How Long Do Microorganisms Survive and Persist in Food? A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and PRISMA Guidelines
2.2. Search Strategy
2.3. The Study Selection Process
2.4. Data Extraction
2.5. Quality Assessment
2.6. Data Synthesis
3. Results
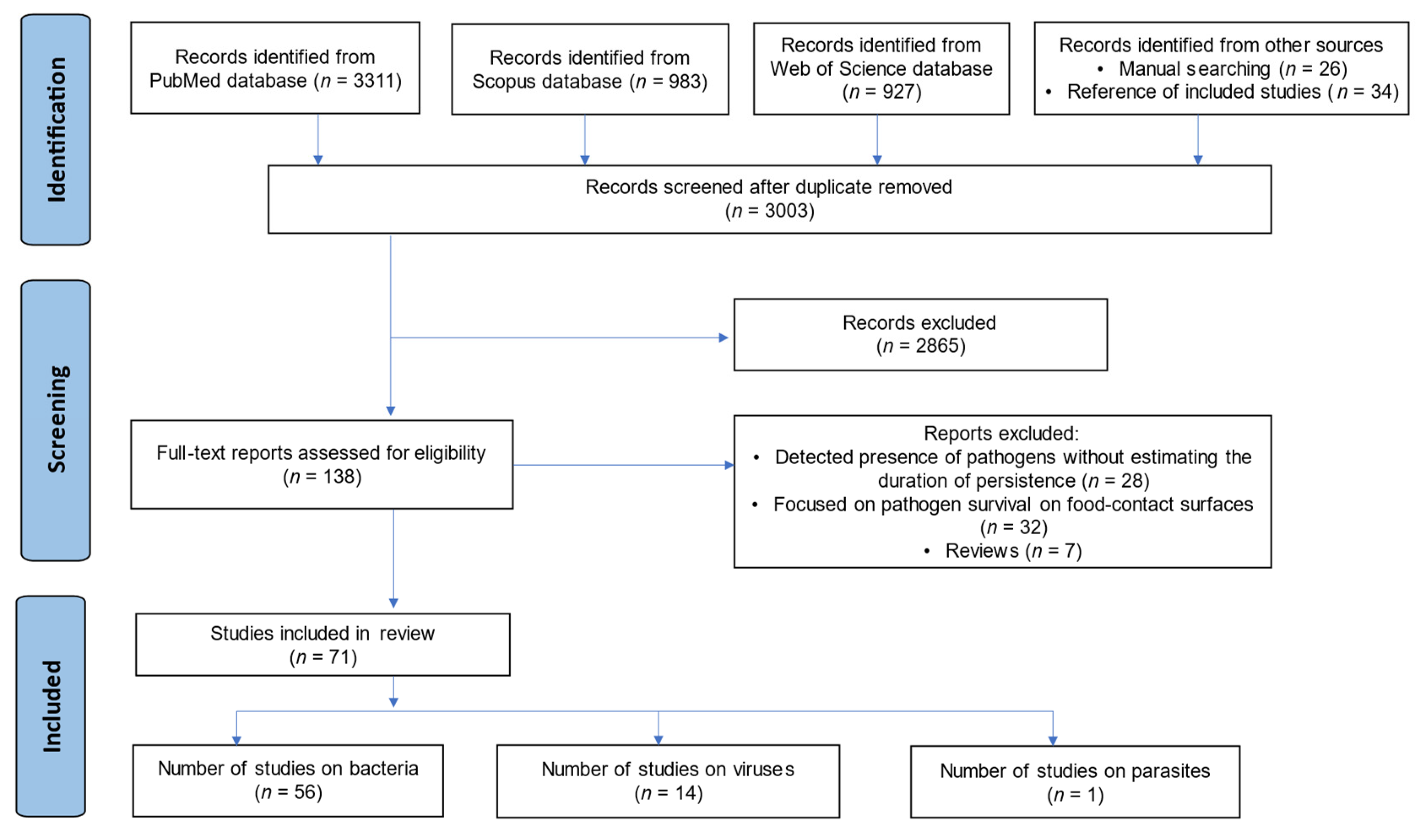
3.1. Search Results
3.2. Survival and Persistence of Bacteria
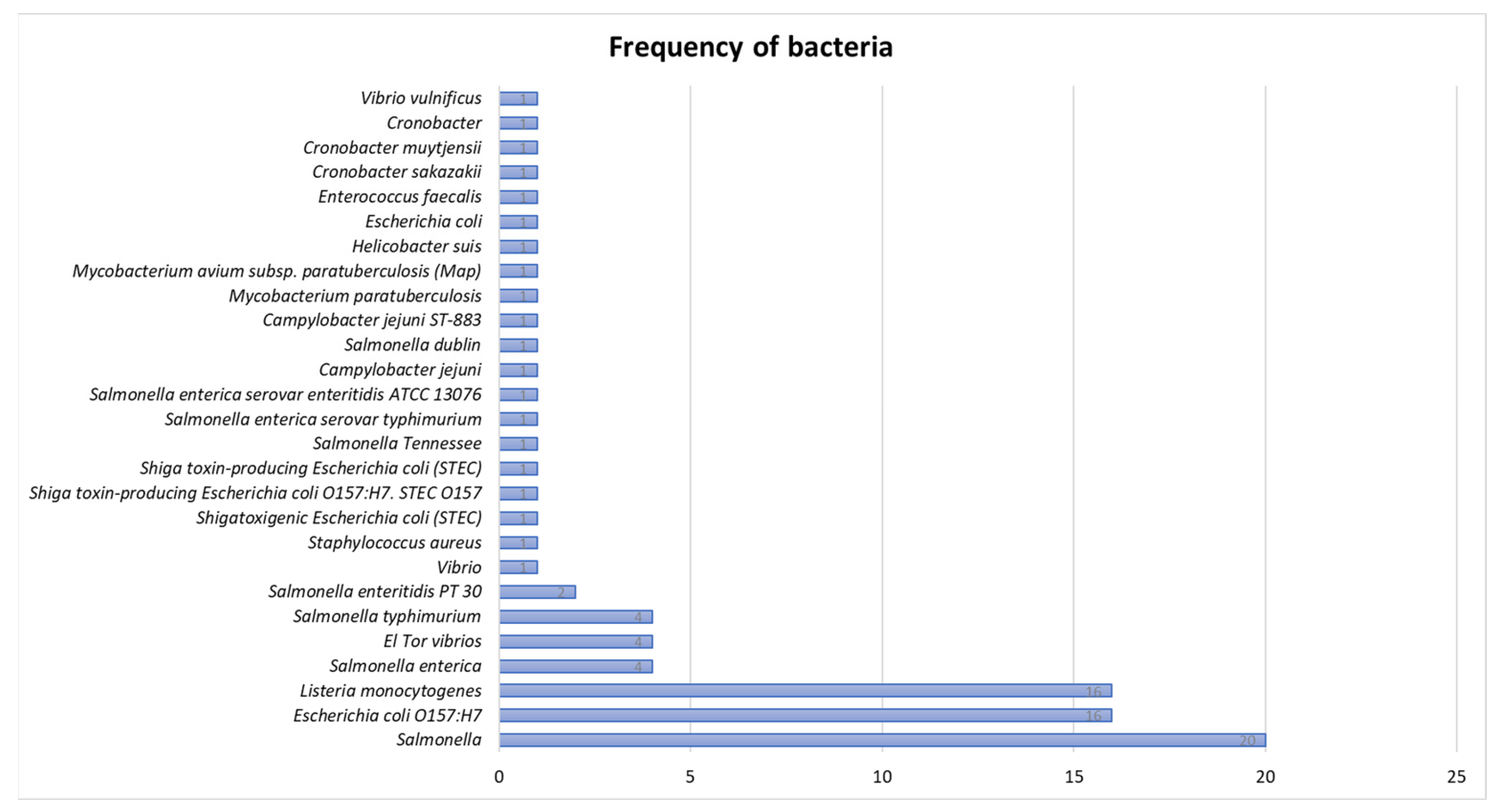
3.2.1. Frequency of Bacteria
3.2.2. Survival and Persistence Durations of Bacteria
3.2.3. Common Conditions Under Which Bacteria Show Survival and Persistence
Influence of Temperature on Bacterial Survival and Persistence
Influence of Humidity on Bacterial Survival and Persistence
Influence of pH on Bacterial Survival and Persistence
Influence of Food Matrix on Bacterial Survival and Persistence
3.3. Survival and Persistence of Viruses
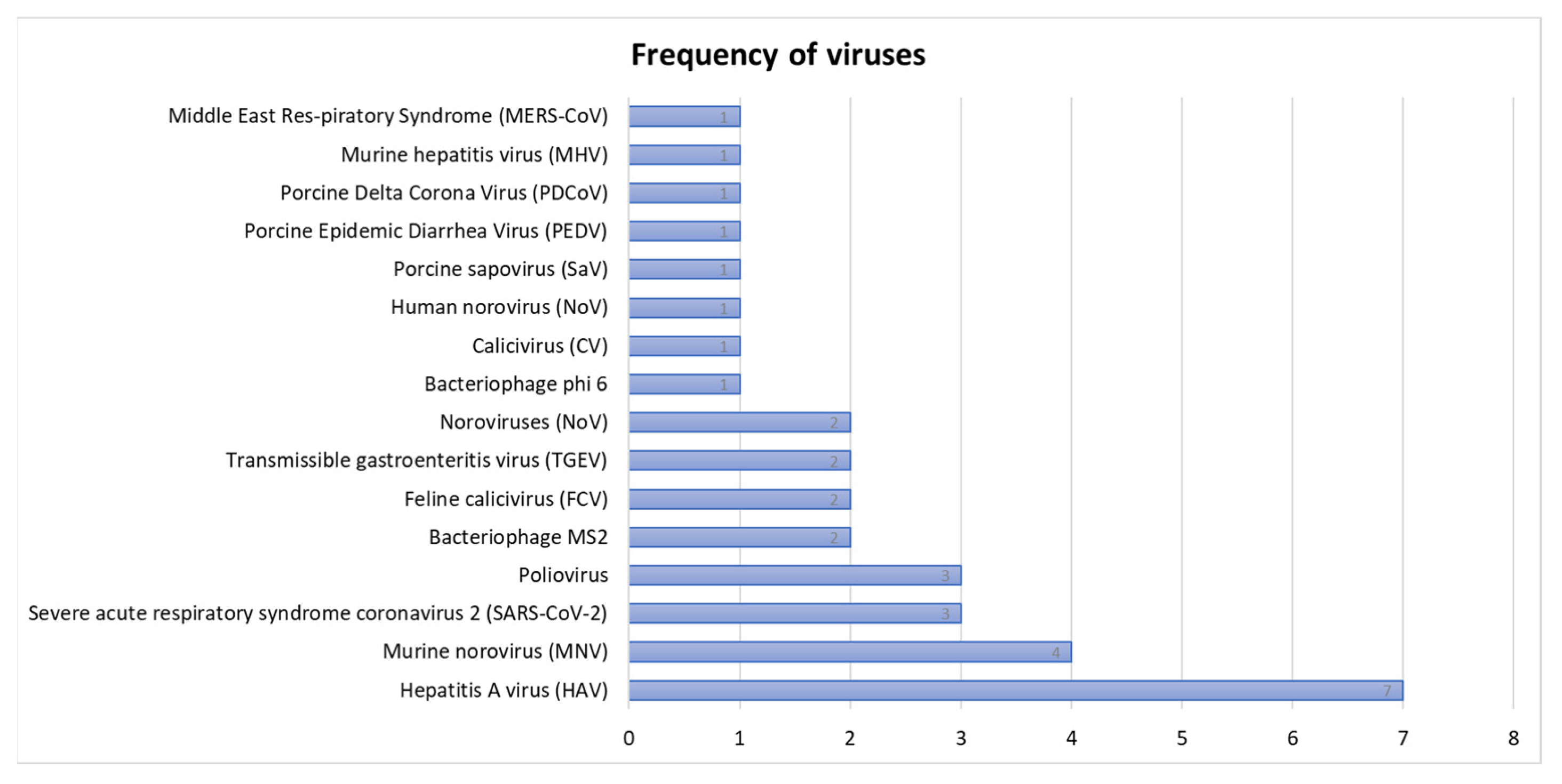
3.3.1. The Frequency of Viruses
3.3.2. Survival Durations of Viruses
3.3.3. Common Conditions Under Which Viruses Show Survival
Influence of Temperature on Virus Survival
Influence of Humidity on Virus Survival
Influence of pH on Virus Survival
Influence of Food Matrix on Virus Survival
3.4. The Survival and Persistence of Protozoa Parasites
3.5. Risk of Bias
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Bacteria | Sample | Duration | Condition | Survival/Persistence | Reference |
|---|---|---|---|---|---|
| Campylobacter jejuni | Unpasteurized milk | 21 days | 4 °C | Survival | Doyle et al. [20] |
| Campylobacter jejuni ST-883 | Raw milk | 4–6 days | - | Survival | Jaakkonen et al. [21] |
| Cronobacter | Powdered infant formula (PIF) | 3 months | - | Persistence | Bennour Hennekinne et al. [22] |
| Cronobacter muytjensii | Infant wheat-based formulas reconstituted with water, milk, grape juice, or apple juice | 24 h | 4, 25, or 37 °C | Survival | Osaili et al. [23] |
| Cronobacter sakazakii | Infant wheat-based formulas reconstituted with water, milk, grape juice, or apple juice | 24 h | 4, 25, or 37 °C | Survival | Osaili et al. [23] |
| Vibrio cholerae (El Tor) | Parsley | 24 h | - | Survival | Sechter et al. [24] |
| Vibrio cholerae (El Tor) | Tomatoes and carrots | 24 to 30 h | - | Survival | Sechter et al. [24] |
| Vibrio cholerae (El Tor) | Cucumbers, peppers, and okra | 24–48 h | - | Survival | Sechter et al. [24] |
| Vibrio cholerae (El Tor) | Lettuce | 2–3 days | - | Survival | Sechter et al. [24] |
| Enterococcus faecalis | Poultry and cattle feed | 7 days | 22 °C, 65% relative humidity | Survival | Channaiah et al. [25] |
| Escherichia coli | Raw carrot and cucumber juice | 10 days | 20 °C | Survival | Van Beeck et al. [26] |
| Escherichia coli O157:H7 | Bruised and unbruised tomatoes | 7 days | 10 and 20 °C | Survival | Tokarskyy et al. [27] |
| Escherichia coli O157:H7 | In-shell hazelnuts | 12 months | 24 ± 1 °C, 40 ± 3% relative humidity | Persistence | Feng et al. [28] |
| Escherichia coli O157:H7 | Black carrot juice | 7 days | 4 and 37 °C | Survival | Degirmenci et al. [29] |
| Escherichia coli O157:H7 | The rhizosphere and leaf surfaces of lettuce | 7–21 days | - | Survival | Mark Ibekwe et al. [30] |
| Escherichia coli O157:H7 | Lettuce cultivars | 12 days | - | Survival | Erickson et al. [32] |
| Escherichia coli O157:H7 | Cabbage cultivars | 9 days | - | Survival | Erickson et al. [31] |
| Escherichia coli O157:H7 | Vegetables (romaine lettuce, iceberg lettuce, perilla leaves, and sprouts) | 7 days | 15 °C | Survival | Tian et al. [33] |
| Escherichia coli O157:H7 | Grape pulp | 30 days | 4 °C, pH 2.51–3.26 | Survival | Marques et al. [34] |
| Escherichia coli O157:H7 | Whole and diced yellow onions (Allium cepa) | 6 days | 4 °C, 30–50% relative humidity | Survival | Lieberman et al. [35] |
| Escherichia coli O157:H7 | Walnut kernels | 3 weeks to more than 1 year | 23 °C | Persistence | Blessington et al. [36] |
| Escherichia coli O157:H7 | Raw peanut and pecan kernels | 365 days | −24 ± 1, 4 ± 2, and 22 ± 1 °C | Persistence | Brar et al. [37] |
| Escherichia coli O157:H7 | Cheddar cheese whey | 21 days | 4, 10 or 15 °C, pH 5.5 | Survival | Marek et al. [38] |
| Escherichia coli O157:H7 | Lamb meat | 12 days | 4 and 12 ± 1 °C | Survival | Barrera et al. [39] |
| Escherichia coli O157:H7 | Galotyri cheese | 28 days | 4 and 12 °C | Survival | Lekkas et al. [40] |
| Escherichia coli O157:H7 | Raw goat milk lactic cheeses | 42 days | - | Survival | Vernozy-Rozand et al. [41] |
| Escherichia coli O157:H7 | Cheese | 90 days | - | Persistence | Maher et al. [42] |
| Helicobacter suis | Retail pork samples | at least 48 h | - | Survival | De Cooman et al. [43] |
| Listeria monocytogenes | Whole mango | 28 days | 12 ± 2 °C | Survival | Saha et al. [44] |
| Listeria monocytogenes | Mixed vegetables (containing green beans, corn, and peas) | 12 months | −18 or −10 °C | Persistence | Fay et al. [45] |
| Listeria monocytogenes | Edible seaweed | 7 days | 4, 10, and 22 °C | Survival | Akomea-Frempong et al. [46] |
| Listeria monocytogenes | Raw carrot and cucumber juice | 8 days | 20 °C | Survival | Van Beeck et al. [26] |
| Listeria monocytogenes | Black carrot juice | 7 days | 4 and 37 °C | Survival | Degirmenci et al. [29] |
| Listeria monocytogenes | Vegetables (romaine lettuce, iceberg lettuce, perilla leaves, and sprouts) | 7 days | 15 °C | Survival | Tian et al. [33] |
| Listeria monocytogenes | Walnut kernels | 3 weeks to more than 1 year | 23 °C | Persistence | Blessington et al. [36] |
| Listeria monocytogenes | Raw peanut and pecan kernels | 28 or 365 days | −24 ± 1, 4 ± 2, and 22 ± 1 °C | Persistence | Brar et al. [37] |
| Listeria monocytogenes | Chickpeas, sesame seeds, pine nuts, and black pepper kernels | 180 days | 25 °C, 25, 45, and 75% relative humidity | Persistence | Salazar et al. [47] |
| Listeria monocytogenes | Nut-, seed-, legume-, and chocolate-containing butters | 6 months | 5 or 25 °C | Persistence | Fay et al. [48] |
| Listeria monocytogenes | Chocolate liquor, corn flakes, and shelled, dry-roasted pistachios | 336 days | 4 and 25 °C, 35%relative humidity | Persistence | Ly et al. [49] |
| Listeria monocytogenes | Dried apples, raisins, and dried strawberries | 336 days | 4 and 23 °C | Persistence | Cuzzi et al. [50] |
| Listeria monocytogenes | Fresh strawberries | 4 weeks | −20 ± 2 °C | Survival | Flessa et al. [51] |
| Listeria monocytogenes | Bell peppers | 14 days | 4 °C | Survival | Moreira et al. [52] |
| Listeria monocytogenes | Cantaloupe rind | 7 days | 24 °C | Survival | Moreira et al. [52] |
| Listeria monocytogenes | Kale, cauliflower, and broccoli | 6 days | 13 °C | Survival | Moreira et al. [52] |
| Mycobacterium avium subsp. paratuberculosis (Map) | Ultrafiltered white cheese | 60 days | - | Survival | Hanifian [53] |
| Mycobacterium paratuberculosis | Cheddar cheese | 27 weeks | - | Persistence | Donaghy et al. [54] |
| Salmonella | Whole mango | 28 days | 12 ± 2 °C | Survival | Saha et al. [44] |
| Salmonella | Bruised and unbruised tomatoes | 7 days | 10 and 20 °C | Survival | Tokarskyy et al. [27] |
| Salmonella | Peanut oil | 96 ± 8 days | - | Persistence | Fong et al. [55] |
| Salmonella | Chia seeds | 94 ± 46 days | - | Persistence | Fong et al. [55] |
| Salmonella | Peanut shell | 42 ± 49 h | - | Survival | Fong et al. [55] |
| Salmonella | Edible seaweeds | 7 days | 4, 10, and 22 °C | Survival | Akomea-Frempong et al. [46] |
| Salmonella | Lettuce cultivars | 12 days | - | Survival | Erickson et al. [32] |
| Salmonella | Cabbage cultivars | 9 days | - | Survival | Erickson et al. [31] |
| Salmonella | Dry- and wet-inoculated sucrose | 52 weeks | 5 and 25 °C | Persistence | Beuchat et al. [56] |
| Salmonella | Boil-in-bag eggs | 36 months | 40 °C | Persistence | Flock et al. [57] |
| Salmonella | Chocolate protein drink and peanut butter | 12 months | 40 °C | Persistence | Flock et al. [57] |
| Salmonella | Whole and diced yellow onions (Allium cepa) | 6 days | 4 °C, 30–50% relative humidity | Survival | Lieberman et al. [35] |
| Salmonella | Walnut kernels | 3 weeks to more than 1 year | 23 °C | Persistence | Blessington et al. [36] |
| Salmonella | Raw peanut and pecan kernels | 28 or 365 days | −24 ± 1, 4 ± 2, and 22 ± 1 °C | Persistence | Brar et al. [37] |
| Salmonella | Dehydrated garlic flakes | 88 days | 25 °C, ambient relative humidity | Survival | Zhang et al. [58] |
| Salmonella | Ground black pepper (Piper nigrum) | 45 or 100 days | 25 or 35 °C, high relative humidity | Persistence | Keller et al. [59] |
| Salmonella | Ground ginger | 170 or 365 days | 25 and 37 °C, 33% (low) and 97% (high) relative humidity | Persistence | Gradl et al. [60] |
| Salmonella | Whole almond kernels | 28 days | 35, 22, 4, or −18 °C | Survival | Xu et al. [61] |
| Salmonella | Strawberries, cranberries, date paste, and raisins | 182–242 days | 4 and 25 °C | Persistence | Beuchat et al. [62] |
| Salmonella | Ground black pepper (Piper nigrum) | 8 months | 25 and 35 °C, ambient humidity | Persistence | Keller et al. [59] |
| Salmonella dublin | Beef–pork pepperoni | 42–43 days | - | Survival | Smith et al. [63] |
| Salmonella enterica | Tomato | 14 days | −16.7 °C | Survival | Zhou et al. [64] |
| Salmonella enterica | Raw carrot and cucumber juice | 10 days | 20 °C | Survival | Van Beeck et al. [26] |
| Salmonella enterica | Whey protein powder | 6 months | 36 °C | Persistence | Farakos et al. [65] |
| Salmonella enterica | Catfish mucus | 63 days | 10–22 °C | Survival | Dhowlaghar et al. [66] |
| Salmonella enterica serovar enteritidis ATCC 13076 | Colonial cheese | 28 days | - | Survival | Degenhardt et al. [67] |
| Salmonella enterica serovar typhimurium | Vegetables (romaine lettuce, iceberg lettuce, perilla leaves, and sprouts) | 7 days | 15 °C | Survival | Tian et al. [33] |
| Salmonella enteritidis PT 30 | Almond kernels | 68 weeks | 23 ± 0.5 °C | Persistence | Limcharoenchat et al. [68] |
| Salmonella enteritidis PT 30 | Whole Nonpareil variety almonds | 48 weeks | 4 or 23 °C | Persistence | Abd et al. [69] |
| Salmonella tennessee | Peanut butter | 14 days | - | Survival | Matak et al. [70] |
| Salmonella typhimurium | Peanut butter | 14 days | - | Survival | Matak et al. [70] |
| Salmonella typhimurium | Black carrot juice | 7 days | 4 and 37 °C | Survival | Degirmenci et al. [29] |
| Salmonella typhimurium | Pacific oyster | 30 days | - | Survival | Chakroun et al. [71] |
| Salmonella typhimurium | Cheddar, Swiss, and mozzarella cheeses | 2 months | 5 °C, pH 5.8 | Survival | Leyer et al. [72] |
| Shiga toxin-producing Escherichia coli (STEC) | Fermented sausages | 28 days | - | Survival | Böhnlein et al. [73] |
| Shiga toxin-producing Escherichia coli O157:H7. STEC O157 | Korean-style kimchi | 8 weeks | 4 °C | Survival | Gill et al. [74] |
| Shigatoxigenic Escherichia coli (STEC) | Edible seaweeds | 7 days | 4, 10, and 22 °C | Survival | Akomea-Frempong et al. [46] |
| Staphylococcus aureus | Vegetables (romaine lettuce, iceberg lettuce, perilla leaves, and sprouts) | 7 days | 15 °C | Survival | Tian et al. [33] |
| Vibrio | Edible seaweed | 7 days | 4, 10, and 22 °C | Survival | Akomea-Frempong et al. [46] |
| Vibrio cholerae (El Tor) | Parsley | 24 h | - | Survival | Sechter et al. [24] |
| Vibrio cholerae (El Tor) | Tomatoes and carrots | 24 to 30 h | - | Survival | Sechter et al. [24] |
| Vibrio cholerae (El Tor) | Cucumbers, peppers, and okra | 24–48 h | - | Survival | Sechter et al. [24] |
| Vibrio cholerae (El Tor) | Lettuce | 2–3 days | - | Survival | Sechter et al. [24] |
| Vibrio vulnificus | Oysters | 2 weeks | Refrigeration conditions | Survival | Wood et al. [75] |
| Viruses | Sample | Duration | Condition | Survival/Persistence | Reference |
|---|---|---|---|---|---|
| Bacteriophage MS2 | Oysters, fresh peppers | 2 weeks | 4, 15, 25, and 40 °C, 50% and 70% relative humidity | Survival | Lee et al. [76] |
| Bacteriophage MS2 | Eastern oysters (Crassostrea virginica) | 6 weeks | 7, 15, or 24 °C | Survival | Kingsley et al. [84] |
| Bacteriophage phi 6 | Meat, fish products | 30 days | refrigerated and frozen temperatures | Survival | Bailey et al. [77] |
| Calicivirus (CV) | Lettuce leaves | 7–14 days | 4 °C | Survival | Esseili et al. [78] |
| Feline calicivirus (FCV) | Cereal, chocolate, pistachios | 4 weeks | room temperature | Survival | Nasheri et al. [79] |
| Feline calicivirus (FCV) | Marinated mussels | 4 weeks | 4 °C | Survival | Hewitt et al. [80] |
| Hepatitis A virus (HAV) | Alfalfa seeds | 50 days | 22 °C | Survival | Wang et al. [81] |
| Hepatitis A virus (HAV) | Cereal, chocolate, pistachios | 4 weeks | room temperature | Survival | Nasheri et al. [79] |
| Hepatitis A virus (HAV) | Marinated mussels | 4 weeks | 4 °C, pH 3.75 | Survival | Hewitt et al. [80] |
| Hepatitis A virus (HAV) | Oysters or the surface of fresh peppers | 2 weeks | 4, 15, 25, and 40 °C, 50% and 70% relative humidity (RH) | Survival | Lee et al. [76] |
| Hepatitis A virus (HAV) | Lettuce | 9 days | 4 °C | Survival | Croci et al. [82] |
| Hepatitis A virus (HAV) | Carrot | 4 days | 4 °C | Survival | Croci et al. [82] |
| Hepatitis A virus (HAV) | Fennel | 7 days | 4 °C | Survival | Croci et al. [82] |
| Human norovirus (NoV) | Cereal, chocolate, pistachios | 4 weeks | room temperature | Survival | Nasheri et al. [79] |
| Middle East respiratory syndrome (MERS-CoV) | Apples, tomatoes, cucumbers | 72 hrs | 22 °C 30–40% relative humidity | Survival | Blondin-Brosseau et al. [83] |
| Murine hepatitis virus (MHV) | Meat and fish products | 30 days | refrigerated and frozen temperatures | Survival | Bailey et al. [77] |
| Murine norovirus (MNV) | Alfalfa seeds | 50 days | 22 °C | Survival | Wang et al. [81] |
| Murine norovirus (MNV) | Cereal, chocolate, pistachios | 4 weeks | room temperature | Survival | Nasheri et al. [79] |
| Murine norovirus (MNV) | Lettuce leaves | 7–14 days | 4 °C | Survival | Esseili et al. [78] |
| Murine norovirus (MNV) | Oysters or the surface of fresh peppers | 2 weeks | 4, 15, 25, and 40 °C, 50% and 70% relative humidity | Survival | Lee et al. [76] |
| Noroviruses (NoV) | Lettuce and turkey | at least 10 days | 7 °C | Survival | Lamhoujeb et al. [85] |
| Noroviruses (NoV) | Marinated mussels | 4 weeks | 4 °C | Survival | Hewitt et al. [80] |
| Poliovirus | Lettuce, green onion, white cabbage | 15 days | 4 °C | Survival | Kurdziel et al. [86] |
| Poliovirus | Strawberries | 15 days | −20 °C | Survival | Kurdziel et al. [86] |
| Poliovirus | Raspberries | 9 days | 4 °C | Survival | Kurdziel et al. [86] |
| Porcine Delta Corona Virus (PDCoV) | Feed ingredient matrices | 56 days | - | Survival | Trudeau et al. [87] |
| Porcine Epidemic Diarrhea Virus (PEDV) | Feed ingredient matrices | 56 days | - | Survival | Trudeau et al. [87] |
| Porcine sapovirus (SaV) | Lettuce leaves | 7–14 days | 4 °C | Survival | Esseili et al. [78] |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | Ready-to-eat deli items, fresh produce, and meats (including seafood) | 21 days | - | Survival | Jia et al. [88] |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | Apples, tomatoes, cucumbers | 72 hrs | 22 °C, 30–40% relative humidity | Survival | Blondin-Brosseau et al. [83] |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | Stew-cut beef and ground beef | 2 days | - | Survival | Featherstone et al. [89] |
| Transmissible gastroenteritis virus (TGEV) | Feed ingredient matrices | 56 days | - | Survival | Trudeau et al. [87] |
| Transmissible gastroenteritis virus (TGEV) | Meat and fish products | 30 days | refrigerated and frozen temperatures | Survival | Bailey et al. [77] |
References
- Foodborne Diseases Estimates. Available online: https://www.who.int/data/gho/data/themes/who-estimates-of-the-global-burden-of-foodborne-diseases (accessed on 1 August 2024).
- Hoffmann, S.; Ahn, J.W. Updating Economic Burden of Foodborne Diseases Estimates for Inflation and Income Growth, ERR-297; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2021.
- McPherson, M.; Kirk, M.D.; Raupach, J.; Combs, B.; Butler, J.R.G. Economic Costs of Shiga Toxin–Producing Escherichia coli Infection in Australia. Foodborne Pathog. Dis. 2011, 8, 55–62. [Google Scholar] [CrossRef]
- Lake, R.J.; Cressey, P.J.; Campbell, D.M.; Oakley, E. Risk Ranking for Foodborne Microbial Hazards in New Zealand: Burden of Disease Estimates. Risk Anal. 2010, 30, 743–752. [Google Scholar] [CrossRef]
- Aladhadh, M. A Review of Modern Methods for the Detection of Foodborne Pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Li, J.; Hu, J.; Lin, G.; Tan, B.K.; Lin, S. Current Perspectives on Viable but Non-Culturable Foodborne Pathogenic Bacteria: A Review. Foods 2023, 12, 1179. [Google Scholar] [CrossRef]
- Lotoux, A.; Milohanic, E.; Bierne, H. The Viable But Non-Culturable State of Listeria monocytogenes in the One-Health Continuum. Front. Cell. Infect. Microbiol. 2022, 12, 849915. [Google Scholar] [CrossRef]
- Schottroff, F.; Fröhling, A.; Zunabovic-Pichler, M.; Krottenthaler, A.; Schlüter, O.; Jäger, H. Sublethal Injury and Viable but Non-culturable (VBNC) State in Microorganisms During Preservation of Food and Biological Materials by Non-thermal Processes. Front. Microbiol. 2018, 9, 2773. [Google Scholar] [CrossRef]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, F.A.J.; Opara, U.L. Modified Atmosphere Packaging Technology of Fresh and Fresh-cut Produce and the Microbial Consequences—A Review. Food Bioproc. Tech. 2013, 6, 303–329. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Lianou, A.; Sofos, J.N. Food Safety: Emerging Pathogens. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 250–272. [Google Scholar]
- Koutsoumanis, K.; Allende, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; Nauta, M. Persistence of microbiological hazards in food and feed production and processing environments. EFSA J. 2024, 22, e8521. [Google Scholar]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Gruzdev, N.; Pinto, R.; Sela (Saldinger), S. Persistence of Salmonella enterica during dehydration and subsequent cold storage. Food Microbiol. 2012, 32, 415–422. [Google Scholar] [CrossRef]
- Dawoud, T.M.; Davis, M.L.; Park, S.H.; Kim, S.A.; Kwon, Y.M.; Jarvis, N.; O’bryan, C.A.; Shi, Z.; Crandall, P.G.; Ricke, S.C. The Potential Link between Thermal Resistance and Virulence in Salmonella: A Review. Front. Vet. Sci. 2017, 4, 93. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Risk of Bias Tools—Robvis (visualization tool). Available online: https://www.riskofbias.info/welcome/robvis-visualization-tool (accessed on 1 August 2024).
- Doyle, M.P.; Roman, D.J. Prevalence and survival of Campylobacter jejuni in unpasteurized milk. Appl. Environ. Microbiol. 1982, 44, 1154–1158. [Google Scholar] [CrossRef]
- Jaakkonen, A.; Kivistö, R.; Aarnio, M.; Kalekivi, J.; Hakkinen, M. Persistent contamination of raw milk by Campylobacter jejuni ST-883. PLoS ONE 2020, 15, e0231810. [Google Scholar] [CrossRef]
- Bennour Hennekinne, R.; Guillier, L.; Fazeuilh, L.; Ells, T.; Forsythe, S.; Jackson, E.; Meheut, T.; Besse, N.G. Survival of Cronobacter in powdered infant formula and their variation in biofilm formation. Lett. Appl. Microbiol. 2018, 66, 496–505. [Google Scholar] [CrossRef]
- Osaili, T.M.; Shaker, R.R.; Ayyash, M.M.; Al-Nabulsi, A.A.; Forsythe, S.J. Survival and growth of Cronobacter species (Enterobacter sakazakii) in wheat-based infant follow-on formulas. Lett. Appl. Microbiol. 2009, 48, 408–412. [Google Scholar] [CrossRef]
- Sechter, I.; Gerichter, C.h.B.; Cahan, D. Method for Detecting Small Numbers of Vibrio cholerae in Very Polluted Substrates. Appl. Microbiol. 1975, 29, 814–818. [Google Scholar] [CrossRef]
- Channaiah, L.H.; Subramanyam, B.; Zurek, L. Survival of Enterococcus faecalis OG1RF:pCF10 in Poultry and Cattle Feed: Vector Competence of the Red Flour Beetle, Tribolium castaneum (Herbst). J. Food Prot. 2010, 73, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Van Beeck, W.; Verschueren, C.; Wuyts, S.; van den Broek, M.F.L.; Uyttendaele, M.; Lebeer, S. Robustness of fermented carrot juice against Listeria monocytogenes, Salmonella Typhimurium and Escherichia coli O157:H7. Int. J. Food Microbiol. 2020, 335, 108854. [Google Scholar] [CrossRef]
- Tokarskyy, O.; De, J.; Fatica, M.K.; Brecht, J.; Schneider, K.R. Survival of Escherichia coli O157:H7 and Salmonella on Bruised and Unbruised Tomatoes from Three Ripeness Stages at Two Temperatures. J. Food Prot. 2018, 81, 2028–2033. [Google Scholar] [CrossRef]
- Feng, L.; Muyyarikkandy, M.S.; Brown, S.R.B.; Amalaradjou, M.A. Attachment and Survival of Escherichia coli O157:H7 on In-Shell Hazelnuts. Int. J. Environ. Res. Public Health 2018, 15, 1122. [Google Scholar] [CrossRef]
- Degirmenci, H.; Karapinar, M.; Karabiyikli, S. The survival of E. coli O157:H7, S. Typhimurium and L. monocytogenes in black carrot (Daucus carota) juice. Int. J. Food Microbiol. 2012, 153, 212–215. [Google Scholar] [CrossRef]
- Mark Ibekwe, A.; Grieve, C.M.; Papiernik, S.K.; Yang, C.H. Persistence of Escherichia coli O157:H7 on the rhizosphere and phyllosphere of lettuce. Lett. Appl. Microbiol. 2009, 49, 784–790. [Google Scholar] [CrossRef]
- Erickson, M.C.; Liao, J.; Payton, A.S.; Cook, P.W.; Ortega, Y.R. Survival and internalization of Salmonella and Escherichia coli O157:H7 sprayed onto different cabbage cultivars during cultivation in growth chambers. J. Sci. Food Agric. 2019, 99, 3530–3537. [Google Scholar] [CrossRef]
- Erickson, M.C.; Liao, J.Y.; Payton, A.S.; Cook, P.W.; Den Bakker, H.C.; Bautista, J.; Pérez, J.C.D. Pre-harvest internalization and surface survival of Salmonella and Escherichia coli O157:H7 sprayed onto different lettuce cultivars under field and growth chamber conditions. Int. J. Food Microbiol. 2019, 291, 197–204. [Google Scholar] [CrossRef]
- Tian, J.Q.; Bae, Y.M.; Choi, N.Y.; Kang, D.H.; Heu, S.; Lee, S.Y. Survival and Growth of Foodborne Pathogens in Minimally Processed Vegetables at 4 and 15 °C. J. Food Sci. 2012, 77, M48–M50. [Google Scholar] [CrossRef]
- Marques, P.A.; Worcman-Barninka, D.; Lannes, S.C.; Landgraf, M. Acid tolerance and survival of Escherichia coli O157:H7 inoculated in fruit pulps stored under refrigeration. J. Food Prot. 2001, 64, 1674–1678. [Google Scholar] [CrossRef]
- Lieberman, V.M.; Zhao, I.Y.; Schaffner, D.W.; Danyluk, M.D.; Harris, L.J. Survival or Growth of Inoculated Escherichia coli O157:H7 and Salmonella on Yellow Onions (Allium cepa) under Conditions Simulating Food Service and Consumer Handling and Storage. J. Food Prot. 2015, 78, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Blessington, T.; Mitcham, E.J.; Harris, L.J. Survival of Salmonella enterica, Escherichia coli O157:H7, and Listeria monocytogenes on Inoculated Walnut Kernels during Storage. J. Food Prot. 2012, 75, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Brar, P.K.; Proano, L.G.; Friedrich, L.M.; Harris, L.J.; Danyluk, M.D. Survival of Salmonella, Escherichia coli O157:H7, and Listeria monocytogenes on Raw Peanut and Pecan Kernels Storedat −24, 4, and 22 °C. J. Food Prot. 2015, 78, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Marek, P.; Nair, M.K.M.; Hoagland, T.; Venkitanarayanan, K. Survival and growth characteristics of Escherichia coli O157:H7 in pasteurized and unpasteurized Cheddar cheese whey. Int. J. Food Microbiol. 2004, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Barrera, O.; Rodriguezcalleja, J.; Santos, J.; Otero, A.; Garcialopez, M. Effect of different storage conditions on E. coli O157:H7 and the indigenous bacterial microflora on lamb meat. Int. J. Food Microbiol. 2007, 115, 244–251. [Google Scholar] [CrossRef]
- Lekkas, C.; Kakouri, A.; Paleologos, E.; Voutsinas, L.P.; Kontominas, M.G.; Samelis, J. Survival of Escherichia coli O157:H7 in Galotyri cheese stored at 4 and 12 °C. Food Microbiol. 2006, 23, 268–276. [Google Scholar] [CrossRef]
- Vernozy-Rozand, C.; Mazuy-Cruchaudet, C.; Bavai, C.; Montet, M.P.; Bonin, V.; Dernburg, A.; Richard, Y. Growth and survival of Escherichia coli O157:H7 during the manufacture and ripening of raw goat milk lactic cheeses. Int. J. Food Microbiol. 2005, 105, 83–88. [Google Scholar] [CrossRef]
- Maher, M.M.; Jordan, K.N.; Upton, M.E.; Coffey, A. Growth and survival of E. coli O157:H7 during the manufacture and ripening of a smear-ripened cheese produced from raw milk. J. Appl. Microbiol. 2001, 90, 201–207. [Google Scholar] [CrossRef]
- De Cooman, L.; Flahou, B.; Houf, K.; Smet, A.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F. Survival of Helicobacter suis bacteria in retail pig meat. Int. J. Food Microbiol. 2013, 166, 164–167. [Google Scholar] [CrossRef]
- Saha, J.; Topalcengiz, Z.; Sharma, V.; Friedrich, L.M.; Danyluk, M.D. Fate and Growth Kinetics of Salmonella and Listeria monocytogenes on Mangoes During Storage. J. Food Prot. 2023, 86, 100151. [Google Scholar] [CrossRef]
- Fay, M.L.; Salazar, J.K.; Stewart, D.S.; Khouja, B.A.; Zhou, X.; Datta, A.R. Survival of Listeria monocytogenes on Frozen Vegetables during Long-term Storage at −18 and −10 °C. J. Food Prot. 2024, 87, 100224. [Google Scholar] [CrossRef] [PubMed]
- Akomea-Frempong, S.; Skonberg, D.I.; Arya, R.; Perry, J.J. Survival of Inoculated Vibrio spp., Shigatoxigenic Escherichia coli, Listeria monocytogenes, and Salmonella spp. on Seaweed (Sugar Kelp) During Storage. J. Food Prot. 2023, 86, 100096. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.K.; Natarajan, V.; Stewart, D.; Suehr, Q.; Mhetras, T.; Gonsalves, L.J.; Tortorello, M.L. Survival kinetics of Listeria monocytogenes on chickpeas, sesame seeds, pine nuts, and black pepper as affected by relative humidity storage conditions. PLoS ONE 2019, 14, e0226362. [Google Scholar] [CrossRef]
- Fay, M.L.; Salazar, J.K.; Zhang, X.; Zhou, X.; Stewart, D.S. Long-Term Survival of Listeria monocytogenes in Nut, Seed, and Legume Butters. J. Food Prot. 2023, 86, 100094. [Google Scholar] [CrossRef]
- Ly, V.; Parreira, V.R.; Sanchez-Maldonado, A.F.; Farber, J.M. Survival and Virulence of Listeria monocytogenes during Storage on Chocolate Liquor, Corn Flakes, and Dry-Roasted Shelled Pistachios at 4 and 23 °C. J. Food Prot. 2020, 83, 1852–1862. [Google Scholar] [CrossRef]
- Cuzzi, R.; Ly, V.; Parreira, V.R.; Sanchez-Maldonado, A.F.; Farber, J.M. Survival of Listeria monocytogenes during storage on dried apples, strawberries, and raisins at 4 °C and 23 °C. Int. J. Food Microbiol. 2021, 339, 108991. [Google Scholar] [CrossRef]
- Flessa, S.; Lusk, D.M.; Harris, L.J. Survival of Listeria monocytogenes on fresh and frozen strawberries. Int. J. Food Microbiol. 2005, 101, 255–262. [Google Scholar] [CrossRef]
- Moreira, J.; Mera, E.; Singh Chhetri, V.; King, J.M.; Gentimis, T.; Adhikari, A. Effect of storage temperature and produce type on the survival or growth of Listeria monocytogenes on peeled rinds and fresh-cut produce. Front. Microbiol. 2023, 14, 1151819. [Google Scholar] [CrossRef]
- Hanifian, S. Survival of Mycobacterium avium subsp. Paratuberculosis in ultra-filtered white cheese. Lett. Appl. Microbiol. 2014, 58, 466–471. [Google Scholar] [CrossRef]
- Donaghy, J.A.; Totton, N.L.; Rowe, M.T. Persistence of Mycobacterium paratuberculosis during manufacture and ripening of cheddar cheese. Appl. Environ. Microbiol. 2004, 70, 4899–4905. [Google Scholar] [CrossRef]
- Fong, K.; Wang, S. Strain-Specific Survival of Salmonella enterica in Peanut Oil, Peanut Shell, and Chia Seeds. J. Food Prot. 2016, 79, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R.; Mann, D.A.; Kelly, C.A.; Ortega, Y.R. Retention of Viability of Salmonella in Sucrose as Affected by Type of Inoculum, Water Activity, and Storage Temperature. J. Food Prot. 2017, 80, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Flock, G.; Richardson, M.; Pacitto-Reilly, D.; Anderson, N.; Chen, F.; Ahnrud, G.P.; Mendoza, A.J.; Senecal, A.G. Survival of Salmonella enterica in Military Low-Moisture Food Products during Long-Term Storage at 4, 25, and 40 °C. J. Food Prot. 2022, 85, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, Y.; Wang, L.; Zhang, S.; Deng, X. Salmonella survival during thermal dehydration of fresh garlic and storage of dehydrated garlic products. Int. J. Food Microbiol. 2017, 263, 26–31. [Google Scholar] [CrossRef]
- Keller, S.E.; VanDoren, J.M.; Grasso, E.M.; Halik, L.A. Growth and survival of Salmonella in ground black pepper (Piper nigrum). Food Microbiol. 2013, 34, 182–188. [Google Scholar] [CrossRef]
- Gradl, D.R.; Sun, L.; Larkin, E.L.; Chirtel, S.J.; Keller, S.E. Survival of Salmonella during Drying of Fresh Ginger Root (Zingiber officinale) and Storage of Ground Ginger. J. Food Prot. 2015, 78, 1954–1960. [Google Scholar] [CrossRef]
- Xu, S.; Chen, H. The influence of almond’s water activity and storage temperature on Salmonella survival and thermal resistance. Food Microbiol. 2023, 113, 104269. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Mann, D.A. Survival of Salmonella on Dried Fruits and in Aqueous Dried Fruit Homogenates as Affected by Temperature. J. Food Prot. 2014, 77, 1102–1109. [Google Scholar] [CrossRef]
- Smith, J.L.; Huhtanen, C.N.; Kissinger, J.C.; Palumbo, S.A. Survival of Salmonellae During Pepperoni Manufacture. Appl. Microbiol. 1975, 30, 759–763. [Google Scholar] [CrossRef]
- Zhou, B.; Luo, Y.; Nou, X.; Yang, Y.; Wu, Y.; Wang, Q. Effects of Postharvest Handling Conditions on Internalization and Growth of Salmonella enterica in Tomatoes. J. Food Prot. 2014, 77, 365–370. [Google Scholar] [CrossRef]
- Farakos, S.M.S.; Hicks, J.W.; Frye, J.G.; Frank, J.F. Relative Survival of Four Serotypes of Salmonella enterica in Low-Water Activity Whey Protein Powder Held at 36 and 70 °C at Various Water Activity Levels. J. Food Prot. 2014, 77, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Dhowlaghar, N.; Bansal, M.; Schilling, M.W.; Nannapaneni, R. Scanning electron microscopy of Salmonella biofilms on various food-contact surfaces in catfish mucus. Food Microbiol. 2018, 74, 143–150. [Google Scholar] [CrossRef]
- Degenhardt, R.; Carvalho, M.M.; Voidaleski, M.F.; Daros, G.F.; Guaragni, A.; de Melo Pereira, G.V.; De Dea Lindner, J. Brazilian artisanal Colonial cheese: Characterization, microbiological safety, and survival of Salmonella enterica serovar Enteritidis during ripening. Braz. J. Microbiol. 2023, 54, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Limcharoenchat, P.; James, M.K.; Marks, B.P. Survival and Thermal Resistance of Salmonella Enteritidis PT 30 on Almonds after Long-Term Storage. J. Food Prot. 2019, 82, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Abd, S.J.; McCarthy, K.L.; Harris, L.J. Impact of Storage Time and Temperature on Thermal Inactivation of Salmonella Enteritidis PT 30 on Oil-Roasted Almonds. J. Food Sci. 2012, 77, M42–M47. [Google Scholar] [CrossRef]
- Matak, K.E.; Hvizdzak, A.L.; Beamer, S.; Jaczynski, J. Recovery of Salmonella enterica Serovars Typhimurium and Tennessee in Peanut Butter after Electron Beam Exposure. J. Food Sci. 2010, 75, M462–M467. [Google Scholar] [CrossRef]
- Chakroun, I.; Fedhila, K.; Mahdhi, A.; Mzoughi, R.; Saidane, D.; Esteban, M.A.; Bakhrouf, A. Atypical Salmonella Typhimurium persistence in the pacific oyster, Crassostrea gigas, and its effect on the variation of gene expression involved in the oyster’s immune system. Microb. Pathog. 2021, 160, 105181. [Google Scholar] [CrossRef]
- Leyer, G.J.; Johnson, E.A. Acid adaptation promotes survival of Salmonella spp. in cheese. Appl. Environ. Microbiol. 1992, 58, 2075–2080. [Google Scholar] [CrossRef]
- Böhnlein, C.; Kabisch, J.; Müller-Herbst, S.; Fiedler, G.; Franz, C.M.A.P.; Pichner, R. Persistence and reduction of Shiga toxin-producing Escherichia coli serotype O26:H11 in different types of raw fermented sausages. Int. J. Food Microbiol. 2017, 261, 82–88. [Google Scholar] [CrossRef]
- Gill, A.; McMahon, T.; Ferrato, C.; Chui, L. Survival of O157 and non-O157 shiga toxin-producing Escherichia coli in Korean style kimchi. Food Microbiol. 2024, 121, 104526. [Google Scholar] [CrossRef]
- Wood, R.R.; Arias, C.R. Distribution and survival of Vibrio vulnificus genotypes in postharvest Gulf Coast (USA) oysters under refrigeration. J. Appl. Microbiol. 2012, 113, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Si, J.; Yun, H.S.; Ko, G. Effect of temperature and relative humidity on the survival of foodborne viruses during food storage. Appl. Environ. Microbiol. 2015, 81, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.S.; Curcic, M.; Sobsey, M.D. Persistence of Coronavirus Surrogates on Meat and Fish Products during Long-Term Storage. Appl. Environ. Microbiol. 2022, 88, e0050422. [Google Scholar] [CrossRef] [PubMed]
- Esseili, M.A.; Saif, L.J.; Farkas, T.; Wang, Q. Feline Calicivirus, Murine Norovirus, Porcine Sapovirus, and Tulane Virus Survival on Postharvest Lettuce. Appl. Environ. Microbiol. 2015, 81, 5085–5092. [Google Scholar] [CrossRef]
- Nasheri, N.; Harlow, J.; Chen, A.; Corneau, N.; Bidawid, S. Survival and Inactivation by Advanced Oxidative Process of Foodborne Viruses in Model Low-Moisture Foods. Food Environ. Virol. 2021, 13, 107–116. [Google Scholar] [CrossRef]
- Hewitt, J.; Greening, G.E. Survival and persistence of norovirus, hepatitis A virus, and feline calicivirus in marinated mussels. J. Food Prot. 2004, 67, 1743–1750. [Google Scholar] [CrossRef]
- Wang, Q.; Hirneisen, K.A.; Markland, S.M.; Kniel, K.E. Survival of murine norovirus, Tulane virus, and hepatitis A virus on alfalfa seeds and sprouts during storage and germination. Appl. Environ. Microbiol. 2013, 79, 7021–7027. [Google Scholar] [CrossRef]
- Croci, L.; De Medici, D.; Scalfaro, C.; Fiore, A.; Toti, L. The survival of hepatitis A virus in fresh produce. Int. J. Food Microbiol. 2002, 73, 29–34. [Google Scholar] [CrossRef]
- Blondin-Brosseau, M.; Harlow, J.; Doctor, T.; Nasheri, N. Examining the persistence of human Coronavirus 229E on fresh produce. Food Microbiol. 2021, 98, 103780. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Chen, H.; Meade, G.K. Persistence of MS-2 Bacteriophage Within Eastern Oysters. Food Environ. Virol. 2017, 10, 83–88. [Google Scholar] [CrossRef]
- Lamhoujeb, S.; Fliss, I.; Ngazoa, S.E.; Jean, J. Evaluation of the persistence of infectious human noroviruses on food surfaces by using real-time nucleic acid sequence-based amplification. Appl. Environ. Microbiol. 2008, 74, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Kurdziel, A.S.; Wilkinson, N.; Langton, S.; Cook, N. Survival of poliovirus on soft fruit and salad vegetables. J. Food Prot. 2001, 64, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, M.P.; Verma, H.; Sampedro, F.; Urriola, P.E.; Shurson, G.C.; Goyal, S.M. Environmental persistence of porcine coronaviruses in feed and feed ingredients. PLoS ONE 2017, 12, e0178094. [Google Scholar] [CrossRef]
- Jia, M.; Taylor, T.M.; Senger, S.M.; Ovissipour, R.; Bertke, A.S. SARS-CoV-2 Remains Infectious on Refrigerated Deli Food, Meats, and Fresh Produce for up to 21 Days. Foods 2022, 11, 286. [Google Scholar] [CrossRef]
- Featherstone, A.B.; Brown, A.C.; Chitlapilly Dass, S. Murine Hepatitis Virus, a Biosafety Level 2 Model for SARS-CoV-2, Can Remain Viable on Meat and Meat Packaging Materials for at Least 48 Hours. Microbiol. Spectr. 2022, 10, e0186222. [Google Scholar] [CrossRef]
- Kubina, S.; Costa, D.; Cazeaux, C.; Villena, I.; Favennec, L.; Razakandrainibe, R.; La Carbona, S. Persistence and survival of Cryptosporidium parvum oocysts on lamb’s lettuce leaves during plant growth and in washing conditions of minimally-processed salads. Int. J. Food Microbiol. 2023, 388, 110085. [Google Scholar] [CrossRef]
- Ryan, U.; Hijjawi, N.; Xiao, L. Foodborne cryptosporidiosis. Int. J. Parasitol. 2018, 48, 1–12. [Google Scholar] [CrossRef]
- Nan, M.; Xue, H.; Bi, Y. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef]
- Bosch, A.; Gkogka, E.; Le Guyader, F.S.; Loisy-Hamon, F.; Lee, A.; van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C.; et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018, 285, 110–128. [Google Scholar] [CrossRef]
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.Á.; Carrasco Jiménez, E.; Rodríguez, F.P.; Márquez, R.J.A. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M. Human Salmonellosis: A Continuous Global Threat in the Farm-to-Fork Food Safety Continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J., II; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Alrefaei, R.; Bushlaibi, M.; Ndegwa, E.; Kaseloo, P.; Wynn, C. Influence of growth temperature on thermal tolerance of leading foodborne pathogens. Food Sci. Nutr. 2019, 7, 4027–4036. [Google Scholar] [CrossRef]
- Awad, D.A.; Masoud, H.A.; Hamad, A. Climate changes and food-borne pathogens: The impact on human health and mitigation strategy. Clim. Change 2024, 177, 92. [Google Scholar] [CrossRef]
- Samelis, J.; Sofos, J.N.; Kendall, P.A.; Smith, G.C. Survival or Growth of Escherichia coli O157:H7 in a Model System of Fresh Meat Decontamination Runoff Waste Fluids and Its Resistance to Subsequent Lactic Acid Stress. Appl. Environ. Microbiol. 2005, 71, 6228–6234. [Google Scholar] [CrossRef]
- Waltenburg, M.A.; Schwensohn, C.; Madad, A.; Seelman, S.L.; Peralta, V.; Koske, S.E.; Boyle, M.M.; Arends, K.; Patel, K.; Mattioli, M.; et al. Two multistate outbreaks of a reoccurring Shiga toxin-producing Escherichia coli strain associated with romaine lettuce: USA, 2018–2019. Epidemiol. Infect. 2021, 150, e16. [Google Scholar] [CrossRef]
- Bonadonna, L.; Briancesco, R.; Coccia, A.M.; Meloni, P.; Rosa GLa Moscato, U. Microbial Air Quality in Healthcare Facilities. Int. J. Environ. Res. Public Health 2021, 18, 6226. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, Y.; Chang, Y.; Liang, X.; Zhang, H.; Lin, X.; Qing, K.; Zhou, X.; Luo, Z. The Effects of Ventilation, Humidity, and Temperature on Bacterial Growth and Bacterial Genera Distribution. Int. J. Environ. Res. Public Health 2022, 19, 15345. [Google Scholar] [CrossRef]
- Zoz, F.; Iaconelli, C.; Lang, E.; Iddir, H.; Guyot, S.; Grandvalet, C.; Gervais, P.; Beney, L. Control of Relative Air Humidity as a Potential Means to Improve Hygiene on Surfaces: A Preliminary Approach with Listeria monocytogenes. PLoS ONE 2016, 11, e0148418. [Google Scholar] [CrossRef]
- Hughes, R.A.; Hallett, K.; Cogan, T.; Enser, M.; Humphrey, T. The Response of Campylobacter jejuni to Low Temperature Differs from That of Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 6292–6298. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.; Wiedmann, M.; Boor, K.J. The Alternative Sigma Factor σ B and the Virulence Gene Regulator PrfA Both Regulate Transcription of Listeria monocytogenes Internalins. Appl. Environ. Microbiol. 2007, 73, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Little, A.; Mendonca, A.; Dickson, J.; Fortes-Da-Silva, P.; Boylston, T.; Lewis, B.; Coleman, S.; Thomas-Popo, E. Acid Adaptation Enhances Tolerance of Escherichia coli O157:H7 to High Voltage Atmospheric Cold Plasma in Raw Pineapple Juice. Microorganisms 2024, 12, 1131. [Google Scholar] [CrossRef]
- Chacón-Flores, N.A.; Olivas-Orozco, G.I.; Acosta-Muñiz, C.H.; Gutiérrez-Méndez, N.; Sepúlveda-Ahumada, D.R. Effect of Water Activity, pH, and Lactic Acid Bacteria to Inhibit Escherichia coli during Chihuahua Cheese Manufacture. Foods 2023, 12, 3751. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.d.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, N.Y.; Koh, Y.J.; Lee, H.J. Epidemiology of foodborne Norovirus outbreak in Incheon, Korea. J. Korean Med. Sci. 2010, 25, 1128–1133. [Google Scholar] [CrossRef]
- Sakon, N.; Sadamasu, K.; Shinkai, T.; Hamajima, Y.; Yoshitomi, H.; Matsushima, Y.; Takada, R.; Terasoma, F.; Nakamura, A.; Komano, J.; et al. Foodborne Outbreaks Caused by Human Norovirus GII.P17-GII.17-Contaminated Nori, Japan, 2017. Emerg. Infect. Dis. 2018, 24, 920–923. [Google Scholar] [CrossRef]
- Donnan, E.J.; Fielding, J.E.; Gregory, J.E.; Lalor, K.; Rowe, S.; Goldsmith, P.; Antoniou, M.; Fullerton, K.E.; Knope, K.; Copland, J.G.; et al. A Multistate Outbreak of Hepatitis A Associated With Semidried Tomatoes in Australia, 2009. Clin. Infect. Dis. 2012, 54, 775–781. [Google Scholar] [CrossRef]
- Castro, M.; Soares, K.; Ribeiro, C.; Esteves, A. Evaluation of the Effects of Food Safety Training on the Microbiological Load Present in Equipment, Surfaces, Utensils, and Food Manipulator’s Hands in Restaurants. Microorganisms 2024, 12, 825. [Google Scholar] [CrossRef]
- Putri, M.S.; Susanna, D. Food safety knowledge, attitudes, and practices of food handlers at kitchen premises in the Port “X” area, North Jakarta, Indonesia 2018. Ital. J. Food Saf. 2021, 10, 9215. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, J.B.; Shoyer, B.A.; Jung, Y.; Shane, L.E.; Osoria, M.; Porto-Fett, A.C.S. Viability of Shiga Toxin–producing Escherichia coli, Salmonella, and Listeria monocytogenes Within Plant Versus Beef Burgers During Cold Storage and Following Pan Frying. J. Food Prot. 2020, 83, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Klappholz, A.; Tamber, S. Effectiveness of Preparation Practices on the Inactivation of Salmonella enterica Serovar Enteritidis in Frozen Breaded Chicken Strips. J. Food Prot. 2020, 83, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]

| Survival/Persistence | Mean Duration (Days) | Mean Duration (Days) ± SD |
|---|---|---|
| Persistence | 246.04 | 21.50, 470.58 |
| Survival | 15.69 | 2.35, 33.73 |
| Temperature Category | Average Duration (Days) |
|---|---|
| Below Freezing (<0 °C) | 96.50 |
| Cold (0–10 °C) | 55.25 |
| Moderate (10–25 °C) | 67.92 |
| Warm (>25 °C) | 316.71 |
| Unknown | 35 |
| Temperature Category | Duration Range (Weeks/Days) | Virus | References |
|---|---|---|---|
| Frozen temperatures (<0 °C) | 15 days | Poliovirus | [86] |
| Low and refrigerated temperatures (0–10 °C) | ≤4 weeks and 30 days | Hepatitis A virus Feline calicivirus Norovirus Bacteriophage phi 6 Murine hepatitis virus Transmissible gastroenteritis virus Poliovirus Porcine sapovirus | [77,78,79,80,86] |
| Room temperature (10–24 °C) | ≤6 weeks | Feline calicivirus Human norovirus Hepatitis A virus Bacteriophage MS2 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Middle East respiratory syndrome (MERS-CoV) Murine norovirus | [79,81] |
| Warm (≥25 °C) | 2 weeks | Hepatitis A virus Murine norovirus | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donkor, E.S.; Sosah, F.K.; Odoom, A.; Odai, B.T.; Kunadu, A.P.-H. How Long Do Microorganisms Survive and Persist in Food? A Systematic Review. Microorganisms 2025, 13, 901. https://doi.org/10.3390/microorganisms13040901
Donkor ES, Sosah FK, Odoom A, Odai BT, Kunadu AP-H. How Long Do Microorganisms Survive and Persist in Food? A Systematic Review. Microorganisms. 2025; 13(4):901. https://doi.org/10.3390/microorganisms13040901
Chicago/Turabian StyleDonkor, Eric S., Famous K. Sosah, Alex Odoom, Bernard T. Odai, and Angela Parry-Hanson Kunadu. 2025. "How Long Do Microorganisms Survive and Persist in Food? A Systematic Review" Microorganisms 13, no. 4: 901. https://doi.org/10.3390/microorganisms13040901
APA StyleDonkor, E. S., Sosah, F. K., Odoom, A., Odai, B. T., & Kunadu, A. P.-H. (2025). How Long Do Microorganisms Survive and Persist in Food? A Systematic Review. Microorganisms, 13(4), 901. https://doi.org/10.3390/microorganisms13040901






