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.; Wang, B.; Yang, H. Activation of TGR5 promotes osteoblastic cell differentiation and mineralization. Biomed. Pharmacother. 2018, 108, 1797–1803. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Carson, M.D.; Warner, A.J.; Hathaway-Schrader, J.D.; Geiser, V.L.; Kim, J.; Gerasco, J.E.; Hill, W.D.; Lemasters, J.J.; Alekseyenko, A.V.; Wu, Y.; et al. Minocycline-induced disruption of the intestinal FXR/FGF15 axis impairs osteogenesis in mice. JCI Insight 2023, 8, e160578. [Google Scholar] [CrossRef]
- Liu, M.; Jin, F.; Zhang, S.; Li, S.; Zhu, D.; Cui, Y.; Cai, M.; Liu, X.; Zhang, Y.; Sun, Y.; et al. Activation of farnesoid X receptor signaling by geniposidic acid promotes osteogenesis. Phytomedicine 2022, 103, 154258. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Feng, X.; Lin, Q.; Deng, J.; Yuan, Y.; Li, M.; Zhai, B.; Chen, J. Folic acid supplementation prevents high body fat-induced bone loss through TGR5 signaling pathways. Food Funct. 2024, 15, 4193–4206. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Z.; Lei, H.; Zhang, C.; Wu, M.; Huang, S.; Li, X.; Xie, D.; Liu, M.; Zhang, L.; et al. Microbial Tryptophan Metabolites Ameliorate Ovariectomy-Induced Bone Loss by Repairing Intestinal AhR-Mediated Gut-Bone Signaling Pathway. Adv. Sci. 2024, 11, e2404545. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.W.; Yang, Y.H.; Pang, M.; Liu, X.L.; Li, Y.; Huang, P.J.; Shang, H.T.; Wei, H.; Ye, Z.J. Indole-3-carboxaldehyde ameliorates ionizing radiation-induced hematopoietic injury by enhancing hematopoietic stem and progenitor cell quiescence. Mol. Cell Biochem. 2024, 479, 313–323. [Google Scholar] [CrossRef]
- Fouad, A.M.; El-Senousey, H.K.; Ruan, D.; Wang, S.; Xia, W.; Zheng, C. Tryptophan in poultry nutrition: Impacts and mechanisms of action. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Linh, N.T.; Guntoro, B.; Qui, N.H. Immunomodulatory, behavioral, and nutritional response of tryptophan application on poultry. Vet. World 2021, 14, 2244. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, G.L.; Zheng, Q.X.; Gu, X.Y.; Shi, Q.M.; Su, Y.S.; Chu, Q.F.; Yuan, X.; Bao, Z.Y.; Lu, J. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Mund, M.D.; Riaz, M.; Mirza, M.A.; Rahman, Z.u.; Mahmood, T.; Ahmad, F.; Ammar, A. Effect of dietary tryptophan supplementation on growth performance, immune response and anti-oxidant status of broiler chickens from 7 to 21 days. Vet. Med. Sci. 2020, 6, 48–53. [Google Scholar] [CrossRef]
- Alhassan, U.B.; Zulkifli, I.; Goh, Y.M.; Elmutaz, A.A.; Abdoreza, S.F. Gut microbiota and transportation stress response affected by tryptophan supplementation in broiler chickens. Ital. J. Anim. Sci. 2018, 17, 107–113. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, B.; Lei, H.; Lin, Z.P.; Chen, J.P.; Zhu, Y.W.; Ye, H.; Yang, L.; Wang, W. Effects of Dietary Tryptophan on Growth Performance, Plasma Parameters, and Internal Organs of 1–28-Day-Old Sichuan White Geese. J. Poult. Sci. 2023, 60, 2023008. [Google Scholar] [CrossRef]
- Ouyang, L.; Yu, C.J.; Xie, Z.Y.; Su, X.Y.; Xu, Z.M.; Song, P.; Li, J.; Huang, H.; Ding, Y.; Zou, M.H. Indoleamine 2, 3-Dioxygenase 1 Deletion–Mediated Kynurenine Insufficiency in Vascular Smooth Muscle Cells Exacerbates Arterial Calcification. Circulation 2022, 145, 1784–1798. [Google Scholar] [CrossRef]
- Abdel Magied, H.A.A.; Ali, M.N.; Waly, A.H.; Habib, H.H. Improving the utilization of broiler low protein diets using tyroosine, tryptophan, crtric acid and sulphate. Egypt. Poult. Sci. Vol. 2022, 42, 265–279. [Google Scholar] [CrossRef]
- Elashry, G.M. Effect of dietary zinc and manganese chelated with tryptophan and protein on growth performance and carcass traits of quail. Egypt. Poult. Sci. Vol. 2019, 39, 579–597. Available online: https://api.semanticscholar.org/CorpusID:201223462 (accessed on 19 March 2025).
- Parsaeimehr, K.; Daneshyar, M.; Farhoomand, P.; Janmohammad, H.; Oliyaee, M. Effects of valine and tryptophan in low protein diets on carcass characteristics and immune response of broiler chickens. J. Anim. Sci. 2022, 32, fa1–fa14. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, N.; Wang, M.; Qian, J.; Nie, H.; Ge, R.; Liao, W.; Yan, F. Effects of periodontitis on bone and tryptophan metabolism of gut microbiota in estrogen-deficient mice. Zhonghua Kou Qiang Yi Xue Za Zhi 2024, 59, 354–363. [Google Scholar] [CrossRef]
- Li, Y.K.; Li, Q.X.; Yuan, R.S.; Wang, Y.F.; Guo, C.B.; Wang, L. Bifidobacterium breve-derived indole-3-lactic acid ameliorates colitis-associated tumorigenesis by directing the differentiation of immature colonic macrophages. Theranostics 2024, 14, 2719. [Google Scholar] [CrossRef]
- Dimitrov, V.; White, J.H. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol. Cell. Endocrinol. 2017, 453, 68–78. [Google Scholar] [CrossRef]
- Kitay, A.M.; Geibel, J.P. Stomach and Bone. Adv. Exp. Med. Biol. 2017, 1033, 97–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Koo, Y.; Chae, Y.; Choi, Y.; Yun, T.; Kang, B.T.; Yang, M.P.; Kim, H. Serum 25-hydroxyvitamin D, vitamin D receptor, and vitamin D binding protein concentrations in dogs with acute pancreatitis compared to healthy control dogs. J. Vet. Intern. Med. 2023, 37, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Song, P.R.; Wang, S.C.; Liu, H.; Shi, Z.M.; Su, J.C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, gut microbiome, and bone health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- Kumar, R.; Vallon, V. Reduced renal calcium excretion in the absence of sclerostin expression: Evidence for a novel calcium-regulating bone kidney axis. J. Am. Soc. Nephrol. 2014, 25, 2159–2168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paneru, D.; Sharma, M.K.; Shi, H.; Wang, J.; Kim, W.K. Aflatoxin B1 Impairs Bone Mineralization in Broiler Chickens. Toxins 2024, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, B.; Chorfi, Y.; Montminy, M.L.; Guay, F. Vitamin D Supplementation Impacts Calcium and Phosphorus Metabolism in Piglets Fed a Diet Contaminated with Deoxynivalenol and Challenged with Lipopolysaccharides. Toxins 2023, 15, 394. [Google Scholar] [CrossRef]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, X.; Liu, Y.; Yu, X. Gut microbiota and bone metabolism. FASEB J. 2021, 35, e21740. [Google Scholar] [CrossRef]
- Grüner, N.; Ortlepp, A.L.; Mattner, J. Pivotal Role of Intestinal Microbiota and Intraluminal Metabolites for the Maintenance of Gut-Bone Physiology. Int. J. Mol. Sci. 2023, 24, 5161. [Google Scholar] [CrossRef]
- Bozec, A.; Zaiss, M.M. T Regulatory Cells in Bone Remodelling. Curr. Osteoporos. Rep. 2017, 15, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Wang, Y.; Wang, L.; Lv, X.; Cui, G.; Ji, L.; Huang, Y.; Michiels, J.; Chen, W. Combination of Cinnamaldehyde with Carvacrol or Thymol Improves the Mechanical Properties of Tibia in Post-Peak Laying Hens. Animals 2022, 12, 3108. [Google Scholar] [CrossRef]
- Ohlsson, C.; Nigro, G.; Boneca, I.G.; Bäckhed, F.; Sansonetti, P.; Sjögren, K. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell. Immunol. 2017, 317, 55–58. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Wang, Z.; Wang, Y.; Chen, B.; Du, P.; Zhang, X.; Huang, Y.; Li, P.; Michiels, J.; et al. Acidification of drinking water improved tibia mass of broilers through the alterations of intestinal barrier and microbiota. Anim. Biosci. 2022, 35, 902–915. [Google Scholar] [CrossRef]
- Wang, W.W.; Wang, J.; Zhang, H.J.; Wu, S.G.; Qi, G.H. Supplemental Clostridium butyricum Modulates Lipid Metabolism Through Shaping Gut Microbiota and Bile Acid Profile of Aged Laying Hens. Front. Microbiol. 2020, 11, 600. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Lu, P.P.; Dai, G.C.; Zhang, M.; Wang, H.; Rui, Y.F. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J. Orthop. Transl. 2022, 37, 46–60. [Google Scholar] [CrossRef]
- Peacock, M. Phosphate metabolism in health and disease. Calcif. Tissue Int. 2021, 108, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Renkema, K.Y.; Alexander, R.T.; Bindels, R.J.; Hoenderop, J.G. Calcium and phosphate homeostasis: Concerted interplay of new regulators. Ann. Med. 2008, 40, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Li, T.; Shao, Y.; Zhang, L.; Lu, L.; Zhang, R.; Hou, S.; Luo, X.; Liao, X. Regulation of bone phosphorus retention and bone development possibly by related hormones and local bone-derived regulators in broiler chicks. J. Anim. Sci. Biotechnol. 2021, 12, 88. [Google Scholar] [CrossRef]
- Xie, C.; Sun, Q.; Dong, Y.; Lu, H.; Li, W.; Lin, Z.; Li, K.; Cheng, J.; Liu, Z.; Qi, J.; et al. Calcitriol-Loaded Multifunctional Nanospheres with Superlubricity for Advanced Osteoarthritis Treatment. ACS Nano 2023, 17, 12842–12861. [Google Scholar] [CrossRef]
- Wan, M.; Smith, C.; Shah, V.; Gullet, A.; Wells, D.; Rees, L.; Shroff, R. Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol. Dial. Transplant. 2013, 28, 153–161. [Google Scholar] [CrossRef]
- Rubio-Aliaga, I.; Krapf, R. Phosphate intake, hyperphosphatemia, and kidney function. Pflugers Arch. 2022, 474, 935–947. [Google Scholar] [CrossRef]
- Stathi, D.; Fountoulakis, N.; Panagiotou, A.; Maltese, G.; Corcillo, A.; Mangelis, A.; Ayis, S.; Gnudi, L.; Karalliedde, J. Impact of treatment with active vitamin D calcitriol on bone turnover markers in people with type 2 diabetes and stage 3 chronic kidney disease. Bone 2023, 166, 116581. [Google Scholar] [CrossRef]
- Bar, A. Calcium homeostasis and vitamin D metabolism and expression in strongly calcifying laying birds. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 151, 477–490. [Google Scholar] [CrossRef]
- Lyu, Z.; Li, H.; Li, X.; Wang, H.; Jiao, H.; Wang, X.; Zhao, J.; Lin, H. Fibroblast growth factor 23 inhibits osteogenic differentiation and mineralization of chicken bone marrow mesenchymal stem cells. Poult. Sci. 2023, 102, 102287. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hou, L.; Bao, Q.; Xu, Q.; Chen, G. Tibial Damage Caused by T-2 Toxin in Goslings: Bone Dysplasia, Poor Bone Quality, Hindered Chondrocyte Differentiation, and Imbalanced Bone Metabolism. Animals 2024, 14, 2281. [Google Scholar] [CrossRef]
- Sahin, E.; Ipcak, H.H.; Orhan, C.; Denli, M.; Erten, F.; Ozercan, I.H.; Balci, T.A.; Sahin, K. Impact of the arginine silicate inositol complex on bone metabolism in broiler chickens with tibial dyschondroplasia caused by manganese deficiency. Br. Poult. Sci. 2024, 65, 455–464. [Google Scholar] [CrossRef]
- Houston, B.; Thorp, B.H.; Burt, D.W. Molecular cloning and expression of bone morphogenetic protein-7 in the chick epiphyseal growth plate. J. Mol. Endocrinol. 1994, 13, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Chen, X.C.; Fraley, G.S.; Zhang, K.Y.; Tian, G.; Bai, S.P.; Ding, X.M.; Wang, J.P.; Lv, L.; Xuan, Y.; et al. Effects of different dietary vitamin D combinations during the grower phase and the feed restriction phase on growth performance and sternal morphology, mineralization, and related genes expression of bone metabolism in Pekin ducks. Poult. Sci. 2024, 103, 103291. [Google Scholar] [CrossRef]
- Salmória, L.A.; Ibelli, A.M.G.; Tavernari, F.C.; Peixoto, J.O.; Morés, M.A.Z.; Marcelino, D.E.P.; Pinto, K.D.S.; Coldebella, A.; Surek, D.; Kawski, V.L. CYP24A1 and TRPC3 Gene Expression in Kidneys and Their Involvement in Calcium and Phosphate Metabolism in Laying Hens. Animals 2024, 14, 1407. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xv, J.; Li, Y.; Bi, Y.; Hou, Y.; Ding, B. Interactive effects of dietary vitamin K3 and Bacillus subtilis PB6 on the growth performance and tibia quality of broiler chickens with sex separate rearing. Animal 2020, 14, 1610–1618. [Google Scholar] [CrossRef]
- Guarino, M.; Loperto, I.; Camera, S.; Cossiga, V.; Di Somma, C.; Colao, A.; Caporaso, N.; Morisco, F. Osteoporosis across chronic liver disease. Osteoporos. Int. 2016, 27, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Goubraim, R.; Kabbaj, N.; Salihoun, M.; Chaoui, Z.; Nya, M.; Amrani, N. Metabolic Bone Disease in Viral Cirrhosis: A Prospective Study. ISRN Hepatol. 2013, 2013, 276563. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Hall, C.; McGowan, N.W.A.; Babb, R.; Devlia, V.; Lucas, S.; Meghji, S.; Henderson, B.; Bozec, A.; Schett, G.; et al. Binding Immunoglobulin Protein (BIP) Inhibits TNF-α-Induced Osteoclast Differentiation and Systemic Bone Loss in an Erosive Arthritis Model. ACR Open Rheumatol. 2019, 1, 382–393. [Google Scholar] [CrossRef]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Alshehri, M.A.; Sayed, S.; Shukry, M.; El-Sayed, Y.S. The Impact of Curcumin on Growth Performance, Growth-Related Gene Expression, Oxidative Stress, and Immunological Biomarkers in Broiler Chickens at Different Stocking Densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef]
- Lv, H.; Tang, Y.; Zhang, H.; Li, S.; Fan, Z. Astragalus polysaccharide supplementation improves production performance, egg quality, serum biochemical index and gut microbiota in Chongren hens. Anim. Sci. J. 2021, 92, e13550. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Guo, Y.; Mohamed, T.; Bumbie, G.Z.; Wang, Y.; Zeng, X.; Zhao, J.; Du, H.; Tang, Z.; Xu, Y.; et al. Dietary Lactobacillus reuteri SL001 Improves Growth Performance, Health-Related Parameters, Intestinal Morphology and Microbiota of Broiler Chickens. Animals 2023, 13, 1690. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, J.; Jin, W.; Li, L.; Lin, L.; Shaukat, A.; Zhang, C.; Cao, Q.; Ashraf, M.; Huang, S. Fort Hare Papers. Front. Pharmacol. 2022, 13, 881057. [Google Scholar] [CrossRef]
- Zhang, C.D.; Xu, T.T.; Lin, L.X.; Shaukat, A.; Tong, X.S.; Yue, K.; Cao, Q.Q.; Zhang, C.; Liu, F.; Huang, S.C. Morinda officinalis polysaccharides ameliorates bone growth by attenuating oxidative stress and regulating the gut microbiota in thiram-induced tibial dyschondroplasia chickens. Metabolites 2022, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Bai, G.S.; Gao, Y.; Gao, Q.T.; Zhong, R.Q.; Chen, L.; Wang, Y.L.; Ma, T.; Zhang, H.F. Solid-state fermentation pro-enzymes supplementation benefits growth performance, health, and intestinal microbiota of broiler chickens fed wheat-based diet. Anim. Res. One Health 2024, 1–13. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierová, M.; Taubner, T.; Skřivanová, E. Effects of Dietary Hemp Seed and Flaxseed on Growth Performance, Meat Fatty Acid Compositions, Liver Tocopherol Concentration and Bone Strength of Cockerels. Animals 2020, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lin, X.; Zhao, J.; Wang, X.; Jiao, H.; Li, H.; Sun, S.; Lin, H. High frequency vaccination-induced immune stress reduces bone strength with the involvement of activated osteoclastogenesis in layer pullets. Poult. Sci. 2020, 99, 734–743. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Xie, Y.; Chen, X.; Tian, L.; Liang, Y.; Li, H.; Zhang, J.; Liu, Y.; Yu, X. Interleukin-6 Knockout Inhibits Senescence of Bone Mesenchymal Stem Cells in High-Fat Diet-Induced Bone Loss. Front. Endocrinol. 2020, 11, 622950. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Liu, T.; Gao, P.; Li, H.; Liu, N.; Gao, L.; Wan, G.; Zhang, Y.; Duan, X. Association between osteoporosis and hepatitis B cirrhosis: A case-control study. Afr. Health Sci. 2020, 20, 1610–1616. [Google Scholar] [CrossRef]
- Paré, F.; Tardif, G.; Fahmi, H.; Ouhaddi, Y.; Pelletier, J.P.; Martel-Pelletier, J. In vivo protective effect of adipsin-deficiency on spontaneous knee osteoarthritis in aging mice. Aging 2020, 12, 2880–2896. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, R.; Luo, M.; Zhang, T.; Pan, L.; Xu, S.; Pan, L.; Ren, F.; Ji, C.; Hu, R.; et al. Liver Injury Impaired 25-Hydroxylation of Vitamin D Suppresses Intestinal Paneth Cell defensins, leading to Gut Dysbiosis and Liver Fibrogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G685–G695. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mo, W.; Feng, J.; Li, J.; Yu, Q.; Li, S.; Zhang, J.; Chen, K.; Ji, J.; Dai, W.; et al. Astaxanthin attenuates hepatic damage and mitochondrial dysfunction in non-alcoholic fatty liver disease by up-regulating the FGF21/PGC-1α pathway. Br. J. Pharmacol. 2020, 177, 3760–3777. [Google Scholar] [CrossRef]
- Truong, A.D.; Tran, H.T.T.; Chu, N.T.; Nguyen, H.T.; Vu, T.H.; Hong, Y.; Song, K.D.; Dang, H.V.; Hong, Y.H. Genome-wide identification, organization, and expression profiles of the chicken fibroblast growth factor genes in public databases and Vietnamese indigenous Ri chickens against highly pathogenic avian influenza H5N1 virus infection. Anim. Biosci. 2023, 36, 570–583. [Google Scholar] [CrossRef]
- Razmyar, J.; Abbasi, M.; Mirsalimi, S.M.; Baghkheirati, A.A.; Ahmadian, G.; Yazdani, A. Serologic and Molecular Evidence of Widespread Infection of Avian Hepatitis E Virus in Poultry Farms of Iran. Avian Dis. 2021, 65, 572–577. [Google Scholar] [CrossRef]
- Chowdhury, E.; Roberts, J.; Walz, H.; Hauck, R.; Morey, A.; Morgan, S.; Joiner, K.; Cattley, R.; Sengupta, S.; Wilson, F.; et al. Hepatic Perisinusoidal Myofibroblast Proliferation and Systemic Inflammatory Response Precedes Sep/Tox Hepatitis in Broilers. Avian Dis. 2021, 65, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, B.; Bharadwaz, A.; Unagolla, J.M.; Jayasuriya, A.C. Evaluation of the optimal dosage of BMP-9 through the comparison of bone regeneration induced by BMP-9 versus BMP-2 using an injectable microparticle embedded thermosensitive polymeric carrier in a rat cranial defect model. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112252. [Google Scholar] [CrossRef]
- Hou, J.M.; Chen, E.Y.; Wei, S.C.; Lin, F.; Lin, Q.M.; Lan, X.H.; Xue, Y.; Wu, M. Lactoferrin inhibits apoptosis through insulin-like growth factor I in primary rat osteoblasts. Acta Pharmacol. Sin. 2014, 35, 523–530. [Google Scholar] [CrossRef]
- Drexler, S.; Cai, C.; Hartmann, A.L.; Moch, D.; Gaitantzi, H.; Ney, T.; Kraemer, M.; Chu, Y.; Zheng, Y.; Rahbari, M.; et al. Intestinal BMP-9 locally upregulates FGF19 and is down-regulated in obese patients with diabetes. Mol. Cell. Endocrinol. 2023, 570, 111934. [Google Scholar] [CrossRef]
- Seemann, P.; Brehm, A.; König, J.; Reissner, C.; Stricker, S.; Kuss, P.; Haupt, J.; Renninger, S.; Nickel, J.; Sebald, W.; et al. Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN. PLoS Genet. 2009, 5, e1000747. [Google Scholar] [CrossRef]
- Mazziotti, G.; Lania, A.G.; Canalis, E. Skeletal disorders associated with the growth hormone-insulin-like growth factor 1 axis. Nat. Rev. Endocrinol. 2022, 18, 353–365. [Google Scholar] [CrossRef]
- Guo, J.; Sun, C.; Qu, L.; Shen, M.; Dou, T.; Ma, M.; Wang, K.; Yang, N. Genetic architecture of bone quality variation in layer chickens revealed by a genome-wide association study. Sci. Rep. 2017, 7, 45317. [Google Scholar] [CrossRef]
- Soumeh, E.A.; Mohebodini, H.; Toghyani, M.; Shabani, A.; Ashayerizadeh, A.; Jazi, V. Synergistic effects of fermented soybean meal and mannan-oligosaccharide on growth performance, digestive functions, and hepatic gene expression in broiler chickens. Poult. Sci. 2019, 98, 6797–6807. [Google Scholar] [CrossRef] [PubMed]
- Kewan, A.; Saneyasu, T.; Kamisoyama, H.; Honda, K. Effects of fasting and re-feeding on the expression of CCK, PYY, hypothalamic neuropeptides, and IGF-related genes in layer and broiler chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 257, 110940. [Google Scholar] [CrossRef]
- Carrillo-López, N.; Panizo, S.; Alonso-Montes, C.; Román-García, P.; Rodríguez, I.; Martínez-Salgado, C.; Dusso, A.S.; Naves, M.; Cannata-Andía, J.B. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016, 90, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Drissi, H.; Pouliot, A.; Koolloos, C.; Stein, J.L.; Lian, J.B.; Stein, G.S.; van Wijnen, A.J. 1,25-(OH)2-vitamin D3 suppresses the bone-related Runx2/Cbfa1 gene promoter. Exp. Cell Res. 2002, 274, 323–333. [Google Scholar] [CrossRef]
- Shin, D.K.; Kim, M.H.; Lee, S.H.; Kim, T.H.; Kim, S.Y. Inhibitory effects of luteolin on titanium particle-induced osteolysis in a mouse model. Acta Biomater. 2012, 8, 3524–3531. [Google Scholar] [CrossRef]
- Mora-Raimundo, P.; Lozano, D.; Manzano, M.; Vallet-Regí, M. Nanoparticles to Knockdown Osteoporosis-Related Gene and Promote Osteogenic Marker Expression for Osteoporosis Treatment. ACS Nano 2019, 13, 5451–5464. [Google Scholar] [CrossRef]
- Lu, K.; Shi, T.S.; Shen, S.Y.; Shi, Y.; Gao, H.L.; Wu, J.; Lu, X.; Gao, X.; Ju, H.X.; Wang, W.; et al. Defects in a liver-bone axis contribute to hepatic osteodystrophy disease progression. Cell Metab. 2022, 34, 441–457.e447. [Google Scholar] [CrossRef]
- Xi, G.; Shen, X.; Rosen, C.J.; Clemmons, D.R. IRS-1 Functions as a Molecular Scaffold to Coordinate IGF-I/IGFBP-2 Signaling During Osteoblast Differentiation. J. Bone Miner. Res. 2016, 31, 1300–1314. [Google Scholar] [CrossRef]
- Xu, T.T.; Chen, P.; Zhang, C.D.; Shaukat, A.; Lin, L.X.; Yue, K.; Ding, W.L.; Tong, X.; Liu, K.L.; He, Y.F.; et al. Gut microbiome dysregulation drives bone damage in broiler tibial dyschondroplasia by disrupting glucose homeostasis. NPJ Biofilms Microbiomes 2023, 9, 1. [Google Scholar] [CrossRef]
- Karpińska, M.; Czauderna, M. Pancreas-Its Functions, Disorders, and Physiological Impact on the Mammals’ Organism. Front. Physiol. 2022, 13, 807632. [Google Scholar] [CrossRef]
- Pramojanee, S.N.; Phimphilai, M.; Chattipakorn, N.; Chattipakorn, S.C. Possible roles of insulin signaling in osteoblasts. Endocr. Res. 2014, 39, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Chikazu, D.; Kubota, N.; Terauchi, Y.; Tobe, K.; Azuma, Y.; Ohta, T.; Kadowaki, T.; Nakamura, K.; Kawaguchi, H. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J. Clin. Investig. 2000, 105, 935–943. [Google Scholar] [CrossRef]
- Akune, T.; Ogata, N.; Hoshi, K.; Kubota, N.; Terauchi, Y.; Tobe, K.; Takagi, H.; Azuma, Y.; Kadowaki, T.; Nakamura, K.; et al. Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts. J. Cell Biol. 2002, 159, 147–156. [Google Scholar] [CrossRef]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, J.; Pan, Y.; Ju, H.; Zhu, L.; Liu, Y.; Zhang, Y. The difference between steroid diabetes mellitus and type 2 diabetes mellitus: A whole-body (18)F-FDG PET/CT study. Acta Diabetol. 2020, 57, 1383–1393. [Google Scholar] [CrossRef]
- Schertzer, J.D.; Lam, T.K.T. Peripheral and central regulation of insulin by the intestine and microbiome. Am. J. Physiol. Endocrinol. Metab. 2021, 320, e234–e239. [Google Scholar] [CrossRef]
- Hansen, M.S.; Frost, M. Alliances of the gut and bone axis. Semin. Cell Dev. Biol. 2022, 123, 74–81. [Google Scholar] [CrossRef]
- Mieczkowska, A.; Bouvard, B.; Chappard, D.; Mabilleau, G. Glucose-dependent insulinotropic polypeptide (GIP) directly affects collagen fibril diameter and collagen cross-linking in osteoblast cultures. Bone 2015, 74, 29–36. [Google Scholar] [CrossRef]
- Chen, P.; Liu, K.L.; Yue, T.J.; Lu, Y.N.; Li, S.Y.; Jian, F.C.; Huang, S.C. Plants, plant-derived compounds, probiotics, and postbiotics as green agents to fight against poultry coccidiosis: A review. Anim. Res. One Health 2024, 1–21. [Google Scholar] [CrossRef]
- Miteu, G.D.; Emmanuel, A.A.; Addeh, I.; Ojeokun, O.; Olayinka, T.; Godwin, J.S.; Adeyemo, O.I.; Benneth, E.O. Nanoscience and technology as a pivot for sustainable agriculture and its One Health approach awareness. Sci. One Health 2023, 2, 100020. [Google Scholar] [CrossRef] [PubMed]
| Metabolite | Receptor | Target Cell | Effect | Function | References |
|---|---|---|---|---|---|
| Minocycline | FXR | Osteoblast | Inhibition | Inhibited osteoblast function, reduced bone mass, impaired bone microstructure and fracture resistance | [69] |
| Geniposidic acid | FXR | Osteoblast | Promotion | FXR is activated to promote osteoblast differentiation and stimulate bone formation | [70] |
| Folic acid | TGR5 | Osteoblast | Promotion | Increased expression of LCA and TGR5, increased phosphorylation of AMPK, decreased phosphorylation of NF-κB and ERK, and reduced bone loss | [71] |
| Betulinic acid | TGR5 FXR | Osteoclast | Inhibition | Osteoclast differentiation is regulated by AMPK signaling pathway | [65] |
| Bile acid | TGR5 | Osteoblast | Promotion | Promotes the expression of Runx2 and increases the expression of ALP, osteocalcin, osterix, and other osteogenic genes | [67] |
| Indole acetic acid (IAA) and indole-3-propionic acid (IPA) | Aryl hydrocarbon receptor | M2 macrophage | Promotion | Repair intestinal barrier function, promote osteoblast generation, and inhibit osteoclast generation | [72] |
| Tryptophan | 5-HT | Osteoclast | Promotion | Activates 5-HT/ERK/CREB for bone resorption | [7] |
| Indole-3-carboxaldehyde | \ | Peripheral blood cell | Promotion | Helps mitigate ionizing radiation-induced myelosuppression | [73] |
| Microbial Signal | Source | Disease/Model | Function | References |
|---|---|---|---|---|
| Kidney–Bone Axis | ||||
| Calcitriol | Kidney | Osteoarthritis | Inhibits the breakdown of extracellular matrix in osteoarthritis, early bone remodeling, and cartilage degeneration | [106] |
| Fibroblast growth factor 23 (FGF-23) | Osteoblast | Chronic kidney disease | Increased levels of FGF-23 and PTH and increased bone resorption | [107,145] |
| Bone morphogenetic protein (BMP)-7 | Mesenchymal stem cells | Tibial damage | The number of tibial growth plate chondrocytes decreased, and osteoclast-related genes and enzymes increased. Chondrocyte differentiation was delayed, and BMP-6, BMP-7, SOX9, and Runx2 were downregulated | [112] |
| Osteopraxin (OPG) | Osteoblast | Tibial dyschondroplasia (TD) | Alkaline phosphatase (ALP) and osteocalcin levels were reduced | [113] |
| Parathyroid hormones (PTHs) | Parathyroid cells | Bone phosphorus retention and bone development | The level of FGF-23 was positively correlated with the level of PTH, and the ALP and bone gla protein (BGP) of tibia were increased | [105] |
| 1,25-(OH)2-Vitamin D3 | Kidney | In vitro study | Osteoblast differentiation is regulated by inhibiting Runx2 activity | [146] |
| Liver–Bone Axis | ||||
| BMP-9 | Liver | Cranial defect model | Improves bone regeneration potential and enhances bone mechanical properties | [137] |
| Insulin-like growth factor 1 (IGF-1) | Liver | In vitro study | Upregulation of IGF-1 inhibits osteoblast apoptosis | [138] |
| Lecithin–cholesterol acyltransferase (LCAT) | Liver | Osteolysis | Inhibition of lecithin–cholesterol acyltransferase (IL-6) expression further inhibited the release of inflammatory cytokines from bone marrow macrophages, thereby inhibiting osteoclast formation and bone resorption | [147] |
| Tumor necrosis factor (TNF-α) | Mononuclear macrophage | Erosive arthritis | After treatment with TNF-α and RANKL, Bip blocked monocytes, and osteoclast precursor NF-κB signaling inhibits osteoclast generation | [120] |
| Recombinant sclerostin | Bone | Mouse embryonic fibroblast cell | Sclerostin gene silencing enhances the expression of osteogenic markers and promotes bone formation | [148] |
| LCAT | Liver | Hepatic osteodystrophy | Downregulation of LCAT expression exacerbates bone loss | [149] |
| Pancreatic–Bone Axis | ||||
| Insulin receptors (IRs) | Osteoblasts | Mouse calvarial osteoblasts | IGF-1 and IGFBP-2 coordinate to stimulate IRs-1 phosphorylation, osteoblasts, and AKT differentiation | [150] |
| Insulin | Pancreatic | TD | The level of glucose and pancreatic function were restored, and glycometabolism inhibited the expression of PI3K/AKT/VEGF pathway, which aggravated the lesions of TD | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-N.; Yue, T.-J.; Ding, W.-L.; Xu, B.-W.; Li, A.-Y.; Huang, S.-C. Gut–X Axis and Its Role in Poultry Bone Health: A Review. Microorganisms 2025, 13, 757. https://doi.org/10.3390/microorganisms13040757
Lu Y-N, Yue T-J, Ding W-L, Xu B-W, Li A-Y, Huang S-C. Gut–X Axis and Its Role in Poultry Bone Health: A Review. Microorganisms. 2025; 13(4):757. https://doi.org/10.3390/microorganisms13040757
Chicago/Turabian StyleLu, Ya-Nan, Tao-Jing Yue, Wen-Li Ding, Bo-Wen Xu, Ao-Yun Li, and Shu-Cheng Huang. 2025. "Gut–X Axis and Its Role in Poultry Bone Health: A Review" Microorganisms 13, no. 4: 757. https://doi.org/10.3390/microorganisms13040757
APA StyleLu, Y.-N., Yue, T.-J., Ding, W.-L., Xu, B.-W., Li, A.-Y., & Huang, S.-C. (2025). Gut–X Axis and Its Role in Poultry Bone Health: A Review. Microorganisms, 13(4), 757. https://doi.org/10.3390/microorganisms13040757








