Immobilization of Acinetobacter sp. A-1 and Applicability in Removal of Difenoconazole from Water–Sediment Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Screening, and Identification of DIF-Degrading Bacteria
2.2. Determination of Growth and Degradation Capacity of Free DIF-Degrading Bacteria
2.3. Optimization of Culture Conditions for DIF Degradation by Strain A-1
2.4. Degradation Kinetics Assay
2.5. Immobilization of Strain A-1 and Degradation Assays
2.6. Effect of Culture Conditions on the DIF Degradation Ability of Immobilized Strain A-1
2.7. Degradation Ability of Immobilized and Free Strain A-1 in Water–Sediment System
3. Results and Discussion
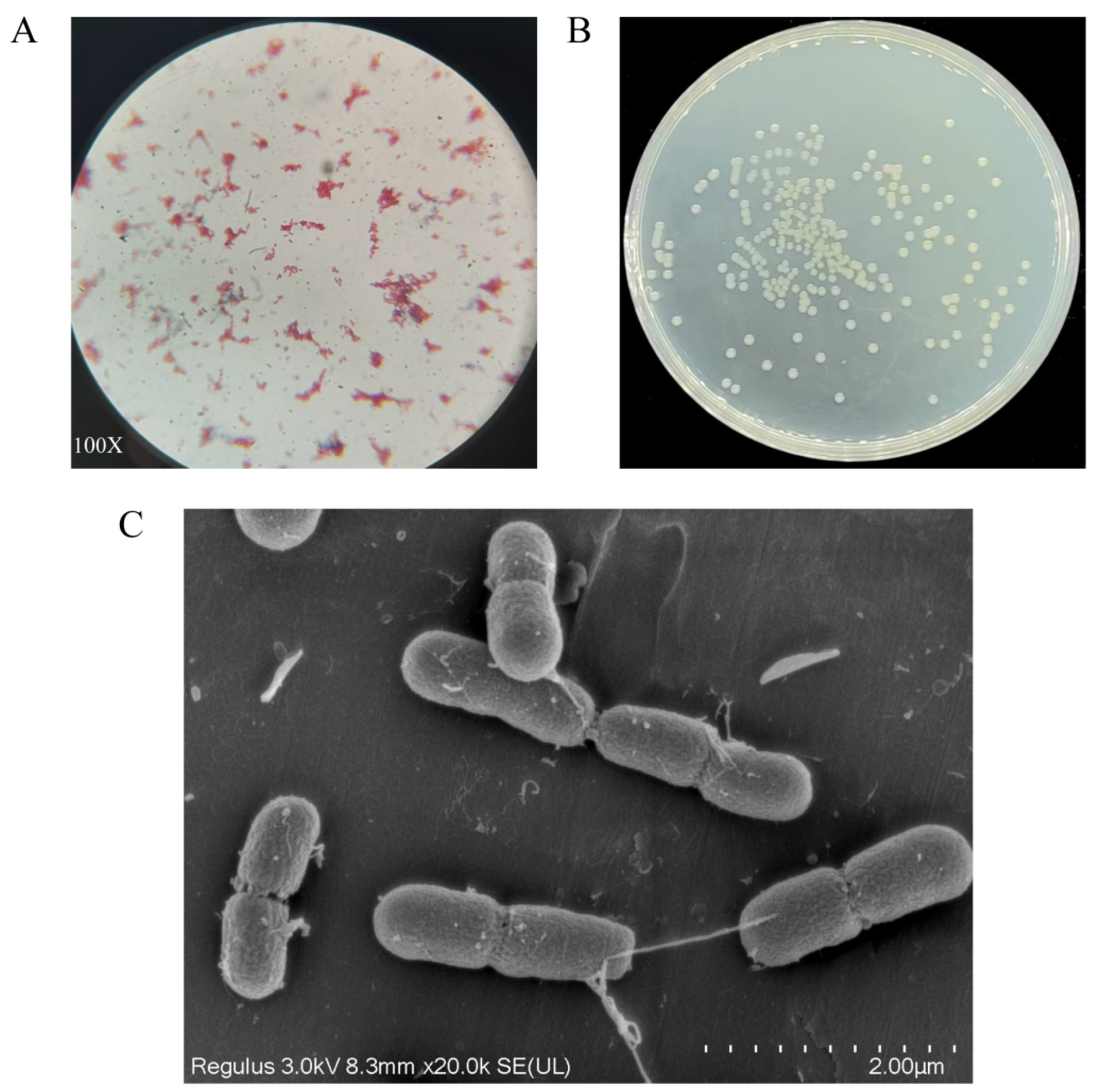
3.1. Isolation, Screening, and Identification of Strain A-1
3.2. The Strain Growth and Degradation Characteristics of Strain A-1
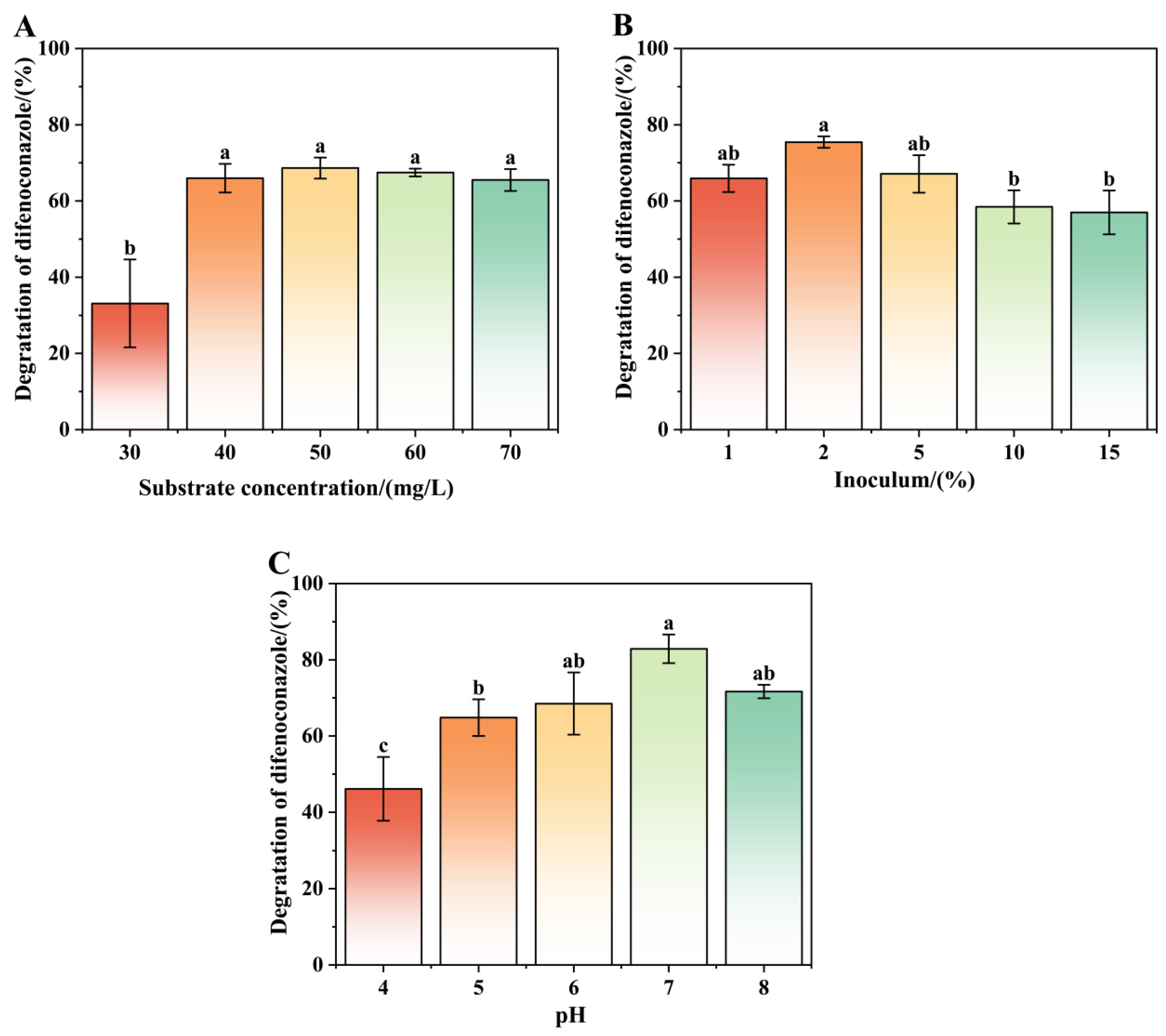
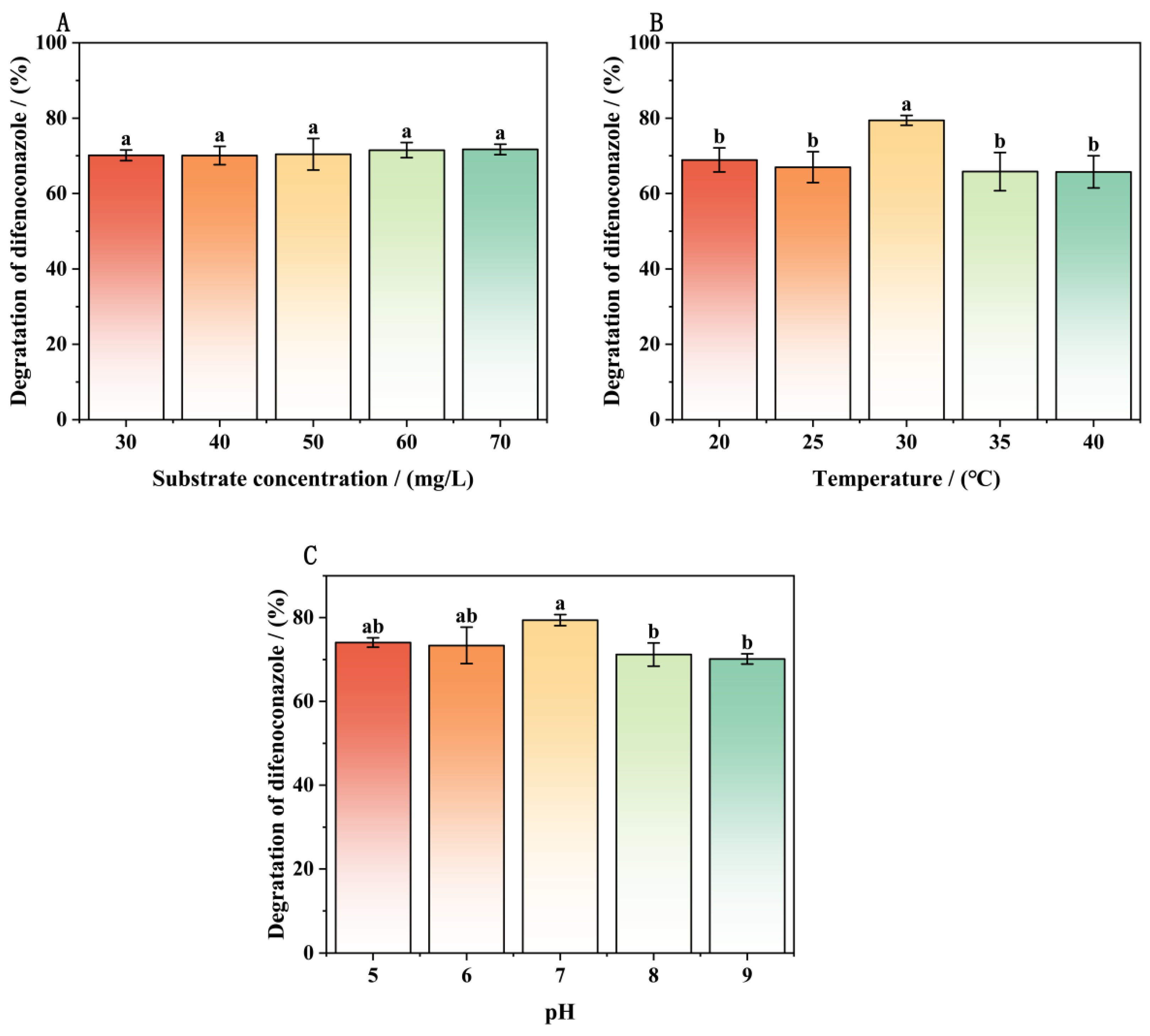
3.3. Effects of Culture Conditions on the Removal of DIF by Strain A-1
3.4. Kinetic Analysis of DIF Degradation
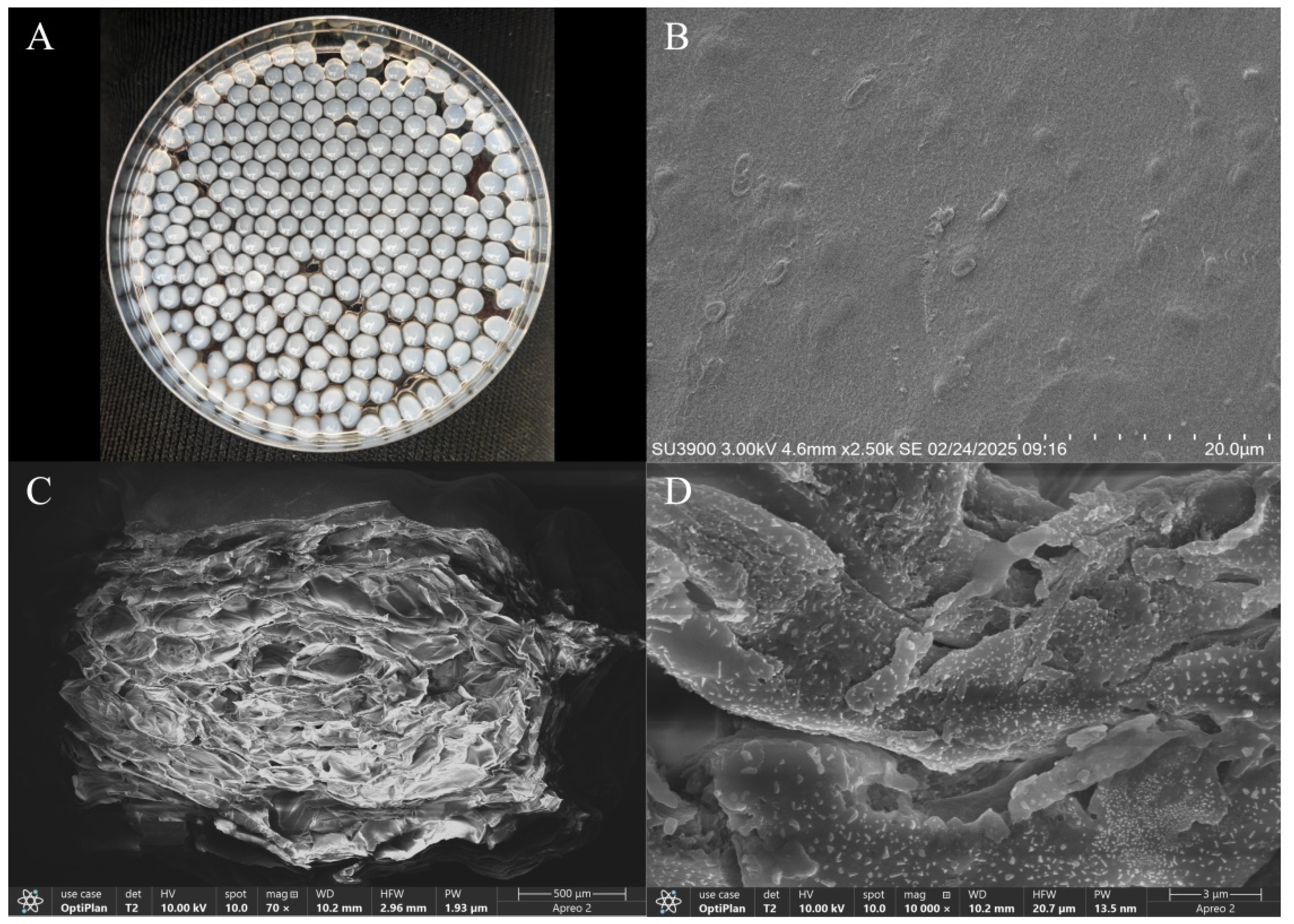
3.5. The DIF Degradation Capacity of Immobilized Strain A-1
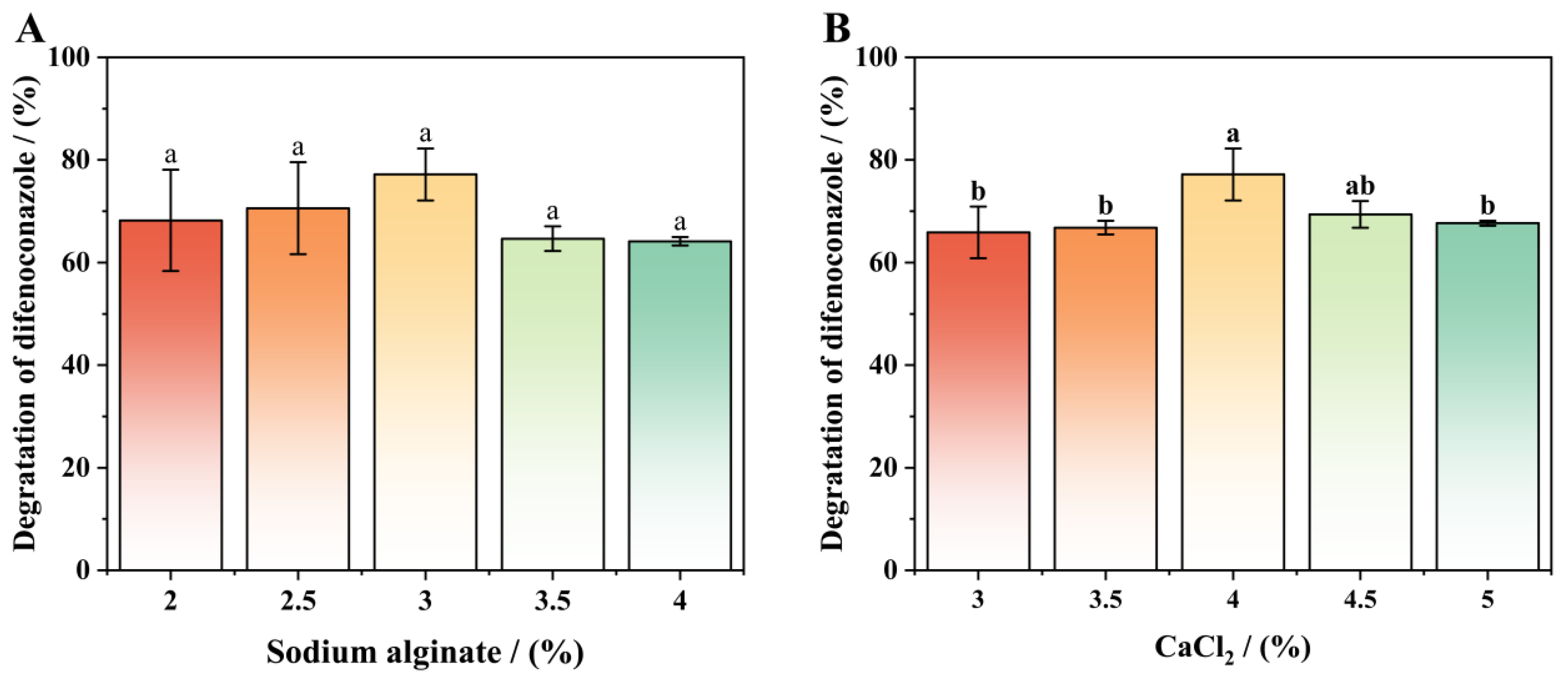
3.6. Components Optimization of Immobilized Conditions
3.7. Determination of Environmental Adaptability of Immobilized Strain A-1
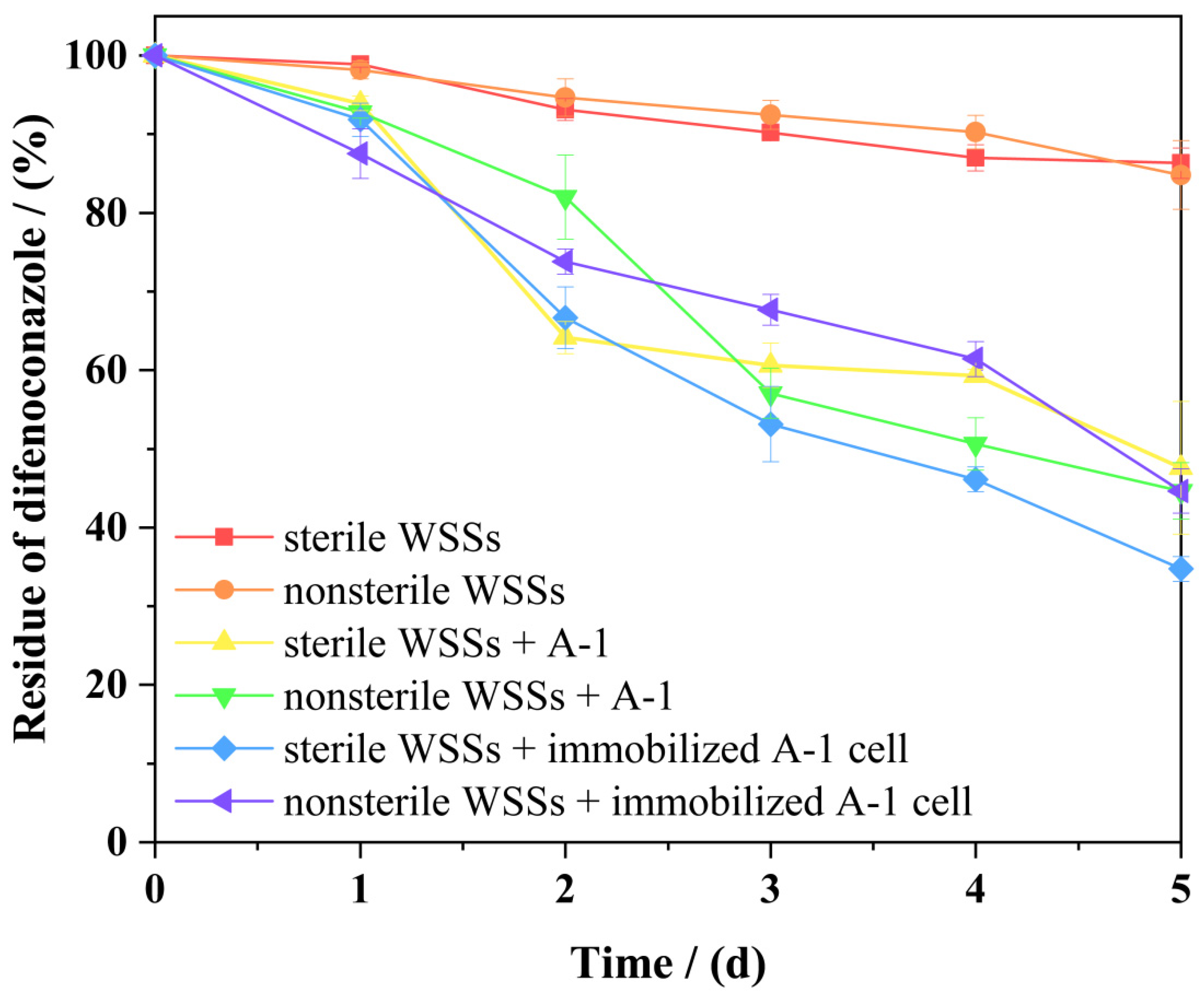
3.8. Comparison of the Bioremediation Effects of Free and Immobilized Strain A-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| DIF | Difenoconazole |
| HPLC | High-performance liquid chromatography |
| LB | Luria-Bertani |
| MSM | Minimum salt medium |
| PCR | Polymerase chain reaction |
| QNC | Quinoline acid |
| rDNA | Ribosomal DNA |
| RSM | Response surface methodology |
| SA | Sodium alginate |
| SEM | Scanning electron microscope |
| SM2 | Sulfamethazine |
| WSS | Water–sediment system |
References
- Dong, B. Recent advances in the toxicological effects of difenoconazole: A focus on toxic mechanisms in fish and mammals. Chemosphere 2024, 368, 143751. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Feng, X.; Cao, M.; Zhang, S.; Huang, Y.; Xu, T.; Jing, J.; Zhang, H. Determination and distribution of pesticides and antibiotics in agricultural soils from northern China. RSC Adv. 2019, 9, 15686–15693. [Google Scholar] [CrossRef] [PubMed]
- Khalidah, A.L.; Kartini, A.B.N.; Nurzawani, M.I. Preliminary study of difenoconazole residues in rice paddy watersheds. Malays. J. Sci. 2010, 29, 73–79. [Google Scholar] [CrossRef]
- Feng, Q.; Han, L.; Wu, Q.; Wu, X. Dissipation, residue and dietary risk assessment of difenoconazole in Rosa roxburghii. J. Environ. Sci. Health Part B 2023, 58, 651–658. [Google Scholar] [CrossRef]
- Liu, Y.; Bei, K.; Zheng, W.; Yu, G.; Sun, C. Multiple pesticide residues and risk assessment of Dendrobium officinale Kimura et Migo: A three-year investigation. Environ. Sci. Pollut. Res. Int. 2023, 30, 107827–107840. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Zhang, J.; Duan, M.; Xu, H.; Zhao, W.; Yang, Y.; Wang, C.; Xu, Y. Exposure to difenoconazole induces reproductive toxicity in zebrafish by interfering with gamete maturation and reproductive behavior. Sci. Total Environ. 2022, 838, 155610. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, L.; Wu, S.; Lv, L.; Liu, X.; Wang, Q.; Zhao, X. Effects of difenoconazole on hepatotoxicity, lipid metabolism and gut microbiota in zebrafish (Danio rerio). Environ. Pollut. 2020, 265, 114844. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, Y.; Wang, L.; Wang, P.; Wang, T.; Ravindran, B.; Mishra, S.; Chen, S.; Cui, X.; Yang, Y.; et al. Elucidating the degradation mechanisms of perfluorooctanoic acid and perfluorooctane sulfonate in various environmental matrices: A review of green degradation pathways. Environ. Geochem. Health 2024, 46, 349. [Google Scholar] [CrossRef]
- Ling, L.; Sun, J.; Fang, J.; Shang, C. Kinetics and mechanisms of degradation of chloroacetonitriles by the UV/H2O2 process. Water Res. 2016, 99, 209–215. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Wang, L.; Sui, J.; Chen, F.; Yang, J.; Chen, S.; Cui, X.; Yang, Y.; Zhang, W. Assessing the biotic and abiotic degradation of malathion in the environment: Current strategies and advances. J. Environ. Chem. Eng. 2025, 13, 115429. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Cheng, H.; Yan, Y.; Iqbal, H.M.N. Multi-point enzyme immobilization, surface chemistry, and novel platforms: A paradigm shift in biocatalyst design. Crit. Rev. Biotechnol. 2019, 39, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Palanivel, T.M.; Sivakumar, N.; Al-Ansari, A.; Victor, R. Bioremediation of copper by active cells of Pseudomonas stutzeri LA3 isolated from an abandoned copper mine soil. J. Environ. Manag. 2020, 253, 109706. [Google Scholar] [CrossRef]
- Gong, Y.Z.; Niu, Q.Y.; Liu, Y.G.; Dong, J.; Xia, M.M. Development of multifarious carrier materials and impact conditions of immobilised microbial technology for environmental remediation: A review. Environ. Pollut. 2022, 314, 120232. [Google Scholar] [CrossRef]
- Luong, T.T.; Nguyen, T.H.T.; Nguyen, T.D.; Le, V.T.; Pham, T.H.T.; Ho, T.T.; Nguyen, N.L. Degradation of Triazole Fungicides by Plant Growth-Promoting Bacteria from Contaminated Agricultural Soil. J. Microbiol. Biotechnol. 2024, 34, 56–64. [Google Scholar] [CrossRef]
- Pinto, A.P.; Serrano, C.; Pires, T.; Mestrinho, E.; Dias, L.; Teixeira, D.M.; Caldeira, A.T. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci. Total Environ. 2012, 435–436, 402–410. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Zhang, B.; Ni, Y.; Liu, J.; Yan, T.; Zhu, X.; Li, Q.X.; Hua, R.; Pan, D.; Wu, X. Bead-immobilized Pseudomonas stutzeri Y2 prolongs functions to degrade s-triazine herbicides in industrial wastewater and maize fields. Sci. Total Environ. 2020, 731, 139183. [Google Scholar] [CrossRef]
- Rui, L.; Bing, W.; Aping, N.; Ning, C.; Miao, C.; Xueyang, Z.; Zebin, Y.; Shengsen, W. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Zhang, M.; Shen, X.; Zhang, X.; Wu, F.; Hu, J.; Wang, B.; Wang, X. Removal of PAHs at high concentrations in a soil washing solution containing TX-100 via simultaneous sorption and biodegradation processes by immobilized degrading bacteria in PVA-SA hydrogel beads. J. Hazard. Mater. 2021, 410, 124533. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, L.; Cao, X. The microorganism and biochar-augmented bioreactive top-layer soil for degradation removal of 2,4-dichlorophenol from surface runoff. Sci. Total Environ. 2020, 733, 139244. [Google Scholar] [CrossRef]
- Partovinia, A.; Rasekh, B. Review of the immobilized microbial cell systems for bioremediation of petroleum hydrocarbons polluted environments. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1–38. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Dai, M.; Ali, I.; Shen, X.; Hou, X.; Alhewairini, S.S.; Peng, C.; Naz, I. Application of microbial immobilization technology for remediation of Cr(VI) contamination: A review. Chemosphere 2022, 286, 131721. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Olamide, A.N.; Rafi, N.; Wahid, S.; Wasiu, I.A.; Sunday, O.O. Sodium alginate/polyvinyl alcohol immobilization of Brevibacillus brevis OZF6 isolated from waste water and its role in the removal of toxic chromate. Biotechnol. J. Int. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Ruan, B.; Wu, P.; Chen, M.; Lai, X.; Chen, L.; Yu, L.; Gong, B.; Kang, C.; Dang, Z.; Shi, Z.; et al. Immobilization of Sphingomonas sp. GY2B in polyvinyl alcohol-alginate-kaolin beads for efficient degradation of phenol against unfavorable environmental factors. Ecotoxicol. Environ. Saf. 2018, 162, 103–111. [Google Scholar] [CrossRef]
- Kadimpati, K.K.; Mondithoka, K.P.; Bheemaraju, S.; Challa, V.R.M. Entrapment of marine microalga, Isochrysis galbana, for biosorption of Cr(III) from aqueous solution: Isotherms and spectroscopic characterization. Appl. Water Sci. 2013, 3, 85–92. [Google Scholar] [CrossRef]
- Tan, L.; Li, H.; Ning, S.; Xu, B. Aerobic decolorization and degradation of azo dyes by suspended growing cells and immobilized cells of a newly isolated yeast Magnusiomyces ingens LH-F1. Bioresour Technol. 2014, 158, 321–328. [Google Scholar] [CrossRef]
- Jiajia, Z.; Jia, W.; Yaw, M.I.; Tangjian, P.; Fei, Y. Immobilization of Microbes for Biodegradation of Microcystins: A Mini Review. Toxins 2022, 14, 573. [Google Scholar] [CrossRef]
- Li, B.; Gan, L.; Owens, G.; Chen, Z. New nano-biomaterials for the removal of malachite green from aqueous solution via a response surface methodology. Water Res. 2018, 146, 55–66. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Wen, S.; Liu, H.; Yang, R.; Wang, L.; Zhu, L.; Wang, J.; Kim, Y.M.; Wang, J. Immobilization of Bacillus thuringiensis and applicability in removal of sulfamethazine from soil. Environ. Pollut. 2023, 333, 122080. [Google Scholar] [CrossRef]
- Xiang, X.; Yi, X.; Zheng, W.; Li, Y.; Zhang, C.; Wang, X.; Chen, Z.; Huang, M.; Ying, G.G. Enhanced biodegradation of thiamethoxam with a novel polyvinyl alcohol (PVA)/sodium alginate (SA)/biochar immobilized Chryseobacterium sp H5. J. Hazard. Mater. 2023, 443, 130247. [Google Scholar] [CrossRef]
- Nazos, T.T.; Ghanotakis, D.F. Biodegradation of phenol by alginate immobilized Chlamydomonas reinhardtii cells. Arch. Microbiol. 2021, 203, 5805–5816. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Geng, P.; Xiao, Y.; Hu, M. Bioremediation of β-cypermethrin and 3-phenoxybenzaldehyde contaminated soils using Streptomyces aureus HP-S-01. Appl. Microbiol. Biotechnol. 2012, 94, 505–515. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Sui, J.; Chen, F.; Wang, T.; Yang, J.; Chen, S.H.; Cui, X.; Yang, Y.; Zhang, W. Pathway elucidation and key enzymatic processes in the biodegradation of difenoconazole by Pseudomonas putida A-3. J. Agric. Food Chem. 2025, 73, 4770–4786. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Alsohim, A.S.; Abidou, H.; Rasheed, Z.; Al Abdulmonem, W. Isolation, identification, biocontrol activity, and plant growth promoting capability of a superior Streptomyces tricolor strain HM10. Pol. J. Microbiol. 2021, 70, 245–256. [Google Scholar] [CrossRef]
- Amor, L.; Kennes, C.; Veiga, M.C. Kinetics of inhibition in the biodegradation of monoaromatic hydrocarbons in presence of heavy metals. Bioresour. Technol. 2001, 78, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.-J.; Zhang, W.; Zhang, Y.; Lei, Q.; Wu, S.; Huang, Y.; Mishra, S.; Bhatt, P.; Chen, S. Effects of free or immobilized bacterium Stenotrophomonas acidaminiphila Y4B on glyphosate degradation performance and indigenous microbial community structure. J. Agric. Food Chem. 2022, 70, 13945–13958. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, W.J.; Li, J.; Ghorab, M.A.; Alansary, N.; El-Hefny, D.E.; El-Sayyad, G.S.; Mishra, S.; Zhang, X.; Bhatt, P.; et al. Novel mechanism and degradation kinetics of allethrin using Bacillus megaterium strain HLJ7 in contaminated soil/water environments. Environ. Res. 2022, 214, 113940. [Google Scholar] [CrossRef]
- Chen, X.; Peng, S.; Liu, M.; Wang, L.; Pang, K.; Zhang, L.; Cui, Z.; Liu, A. Highly efficient in-situ cleaner degradation of difenoconazole by two novel dominant strains: Microflora diversity, monoclonal isolation, growth factor optimization, intermediates, and pathways. Chemosphere 2022, 310, 136863. [Google Scholar] [CrossRef]
- Huang, S.; Pan, J.; Tuwang, M.; Li, H.; Ma, C.; Chen, M.; Lin, X. Isolation, Screening, and degradation characteristics of a quinclorac-degrading bacterium, Strain D, and its potential for bioremediation of rice fields polluted by quinclorac. Microbiol. Spectr. 2021, 9, e0039821. [Google Scholar] [CrossRef]
- Fan, J.; Yang, G.; Zhao, H.; Shi, G.; Geng, Y.; Hou, T.; Tao, K. Isolation, identification and characterization of a glyphosate-degrading bacterium, Bacillus cereus CB4, from soil. J. Gen. Appl. Microbiol. 2012, 58, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Zhang, Y.; Wu, X.; Zhou, Z.; Huang, Y.; Zhao, Y.; Mishra, S.; Bhatt, P.; Chen, S. Characterization of a novel glyphosate-degrading bacterial species, Chryseobacterium sp. Y16C, and evaluation of its effects on microbial communities in glyphosate-contaminated soil. J. Hazard. Mater. 2022, 432, 128689. [Google Scholar] [CrossRef] [PubMed]
- Tithi, M.; Subhabrata, D.; Aditi, B.; Abhijit, C.; Rachana, S.; Srijan, A. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chen Eng. 2021, 9, 5. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, M.; Wang, Y. Immobilization of laccase by alginate–chitosan microcapsules and its use in dye decolorization. World J. Microbiol. Biotechnol. 2007, 23, 159–166. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, W. The neutral protease immobilization: Physical characterization of sodium alginate-chitosan gel beads. Appl. Biochem. Biotechnol. 2022, 194, 2269–2283. [Google Scholar] [CrossRef]
- Won, K.; Kim, S.; Kim, K.-J.; Park, H.W.; Moon, S.-J. Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem. 2004, 40, 2149–2154. [Google Scholar] [CrossRef]
- Xiong, J.; An, T.; Li, G.; Peng, P. Accelerated biodegradation of BPA in water-sediment microcosms with Bacillus sp. GZB and the associated bacterial community structure. Chemosphere 2017, 184, 120–126. [Google Scholar] [CrossRef]
- Yu, Y.; Yin, H.; Peng, H.; Lu, G.; Dang, Z. Proteomic mechanism of decabromodiphenyl ether (BDE-209) biodegradation by Microbacterium Y2 and its potential in remediation of BDE-209 contaminated water-sediment system. J. Hazard. Mater. 2020, 387, 121708. [Google Scholar] [CrossRef]





| Factor | Name | −1 | 0 | 1 |
|---|---|---|---|---|
| A | pH | 6 | 7 | 8 |
| B | Substrate concentration/(mg/L) | 40 | 50 | 60 |
| C | Inoculum/(%) | 1 | 2 | 3 |
| Run Order | Factor | Degradation Rate/(%) | ||
|---|---|---|---|---|
| A: pH | B: Substrate Concentration/(mg/L) | C: Inoculum/(%) | ||
| 1 | 7 | 50 | 2 | 78.61 |
| 2 | 7 | 60 | 3 | 66.50 |
| 3 | 8 | 50 | 3 | 56.70 |
| 4 | 7 | 50 | 2 | 75.48 |
| 5 | 7 | 60 | 1 | 68.20 |
| 6 | 7 | 40 | 3 | 47.90 |
| 7 | 8 | 60 | 2 | 65.10 |
| 8 | 6 | 50 | 1 | 57.50 |
| 9 | 8 | 50 | 1 | 55.40 |
| 10 | 6 | 60 | 2 | 68.90 |
| 11 | 7 | 50 | 2 | 75.71 |
| 12 | 7 | 40 | 1 | 52.50 |
| 13 | 6 | 40 | 2 | 48.80 |
| 14 | 7 | 50 | 2 | 76.78 |
| 15 | 6 | 50 | 3 | 55.20 |
| 16 | 7 | 50 | 2 | 73.81 |
| 17 | 8 | 40 | 2 | 50.20 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1821.67 | 9 | 202.41 | 85.78 | <0.0001 | significant |
| A—pH | 1.13 | 1 | 1.13 | 0.4767 | 0.5121 | |
| B—substrate concentration/(mg/L) | 600.31 | 1 | 600.31 | 254.40 | <0.0001 | |
| C—inoculum | 6.66 | 1 | 6.66 | 2.82 | 0.1368 | |
| AB | 6.76 | 1 | 6.76 | 2.86 | 0.1344 | |
| AC | 3.24 | 1 | 3.24 | 1.37 | 0.2796 | |
| BC | 2.10 | 1 | 2.10 | 0.8910 | 0.3766 | |
| A2 | 438.19 | 1 | 438.19 | 185.70 | <0.0001 | |
| B2 | 244.90 | 1 | 244.90 | 103.78 | <0.0001 | |
| C2 | 394.25 | 1 | 394.25 | 167.07 | <0.0001 | |
| Residual | 16.52 | 7 | 2.36 | |||
| Lack of Fit | 3.98 | 3 | 1.33 | 0.4229 | 0.7473 | not significant |
| Pure error | 12.54 | 4 | 3.14 | |||
| Cor total | 1838.19 | 16 |
| Treatments | Regression Equation | k/(Day−1) | R2 | t1/2/(Days) |
|---|---|---|---|---|
| Sterile WSSs | Ct = 50.18334e−0.0329t | 0.0329 | 0.95996 | 21.07 |
| Nonsterile WSSs | Ct = 50.43438e−0.03142t | 0.03142 | 0.96527 | 22.06 |
| Sterile WSSs +free cells | Ct = 52.91691e−0.17745t | 0.17745 | 0.95852 | 3.91 |
| Nonsterile WSSs + free cells | Ct = 49.45648e−0.14705t | 0.14705 | 0.91153 | 4.71 |
| Sterile WSSs + immobilized A-1 cell | Ct = 52.51469e−0.21653t | 0.21653 | 0.98433 | 3.20 |
| Nonsterile WSSs + immobilized A-1 cell | Ct = 50.83235e−0.14804t | 0.14804 | 0.95793 | 4.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Wang, L.; Zhou, Y.; Sui, J.; Wang, T.; Yang, J.; Cui, X.; Yang, Y.; Zhang, W. Immobilization of Acinetobacter sp. A-1 and Applicability in Removal of Difenoconazole from Water–Sediment Systems. Microorganisms 2025, 13, 802. https://doi.org/10.3390/microorganisms13040802
Chen F, Wang L, Zhou Y, Sui J, Wang T, Yang J, Cui X, Yang Y, Zhang W. Immobilization of Acinetobacter sp. A-1 and Applicability in Removal of Difenoconazole from Water–Sediment Systems. Microorganisms. 2025; 13(4):802. https://doi.org/10.3390/microorganisms13040802
Chicago/Turabian StyleChen, Feiyu, Liping Wang, Yi Zhou, Jingyi Sui, Tianyue Wang, Jia Yang, Xiuming Cui, Ye Yang, and Wenping Zhang. 2025. "Immobilization of Acinetobacter sp. A-1 and Applicability in Removal of Difenoconazole from Water–Sediment Systems" Microorganisms 13, no. 4: 802. https://doi.org/10.3390/microorganisms13040802
APA StyleChen, F., Wang, L., Zhou, Y., Sui, J., Wang, T., Yang, J., Cui, X., Yang, Y., & Zhang, W. (2025). Immobilization of Acinetobacter sp. A-1 and Applicability in Removal of Difenoconazole from Water–Sediment Systems. Microorganisms, 13(4), 802. https://doi.org/10.3390/microorganisms13040802






