Abstract
Ulcerative colitis (UC) is a chronic inflammatory bowel disease closely associated with dysbiosis of the gut microbiome, encompassing not only bacterial communities but also fungal populations. Despite the growing recognition of the gut microbiome’s role in UC pathogenesis, the contribution of intestinal fungi has only recently garnered significant attention. In this review, we comprehensively examine the characteristics of intestinal fungi in both healthy individuals and UC patients, elucidating their role in disease pathogenesis and their interactions with bacterial communities. Additionally, we explore the impact of intestinal fungi on disease severity and therapeutic responses in UC. Furthermore, we evaluate the therapeutic potential of antifungal agents, probiotics, and fecal microbiota transplantation (FMT) in UC management, emphasizing the critical role of fungi in these treatment modalities. Future research should prioritize elucidating the multifunctional roles of fungi in UC pathogenesis and their implications for treatment strategies. Moreover, the identification of fungal biomarkers associated with FMT efficacy could pave the way for precision medicine approaches in FMT, offering novel insights into personalized therapeutic interventions for UC.
1. Introduction
Ulcerative colitis (UC), a chronic inflammatory bowel disease (IBD), is characterized by recurrent episodes and treatment resistance, significantly impairing patients’ quality of life and imposing a substantial economic burden on healthcare systems [1]. The clinical manifestations of UC can be categorized into gastrointestinal symptoms and extraintestinal manifestations. Gastrointestinal symptoms typically present as diarrhea, abdominal pain, and mucoempulent blood stool. Extraintestinal manifestations commonly include primary sclerosing cholangitis and peripheral arthritis, among others [2]. Current therapeutic interventions for UC primarily consist of: 5-aminosalicylic acid, sulfasalazine, corticosteroids, immunosuppressants, and biologics [2]. The pathogenesis of UC is widely attributed to gene–environment interactions, which trigger an aberrant immune response to the intestinal microbiota [3]. While the role of gut bacteria in UC has been extensively investigated [4,5], the contribution of gut fungi has only recently emerged as a critical area of research. The fungal microbiome is an integral part of the gut microbiota, interacting with the host immune system and influencing physiological functions [6,7], and is closely related to the development and prognosis of UC, which is characterized by an imbalance in the intestinal microecology [8]. Studies suggest that fungal microbiota are associated with disease activity in UC [9] and that fungal microbiota signatures may serve as predictive biomarkers of therapeutic response to infliximab in patients with IBD [10]. Notably, intricate interactions exist between intestinal fungi and bacteria [11], yet the mechanisms underlying their cross-talk and its impact on UC pathogenesis remain poorly understood and warrant further exploration.
In recent years, rapid advancements have been made in therapeutic approaches targeting the gut microbiome, including probiotics, prebiotics, and fecal microbiota transplantation (FMT). Unlike single- or multistrain probiotic therapies, FMT involves the transfer of a complex microbial community, including bacteria, fungi, viruses, and their metabolites, from healthy donor stool to recipients. FMT has demonstrated broad therapeutic potential across a range of diseases, such as Clostridium difficile infection (CDI), inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and obesity [12]. However, the clinical response to FMT in IBD patients, especially those with UC, exhibits considerable heterogeneity [13,14,15], making it challenging to predict clinical efficacy. Studies have shown that gut fungi significantly influence the therapeutic effects of FMT [16,17]. For example, a reduction in Candida after FMT has been associated with an improvement in the severity of the disease [18]. Therefore, there is an urgent need to explore the specific mechanisms of intestinal fungi in FMT and to search for fungal biomarkers associated with FMT efficacy.
In this review, we discuss the characterization of fungal communities in healthy individuals and UC patients, the role of fungi in the pathogenesis of UC, and the impact of their interactions with bacteria on UC. We also summarize the therapeutic effects of antifungal drugs, probiotics, and FMT in ulcerative colitis, emphasizing the important role of gut fungi in these treatment modalities.
2. Overview of the Gut Mycobiota
2.1. Types and Distribution of Intestinal Fungi
Fungi are generally considered a relatively minor component of the gut microbiota, accounting for approximately 0.1% of the microbiome in healthy populations [19]. Fungi are categorized into sexual and asexual forms, which may influence their annotation and limit research on them. The number of gut fungi increases sequentially from the ileum to the colon, peaking in the distal colon [20]. A comparison of intestinal luminal and mucosal fungi reveals that the alpha diversity (a metric of microbial community richness and evenness) of the former is higher than that of the latter [21,22]. This discrepancy can be attributed to the influence of environmental factors on fecal fungi, as well as the transient presence of certain fungi in the gut lumen.
The diversity of the gut mycobiome is comparatively lower than that of gut bacteria, with the mycobiome predominantly consisting of yeasts, which are principally from the phyla Ascomycota and Basidiomycota [23,24]. At the genus level, the gut mycobiota of healthy adults is predominantly characterized by Saccharomyces, Malassezia, and Candida [23]. A comprehensive fungal analysis of 3363 stool samples from populations in Europe, North America, and Asia similarly confirmed that Saccharomyces and Candida are the most abundant genera across all samples, followed by Penicillium and Aspergillus [25], whereas Malassezia was less prevalent in these studies, potentially due to geographical and methodological variations. In healthy older adults (aged > 65 years), the gut mycobiome is primarily dominated by Penicillium, Candida, Saccharomyces, and Aspergillus [26]. Among these, Candida consistently emerged as the dominant genus across multiple studies [26]. The most commonly identified Candida species in the human gut include Candida albicans, Candida glabrata, Candida dubliniensis and Candida parapsilosis, while Candida tropicalis is more prevalent in murine models [27].
A core mycobiome may exist within the human gut, as evidenced by studies demonstrating consistent fungal taxa across individuals. In the Human Microbiome Project (HMP) cohort study, gut fungal communities exhibited significant inter-individual variability and longitudinal heterogeneity. However, three fungal species—Saccharomyces cerevisiae, Saccharomyces limpetus, and Candida albicans—were detected in more than 60% of the samples, suggesting their potential role as core members of the gut mycobiome [23]. This finding is further supported by a longitudinal study involving 184 healthy participants, which revealed that 11 genera from the Ascomycota phylum, including Pichia, Alternaria, and Wickerhamiella, demonstrated remarkable stability over a 3.2-year follow-up period [28].
Based on taxonomic profiling, gut fungal communities can be classified into four distinct enterotypes: Saccharomyces_type, dominated by Saccharomyces cerevisiae; Candida_type, dominated by Candida albicans; Aspergillus_type; and mixed Asc_type, which reflects structural variations in fungal colonies due to differences in the amplification regions of ITS1 and ITS2 [25]. Different sequencing technologies for fungi may have biases between sequencing results due to differences in amplification fragmentation, specificity, and accuracy of the primers used, which can lead to different results in subsequent analyses [29]. Notably, the gut fungal community exhibits strong functional synergy with the fecal metabolome [28]. Despite the high taxonomic variability, the metabolic functions of the gut mycobiome are relatively conserved and exhibit low diversity. This functional redundancy, observed across multiple phyla and over time, suggests that defining the gut microbiota based on metabolic functions—rather than taxonomy—may provide a more robust framework for understanding its role in host physiology [30]. Such functional conservation is further supported by the identification of inferred metabolic modules derived from proteomic data, as mapped to KEGG homolog groups [30].
2.2. Factors Affecting Intestinal Fungal Communities
Diet is one of the main factors influencing intestinal fungal colonization [31,32,33]. Saccharomyces cerevisiae, a species commonly present in fermented foods and bread, is often one of the most abundant species in the human mycobiome. Moreover, carbohydrate-rich diets have been shown to positively correlate with the abundance of intestinal Candida, whereas high-protein diets are inversely associated with the levels of Methanobrevibacter and Candida populations in healthy volunteers [34]. Further studies have indicated that high levels of animal protein can exacerbate dextran sulfate solution (DSS)-induced colitis in mice by promoting proinflammatory responses in monocytes, but high plant protein does not [35]. Vitamins A and D exhibit antifungal properties, with vitamin D3 being considered a “good antifungal therapy” [36].
The composition of human gut fungal communities exhibits significant variation across geographic locations [25,28]. A comparative study on the distribution of gut fungi among residents of Hong Kong and Yunnan revealed pronounced differences, with a ratio of 15:2 in the enrichment of different fungal species in the feces of Yunnan and Hong Kong residents, and similar results were obtained in the validation cohort [37]. Intestinal fungi are influenced not only by the above factors but also by various other elements. Age is correlated with certain fungal genera; for example, Candida is positively correlated with older individuals, whereas the genera Saccharomyces and Aspergillus are preferentially enriched in younger cohorts [25]. In contrast, body mass index (BMI) and sex are not correlated with the distribution of fungal enterotypes [25]. However, the functional composition of gut bacteria correlates with host BMI and sex. For example, the ATPase complex for energy acquisition is associated with obesity, and specific bacteria linked to aspartic acid biosynthesis are more abundant in males [38]. Saccharomyces may be beneficial for human metabolic health, as its relative abundance is negatively correlated with fasting blood glucose and positively correlated with high-density lipoprotein cholesterol [28].
3. The Role of Intestinal Fungi in the Pathogenesis of Ulcerative Colitis
3.1. Impact of Fungi on the Intestinal Mucosal Barrier
The intestinal mucosal barrier functions as a critical protective shield against microbial invasion, comprising three primary components: the mucus layer, epithelial cells, and intestinal immune cells. First, the mucus layer is formed by goblet cells that secrete two layers of mucus, with mucin 2 as its main component, and it also contains secretory IgA (sIgA) and other proteins, such as antimicrobial peptides [39,40]. In active ulcerative colitis, the mucus layer is thinner and more permeable [41,42]. This compromised barrier facilitates fungal interactions with epithelial cells, thereby initiating immune responses and exacerbating intestinal inflammation. Research has shown that the filamentous form of Candida albicans has been strongly associated with enhanced pathogenicity, as this morphological state promotes the proliferation and migration of intestinal epithelial cells, as well as the secretion of proinflammatory cytokines such as IL-8 [43]. Furthermore, sIgA within the mucus layer preferentially targets the filamentous form of Candida albicans, with adhesion molecules expressed by the filaments serving as direct epitopes. A deficiency in intestinal sIgA has been linked to an increased prevalence of the filamentous form of Candida albicans, further highlighting its role in maintaining mucosal homeostasis [44,45]. Second, the epithelial cell barrier consists of a monolayer of tightly interconnected epithelial cells, bound by tight junctions to form a robust physical barrier. The adherence of Candida to intestinal epithelial cells is a critical initial step in infection, followed by invasion of this barrier by active penetration [34]. Key regulatory factors, such as HIF-1α and IL-37, have been identified as essential mediators in resisting Candida colonization, underscoring their protective roles in maintaining epithelial integrity [46]. For intestinal immune cells, distal colonic macrophages, called “balloon-like” protrusions (BLPs) + macrophages, extend specialized “balloon-like” protrusions into the intestinal epithelium, a unique cellular adaptation that critically prevents toxin absorption and maintains epithelial barrier integrity [47]. Additionally, gut B cells also regulate the morphology of Candida in the intestine, effectively modulating its pathogenic potential [44].
Changes in the structure and function of the intestinal microbiota can disrupt mucosal homeostasis and lead to an exaggerated and sustained immune response to specific microbial components [48], ultimately resulting in disease progression. Multiple analyses of fecal microbial profiles from UC patients have revealed an increase in Candida species, which can disrupt the intestinal mucosal barrier through various mechanisms. For instance, Candida albicans secretes aspartyl proteases that degrade mucin, compromising the protective mucus layer of the intestinal epithelium [43]. Similarly, Candida tropicalis can exacerbate disease in wild-type animals by increasing gut permeability and damaging tight junctions [49]. Moreover, the Candida-dominated microbial community is associated with the biosynthesis of hemoglobin, a key iron source for pathogenic bacteria [50]. This interaction may indirectly damage the intestinal barrier by promoting the growth of other pathogenic microorganisms [25].
Despite the association of gut fungi with disease development, certain fungi exhibit protective effects under specific conditions. A study demonstrated that mucosa-associated fungi (MUC), including Candida albicans, Saccharomyces cerevisiae, and Saccharomycopsis fibuligera, can reduce intestinal permeability, preventing colon damage induced by DSS and decreasing mortality rates in mice [21]. IL-22 is widely recognized as a pivotal cytokine in promoting intestinal mucosal barrier repair and maintaining gut homeostasis [51]. Mucosa-associated fungi effectively stimulate IL-22 production from CD4+ T cells, enhancing the functionality of the intestinal barrier and participating in JAK/STAT signaling and the transcription of epithelial genes involved in DNA repair, thus playing an immune-protective role in the intestine [21].
Therefore, understanding the interactions between fungi and the intestinal mucosal barrier may be crucial for elucidating the pathogenesis of intestinal diseases.
3.2. Intestinal Fungi and Immunity in UC Patients
The fungal cell wall is critical for fungal survival and reproduction and is a key target for antifungal drugs and the host immune system [52]. Fungal cell walls are divided into two layers, with the inner layer consisting mainly of β-glucans and chitin and the outer layer consisting mainly of a diverse range of glycoproteins. For example, the outer layers of the cell walls of Saccharomyces cerevisiae and Candida albicans are enriched in mannose glycosylated glycoproteins [53]. In response to fungal invasion, various host receptors can initiate an immune response by recognizing components of the fungal cell wall. Genetic evidence and experimental studies highlight the central role of C-type lectin receptors (CLRs) in antifungal immunity, whereas Toll-like receptors (TLRs) and nucleotide oligomerization domain (NOD)-like receptors (NLRs) generally play secondary roles [54]. CLRs are expressed primarily by phagocytic cells such as macrophages and dendritic cells, with common types including Dectin-1, Dectin-2, Dectin-3, and Mincle.
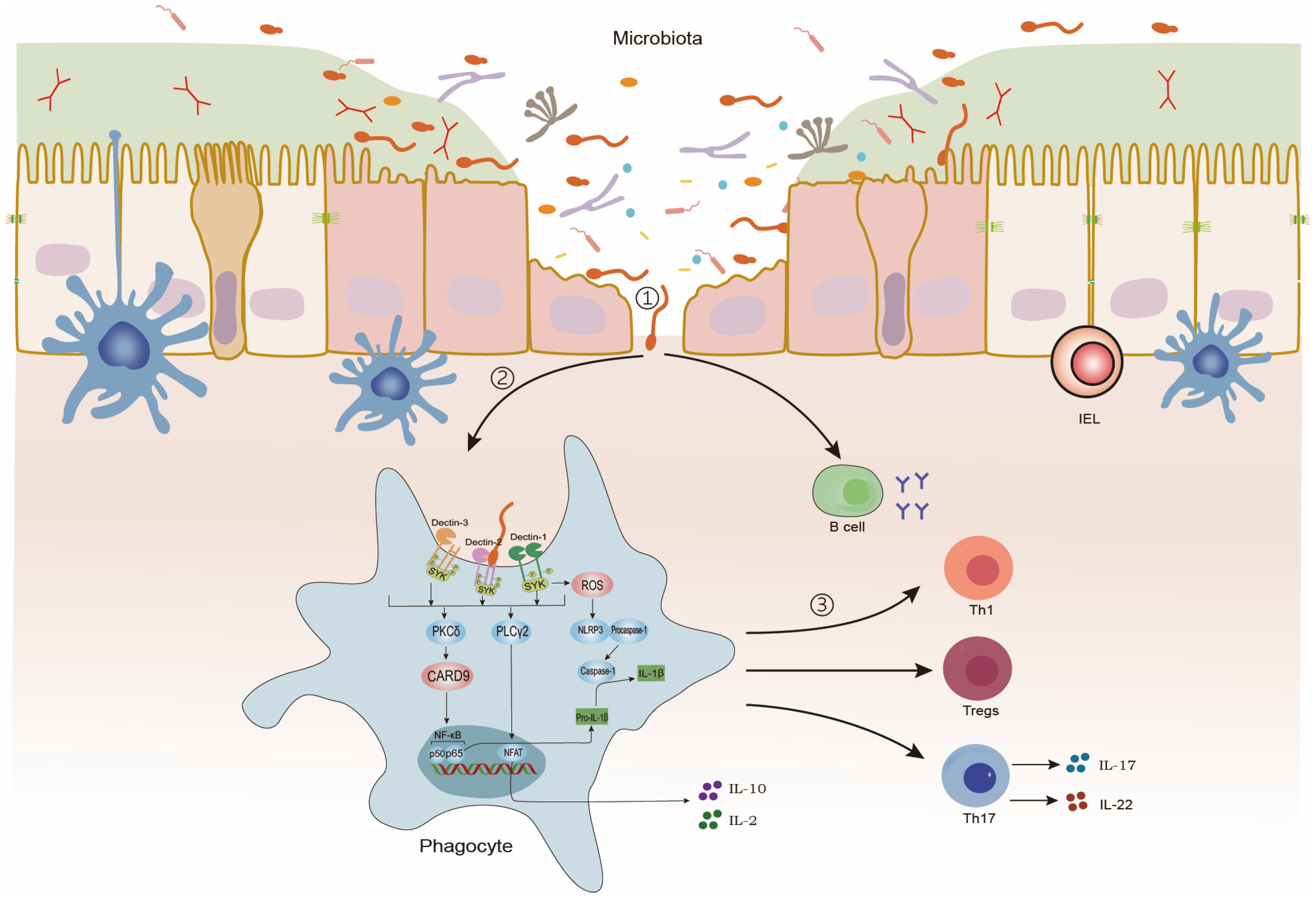
Within the intestinal mucosa, CLRs recognize fungal cell wall components, triggering a series of immune responses. This interaction initiates caspase recruitment domain-containing protein 9 (CARD9)-dependent nuclear factor kappa-B (NF-κB) activation, leading to the production of IL-1β, a cytokine critical for protection against colitis. Concurrently, it promotes the secretion of cytokines such as IL-2 and IL-10. Phagocytes recognize, internalize, and process fungal antigens, subsequently directing the differentiation of CD4+ T cells into functionally distinct subsets—such as Th17, Th1, and regulatory T cells (Tregs)—via divergent intracellular signaling cascades. Unlike T cells, B cells directly recognize fungal antigens, producing immunoglobulins [7,54,55,56,57,58,59] (Figure 1).
Figure 1.
Intestinal immunity against intestinal fungi. ①: Intestinal fungi invade the intestinal epithelium. ②: Phagocytes recognize and present intestinal fungi. ③: B-cell and T-cell immune response.
Phagocytosis in response to fungi initiating intestinal fungal immunity plays a crucial part in the immune response. CX3CR1+ mononuclear phagocytes (CX3CR1+ MNPs) are key mediators in orchestrating the Th17 immune response against fungal infections. These cells recognize both commensal and pathogenic fungi, such as Candida, through CLRs [60]. Additionally, CX3CR1+ MNPs regulate early Candida infections by suppressing caspase-dependent apoptosis and enhancing Akt phosphorylation [34]. Moreover, CX3CR1+ MNPs facilitate the generation of antifungal IgG antibodies in serum through T-cell-dependent mechanisms and the SYK-CARD9 signaling pathway, with IgG antibodies in healthy individuals showing preferential specificity for symbiotic Candida [61]. Moreover, CX3CR1+ MNPs facilitate the generation of antifungal IgG antibodies in serum through T-cell-dependent mechanisms and the SYK-CARD9 signaling pathway [62], which is closely related to the impact of gut fungi on the host.
In addition to direct recognition by the host, gut-fungi-derived metabolites play a pivotal role in modulating host immune responses. Candida albicans, for instance, induces phagocyte lysis through both inflammasome-dependent pathways and the formation of membrane-penetrating hyphae. Furthermore, its secreted toxin, candidalysin, facilitates phagocyte dissolution via a non-inflammasome-dependent mechanism, thereby enabling immune evasion [63]. Additionally, lactate signaling contributes to immune escape by regulating β-glucan masking [64]. Candidalysin also drives the production of interleukin-1β (IL-1) [65], which exacerbates colitis through the modulation of Th17 cell differentiation. Intestinal fungi further influence immune dynamics by secreting prostaglandins (PGs) or converting exogenous arachidonic acid (AA) into PGs. These PGs suppress antifungal Th1 responses, inhibit lymphocyte proliferation, and impair phagocytosis by promoting Th2 cell responses, thereby skewing adaptive immunity to favor Candida survival [66,67]. Collectively, these interactions between gut fungi and the immune system are intricately linked to the pathogenesis of various diseases.
The interaction between gut fungi and the host immune system is complex and multifaceted, with different fungal species exerting varying effects on immune responses. For instance, Saccharomyces cerevisiae, recognized for its anti-inflammatory properties, has been shown to significantly elevate levels of the anti-inflammatory cytokine IL-10 [8]. In contrast, another study demonstrated that Saccharomyces cerevisiae exacerbates colitis without inducing a prominent T-cell inflammatory response in the gut or in vitro. This effect may be mediated through the upregulation of uric acid via purine metabolism, thereby influencing colitis progression [68].
Disruptions in any component of the immune response to intestinal fungi can predispose the host to disease development. Dectin-1, Dectin-2, Dectin-3, and CARD9 are key molecules responsible for host defense against fungi, and mutations in any of the genes encoding these molecules correlate with human susceptibility to fungal infections [27]. Furthermore, defects in these molecules exacerbate the severity of dextran sulfate sodium (DSS)-induced colitis in murine models [49,69]. Dectin-1, a receptor for fungal β-glucans [70], plays a critical role in fungal recognition. Knockout of the CLEC7A gene, which encodes Dectin-1, results in heightened susceptibility to chemically induced colitis due to altered responses to resident fungi. Additionally, polymorphisms in the Dectin-1 gene have been correlated with the severity of UC [49]. However, Tang et al. reported that the absence of Dectin-1 may provide protection against DSS-induced colitis, potentially because of the original fungal characteristics in the gut [71]. Intriguingly, dual deficiency of Dectin-1 and Dectin-2 has been shown to protect against DSS-induced colitis, with this protective effect mediated by gut bacteria rather than fungi. Specifically, the Lachnospiraceae family has been identified as a key protective bacterial group in this context [72]. In addition to the strong association of Dectin-1 and Dectin-2 with fungal immunity, studies have shown that Dectin-3-deficient mice are more susceptible to the induction of DSS-induced colitis and are defective in promoting tissue repair in colonic epithelial cells, possibly related to defective NF-κB activation and reduced IL-6 production [55]. Similarly, CARD9 is essential for the host immune response to fungi. CARD9 knockout in mice leads to mitochondrial dysfunction and apoptosis, which may explain the heightened susceptibility to intestinal inflammation and fungal infections observed in CARD9-deficient individuals [73].
3.3. The Effects of Intestinal Fungal–Bacterial Interactions on UC
Studies have shown that the positive correlation between bacteria and fungi is greater in UC patients than in healthy individuals and that the negative correlation is greater in UC patients than in those with Crohn’s disease (CD) [8]. Furthermore, prolonged antibiotic use has been shown to disrupt the microbial balance, leading to fungal overgrowth and increased susceptibility to fungal infections due to the depletion of bacterial populations [74,75]. These findings underscore the intricate interplay between fungi and bacteria, suggesting that cross-kingdom interactions play a pivotal role in disease pathogenesis [76,77]. First, one key mechanism underlying this interaction involves the modulation of the fungal colonization environment by bacterial metabolites. Short-chain fatty acids (SCFAs), which are primarily produced by gut bacteria, are crucial for maintaining intestinal homeostasis and mucosal immunity [78,79]. Some studies have shown that SCFAs possess antifungal properties and that SCFA levels are reduced in the intestines of UC patients [46,80,81], potentially facilitating changes in the gut fungal community. Supporting this, a large-scale analysis of gut fungal communities in 1244 participants with an average age of 64.9 years revealed that Saccharomyces bacteria exhibited a positive association with SCFA-producing bacteria, such as Clostridium sensu stricto 1, Faecalitalea, and Megamonas [28], whereas Candida bacteria were negatively correlated with major SCFA-producing gut bacteria [24,81]. Second, bacterial communities influence the immune landscape, which in turn regulates fungal survival and colonization. Bacterium-induced immune responses can limit Candida colonization of the gut [46]. Conversely, fungi can also impact bacteria. For example, Bacteroides produce specialized enzymes that degrade the mannan present on the surface of Candida, thereby facilitating their own proliferation through nutrient acquisition [82].
From the perspective of fungal diversity, UC patients with a relatively high baseline abundance of Candida exhibit increased bacterial alpha diversity, which persists for eight weeks after FMT [83]. This observation may be attributed to alterations in the bacterial production environment, which create a favorable ecological niche for Candida albicans colonization. Additionally, Crohn’s disease (CD) is characterized by an elevated ratio of fungal-to-bacterial diversity [8], but the specific dynamics in UC remain to be further elucidated. At the species level, Candida are the most extensively studied opportunistic pathogens within the gut fungal community of UC patients. The relative abundance of Candida in fecal samples from UC patients shows a positive correlation with Parabacteroides diastonis and a negative correlation with Eubacterium hallii and Bifidobacterium adolescentis [9]. Further stratification of UC patients into active and remission phases revealed that Candida is positively associated with P. diastonis, Faecalibacterium prausnitzii, and Bacteroides dorei during remission, whereas no significant bacterial correlations were observed during active phases [9]. The interplay between fungi and bacteria has been corroborated not only in clinical studies but also in murine models of colitis. The colonization of germ-free mice with specific fungi alone is insufficient to induce significant colitis under dextran sulfate sodium (DSS) challenge; however, co-colonization with specific fungi and bacteria exacerbates colitis severity [76,84].
Both synergistic and competitive interactions exist between fungi and bacteria. Research indicates that the detrimental effects of Candida albicans on colitis, as well as the beneficial effects of Saccharomyces boulardii, are contingent upon the presence of mucin-sensitive bacteria, particularly those from the Enterobacteriaceae, which can sustain fungal loads in the gut [76]. Conversely, competition between fungi and bacteria is also evident. In adult mice, commensal bacteria, especially those from the Bacteroidetes and Firmicutes, play a critical role in limiting Candida colonization [46]. For example, Lactobacillus rhamnosus can deplete essential nutrients (nitrogen, carbon, etc.) required by Candida, leading to alterations in its gene expression and metabolic activity, thereby reducing its pathogenicity [85]. Additionally, Faecalibacterium prausnitzii can inhibit Candida albicans reproduction, colonization, and pathogenicity to improve DSS-induced colitis, whereas Escherichia coli primarily suppresses Candida albicans quantity without affecting its virulence [86]. Furthermore, bacteria can modulate the morphological transition of Candida albicans between yeast and hyphal forms [87,88,89], thereby influencing its virulence. The reciprocal interactions between gut fungi and bacteria are both causative and dynamic, contributing to the ecological dysbiosis of intestinal microbial communities. The dual roles of fungi and bacteria in these interactions underscore their collective impact on disease progression.
4. Influence of Intestinal Fungi on the Severity and Treatment Response of UC
4.1. Characteristics of Intestinal Fungi in UC Patients
In terms of intestinal fungal diversity, the alpha diversity of fungi in the feces of UC patients is lower than that in healthy subjects [8]. However, no significant differences in fungal α-diversity or β-diversity were observed between the active and remission phases of UC [9,90]. Regarding gut fungal composition, a prospective cohort study of 421 UC patients revealed that 86% of the fungi analyzed in feces were Ascomycetes and 3% were Basidiomycetes, with Saccharomyces and Candida being the most abundant intestinal fungal genera [9]. However, another study indicated that, among IBD patients, the most abundant genus was Candida, followed by Clavispora [10]. These discrepancies may be attributed to the inclusion of CD patients in the latter study, as well as variations in disease severity among participants. Within the Ascomycota phylum, the majority of fungi belong to the Saccharomycetales order, which includes genera such as Candida, Pichia, and Saccharomyces [90]. At the species level, both the relative and absolute numbers of Saccharomyces cerevisiae in the feces of UC patients decreased, whereas the relative quantity of Candida albicans increased, with its absolute quantity remaining unchanged, which were unrelated to the disease phenotype [8]. However, the relative abundance of Candida was linked to disease activity [9,90].
Alterations in the composition of the intestinal fungal community, referred to as “fungal ecological dysbiosis”, have been implicated in the pathogenesis of both intestinal and extraintestinal disorders, including colitis, alcoholic liver disease, and hypersensitivity pneumonitis [21]. The ratio of the abundance of Basidiomycota to that of Ascomycota can represent an index of fungal ecological dysbiosis. Compared with healthy individuals, UC patients presented an increased ratio of Ascomycota to Basidiomycota, although this difference was not statistically significant [16]. Conversely, a study utilizing ITS2 sequencing of fecal fungi from 235 IBD patients and 38 healthy controls indicated that the abundance ratio of Basidiomycota to Ascomycota varied greatly among different disease phenotypes, with higher ratios during IBD flare-ups than during remission and healthy states [8].
4.2. Influence of Intestinal Fungi on UC Severity and Treatment Response
Dectin-1 is a host receptor that recognizes fungi. Polymorphisms in the dectin-1 gene have been linked to the severity of UC, and experimental studies have demonstrated that mice deficient in dectin-1 exhibit heightened susceptibility to colitis [49]. In murine models of colitis, the co-administration of Candida and DSS exacerbates disease severity, promoting systemic inflammation and intestinal dysbiosis through increased intestinal permeability [91]. Similarly, Saccharomyces cerevisiae also enhances intestinal permeability and aggravates colitis [68]. Clinical studies have also demonstrated a correlation between intestinal fungi and UC disease severity. The relative abundance of Candida correlates with disease severity indices, and its temporal dynamics align with the clinical activity of UC over time [9]. In patients with endoscopically active UC, elevated levels of Saccharomyces and Candida are observed, whereas Penicillium predominates in patients with endoscopically quiescent UC [90].
Additionally, fungi also appear to influence treatment response in UC. For instance, non-responders to infliximab therapy for IBD exhibit higher abundances of Candida [10]. Additionally, Debaryomyces hansenii has been shown to colonize injured tissue and impair mucosal healing via the myeloid cell-specific type 1 interferon-CCL5 axis, contributing to intestinal tissue damage in CD [92]. While similar mechanisms may influence tissue healing and treatment outcomes in UC, further investigation is required to elucidate these relationships.
5. Treatment of Ulcerative Colitis with Intestinal Fungi
5.1. Antifungal Drugs
Antifungal drugs, which are not conventionally used to treat IBD, are primarily utilized to manage fungal infections such as candidiasis or candidemia in immunocompromised IBD patients. Nevertheless, their therapeutic efficacy in colitis is controversial. Miconazole effectively alleviates acetic-acid-induced colitis in mice by activating nuclear factor erythroid derived 2-like 2 (Nrf2)-regulated expression of cytoprotective proteins [93]. A study was conducted in which the antifungal drug fluconazole was administered to recipient mice that had been colonized with Candida albicans prior to human stool infusion. This treatment resulted in the restoration of the efficacy of FMT in eradicating Clostridium difficile infection [94]. Conversely, Wheeler et al. reported that fluconazole disrupts intestinal fungal communities in mice, exacerbating colitis and even aggravating dust-mite-induced allergic airway disease [95]. There may be a correlation between the duration of antifungal use and the effectiveness of treatment, and the overuse of antifungals leads to gut microbial dysbiosis. While antifungal therapy reduces the prevalence of Candida, it may also promote the relative expansion of other fungal species and alter bacterial communities [95]. However, whether the observed therapeutic effects are directly mediated by changes in the fungal microbiota or indirectly through secondary impacts on bacterial populations remains unclear.
Clinical studies have demonstrated that fluconazole treatment improves both endoscopic and histological indices in UC patients with confirmed fungal infections, findings further corroborated in murine models [48]. Another study also demonstrated that fluconazole therapy enhances clinical, histological, and calreticulin levels in UC patients with Candida infections, with Candida colonization being associated with steroid use and disease activity [96].
5.2. Probiotics and Prebiotics
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer health benefits to the host” [97]. Prebiotics are substrates that provide health benefits and are selectively utilized by host microbes. A randomized, double-blind, controlled trial investigating the efficacy of a combined probiotic and prebiotic treatment for UC demonstrated significant improvements in the experimental group one month posttreatment. These improvements included reduced endoscopic scores, decreased levels of human β-defensin, tumor necrosis factor-α (TNF-α), and IL-1α, and amelioration of intestinal inflammation [98]. Furthermore, a probiotic prebiotic mixture (containing Lactobacillus casei Zhang, Lactobacillus plantarum P-8, and Bifidobacterium animalis subsp. lactis V9) in combination with mesalazine reduced the UC disease activity index better than mesalazine alone and resulted in a higher remission rate [99]. A meta-analysis of 18 studies demonstrated that probiotics have significant effects on both the active and remission phases of ulcerative colitis [100]. Probiotics enhance intestinal mucosal barrier function by upregulating the expression of gut mucin-related genes, such as mucin-2 (MUC-2), and epithelial cell adhesion molecules, thereby promoting the enrichment of beneficial bacteria [101]. These alterations significantly reshape the fungal gut environment, inhibiting the growth and pathogenicity of harmful fungi.
Treatment with the probiotic formulation VSL#3 has been shown to increase the abundance and diversity of bacterial microbiota, particularly anaerobic bacteria, while simultaneously reducing fungal diversity and reinforcing intestinal epithelial barrier function [102,103]. Under TNF-α-induced intestinal mucosal injury, Lactobacillus reuteri has been shown to preserve the population of Lgr5+ cells, facilitate the repair of intestinal epithelial damage, attenuate the secretion of proinflammatory cytokines, and activate the Wnt/β-catenin signaling pathway through the upregulation of R-spondins, thereby promoting the proliferation of intestinal epithelial cells [104]. Additionally, Lactobacillus rhamnosus has been reported to mitigate disease severity in both DSS- and DSS + Candida-induced colitis models [91]. Notably, Lactobacillus also possesses anti-Candida properties [91,105].
Like bacteria, fungi are histoprotective and can enhance systemic and local immunity [106]. Moreover, fungal probiotics offer distinct advantages over bacterial counterparts, including inherent antibiotic resistance and the absence of antibiotic resistance gene transfer to bacterial populations [107]. Saccharomyces cerevisiae can effectively alleviate DSS-induced colitis through multiple mechanisms, such as reinforcing the intestinal mucosal barrier, attenuating tissue inflammation, modulating gut microbiota composition, and regulating microbial metabolism [108,109,110]. Saccharomyces boulardii, a member of the genus Saccharomyces, also has these effects [110,111,112,113]. Specifically, Saccharomyces boulardii activates peroxisome proliferator-activated receptor-γ (PPAR-γ) expression to prevent intestinal inflammation and IBD [114].
Despite the therapeutic potential of naturally occurring bacterial and fungal probiotics, their application is not without limitations. Consequently, engineered probiotics have attracted interest. High levels of the human P2Y2 purinergic receptor in engineered yeast increase the secretion of ATP-degrading enzymes, leading to increased breakdown of eATP, thereby limiting the eATP-driven inflammatory response, improving intestinal inflammation, and reducing intestinal fibrosis and microbial dysbiosis [115]. Similarly, engineered Escherichia coli strains expressing high levels of catalase and superoxide dismutase exhibit comparable effects by scavenging ROS in inflamed areas [116].
5.3. FMT
Currently, the primary therapeutic strategies for UC encompass 5-aminosalicylic acid, sulfasalazine, corticosteroids, immunosuppressants, and biological agents. However, these treatments are not universally effective and are often associated with adverse effects [11]. This limitation has spurred the exploration of novel therapeutic approaches. FMT has been successfully used for the treatment of CDI [117], but the clinical remission rate after FMT in patients with UC is approximately 35.0% [118]. Consequently, there is a pressing need to elucidate the key factors influencing the therapeutic efficacy of FMT to enhance its clinical outcomes in UC management.
Unlike therapies that selectively target gut bacteria, FMT addresses the entire gut microbiota, including fungi. Studies have demonstrated that targeting a healthy gut fungal community exacerbates experimental colitis in hosts with intact microbiota, which may be attributed to the protective functions performed by specific members of the gut fungal community [16]. Therefore, the therapeutic efficacy of FMT may be closely linked to gut fungi.
In studies of FMT for CDI, higher baseline relative abundances of Penicillium were associated with treatment failure, whereas Dorea longicatena and Fecalibacillus enteris were enriched in donors and responders to FMT [119]. Additionally, an exclusionary relationship was observed between Dorea longicatena, Faecalibacillus enteris, and Candida [119]. Furthermore, the abundance of Candida albicans in fecal samples from either FMT donors or CDI patients prior to treatment was linked to poor treatment outcomes, and eradication of Candida albicans from the intestinal tracts of mice via antifungal drugs prior to FMT restores the efficacy of FMT [94]. These findings support the idea that gut fungal communities play an important role in FMT treatment outcomes. In a longitudinal study of FMT-treated graft-versus-host disease (GVHD) patients, intestinal bacterial diversity increased in patients after FMT, whereas intestinal fungal diversity increased in only a few species, such as Candida dubliniensis, followed by a decrease in diversity [17].
Similarly, intestinal fungi play an important role in the treatment of UC via FMT. A high abundance of Candida albicans in the feces before FMT was associated with positive clinical findings with FMT, and a decrease in the relative abundance of Candida after FMT was positively associated with a decrease in disease severity. Interestingly, serum concentrations of anti-Candida albicans IgG remained stable in UC patients before and after FMT, whereas they increased in the control group. These observations suggest that FMT may exert its therapeutic effects, in part, by reducing Candida abundance and suppressing the proinflammatory immune responses triggered by fungal colonization during intestinal inflammation [16]. Another study demonstrated that encapsulated FMT improved microbial fungal diversity and composition, and patients in remission had a reduction in fungal diversity similar to that of donors. Additionally, reduced levels of pathogenic fungi, such as Candida and Aspergillus hansen, are associated with remission after encapsulated FMT in UC patients [18]. Patients who achieved remission after encapsulated FMT had specific enrichment of Kazachstania naganishii, Pyricularia grisea, Lachancea thermotolerans, and Schizosaccharomyces pombe compared with patients who did not remit [18]. Filobasidium is associated with clinical remission and endoscopic response after FMT in patients with mild to moderate UC, with F. floriforme being able to promote the release of the anti-inflammatory factor IL-10 from macrophages in vitro [120] (Table 1).

Table 1.
Correlations between intestinal fungi and FMT.
6. Summary and Outlook
Numerous studies have shown that the intestinal fungal community plays an important role in ulcerative colitis, intervening in various aspects of the disease through roles with the host immune system and cross-border interactions with bacteria, but many unknowns still need to be explored and added. A deeper understanding of the gut fungi of UC patients could help reveal the mechanisms of disease onset, which will guide and facilitate the precise treatment of FMT in this disease.
Author Contributions
Y.Z. conceived the manuscript and wrote the manuscript. All the authors revised the manuscript critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| UC | Ulcerative colitis |
| FMT | Fecal microbiota transplantation |
| CDI | Clostridium difficile infection |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| HMP | Human Microbiome Project |
| DSS | Dextran sulfate solution |
| BMI | Body mass index |
| sIgA | Secretory IgA |
| BLPs | “Balloon-like” protrusions |
| MUC | Mucosa-associated fungi |
| CLRs | C-type lectin receptors |
| TLRs | Toll-like receptors |
| NOD | Nucleotide oligomerization domain |
| NLRs | NOD-like receptors |
| SYK | Spleen tyrosine kinase |
| PKCδ | Protein kinase C delta |
| PLCγ2 | Phospholipase C gamma 2 |
| CARD9 | Caspase recruitment domain-containing protein 9 |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| NFAT | Nuclear factor of activated T cells |
| ROS | Reactive oxygen species |
| CX3CR1+ MNP | CX3CR1+ mononuclear phagocytes |
| PG | Prostaglandin |
| AA | Arachidonic acid |
| CLEC7A | C-type lectin domain-containing 7A |
| CD | Crohn’s disease |
| SCFAs | Short-chain fatty acids |
| Nrf2 | Nuclear factor erythroid derived 2-like 2 |
| TNF-α | Tumor necrosis factor-α |
| MUC-2 | Mucin-2 |
| PPAR-γ | Peroxisome proliferator-activated receptor-γ |
| GVHD | Graft-versus-host disease |
References
- Le Berre, C.; Ananthakrishnan, A.N.; Danese, S.; Singh, S.; Peyrin-Biroulet, L. Ulcerative Colitis and Crohn’s Disease Have Similar Burden and Goals for Treatment. Clin. Gastroenterol. Hepatol. 2020, 18, 14–23. [Google Scholar] [CrossRef]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e1442. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients with vs. Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.e931. [Google Scholar] [CrossRef] [PubMed]
- Li, X.V.; Leonardi, I.; Iliev, I.D. Gut Mycobiota in Immunity and Inflammatory Disease. Immunity 2019, 50, 1365–1379. [Google Scholar] [CrossRef]
- Iliev, I.D.; Cadwell, K. Effects of Intestinal Fungi and Viruses on Immune Responses and Inflammatory Bowel Diseases. Gastroenterology 2021, 160, 1050–1066. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Jangi, S.; Hsia, K.; Zhao, N.; Kumamoto, C.A.; Friedman, S.; Singh, S.; Michaud, D.S. Dynamics of the Gut Mycobiome in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2023, 22, 821–830.e7. [Google Scholar] [CrossRef]
- Ventin-Holmberg, R.; Eberl, A.; Saqib, S.; Korpela, K.; Virtanen, S.; Sipponen, T.; Salonen, A.; Saavalainen, P.; Nissila, E. Bacterial and Fungal Profiles as Markers of Infliximab Drug Response in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 1019–1031. [Google Scholar] [CrossRef]
- Ost, K.S.; Round, J.L. Commensal fungi in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 723–734. [Google Scholar] [CrossRef]
- Green, J.E.; Davis, J.A.; Berk, M.; Hair, C.; Loughman, A.; Castle, D.; Athan, E.; Nierenberg, A.A.; Cryan, J.F.; Jacka, F.; et al. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: A systematic review and meta-analysis. Gut Microbes 2020, 12, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Haifer, C.; Paramsothy, S.; Kaakoush, N.O.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Lowenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e114. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.F.; et al. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe 2020, 27, 823–829.e823. [Google Scholar] [CrossRef]
- Zhang, F.; Zuo, T.; Yeoh, Y.K.; Cheng, F.W.T.; Liu, Q.; Tang, W.; Cheung, K.C.Y.; Yang, K.; Cheung, C.P.; Mo, C.C.; et al. Longitudinal dynamics of gut bacteriome, mycobiome and virome after fecal microbiota transplantation in graft-versus-host disease. Nat. Commun. 2021, 12, 65. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, Y.; Zhang, B.; Yan, C.; Chen, Z.; Wang, L.; Hu, Y.; Huang, Q.; Su, J.; Ren, J.; et al. Specific fungi associated with response to capsulized fecal microbiota transplantation in patients with active ulcerative colitis. Front. Cell Infect. Microbiol. 2022, 12, 1086885. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Tian, G.; Huang, Z.; et al. Fungi in Gastrointestinal Tracts of Human and Mice: From Community to Functions. Microb. Ecol. 2018, 75, 821–829. [Google Scholar] [CrossRef]
- Leonardi, I.; Gao, I.H.; Lin, W.Y.; Allen, M.; Li, X.V.; Fiers, W.D.; De Celie, M.B.; Putzel, G.G.; Yantiss, R.K.; Johncilla, M.; et al. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell 2022, 185, 831–846.e814. [Google Scholar] [CrossRef]
- Alcazar, C.G.-M.; Paes, V.M.; Shao, Y.; Oesser, C.; Miltz, A.; Lawley, T.D.; Brocklehurst, P.; Rodger, A.; Field, N. The association between early-life gut microbiota and childhood respiratory diseases: A systematic review. Lancet Microbe 2022, 3, e867–e880. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Manichanh, C. FunOMIC: Pipeline with built-in fungal taxonomic and functional databases for human mycobiome profiling. Comput. Struct. Biotechnol. J. 2022, 20, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Yan, Y.; Pu, Y.; Lin, S.; Qiu, J.G.; Jiang, B.H.; Keller, M.I.; Wang, M.; Bork, P.; Chen, W.H.; et al. Enterotypes of the human gut mycobiome. Microbiome 2023, 11, 179. [Google Scholar] [CrossRef]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Underhill, D.M.; Iliev, I.D. The mycobiota: Interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 2014, 14, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Shuai, M.; Fu, Y.; Zhong, H.L.; Gou, W.; Jiang, Z.; Liang, Y.; Miao, Z.; Xu, J.J.; Huynh, T.; Wahlqvist, M.L.; et al. Mapping the human gut mycobiome in middle-aged and elderly adults: Multiomics insights and implications for host metabolic health. Gut 2022, 71, 1812–1820. [Google Scholar] [CrossRef]
- Li, S.; Deng, Y.; Wang, Z.; Zhang, Z.; Kong, X.; Zhou, W.; Yi, Y.; Qu, Y. Exploring the accuracy of amplicon-based internal transcribed spacer markers for a fungal community. Mol. Ecol. Resour. 2019, 20, 170–184. [Google Scholar] [CrossRef]
- Blakeley-Ruiz, J.A.; Erickson, A.R.; Cantarel, B.L.; Xiong, W.; Adams, R.; Jansson, J.K.; Fraser, C.M.; Hettich, R.L. Metaproteomics reveals persistent and phylum-redundant metabolic functional stability in adult human gut microbiomes of Crohn’s remission patients despite temporal variations in microbial taxa, genomes, and proteomes. Microbiome 2019, 7, 18. [Google Scholar] [CrossRef]
- Szóstak, N.; Handschuh, L.; Samelak-Czajka, A.; Tomela, K.; Schmidt, M.; Pruss, Ł.; Milanowska-Zabel, K.; Kozlowski, P.; Philips, A. Host Factors Associated with Gut Mycobiome Structure. mSystems 2023, 8, e0098622. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. The Healthy Microbiome-What Is the Definition of a Healthy Gut Microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef]
- Xie, Z.; Canalda-Baltrons, A.; d’Enfert, C.; Manichanh, C. Shotgun metagenomics reveals interkingdom association between intestinal bacteria and fungi involving competition for nutrients. Microbiome 2023, 11, 275. [Google Scholar] [CrossRef]
- Wu, X.; Xia, Y.; He, F.; Zhu, C.; Ren, W. Intestinal mycobiota in health and diseases: From a disrupted equilibrium to clinical opportunities. Microbiome 2021, 9, 60. [Google Scholar] [CrossRef]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Hsu, C.; Ghannoum, M.; Cominelli, F.; Martino, L.D. Mycobiome and Inflammatory Bowel Disease: Role in Disease Pathogenesis, Current Approaches and Novel Nutritional-based Therapies. Inflamm. Bowel Dis. 2023, 29, 470–479. [Google Scholar] [CrossRef]
- Sun, Y.; Zuo, T.; Cheung, C.P.; Gu, W.; Wan, Y.; Zhang, F.; Chen, N.; Zhan, H.; Yeoh, Y.K.; Niu, J.; et al. Population-Level Configurations of Gut Mycobiome Across 6 Ethnicities in Urban and Rural China. Gastroenterology 2021, 160, 272–286.e211. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef]
- Cai, R.; Cheng, C.; Chen, J.; Xu, X.; Ding, C.; Gu, B. Interactions of commensal and pathogenic microorganisms with the mucus layer in the colon. Gut Microbes 2020, 11, 680–690. [Google Scholar] [CrossRef]
- Johansson, M.E.; Gustafsson, J.K.; Holmen-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjovall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef]
- Pullan, R.D.; Thomas, G.A.; Rhodes, M.; Newcombe, R.G.; Williams, G.T.; Allen, A.; Rhodes, J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 1994, 35, 353–359. [Google Scholar] [CrossRef]
- Schirbel, A.; Shouval, D.S.; Hebecker, B.; Hube, B.; Sturm, A.; Werner, L. Intestinal epithelial cells and T cells differentially recognize and respond to Candida albicans yeast and hypha. Eur. J. Immunol. 2018, 48, 1826–1837. [Google Scholar] [CrossRef]
- Ost, K.S.; O’Meara, T.R.; Stephens, W.Z.; Chiaro, T.; Zhou, H.; Penman, J.; Bell, R.; Catanzaro, J.R.; Song, D.; Singh, S.; et al. Adaptive immunity induces mutualism between commensal eukaryotes. Nature 2021, 596, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Doron, I.; Mesko, M.; Li, X.V.; Kusakabe, T.; Leonardi, I.; Shaw, D.G.; Fiers, W.D.; Lin, W.Y.; Bialt-DeCelie, M.; Roman, E.; et al. Mycobiota-induced IgA antibodies regulate fungal commensalism in the gut and are dysregulated in Crohn’s disease. Nat. Microbiol. 2021, 6, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015, 21, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Chikina, A.S.; Nadalin, F.; Maurin, M.; San-Roman, M.; Thomas-Bonafos, T.; Li, X.V.; Lameiras, S.; Baulande, S.; Henri, S.; Malissen, B.; et al. Macrophages Maintain Epithelium Integrity by Limiting Fungal Product Absorption. Cell 2020, 183, 411–428.e416. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339.e324. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Seiwert, N.; Heylmann, D.; Hasselwander, S.; Fahrer, J. Mechanism of colorectal carcinogenesis triggered by heme iron from red meat. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2020, 1873, 188334. [Google Scholar] [CrossRef]
- Keir, M.; Yi, Y.; Lu, T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Lenardon, M.D. Architecture of the dynamic fungal cell wall. Nat. Rev. Microbiol. 2023, 21, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.C.G.; Curto, M.A.; Carvalho, V.S.D.; Perez, P.; Ribas, J.C. The fungal cell wall as a target for the development of new antifungal therapies. Biotechnol. Adv. 2019, 37, 107352. [Google Scholar] [CrossRef]
- Iliev, I.D.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef]
- Wang, T.; Pan, D.; Zhou, Z.; You, Y.; Jiang, C.; Zhao, X.; Lin, X. Dectin-3 Deficiency Promotes Colitis Development due to Impaired Antifungal Innate Immune Responses in the Gut. PLoS Pathog. 2016, 12, e1005662. [Google Scholar] [CrossRef]
- Roth, S.; Ruland, J. Caspase recruitment domain-containing protein 9 signaling in innate immunity and inflammation. Trends Immunol. 2013, 34, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Levitz, S.M. Innate recognition of fungal cell walls. PLoS Pathog. 2010, 6, e1000758. [Google Scholar] [CrossRef]
- Verma, A.; Wuthrich, M.; Deepe, G.; Klein, B. Adaptive Immunity to Fungi. Cold Spring Harb. Perspect. Med. 2014, 5, a019612. [Google Scholar] [CrossRef]
- Malik, A.; Sharma, D.; Malireddi, R.K.S.; Guy, C.S.; Chang, T.C.; Olsen, S.R.; Neale, G.; Vogel, P.; Kanneganti, T.D. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity 2018, 49, 515–530.e515. [Google Scholar] [CrossRef]
- Leonardi, I.; Li, X.; Semon, A.; Li, D.; Doron, I.; Putzel, G.; Bar, A.; Prieto, D.; Rescigno, M.; McGovern, D.P.B.; et al. CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi. Science 2018, 359, 232–236. [Google Scholar] [CrossRef]
- Spencer, J.; Bemark, M. Human intestinal B cells in inflammatory diseases. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, P.; Zelante, T.; D’Angelo, C.; De Luca, A.; Moretti, S.; Bozza, S.; Perruccio, K.; Iannitti, R.G.; Giovannini, G.; Volpi, C.; et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009, 2, 362–374. [Google Scholar] [CrossRef]
- Kasper, L.; Konig, A.; Koenig, P.A.; Gresnigt, M.S.; Westman, J.; Drummond, R.A.; Lionakis, M.S.; Gross, O.; Ruland, J.; Naglik, J.R.; et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018, 9, 4260. [Google Scholar] [CrossRef] [PubMed]
- Ballou, E.R.; Avelar, G.M.; Childers, D.S.; Mackie, J.; Bain, J.M.; Wagener, J.; Kastora, S.L.; Panea, M.D.; Hardison, S.E.; Walker, L.A.; et al. Lactate signalling regulates fungal beta-glucan masking and immune evasion. Nat. Microbiol. 2016, 2, 16238. [Google Scholar] [CrossRef]
- Li, X.V.; Leonardi, I.; Putzel, G.G.; Semon, A.; Fiers, W.D.; Kusakabe, T.; Lin, W.Y.; Gao, I.H.; Doron, I.; Gutierrez-Guerrero, A.; et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 2022, 603, 672–678. [Google Scholar] [CrossRef]
- Tan, T.G.; Lim, Y.S.; Tan, A.; Leong, R.; Pavelka, N. Fungal Symbionts Produce Prostaglandin E(2) to Promote Their Intestinal Colonization. Front. Cell Infect. Microbiol. 2019, 9, 359. [Google Scholar] [CrossRef]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 2003, 16, 517–533. [Google Scholar] [CrossRef]
- Chiaro, T.R.; Soto, R.; Zac Stephens, W.; Kubinak, J.L.; Petersen, C.; Gogokhia, L.; Bell, R.; Delgado, J.C.; Cox, J.; Voth, W.; et al. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci. Transl. Med. 2017, 9, eaaf9044. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef]
- Tang, C.; Kamiya, T.; Liu, Y.; Kadoki, M.; Kakuta, S.; Oshima, K.; Hattori, M.; Takeshita, K.; Kanai, T.; Saijo, S.; et al. Inhibition of Dectin-1 Signaling Ameliorates Colitis by Inducing Lactobacillus-Mediated Regulatory T Cell Expansion in the Intestine. Cell Host Microbe 2015, 18, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spatz, M.; Da Costa, G.; Michaudel, C.; Lapiere, A.; Danne, C.; Agus, A.; Michel, M.L.; Netea, M.G.; Langella, P.; et al. Deletion of both Dectin-1 and Dectin-2 affects the bacterial but not fungal gut microbiota and susceptibility to colitis in mice. Microbiome 2022, 10, 91. [Google Scholar] [CrossRef]
- Danne, C.; Michaudel, C.; Skerniskyte, J.; Planchais, J.; Magniez, A.; Agus, A.; Michel, M.-L.; Lamas, B.; Da Costa, G.; Spatz, M.; et al. CARD9 in neutrophils protects from colitis and controls mitochondrial metabolism and cell survival. Gut 2023, 72, 1081–1092. [Google Scholar] [CrossRef]
- Jacobsen, I.D.; Dollive, S.; Chen, Y.-Y.; Grunberg, S.; Bittinger, K.; Hoffmann, C.; Vandivier, L.; Cuff, C.; Lewis, J.D.; Wu, G.D.; et al. Fungi of the Murine Gut: Episodic Variation and Proliferation during Antibiotic Treatment. PLoS ONE 2013, 8, e71806. [Google Scholar] [CrossRef]
- Noverr, M.C.; Noggle, R.M.; Toews, G.B.; Huffnagle, G.B. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 2004, 72, 4996–5003. [Google Scholar] [CrossRef] [PubMed]
- Sovran, B.; Planchais, J.; Jegou, S.; Straube, M.; Lamas, B.; Natividad, J.M.; Agus, A.; Dupraz, L.; Glodt, J.; Da Costa, G.; et al. Enterobacteriaceae are essential for the modulation of colitis severity by fungi. Microbiome 2018, 6, 152. [Google Scholar] [CrossRef]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. mBio 2016, 7, e01250-16. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Zeise, K.D.; Woods, R.J.; Huffnagle, G.B. Interplay between Candida albicans and Lactic Acid Bacteria in the Gastrointestinal Tract: Impact on Colonization Resistance, Microbial Carriage, Opportunistic Infection, and Host Immunity. Clin. Microbiol. Rev. 2021, 34, e0032320. [Google Scholar] [CrossRef]
- Seelbinder, B.; Chen, J.; Brunke, S.; Vazquez-Uribe, R.; Santhaman, R.; Meyer, A.C.; de Oliveira Lino, F.S.; Chan, K.F.; Loos, D.; Imamovic, L.; et al. Antibiotics create a shift from mutualism to competition in human gut communities with a longer-lasting impact on fungi than bacteria. Microbiome 2020, 8, 133. [Google Scholar] [CrossRef]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef]
- Fritsch, J.; Abreu, M.T. Candida in IBD: Friend or Foe? Cell Host Microbe 2020, 27, 689–691. [Google Scholar] [CrossRef]
- van Tilburg Bernardes, E.; Pettersen, V.K.; Gutierrez, M.W.; Laforest-Lapointe, I.; Jendzjowsky, N.G.; Cavin, J.B.; Vicentini, F.A.; Keenan, C.M.; Ramay, H.R.; Samara, J.; et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat. Commun. 2020, 11, 2577. [Google Scholar] [CrossRef]
- Alonso-Roman, R.; Last, A.; Mirhakkak, M.H.; Sprague, J.L.; Moller, L.; Grossmann, P.; Graf, K.; Gratz, R.; Mogavero, S.; Vylkova, S.; et al. Lactobacillus rhamnosus colonisation antagonizes Candida albicans by forcing metabolic adaptations that compromise pathogenicity. Nat. Commun. 2022, 13, 3192. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ma, J.; Jiao, C.; Tang, N.; Zhao, X.; Wang, D.; Zhang, Y.; Ye, Z.; Xu, C.; Jiang, J.; et al. Faecalibacterium prausnitzii Attenuates DSS-Induced Colitis by Inhibiting the Colonization and Pathogenicity of Candida albicans. Mol. Nutr. Food Res. 2021, 65, e2100433. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Lee, R.T.; Fang, H.M.; Wang, Y.M.; Li, R.; Zou, H.; Zhu, Y.; Wang, Y. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 2008, 4, 28–39. [Google Scholar] [CrossRef] [PubMed]
- MacAlpine, J.; Daniel-Ivad, M.; Liu, Z.; Yano, J.; Revie, N.M.; Todd, R.T.; Stogios, P.J.; Sanchez, H.; O’Meara, T.R.; Tompkins, T.A.; et al. A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat. Commun. 2021, 12, 6151. [Google Scholar] [CrossRef]
- Graham, C.E.; Cruz, M.R.; Garsin, D.A.; Lorenz, M.C. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 2017, 114, 4507–4512. [Google Scholar] [CrossRef]
- Hsia, K.; Zhao, N.; Chung, M.; Algarrahi, K.; Montaser Kouhsari, L.; Fu, M.; Chen, H.; Singh, S.; Michaud, D.S.; Jangi, S. Alterations in the Fungal Microbiome in Ulcerative Colitis. Inflamm. Bowel Dis. 2023, 29, 1613–1621. [Google Scholar] [CrossRef]
- Panpetch, W.; Hiengrach, P.; Nilgate, S.; Tumwasorn, S.; Somboonna, N.; Wilantho, A.; Chatthanathon, P.; Prueksapanich, P.; Leelahavanichkul, A. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes 2020, 11, 465–480. [Google Scholar] [CrossRef]
- Jain, U.; Ver Heul, A.M.; Xiong, S.; Gregory, M.H.; Demers, E.G.; Kern, J.T.; Lai, C.W.; Muegge, B.D.; Barisas, D.A.G.; Leal-Ekman, J.S.; et al. Debaryomyces is enriched in Crohn’s disease intestinal tissue and impairs healing in mice. Science 2021, 371, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, I.A.; Fayed, H.M.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A. Miconazole Mitigates Acetic Acid-Induced Experimental Colitis in Rats: Insight into Inflammation, Oxidative Stress and Keap1/Nrf-2 Signaling Crosstalk. Biology 2022, 11, 303. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat. Commun. 2018, 9, 3663. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Dutta, U.; Shah, J.; Sharma, V.; Prasad, K.K.; Shivaprakash, R.M.; Mandavdhare, H.S.; Samanta, J.; Sharma, P.; Popli, P.; et al. Oral Fluconazole Therapy in Patients With Active Ulcerative Colitis Who Have Detectable Candida in the Stool: A Double-Blind Randomized Placebo-controlled Trial. J. Clin. Gastroenterol. 2022, 56, 705–711. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’Neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef]
- Chen, P.; Xu, H.; Tang, H.; Zhao, F.; Yang, C.; Kwok, L.Y.; Cong, C.; Wu, Y.; Zhang, W.; Zhou, X.; et al. Modulation of gut mucosal microbiota as a mechanism of probiotics-based adjunctive therapy for ulcerative colitis. Microb. Biotechnol. 2020, 13, 2032–2043. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, Y.; Zheng, L.; Rong, N.; Yang, Y.; Gong, P.; Yang, Y.; Siwu, X.; Zhang, C.; Zhu, L.; et al. Bifidobacterium and Lactobacillus improve inflammatory bowel disease in zebrafish of different ages by regulating the intestinal mucosal barrier and microbiota. Life Sci. 2023, 324, 121699. [Google Scholar] [CrossRef]
- Kuhbacher, T.; Ott, S.J.; Helwig, U.; Mimura, T.; Rizzello, F.; Kleessen, B.; Gionchetti, P.; Blaut, M.; Campieri, M.; Folsch, U.R.; et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut 2006, 55, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Itapary Dos Santos, C.; Ramos Franca, Y.; Duarte Lima Campos, C.; Quaresma Bomfim, M.R.; Oliveira Melo, B.; Assuncao Holanda, R.; Santos, V.L.; Gomes Monteiro, S.; Buozzi Moffa, E.; Souza Monteiro, A.; et al. Antifungal and Antivirulence Activity of Vaginal Lactobacillus Spp. Products against Candida Vaginal Isolates. Pathogens 2019, 8, 150. [Google Scholar] [CrossRef]
- Jiang, T.T.; Shao, T.Y.; Ang, W.X.G.; Kinder, J.M.; Turner, L.H.; Pham, G.; Whitt, J.; Alenghat, T.; Way, S.S. Commensal Fungi Recapitulate the Protective Benefits of Intestinal Bacteria. Cell Host Microbe 2017, 22, 809–816.e804. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, L.; Zhai, Q.; Zhao, J.; Tian, F. Anti-Inflammatory, Barrier Maintenance, and Gut Microbiome Modulation Effects of Saccharomyces cerevisiae QHNLD8L1 on DSS-Induced Ulcerative Colitis in Mice. Int. J. Mol. Sci. 2023, 24, 6721. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, Y.; Xia, Y.; Wang, F.; Sun, Y.; Yang, Y.; Ai, L. Probiotic yeast BR14 ameliorates DSS-induced colitis by restoring the gut barrier and adjusting the intestinal microbiota. Food Funct. 2021, 12, 8386–8398. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Jin, Y.; Huang, K.; Zhang, Y.; Liang, Z. Both Saccharomyces boulardii and Its Postbiotics Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, Association with Modulating Inflammation and Intestinal Microbiota. Nutrients 2023, 15, 1484. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.; Xu, J.; Zuo, X.; Yue, T.; Xu, H.; Sun, J.; Wang, M.; Ye, T.; Yu, Y.; et al. Saccharomyces boulardii protects against murine experimental colitis by reshaping the gut microbiome and its metabolic profile. Front. Microbiol. 2023, 14, 1204122. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Shi, L.; Li, R.; Luo, Y.; Deng, Y.; Li, S.; Li, R.; Liu, Z. Saccharomyces boulardii alleviates DSS-induced intestinal barrier dysfunction and inflammation in humanized mice. Food Funct. 2022, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.P.; Zheng, Y.; Wu, T.; He, Q.; Teng, G.G.; Wang, H.H. Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chin. Med. J. 2019, 132, 1951–1958. [Google Scholar] [CrossRef]
- Pothoulakis, C. Review article: Anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2009, 30, 826–833. [Google Scholar] [CrossRef]
- Scott, B.M.; Gutierrez-Vazquez, C.; Sanmarco, L.M.; da Silva Pereira, J.A.; Li, Z.; Plasencia, A.; Hewson, P.; Cox, L.M.; O’Brien, M.; Chen, S.K.; et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med. 2021, 27, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef] [PubMed]
- Borody, T.J.; Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 88–96. [Google Scholar] [CrossRef]
- Lam, S.; Bai, X.; Shkoporov, A.N.; Park, H.; Wu, X.; Lan, P.; Zuo, T. Roles of the gut virome and mycobiome in faecal microbiota transplantation. Lancet Gastroenterol. Hepatol. 2022, 7, 472–484. [Google Scholar] [CrossRef]
- Haifer, C.; Paramsothy, S.; Borody, T.J.; Clancy, A.; Leong, R.W.; Kaakoush, N.O. Long-Term Bacterial and Fungal Dynamics following Oral Lyophilized Fecal Microbiota Transplantation in Clostridioides difficile Infection. mSystems 2021, 6, e00905-20. [Google Scholar] [CrossRef]
- van Thiel, I.A.M.; Rahman, S.; Hakvoort, T.B.M.; Davids, M.; Verseijden, C.; van Hamersveld, P.H.P.; Bénard, M.V.; Lodders, M.H.; Boekhout, T.; van den Wijngaard, R.M.; et al. Fecal Filobasidium Is Associated with Clinical Remission and Endoscopic Response following Fecal Microbiota Transplantation in Mild-to-Moderate Ulcerative Colitis. Microorganisms 2022, 10, 737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).