Silver Nanoparticles and Antibiotics: A Promising Synergistic Approach to Multidrug-Resistant Infections
Abstract
:1. Introduction
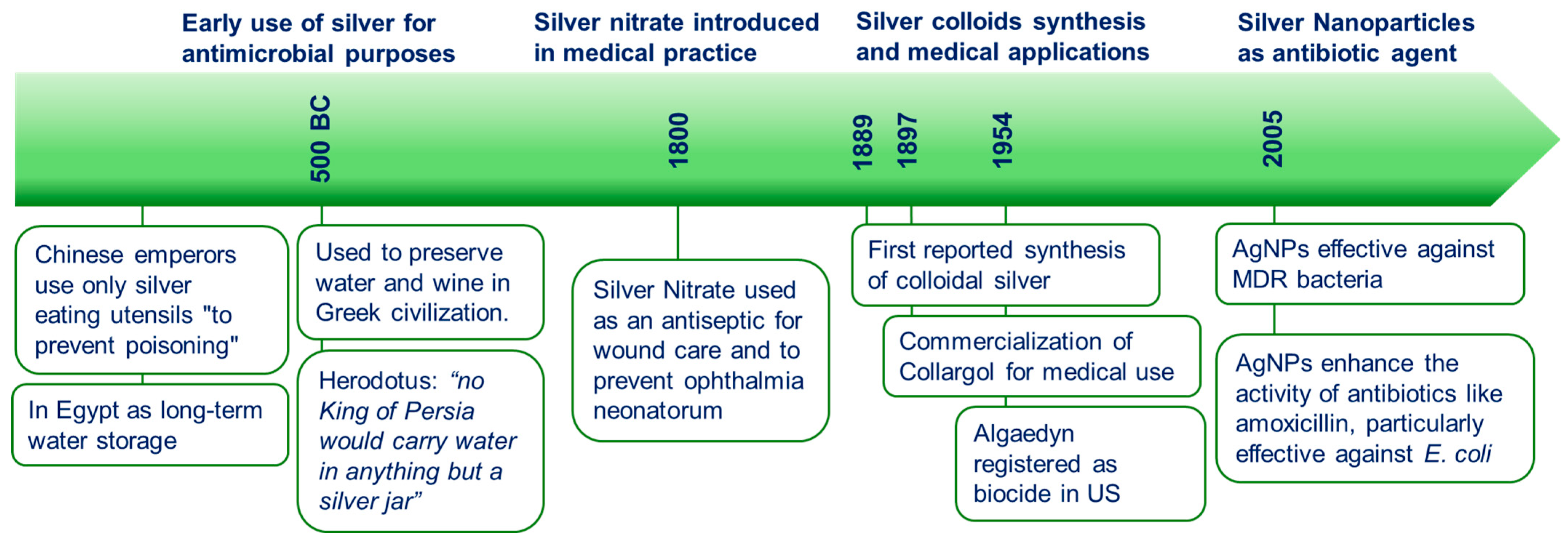
2. Silver Colloids as Antibiotics: An Historical Perspective
3. Mechanisms of Action and AgNPs Properties Influencing Antimicrobial Synergy
3.1. Mechanisms of Action of AgNPs and AgNPs-Based Antibiotic Therapies
3.2. Influence of AgNP Physicochemical Properties on Ion Release and Antimicrobial Synergy
4. Advances in the Combined Use of Silver and Antibiotics
4.1. Overview of Early Research on Silver–Antibiotic Synergy
4.2. Recent Advances in Silver and Antibiotic Combinations (2015–2025)
4.3. Restoring Antibiotic Susceptibility
4.4. Antifungal Effects
4.5. Biofilm Eradication
5. Conclusions and Future Perspectives
- The potential for resistance development against silver. While AgNPs exhibit broad-spectrum antimicrobial activity, prolonged exposure to sub-lethal concentrations may lead to bacterial adaptation and silver resistance. Studies have shown that bacteria exposed to AgNPs can develop increased tolerance, potentially affecting the efficacy of both silver-based and conventional antibiotic treatments. Mechanistically, silver resistance has been linked to genetic determinants such as the sil operon, which encodes proteins involved in silver ion efflux (SilP, SilCBA) and periplasmic sequestration (SilE), thereby reducing intracellular Ag+ accumulation [82,83]. Additionally, endogenous efflux systems like SilCFBA, as well as CusCFBA (originally characterized for copper), have been implicated in silver detoxification [82,84].Biofilm-mediated tolerance also contributes to resistance by limiting the penetration of AgNPs and silver ions through the extracellular polymeric matrix and mitigating oxidative stress via localized redox buffering. Furthermore, horizontal gene transfer and co-selection with antibiotic resistance genes may accelerate the spread of silver resistance, especially in clinical and environmental settings. These findings underscore the need for prudent AgNP use and the development of dosing strategies that minimize resistance development while preserving therapeutic efficacy. To mitigate all these risks, future research should focus on optimizing dosing strategies, elucidating resistance mechanisms at the molecular level, and designing AgNP formulations that minimize resistance selection while maximizing synergy with antibiotics.
- Scalable Production. As with many other nanomaterials, the clinical translation of AgNP-antibiotic therapies requires regulatory approval, which demands adherence to Good Manufacturing Practice (GMP) standards. Challenges such as ensuring batch consistency, characterizing physicochemical properties, and sourcing GMP-compliant reagents must be addressed. Streamlining production protocols and establishing standardized evaluation frameworks will be crucial for advancing AgNP-based therapeutics to clinical application.
- Regulatory approval. One significant limitation for the clinical development of AgNP-based therapies is the current lack of harmonized regulatory frameworks and well-defined cytotoxicity thresholds for human applications. Despite increasing evidence of AgNPs’ antimicrobial potential, their physicochemical variability leads to inconsistent toxicity profiles, complicating risk assessment and regulatory approval. Although different studies suggest that concentrations below 10 µg/mL are safe for human cells, the variability in formulations and testing approaches remains a significant challenge in establishing universal safety limits. Moreover, stabilizing agents used to ensure colloidal stability may themselves contribute to cytotoxicity or immunogenicity. To address these challenges, it is essential to implement standardized evaluation protocols for AgNP formulations, including in vitro and in vivo toxicity screening under conditions that mimic physiological environments. Establishing universal parameters, such as maximum tolerable dose, exposure limits, and biocompatibility standards, would enable more consistent safety assessments. Surface engineering approaches that use biocompatible, FDA-approved coatings (e.g., PEG, albumin, or phospholipid layers) can reduce toxicity while maintaining stability and efficacy.
- Long-Term Toxicity, Organ Accumulation. Beyond acute cytotoxicity, the long-term safety profile of NPs, including AgNPs, remains an area of concern. Multiple studies have shown that systemically administered NPs accumulate mainly in the liver and spleen, and much less in kidneys and lungs, with retention times influenced by NP size, surface coating, and administration route. Persistent exposure may lead to oxidative stress, inflammatory responses, or subtle immunomodulatory effects, particularly with non-degradable coatings. Although some formulations appear to be well tolerated in animal models, comprehensive long-term studies are still lacking.
- Variability in reported synergistic effects. Factors such as nanoparticle size, surface chemistry, and the choice of antibiotic play a critical role in determining synergistic effects. Optimizing these parameters will not only enable the development of tailored AgNP formulations but also enhance the interpretation of findings, contributing to the advancement of the field.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sir Alexander Fleming—Nobel Lecture. NobelPrize.org. Nobel Prize Outreach 2025. Available online: https://www.nobelprize.org/prizes/medicine/1945/fleming/lecture/ (accessed on 19 March 2025).
- World Health Organization. The Evolving Threat of Antimicrobial Resistance: Options for Action; World Health Organization: Geneva, Switzerland, 2012; Available online: https://iris.who.int/bitstream/handle/10665/44812/9789241503181_eng.pdf;jsessionid=BB2065300EFF26DF8B37D91247DC6E58?sequence=1 (accessed on 19 March 2025).
- Bäumler, A.J. The coming microbial crisis: Our antibiotic bubble is about to burst. Science 2024, 385, eads3473. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Poudel, A.N.; Zhu, S.; Cooper, N.; Little, P.; Tarrant, C.; Hickman, M.; Yao, G. The economic burden of antibiotic resistance: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0285170. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Najafpour, Z.; Cheraghian, B.; Keliddar, I.; Mombeyni, R. The Extra Length of Stay, Costs, and Mortality Associated With Healthcare-Associated Infections: A Case-Control Study. Health Sci. Rep. 2024, 7, e70168. [Google Scholar] [CrossRef]
- Shambhu, S.; Gordon, A.S.; Liu, Y.; Pany, M.; Padula, W.V.; Pronovost, P.J.; Hsu, E. The Burden of Health Care Utilization, Cost, and Mortality Associated with Select Surgical Site Infections. Jt. Comm. J. Qual. Patient Saf. 2024, 50, 857–866. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Rello, J.; Bunsow, E.; Perez, A. What if there were no new antibiotics? A look at alternatives. Expert Rev. Clin. Pharmacol. 2016, 9, 1547–1555. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Hickson, S.M.; Ledger, E.L.; Wells, T.J. Emerging antimicrobial therapies for Gram-negative infections in human clinical use. npj Antimicrob. Resist. 2025, 3, 16. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Antimicrobial Peptides Therapy: An Emerging Alternative for Treating Drug-Resistant Bacteria. Yale J. Biol. Med. 2022, 95, 445–463. [Google Scholar] [PubMed]
- Available online: https://www.nano.gov/sites/default/files/pub_resource/nni_implementation_plan_2000.pdf (accessed on 19 March 2025).
- European Commission. Directorate-General for Health and Consumers, Opininon on Nanosilver: Safety, Health and Environmental Effects and Role in Antimicrobial Resistance, European Commission. 2014. Available online: https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_039.pdf (accessed on 19 March 2025).
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic Study of the Synergistic Antibacterial Activity of Combined Silver Nanoparticles and Common Antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver Enhances Antibiotic Activity Against Gram-Negative Bacteria. Sci. Transl. Med. 2013, 5, 190ra181. [Google Scholar] [CrossRef]
- Maklakova, M.; Villarreal-Gómez, L.J.; Nefedova, E.; Shkil, N.; Luna Vázquez-Gómez, R.; Pestryakov, A.; Bogdanchikova, N. Potential Antibiotic Resurgence: Consecutive Silver Nanoparticle Applications Gradually Increase Bacterial Susceptibility to Antibiotics. ACS Omega 2025, 10, 4624–4635. [Google Scholar] [CrossRef]
- Hochvaldová, L.; Panáček, D.; Válková, L.; Prucek, R.; Kohlová, V.; Večeřová, R.; Kolář, M.; Kvítek, L.; Panáček, A. Restoration of antibacterial activity of inactive antibiotics via combined treatment with a cyanographene/Ag nanohybrid. Sci. Rep. 2022, 12, 5222. [Google Scholar] [CrossRef]
- Dove, A.S.; Dzurny, D.I.; Dees, W.R.; Qin, N.; Nunez Rodriguez, C.C.; Alt, L.A.; Ellward, G.L.; Best, J.A.; Rudawski, N.G.; Fujii, K.; et al. Silver nanoparticles enhance the efficacy of aminoglycosides against antibiotic-resistant bacteria. Front. Microbiol. 2023, 13, 1064095. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Palau, M.; Muñoz, E.; Gusta, M.F.; Larrosa, N.; Gomis, X.; Gilabert, J.; Almirante, B.; Puntes, V.; Texidó, R.; Gavaldà, J. In Vitro Antibacterial Activity of Silver Nanoparticles Conjugated with Amikacin and Combined with Hyperthermia against Drug-Resistant and Biofilm-Producing Strains. Microbiol. Spectr. 2023, 11, e0028023. [Google Scholar] [CrossRef]
- Kędziora, A.; Wieczorek, R.; Speruda, M.; Matolínová, I.; Goszczyński, T.M.; Litwin, I.; Matolín, V.; Bugla-Płoskońska, G. Comparison of Antibacterial Mode of Action of Silver Ions and Silver Nanoformulations With Different Physico-Chemical Properties: Experimental and Computational Studies. Front. Microbiol. 2021, 12, 659614. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.; Skiba-Kurek, I.; Mrowiec, P.; Karczewska, E.; Drożdż, R. Synergistic ROS-Associated Antimicrobial Activity of Silver Nanoparticles and Gentamicin Against Staphylococcus epidermidis. Int. J. Nanomed. 2020, 15, 3551–3562. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.Y.; Kim, J.; Lee, J.H.; Hahn, J.S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Cano Aristizábal, V.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, M.A.; Viviana, C.A.; Onnainty, R.; Mary, V.S.; Theumer, M.G.; Granero, G.E.; Paraje, M.G.; Páez, P.L. Biosynthesized silver nanoparticles: Decoding their mechanism of action in Staphylococcus aureus and Escherichia coli. Int. J. Biochem. Cell Biol. 2018, 104, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Marchioni, M.; Gallon, T.; Worms, I.; Jouneau, P.-H.; Lebrun, C.; Veronesi, G.; Boutry, D.; Mintz, E.; Delangle, P.; Deniaud, A.; et al. Insights into polythiol-assisted AgNP dissolution induced by bio-relevant molecules. Environ. Sci. Nano 2018, 5, 1911–1920. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Hossain, Z.; Huq, F. Studies on the interaction between Ag+ and DNA. J. Inorg. Biochem. 2002, 91, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Hoz, A.d.l.; Navarro, A.; Aviñó, A.; Eritja, R.; Gargallo, R. Studies on the interactions of Ag(i) with DNA and their implication on the DNA-templated synthesis of silver nanoclusters and on the interaction with complementary DNA and RNA sequences. RSC Adv. 2021, 11, 9029–9042. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- ‘Sleeping Beauties’: Yesterday’s Findings Fuel Today’s Research Breakthroug. Available online: https://clarivate.com/blog/sleeping-beauties-yesterdays-findings-fuel-todays-research-breakthroughs/ (accessed on 16 September 2024).
- Casals, E.; Vitali, M.; Puntes, V. The nanoparticle-Protein Corona untold history (1907–2007). Nano Today 2024, 58, 102435. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.C. Properties of allotropic silver. Am. J. Sci. 1889, 3, 237–240. [Google Scholar] [CrossRef]
- Nowack, B.; Krug, H.F.; Height, M. 120 Years of Nanosilver History: Implications for Policy Makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef]
- Chambers, C.W.; Chambers, C.A.; Kabler, P. New Colloidal Silver Disinfectant. Ind. Eng. Chem. 1953, 45, 2569–2571. [Google Scholar] [CrossRef]
- Frens, G.; Overbeek, J.T.G. Carey Lea’s colloidal silver. Kolloid-Z. Z. Polym. 1969, 233, 922–929. [Google Scholar] [CrossRef]
- Hoffman, R.K.; Surkiewicz, B.F.; Chambers, L.A.; Chambers, C.R. Bactericidal Action of Movidyn. Ind. Eng. Chem. 1953, 45, 2571–2573. [Google Scholar] [CrossRef]
- Moudry, Z.V. Process of Producing Oligodynamic Metal Biocides. U.S. Patent No. 2,927,052, 1 March 1953. [Google Scholar]
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 2005, 16, 1912. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in silver nanoparticles: A comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Malawong, S.; Thammawithan, S.; Sirithongsuk, P.; Daduang, S.; Klaynongsruang, S.; Wong, P.T.; Patramanon, R. Silver Nanoparticles Enhance Antimicrobial Efficacy of Antibiotics and Restore That Efficacy against the Melioidosis Pathogen. Antibiotics 2021, 10, 839. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef]
- O’Toole, A.; Ricker, E.B.; Nuxoll, E. Thermal mitigation of Pseudomonas aeruginosa biofilms. Biofouling 2015, 31, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Ershov, B.; Ershov, V. Electrochemical Mechanism of Oxidative Dissolution of Silver Nanoparticles in Water: Effect of Size on Electrode Potential and Solubility. Nanomaterials 2023, 13, 1907. [Google Scholar] [CrossRef]
- Ershov, V.A.; Ershov, B.G. Effect of Silver Nanoparticle Size on Antibacterial Activity. Toxics 2024, 12, 801. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Derakhshi, M.; Ashkarran, A.A.; Bahari, A.; Bonakdar, S. Shape selective silver nanostructures decorated amine-functionalized graphene: A promising antibacterial platform. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 101–109. [Google Scholar] [CrossRef]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 739–749. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Ershov, V.; Tarasova, N.; Abkhalimov, E.; Safonov, A.; Sorokin, V.; Ershov, B. Photochemical Synthesis of Silver Hydrosol Stabilized by Carbonate Ions and Study of Its Bactericidal Impact on Escherichia coli: Direct and Indirect Effects. Int. J. Mol. Sci. 2022, 23, 949. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Gusta, M.F.; Piella, J.; Casals, G.; Jiménez, W.; Puntes, V. Intrinsic and Extrinsic Properties Affecting Innate Immune Responses to Nanoparticles: The Case of Cerium Oxide. Front. Immunol. 2017, 8, 970. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar] [CrossRef]
- Herisse, M.; Duverger, Y.; Martin-Verstraete, I.; Barras, F.; Ezraty, B. Silver potentiates aminoglycoside toxicity by enhancing their uptake. Mol. Microbiol. 2017, 105, 115–126. [Google Scholar] [CrossRef]
- Khleifat, K.; Qaralleh, H.; Al-Limoun, M.; Alqaraleh, M.; Abu-Hajleh, M.; Al-Frouhk, R.; Al-Omari, L.; Al-Buqain, R.; Dmour, S. Antibacterial Activity of Silver Nanoparticles Synthesized by Aspergillus flavus and its Synergistic Effect with Antibiotics. J. Pure Appl. Microbiol. 2022, 16, 1722–1735. [Google Scholar] [CrossRef]
- Khaled, J.M.; Alharbi, N.S.; Siddiqi, M.Z.; Alobaidi, A.S.; Nauman, K.; Alahmedi, S.; Almazyed, A.O.; Almosallam, M.A.; Al Jurayyan, A.N. A synergic action of colistin, imipenem, and silver nanoparticles against pandrug-resistant Acinetobacter baumannii isolated from patients. J. Infect. Public Health 2021, 14, 1679–1685. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Wang, M.; Liu, C.; Koohi-Moghadam, M.; Wang, H.; Ho, P.L.; Li, H.; Sun, H. Re-sensitization of mcr carrying multidrug resistant bacteria to colistin by silver. Proc. Natl. Acad. Sci. USA 2022, 119, e2119417119. [Google Scholar] [CrossRef]
- Leonhard, V.; Alasino, R.V.; Munoz, A.; Beltramo, D.M. Silver Nanoparticles with High Loading Capacity of Amphotericin B: Characterization, Bactericidal and Antifungal Effects. Curr. Drug Deliv. 2018, 15, 850–859. [Google Scholar] [CrossRef]
- Tawre, M.S.; Shiledar, A.; Satpute, S.K.; Ahire, K.; Ghosh, S.; Pardesi, K. Synergistic and antibiofilm potential of Curcuma aromatica derived silver nanoparticles in combination with antibiotics against multidrug-resistant pathogens. Front. Chem. 2022, 10, 1029056. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, B.; Liu, R.; Dong, Y.; Zhao, Y.; Wu, Y. Development of pH-responsive nanocomposites with remarkably synergistic antibiofilm activities based on ultrasmall silver nanoparticles in combination with aminoglycoside antibiotics. Colloids Surf. B Biointerfaces 2021, 208, 112112. [Google Scholar] [CrossRef] [PubMed]
- Wali, N.; Shabbir, A.; Wajid, N.; Abbas, N.; Naqvi, S.Z.H. Synergistic efficacy of colistin and silver nanoparticles impregnated human amniotic membrane in a burn wound infected rat model. Sci. Rep. 2022, 12, 6414. [Google Scholar] [CrossRef]
- Kadirvelu, L.; Sivaramalingam, S.S.; Jothivel, D.; Chithiraiselvan, D.D.; Karaiyagowder Govindarajan, D.; Kandaswamy, K. A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants. Curr. Res. Microb. Sci. 2024, 6, 100231. [Google Scholar] [CrossRef]
- Feizi, S.; Cooksley, C.M.; Nepal, R.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Silver nanoparticles as a bioadjuvant of antibiotics against biofilm-mediated infections with methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in chronic rhinosinusitis patients. Pathology 2022, 54, 453–459. [Google Scholar] [CrossRef]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Min, C.; Xia, F.; Tang, M.; Li, J.; Hu, Y.; Zou, M. Characterization of Silver Resistance and Coexistence of sil Operon with Antibiotic Resistance Genes Among Gram-Negative Pathogens Isolated from Wound Samples by Using Whole-Genome Sequencing. Infect. Drug Resist. 2022, 15, 1425–1437. [Google Scholar] [CrossRef]
- Andrade, L.N.; Siqueira, T.E.S.; Martinez, R.; Darini, A.L.C. Multidrug-Resistant CTX-M-(15,9,2)- and KPC-2-Producing Enterobacter hormaechei and Enterobacter asburiae Isolates Possessed a Set of Acquired Heavy Metal Tolerance Genes Including a Chromosomal sil Operon (for Acquired Silver Resistance). Front. Microbiol. 2018, 9, 539. [Google Scholar] [CrossRef]

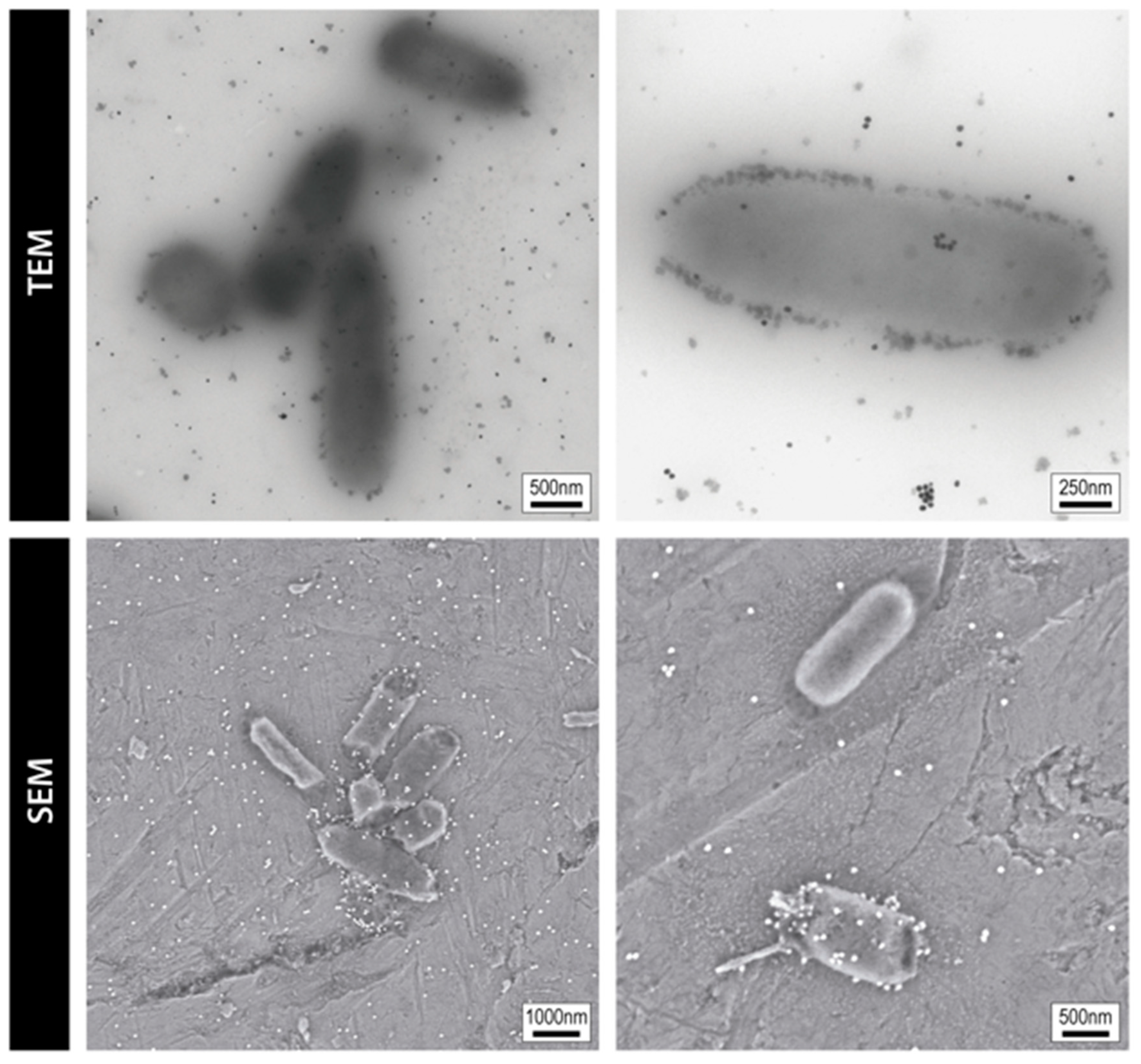
| Type | Members | Characteristics | Mode of Action | Spectrum |
|---|---|---|---|---|
| β-Lactams | Penicillins, Cephalosporins, Carbapenems, Monobactams | β-lactam ring | Inhibits cell wall synthesis (peptidoglycan) | Gram-positive, some Gram-negative |
| Aminoglycosides | Kanamycin, Streptomycin, Gentamicin, Neomycin, Amikacin, Netilmicin, Tobramycin | Amino sugars linked together | Inhibits protein synthesis (30S ribosomal subunit) | Gram-negative |
| Tetracyclines | Tetracycline, Oxytetracycline, Chlortetracycline, Doxycycline, Minocycline | Naphthalene tetracyclic structure (four rings) | Inhibits protein synthesis (30S ribosomal subunit) | Gram-positive and Gram-negative |
| Macrolides | Erythromycin, Oleandomycin, Azithromycin, Clarithromycin, Josamycin, Telithromycin | Large macrolactone rings with aminated sugars | Inhibits protein synthesis (50S ribosomal subunit) | Gram-negative |
| Chloramphenicol | — | Synthesized in the laboratory | Inhibits protein synthesis (50S ribosomal subunit) | Gram-positive and Gram-negative |
| Rifamycins | Rifampin, Rifabutin | — | Inhibits RNA synthesis (RNA polymerase) | Gram-positive and Gram-bacteria, Tuberculosis |
| Sulfonamides | Sulfacetamide, Silver sulfadiazine | Chemotherapeutic, synthesized in the laboratory | Inhibits PABA (folic acid) synthesis | Gram-positive and Gram-negative |
| Quinolones | Nalidixic acid, Ciprofloxacin, Moxifloxacin, Levofloxacin, Ofloxacin | Synthesized in the laboratory | Inhibits DNA replication | Gram-positive and Gram-negative |
| Polypeptides | Bacitracin, Colistin, Polymyxin B | — | Inhibits cell wall synthesis (peptidoglycan) and alters plasma membrane permeability | Gram-positive and Gram-negative |
| Property | Effect on Ag+ Ion Release | Impact on Antimicrobial Synergy |
|---|---|---|
| Size | Smaller size increases surface area and dissolution rate | Enhances Ag+ availability; boosts antibiotic penetration and efficacy |
| Shape | Triangular/cubic shapes expose high-energy facets (e.g., {111}) | Higher Ag+ release and membrane interaction; shape-dependent synergy |
| Surface charge | Positive or mildly negative charge enhances bacterial membrane binding | Increases local NP and Ag+ accumulation; facilitates antibiotic uptake |
| Surface coating | Functional groups (e.g., citrate, PEG) modulate ion release | Affects colloidal stability and pharmacokinetics of NP-antibiotic conjugates |
| Aggregation state | Aggregated NPs release fewer ions due to lower exposed surface | Reduced efficacy in biological fluids and biofilms |
| Antibiotic Class | Antibiotic Combined with AgNPs | Bacterial Strains | Results | Author-Year |
|---|---|---|---|---|
| β-lactam | Amoxicillin | E. coli | AgNPs enhanced antibacterial activity; 0.150 mg/mL amoxicillin and 5 µg/mL AgNPs showed same effect as higher doses | Li et al. [49] |
| β-lactam, Macrolide | Piperacillin, Erythromycin, Chloramphenicol, Vancomycin | A. baumannii, P. aeruginosa | 3.6-fold enhancement with piperacillin, 4.9-fold with chloramphenicol, 4.2-fold with vancomycin | Gosh et al. [71] |
| β-lactam, Macrolide | Penicillin G, Amoxicillin, Erythromycin, Clindamycin, Vancomycin | E. coli, S.aureus | Increased zone of inhibition, strongest with Vancomycin, Amoxicillin, Penicillin G | Shahverdi et al. [63] |
| β-lactam, Aminoglycoside | Ceftazidime, Imipenem, Meropenem, Gentamicin | e | Gentamicin showed highest enhancement | Malawong et al. [55] |
| β-lactam, Macrolide, Aminoglycoside | Ampicillin, Kanamycin, Erythromycin, Chloramphenicol | S.aureus, B. subtilis, E. coli, P. aeruginosa | Ampicillin showed the strongest synergistic effect | Fayaz et al. [70] |
| β-lactam, Quinolone, Aminoglycoside | Ampicillin, Ofloxacin, Gentamicin, Vancomycin | E. coli | Silver enhances ROS production, increases membrane permeability, and restores antibiotic efficacy | Morones et al. [21] |
| β-lactam, Tetracycline, Aminoglycoside | Ampicillin, Penicillin, Enoxacin, Kanamycin, Neomycin, Tetracycline | Salmonella Typhimurium | Tetracycline formed Ag complexes, enhanced Ag+ release | Deng et al. [17] |
| β-lactam, Aminoglycoside, Macrolide, Fluoroquinolone | Ampicillin, Erythromycin, Ceftriaxone, Vancomycin, Azlocillin, Amoxicillin, Clindamycin, Aztreonam, Ciprofloxacin | S.aureus, P. aeruginosa, E. cloacae, E.e, Shigella sp. | Up to 31-fold enhancement in antimicrobial efficacy | Khleifat et al. [73] |
| Aminoglycoside | Aminoglycosides | E. coli, P. aeruginosa, A. baumannii, S.aureus (methicillin-resistant) | 22-fold reduction in MIC with AgNPs | Dove et al. [24] |
| Aminoglycoside | Amikacin | A. baumannii, E.e. pneumoniae, P. aeruginosa | 10× more effective than amikacin alone | Palau et al. [26] |
| Aminoglycoside | Kanamycin | S.aureus, S. pneumoniae, P. aeruginosa, E. coli BL21 | Enhanced antimicrobial activity, on-demand drug release | Li et al. [78] |
| Aminoglycoside, Tetracycline | Gentamicin, Kanamycin, Tobramycin, Streptomycin, Spectinomycin, Tetracycline | E. coli K12, C. difficile | Silver enhanced efficacy by 10-fold or more for aminoglycosides | Herisse et al. [72] |
| Polymyxin | Colistin | E. coli (mcr-1 positive) | AgNPs restored susceptibility to colistin | Zhang et al. [75] |
| Polymyxin | Colistin | Orthopedic implants or catheters | Prevention of implant-related infections and biofilm formation | Kadirvelu et al. [80] |
| Polymyxin | Colistin | P. aeruginosa, K. pneumoniae | Enhanced antimicrobial activity, faster wound healing | Wali et al. [79] |
| Curcuma aromatica-derived AgNPs | P. aeruginosa, S.aureus | Inhibited biofilm formation and bacterial growth | Tawre et al. [77] | |
| Various antibiotics | Multidrug-resistant bacteria | broad antimicrobial activity | Feizi et al. [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casals, E.; Gusta, M.F.; Bastus, N.; Rello, J.; Puntes, V. Silver Nanoparticles and Antibiotics: A Promising Synergistic Approach to Multidrug-Resistant Infections. Microorganisms 2025, 13, 952. https://doi.org/10.3390/microorganisms13040952
Casals E, Gusta MF, Bastus N, Rello J, Puntes V. Silver Nanoparticles and Antibiotics: A Promising Synergistic Approach to Multidrug-Resistant Infections. Microorganisms. 2025; 13(4):952. https://doi.org/10.3390/microorganisms13040952
Chicago/Turabian StyleCasals, Eudald, Muriel F. Gusta, Neus Bastus, Jordi Rello, and Victor Puntes. 2025. "Silver Nanoparticles and Antibiotics: A Promising Synergistic Approach to Multidrug-Resistant Infections" Microorganisms 13, no. 4: 952. https://doi.org/10.3390/microorganisms13040952
APA StyleCasals, E., Gusta, M. F., Bastus, N., Rello, J., & Puntes, V. (2025). Silver Nanoparticles and Antibiotics: A Promising Synergistic Approach to Multidrug-Resistant Infections. Microorganisms, 13(4), 952. https://doi.org/10.3390/microorganisms13040952







