Adaptation to Long-Term Nitrogen Starvation in a Biocrust-Derived Microalga Vischeria sp. WL1: Insights into Cell Wall Features and Desiccation Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Algal Strain and Nitrogen Starvation Treatment
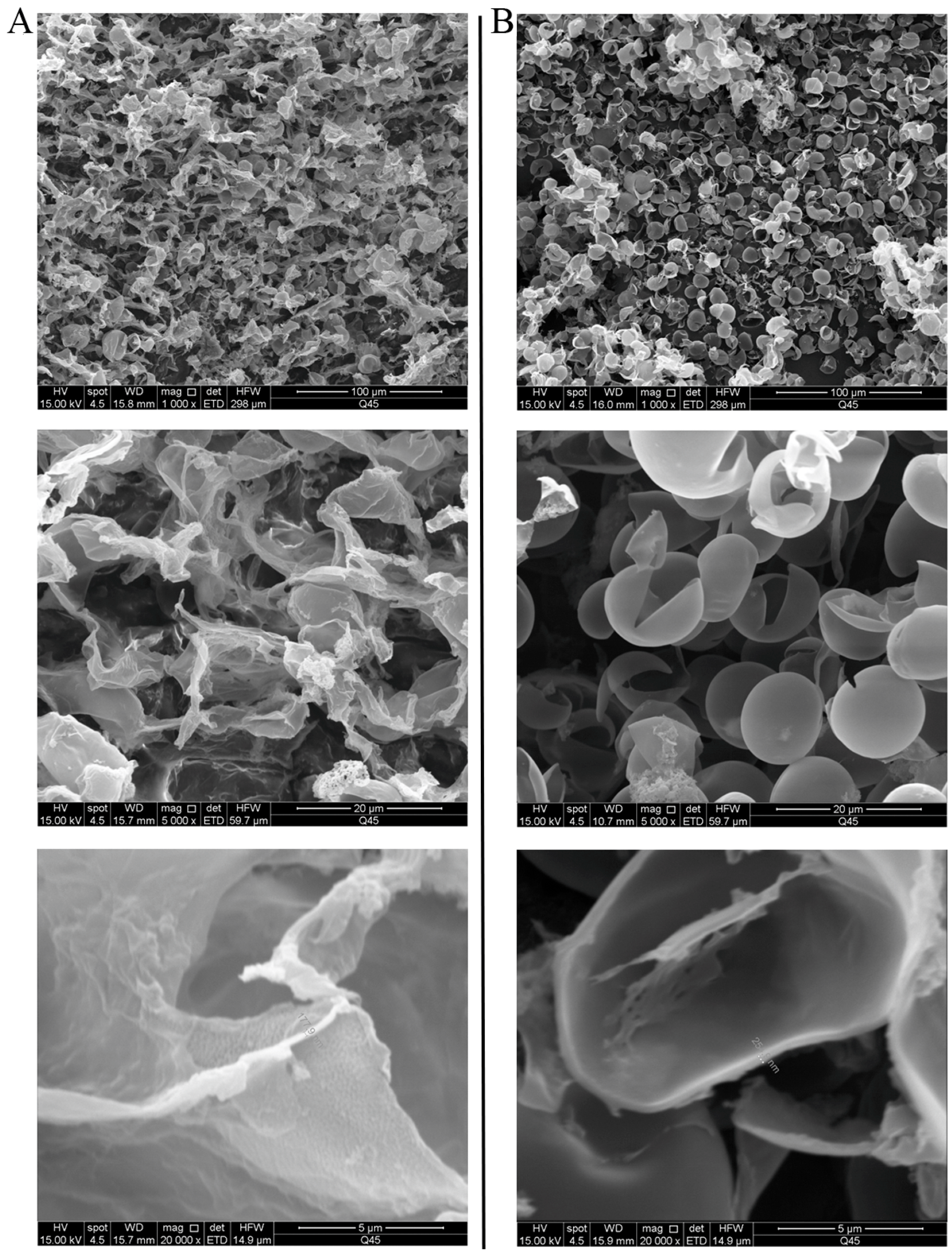
2.2. Microscopic, SEM, and TEM Observations of Cells
2.3. Cell Wall Extraction
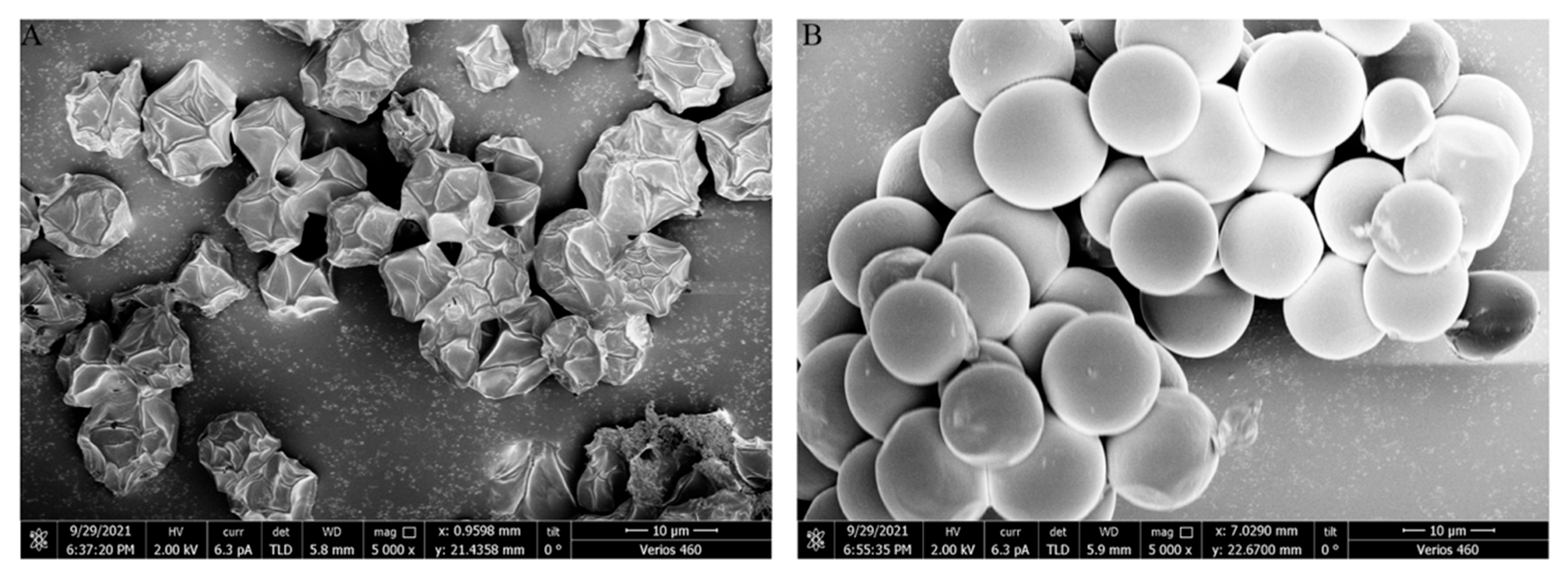
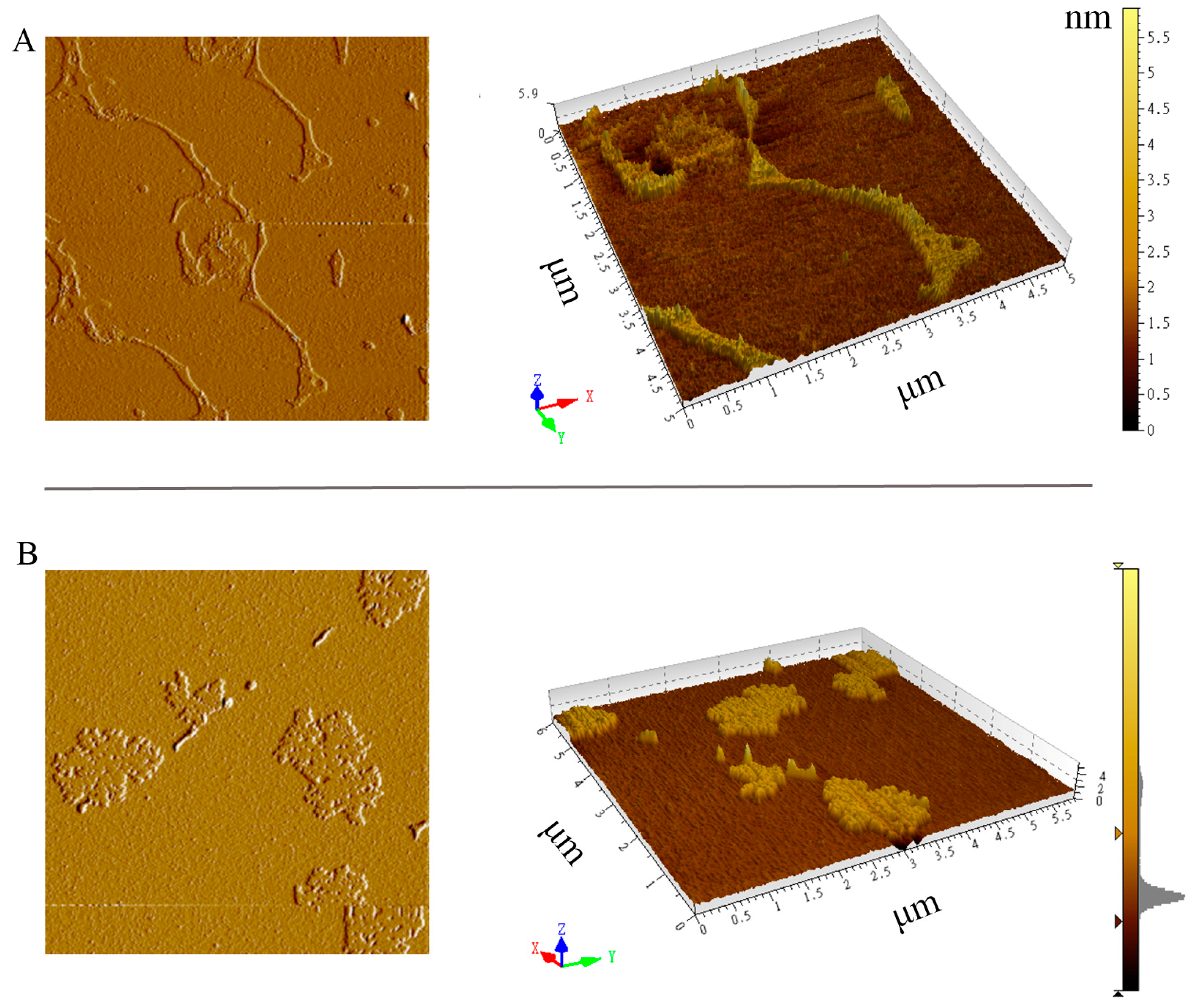
2.4. SEM and AFM Observations of the Extracted Cell Wall
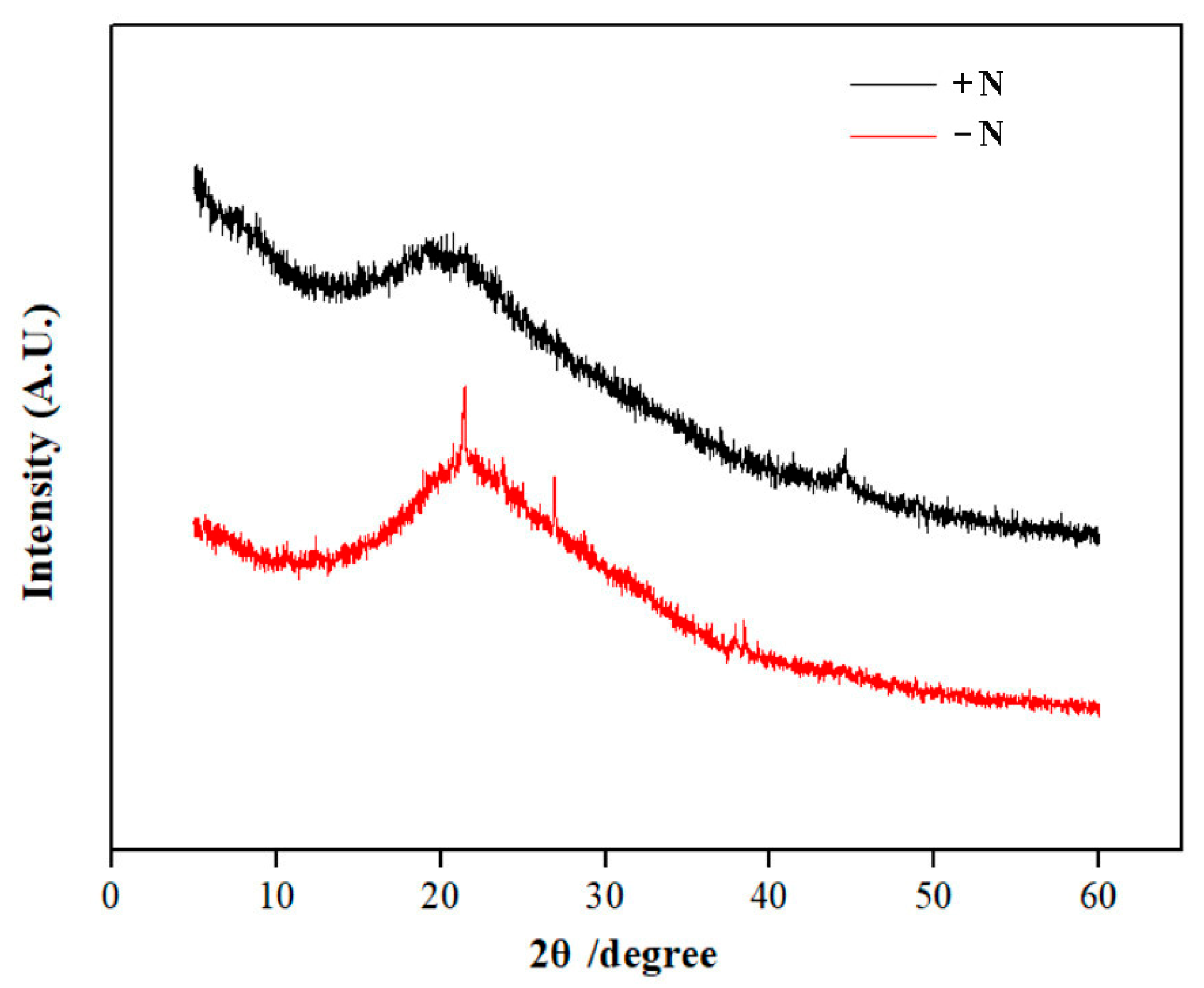
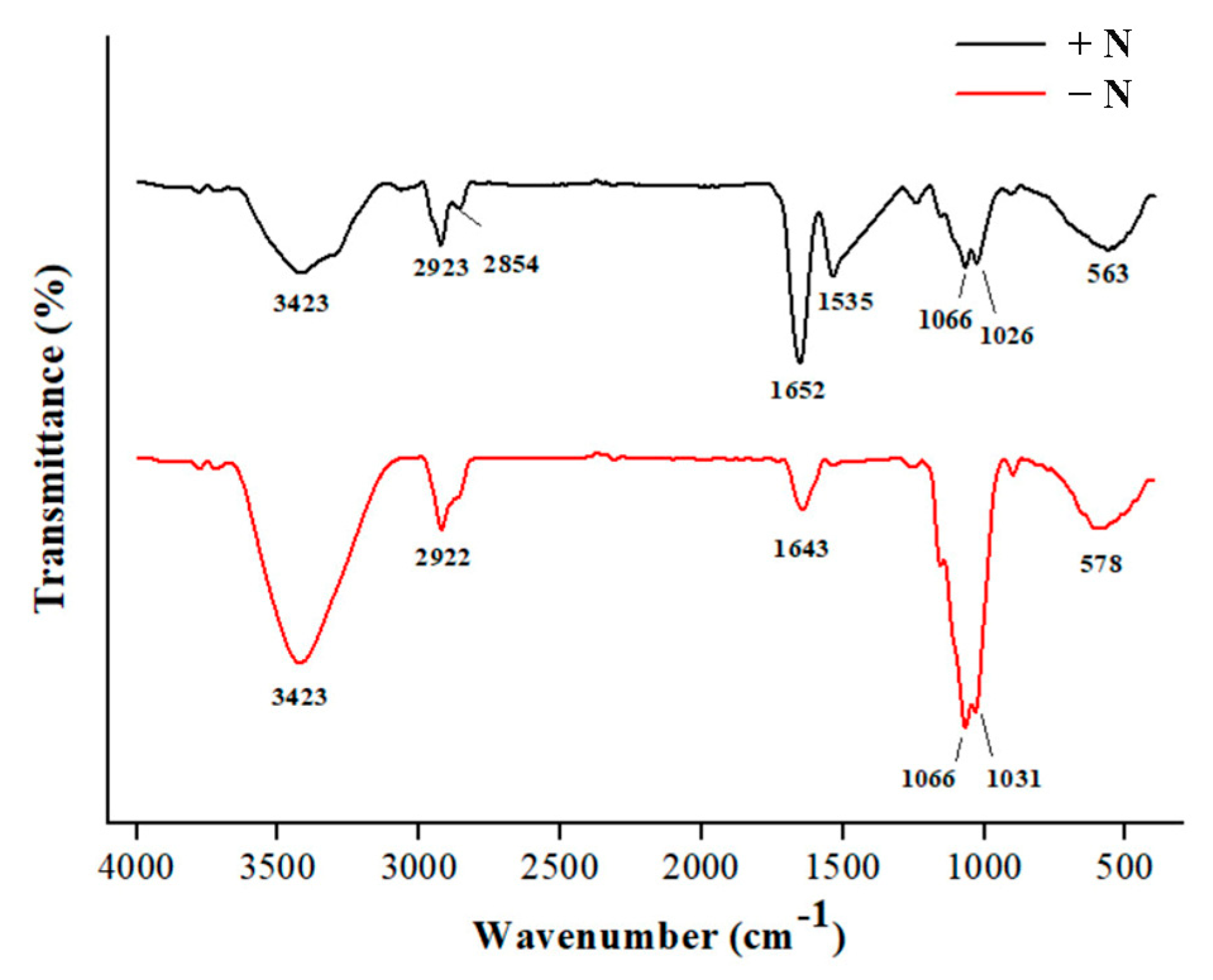
2.5. XRD and FTIR Analyses of the Extracted Cell Wall
2.6. Analysis of the Monosaccharide Composition of Cell Wall
2.7. Cell Survival Observation Through Fluorescence Dye Staining
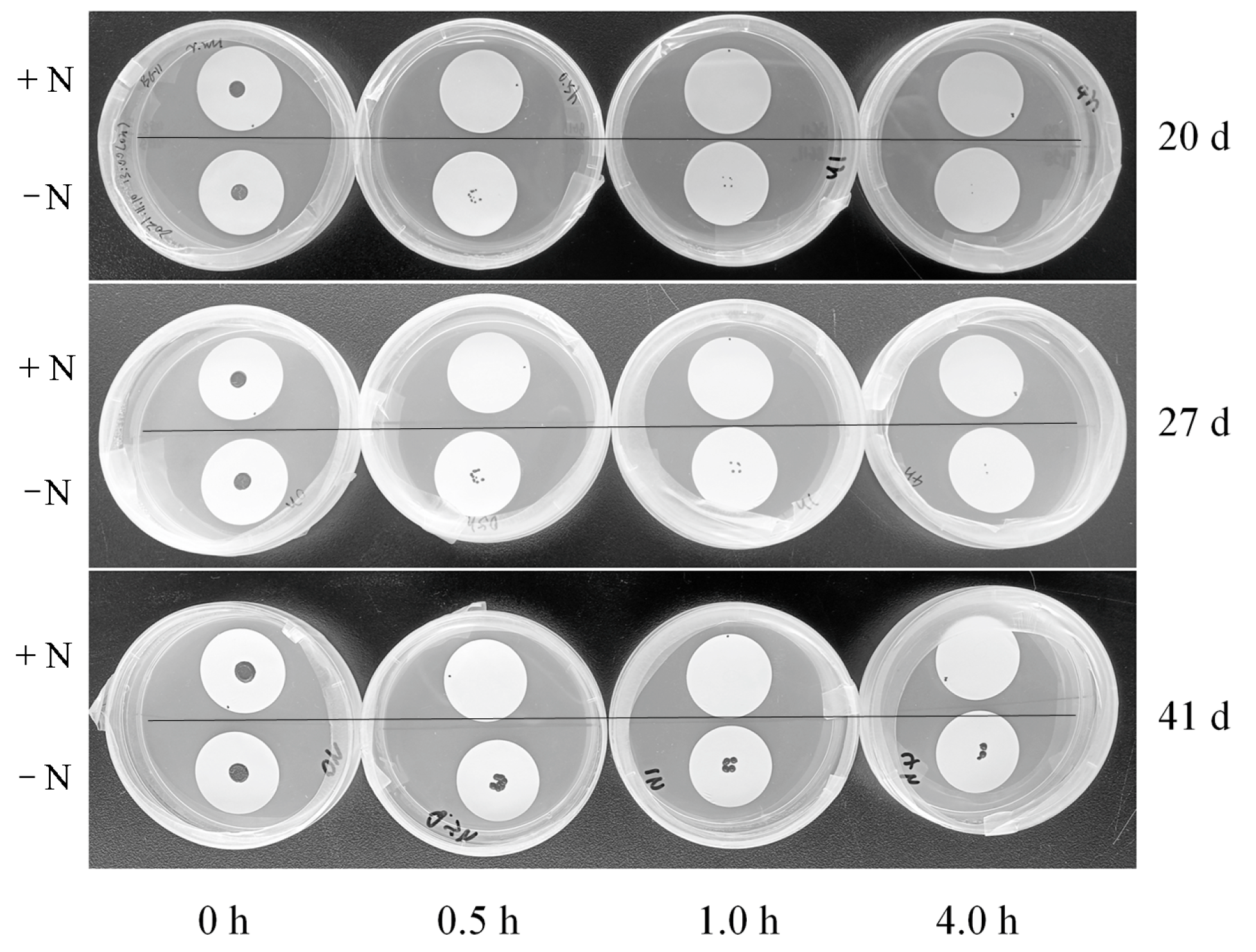
2.8. Cell Survival Testing on Solid Medium
3. Results
3.1. Morphological Integrity of Cells After the Drying Treatment
3.2. Cell Observation by SEM and TEM
3.3. SEM and AFM Observations of Cell Walls
3.4. XRD and FTIR Analyses of Cell Walls
3.5. The Monosaccharide Composition of Cell Walls
3.6. Comparison of Desiccation Resistance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J. Biotechnol. 2012, 162, 21–27. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Li, C.; Zhu, S.; Wang, Z.; Zeng, E. Lipid accumulation and eicosapentaenoic acid distribution in response to nitrogen limitation in microalga Eustigmatos vischeri JHsu-01 (Eustigmatophyceae). Algal Res. 2020, 48, 101910. [Google Scholar] [CrossRef]
- Liang, L.; Wang, Z.; Ding, Y.; Li, Y.; Wen, X. Protein reserves elucidate the growth of microalgae under nitrogen deficiency. Algal Res. 2023, 75, 103269. [Google Scholar] [CrossRef]
- Tibocha-Bonilla, J.D.; Kumar, M.; Richelle, A.; Godoy-Silva, R.D.; Zengler, K.; Zuñiga, C. Dynamic resource allocation drives growth under nitrogen starvation in eukaryotes. NPJ Syst. Biol. Appl. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yang, J.; Lei, X.; Xia, S.; Li, A.; Zhang, C.W. Characterization of cell structural change, growth, lipid accumulation, and pigment profile of a novel oleaginous microalga, Vischeria stellata (Eustigmatophyceae), cultured with different initial nitrate supplies. J. Appl. Phycol. 2016, 28, 821–830. [Google Scholar] [CrossRef]
- Yap, B.H.J.; Crawford, S.A.; Dagastine, R.R.; Scales, P.J.; Martin, G.J.O. Nitrogen deprivation of microalgae: Effect on cell size, cell wall thickness, cell strength, and resistance to mechanical disruption. J. Ind. Microbiol. Biot. 2016, 43, 1671–1680. [Google Scholar] [CrossRef]
- González-Hourcade, M.; Braga, M.R.; Del Campo, E.M.; Ascaso, C.; Patiño, C.; Casano, L.M. Ultrastructural and biochemical analyses reveal cell wall remodelling in lichen-forming microalgae submitted to cyclic desiccation-rehydration. Ann. Bot. 2020, 125, 459–469. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Zheng, H.; Wang, Q.; Li, A. Growth, biochemical composition and photosynthetic performance of Scenedesmus acuminatus under different initial sulfur supplies. Algal Res. 2020, 45, 101728. [Google Scholar] [CrossRef]
- Fattore, N.; Bellan, A.; Pedroletti, L.; Vitulo, N.; Morosinotto, T. Acclimation of photosynthesis and lipids biosynthesis to prolonged nitrogen and phosphorus limitation in Nannochloropsis gaditana. Algal Res. 2021, 58, 102368. [Google Scholar] [CrossRef]
- Şirin, P.A.; Serdar, S. Effects of nitrogen starvation on growth and biochemical composition of some microalgae species. Folia Microbiol. 2024, 69, 889–902. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological, and molecular mechanisms. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef] [PubMed]
- Perera-Castro, A.V.; Flexas, J. Desiccation tolerance in bryophytes relates to elasticity but is independent of cell wall thickness and photosynthesis. Physiol. Plant. 2022, 174, 13661. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, L.; Xu, H.; Zhu, Z.; Gao, X. Flexibility-rigidity coordination of the dense exopolysaccharide matrix in terrestrial cyanobacteria acclimated to periodic desiccation. Appl. Environ. Microb. 2017, 83, e01619-17. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Medwed, C.; Holzinger, A.; Hofer, S.; Hartmann, A.; Michalik, D.; Glaser, C.; Karsten, U. Ecophysiological, morphological, and biochemical traits of free-living Diplosphaera chodatii (Trebouxiophyceae) reveal adaptation to harsh environmental conditions. Protoplasma 2021, 258, 1187–1199. [Google Scholar] [CrossRef]
- Gao, B.; Huang, L.; Wang, F.; Zhang, C. Trachydiscus guangdongensis sp. nov., a new member of Eustigmatophyceae (Stramenopiles) isolated from China: Morphology, phylogeny, fatty acid profile, pigment, and cell wall composition. Hydrobiologia 2019, 835, 37–47. [Google Scholar] [CrossRef]
- She, Y.; Gao, X.; Jing, X.; Wang, J.; Dong, Y.; Cui, J.; Xue, H.; Li, Z.; Zhu, D. Effects of nitrogen source and NaCl stress on oil production in Vischeria sp. WL1 (Eustigmatophyceae) isolated from dryland biological soil crusts in China. J. Appl. Phycol. 2022, 34, 1281–1291. [Google Scholar] [CrossRef]
- Eliáš, M.; Amaral, R.; Fawley, K.P.; Fawley, M.W.; Němcová, Y.; Neustupa, J.; Přibyl, P.; Santos, L.M.A.; Ševčíková, T. Eustigmatophyceae. In Handbook of the Protists; Archibald, J., Simpson, A., Slamovits, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 367–406. [Google Scholar]
- Eslick, E.M.; Beilby, M.J.; Moon, A.R. A study of the native cell wall structures of the marine alga Ventricaria ventricosa (Siphonocladales, Chlorophyceae) using atomic force microscopy. Microscopy 2014, 63, 131–140. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-ray diffraction instrumentation and applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rongpipi, S.; Ye, D.; Gomez, E.D. Progress and opportunities in the characterization of cellulose—An Important regulator of cell wall growth and mechanic. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.; Abbas, L.; Coutinho, O.; Opara, S.; Najaf, H.; Kasperek, D.; Pokhrel, K.; Li, X.; Tiquia-Arashiro, S. Applications of Fourier Transform-Infrared spectroscopy in microbial cell biology and environmental microbiology: Advances, challenges, and future perspectives. Front. Microbiol. 2023, 14, 1304081. [Google Scholar]
- Yuan, X.; Gao, X.; She, Y.; Zheng, T.; Xue, H.; Liu, W. Investigations of solid culture–induced acquisition of desiccation tolerance in liquid suspension culture of Nostoc flagelliforme. J. Appl. Phycol. 2021, 33, 3657–3669. [Google Scholar] [CrossRef]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 6483. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohyd. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Renard, C.M.G.C.; Bourvellec, C.L.; Bureau, S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohyd. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Bourvellec, C.L. Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohyd. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef]
- Jung, P.; Briegel-Williams, L.; Simon, A.; Thyssen, A.; Büdel, B. Uncovering biological soil crusts: Carbon content and structure of intact Arctic, Antarctic and alpine biological soil crusts. Biogeosciences 2018, 15, 1149–1160. [Google Scholar] [CrossRef]
- Gao, X.; Liu, C.; Liang, W. Uncovering the unusual long chains of vegetative cells within single colonies of the dryland nitrogen-fixing cyanobacterium Nostoc flagelliforme. Nitrogen 2024, 5, 144–151. [Google Scholar] [CrossRef]
- Pelusi, A.; Ambrosino, L.; Miralto, M.; Chiusano, M.L.; Rogato, A.; Ferrante, M.I.; Montresor, M. Gene expression during the formation of resting spores induced by nitrogen starvation in the marine diatom Chaetoceros socialis. BMC Genom. 2023, 24, 106. [Google Scholar] [CrossRef] [PubMed]
- Beacham, T.A.; Bradley, C.; White, D.A.; Bond, P.; Ali, S.T. Lipid productivity and cell wall ultrastructure of six strains of Nannochloropsis: Implications for biofuel production and downstream processing. Algal Res. 2014, 6, 64–69. [Google Scholar] [CrossRef]
- Spain, O.; Funk, C. Detailed characterization of the cell wall structure and composition of Nordic green microalgae. J. Agric. Food Chem. 2022, 70, 9711–9721. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.C.; Coe, K.K.; Sparks, J.P.; Housman, D.C.; Zelikova, T.J.; Belnap, J. Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nat. Clim. Change 2012, 2, 752–755. [Google Scholar] [CrossRef]
- Zhang, T.A.; Chen, H.Y.H.; Ruan, H.H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Dou, W.; Xiao, B.; Delgado-Baquerizo, M.; Revillini, D.; Kidron, G.J. Dryland nitrogen deposition induces microbiome-driven increases in biocrust respiration and losses of soil carbon. Land Degrad. Dev. 2023, 35, 647–658. [Google Scholar] [CrossRef]



| Monosaccharides (mol %) | Glucose | Fucose | Mannose | Galactose |
|---|---|---|---|---|
| Nitrogen-replete cells | 0.702 | 0.153 | 0.088 | 0.057 |
| Nitrogen-starved cells | 0.827 | 0.095 | 0.057 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Gao, X.; She, Y.; Jing, X.; Yuan, X.; Zhu, D. Adaptation to Long-Term Nitrogen Starvation in a Biocrust-Derived Microalga Vischeria sp. WL1: Insights into Cell Wall Features and Desiccation Resistance. Microorganisms 2025, 13, 903. https://doi.org/10.3390/microorganisms13040903
Liang W, Gao X, She Y, Jing X, Yuan X, Zhu D. Adaptation to Long-Term Nitrogen Starvation in a Biocrust-Derived Microalga Vischeria sp. WL1: Insights into Cell Wall Features and Desiccation Resistance. Microorganisms. 2025; 13(4):903. https://doi.org/10.3390/microorganisms13040903
Chicago/Turabian StyleLiang, Wensheng, Xiang Gao, Yang She, Xin Jing, Xiaolong Yuan, and Derui Zhu. 2025. "Adaptation to Long-Term Nitrogen Starvation in a Biocrust-Derived Microalga Vischeria sp. WL1: Insights into Cell Wall Features and Desiccation Resistance" Microorganisms 13, no. 4: 903. https://doi.org/10.3390/microorganisms13040903
APA StyleLiang, W., Gao, X., She, Y., Jing, X., Yuan, X., & Zhu, D. (2025). Adaptation to Long-Term Nitrogen Starvation in a Biocrust-Derived Microalga Vischeria sp. WL1: Insights into Cell Wall Features and Desiccation Resistance. Microorganisms, 13(4), 903. https://doi.org/10.3390/microorganisms13040903







