Abstract
Shiga toxin-producing Escherichia coli are important foodborne pathogens. There are several subtypes of the Shiga toxin Stx known, with Stx2 (a–o) being more diverse than Stx1 (a, c, d). Multiple occurrences of stx2 genes as well as combinations of stx1 and stx2 have been reported. However, there is a lack of knowledge on the occurrence of multiple stx1 genes in STEC strains. Here, we report two strains from food and animal feces which show genomic variations in the stx1 operon. The first strain harbors stx1a and stx1c genes, and the second strain shows an inactive stx1 operon due to an insertion in the stxA1a subunit gene. The screening of publicly available complete genome sequences of STEC revealed further strains harboring multiple stx1 genes, indicating that those strains also occur in human infections. This should be kept in mind when applying routine diagnostic methods like PCR, that do not detect multiple occurrences of stx1 genes of the same subtype. Moreover, the impact on the severity of human infections due to multiple stx1 genes has not been investigated well.
1. Introduction
Shiga toxin-producing Escherichia coli are important foodborne pathogens worldwide. They are naturally occurring in the gastrointestinal tracts of cattle and small and wild ruminants, as well as other mammals, fish, birds and some insects [1], and can cause diarrhea and hemolytic uremic syndrome (HUS) in humans [2]. The main virulence factor—the Stx toxin—is an AB5 toxin which is encoded by the stxA and stxB subunit genes [3]. Currently, there are 3 subtypes reported for Stx1 toxins (a, c, d) and 15 subtypes are known for Stx2 toxins (a–o) [4,5,6,7], showing a much higher diversity in stx2 genes than in stx1 genes. The occurrence of multiple stx2 genes has previously been reported in studies on STEC [8]. Also, the combination of stx1 and stx2 genes in one strain has been clearly proven [9]. In contrast, the occurrence of multiple stx1 genes in STEC has not been well investigated and has not been explicitly mentioned in STEC studies to the best of our knowledge. The combination of stx1 genes is also not included in risk analyses on public health risks based on the occurrence of stx subtypes and further virulence genes [9]. When it comes to genomic variation or inactivation in stx genes, the inactivation of stx2 genes via the insertion of transposases into the subunit genes has previously been reported [10,11,12]. However, similar events have not been specifically described for stx1 genes before.
Here, we analyzed two STEC strains which were isolated from food and animal feces in Germany, showing genomic events in terms of stx1 gene combination and genomic variation. We also analyzed sequence data from public repositories concerning further occurrences of multiple stx1 combinations in STEC strains.
2. Materials and Methods
The two STEC strains were isolated by Federal state laboratories from food and animal source programs according to DIN CEN ISO/TS13136:2013-04 [13] in the framework of German food control and Zoonosis monitoring. Strain BfR-EC-20006 (CVUAS 31424.2) was isolated from roe deer meat in 2023 using modified Tryptic Soy Broth with Novobiocin (Merck KGaA, Darmstadt, Germany). Strain BfR-EC-18115 (LVI OL 10-12119-00272) was isolated from a feces sample of a c. eight-month-old cow, taken at a slaughterhouse in 2019. Isolation was performed using buffered peptone water. The strains were characterized at the National Reference Laboratory for Escherichia coli including VTEC (NRL-E. coli) and the National Study Centre for Sequencing in Risk Assessment using short-read and long-read genomic sequencing. For this purpose, whole genomic DNA was isolated using the PureLink® Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturers’ instructions.
The libraries for short-read sequencing were prepared using the llumina DNA Prep, (M) Tagmentation kit (Illumina, San Diego, CA, USA). The libraries were sequenced on the Illumina MiSeq benchtop sequencer using the MiSeq Reagent Kit v3 (600 cycles; Illumina) in 2 × 201 bp cycles or the NextSeq500 benchtop sequencer using the NextSeq 500/550 Mid Output Kit v2.5 (300 Cycles) in 2 × 151 bp cycles (Illumina, San Diego, CA, USA). For long-read sequencing, genomic DNA was subjected to an additional purification step using Ampure XP (Beckman Coulter GmbH, Krefeld; Germany). DNA was adjusted to 2000 ng in 100 µL with ultrapure water in a 1.5 mL reaction tube. The beads were added to the samples (0.45 times the sample volume), mixed by flicking, and briefly centrifuged. The samples were incubated for 5 min at room temperature. The tubes were placed on a magnetic stand, and the supernatant was discarded. The bead pellets were washed twice with 80% ethanol. Remaining ethanol was completely removed and the pellet was dried for 30 s. Elution was performed in 16 µL 10 mM Tris-HCl pH 8.0 for 15 min at 37 °C. For long-read sequencing, library preparation was carried out using the Rapid Barcoding Kit 96 V14 (Oxford Nanopore Technologies, Oxford, UK). Sequencing was performed on a MinIon Mk1C device using a R10.4.1 flow cell (Oxford Nanopore Technologies, Oxford, UK). Signal data (fast5) were basecalled using guppy v6.4.8 in SUP mode. Long-read data were assembled using the MiLongA pipeline (https://gitlab.com/bfr_bioinformatics/milonga). There, reads were trimmed and filtered using porechop v0.2.4 (https://github.com/rrwick/Porechop) and nanofilt v2.8.0 [14]. Subsequently, fastq data were subjected to assembly using flye v2.9 [15]. Resulting assemblies were polished with Illumina short-read data using pilon v1.24 [16].
For the investigation of public data concerning multiple occurrences of stx1 genes, all available complete Escherichia coli genomes from NCBI RefSeq (https://www.ncbi.nlm.nih.gov/refseq/, accessed on 6 March 2025) [17] were retrieved. The presence of stx genes was determined using abricate (https://github.com/tseemann/abricate, with mincov = 50, minid = 80) against a custom database of full-length stx genes. Likewise, complete NCBI Genbank genomes were also scanned. Genomes were selected that carried two or more (nearly) complete stx genes.
Strain characterization was performed using BakCharak (https://gitlab.com/bfr_bioinformatics/bakcharak), which implements the determination of the genoserotype based on the CGE SeroTypeFinder [18] and the EcOH database [19] as well as the MLST typing tool mlst (https://github.com/tseemann/mlst), the virulence finder database [20] and AMRFinderPlus v4.0 [21]. Sequence annotation was performed using bakta [22]. Sequence comparisons of stx genes with respective reference genes were carried out with Geneious Prime® (version 2024.0.7., Biomatters Ltd., Auckland, New Zealand). The flanking inverted repeats of the transposon were identified using ISFinder (https://isfinder.biotoul.fr/) and the stx1 phage integration sites were determined using PHASTEST (https://phastest.ca).
3. Results
3.1. STEC Strains from Food and Livestock in Germany
STEC strains were isolated from food and food-producing animals in the framework of German food control and the Zoonosis monitoring programs (Table 1).

Table 1.
STEC strains from food.
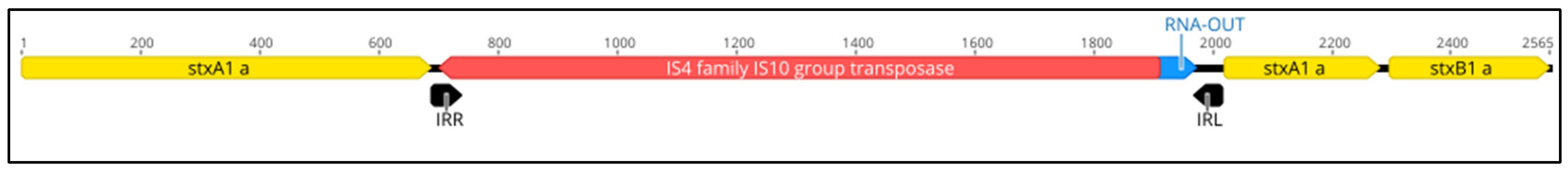
Strain BfR-EC-20006 (CVUAS 31424.2) was isolated from the meat of deer and was assigned as genoserotype O128:H2. For this strain, two different stx1 subtype genes, namely stx1a and stx1c, were detected. Strain BfR-EC-18115 (LVI OL 10-12119-00272) was isolated from cattle feces and was determined as O150:H2. This strain harbors an inactive form of the stx1a operon due to an insertion element in the stxA1a subunit (Figure 1) but also an additional stx2a gene.

Figure 1.
Genomic region of the stx1a operon of strain BfR-EC-18115 showing the integration of an insertion element into the stxA1a subunit gene (yellow: stx1a subunit genes, red: transposase, blue: non-coding RNA, black: inverted repeats, numbers represent base pairs [bp]).
3.2. Sequence Data from Public Repositories
We found indications that stx1a and stx1c can occur in duplicate or even triplicate (Table 2). Moreover, stx1a frequently occurs together with stx2a or stx2c (see Supplementary Table S1). However, only seven genomes could be identified harboring multiple stx1 genes. All of the seven publicly available strains were reported to be isolated from humans between 2008 and 2023 in different world regions. They were associated with different serotypes and MLST and frequently harbored additional virulence factors such as eae, nleB and ehxA.

Table 2.
Overview of STEC strain characteristics derived from public NCBI data.
Strains 180-PT54 and 644-PT8 were sequenced in the frame of an outbreak investigation and were characterized concerning short-term evolution but were not investigated concerning the duplication of stx1 genes [23]. Strains 2013C-3033 and 2013C-4974 were published within a study on PacBio sequencing of STEC [24]. Strain PV0838 was investigated within a study on STEC O26:H11 strains and the authors mentioned the duplication of the stx1a genes and also investigated the respective integrations sites of both phages [25]. Strain PNUSAE145590 (BioSample: SAMN36828866) was isolated during an outbreak which was associated with municipal irrigation water, but the investigators only reported on the serotype O157:H7 and not on the stx genes [26]. All of the public strain sequences harbored multiple genes of the same stx1 subtype. Therefore, strains were also checked for variations within these genes (Table 3). It turned out that strains 180-PT54 and 644-PT8 had different variants of the same stx1 subtype, whereas 2013C-3033, 2013C-4974, PV0838 and PNUSAE145590 harbored identical alleles multiple times in the genomes. In all cases, the locations of the different stx genes were separated by more than 100 kbp. In the case of PNUSAE145590, the second stx1a gene was localized on another contig, which was most likely circularly assembled phage DNA instead of a plasmid.

Table 3.
Comparison of stx1 allele sequences of public data (stx1 duplicated variants only).
4. Discussion
STECs are important food-borne pathogens showing high diversity in terms of stx gene content in their genomes. In recent years, several new subtypes have been identified for Stx2 toxins [4,5,6,7], whereas for Stx1, only three have been discovered in E. coli so far. This indicates a much higher frequency of genomic rearrangement including certain recombination and mutation events in stx2 genes than in stx1 genes.
Risk analysis studies revealed that infections with STEC harboring certain subtypes like stx2a, stx2d and stx2c, especially in combination with the virulence gene eae, are more likely to lead to severe symptoms [9]. Therefore, more research is being conducted and efforts are being invested in studying stx2 variants to gain a better understanding and improve diagnostics. It turned out that other STECs can also be a threat to human health [7,27]. STECs in general can cause a wide variation in symptoms in humans, ranging from no or only mild symptoms to HUS, kidney failure and also death [2].
Routine diagnosis using PCR methods is able to detect a certain stx gene or stx subtype. However, it is not possible to provide information on the number of genes of a particular stx subtype in a single strain. With the increasing use of next generation sequencing (NGS) in bacterial analyses, STEC strains can also be characterized in more detail, including the detection of multiple occurrences of genes of the same stx subtype or the integration of insertion sequences/transposases into stx subunit genes, which are seldom-reported genomic events but might also be underestimated [10,11,12]. For the investigation of gene duplications and insertion sequence/transposon integration, short-read data provide only limited insights, since such events often lead to contig breaks in the assemblies and therefore cannot be resolved. Hence, long-read sequencing must be performed for the detection of such genomic events. Therefore, we screened only complete genomes from public repositories. However, although NGS methods and bioinformatics tools have improved over the years, specific results cannot be reliably assessed without a comprehensive examination of the methods and tools used for each individual sequence.
Here, we report that stx1 genes can also occur multiple times in STEC genomes, and it seems like the subtypes stx1a and stx1c are likely involved in multiplications, but not the subtype stx1d. This topic is yet not well investigated, and raises the question of whether multiple occurrences can have an influence on the severity of human infections. Moreover, we showed that the integration of insertion sequences occurs not only in stx2 subunit genes but also in stx1 subunit genes, giving insights into genomic rearrangements in STECs harboring stx1 genes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13051079/s1, Table S1: Numbers of NCBI RefSeq genomes with multiple stx genes and at least one stx1 gene (the six genomes with multiple stx1 genes are highlighted in bold), https://www.ncbi.nlm.nih.gov/refseq/, accessed on 6 March 2025.
Author Contributions
Conceptualization, M.P., M.B. and C.D.; methodology, M.P., M.B. and C.D.; investigation, M.P., M.C., E.H., C.W. and E.S.; writing—original draft preparation, M.P., M.B. and C.D.; writing—review and editing, M.C., E.H., C.W. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Institute for Risk Assessment, internal grant numbers 42-001 and 4NSZ-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Sequence data are available under BioProject PRJNA1234488 (BioSample number SAMN47290620 and SAMN47290621).
Acknowledgments
We gratefully thank Beatrice Baumann, Annica Seemann, Jana Ade, Mandy Hailer, Karin Pries and Sebastian Steffan for excellent technical assistance in the labs of the different institutions involved in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Persad, A.K.; LeJeune, J.T. Animal Reservoirs of Shiga Toxin-Producing Escherichia coli. Microbiol. Spectr. 2014, 2, EHEC-0027-2014. [Google Scholar] [CrossRef] [PubMed]
- Fruth, A.; Prager, R.; Tietze, E.; Rabsch, W.; Flieger, A. Molecular epidemiological view on Shiga toxin-producing Escherichia coli causing human disease in Germany: Diversity, prevalence, and outbreaks. Int. J. Med. Microbiol. 2015, 305, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Biernbaum, E.N.; Kudva, I.T. AB5 Enterotoxin-Mediated Pathogenesis: Perspectives Gleaned from Shiga Toxins. Toxins 2022, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, F.; Teel, L.D.; Beutin, L.; Piérard, D.; Buvens, G.; Karch, H.; Mellmann, A.; Caprioli, A.; Tozzoli, R.; Morabito, S.; et al. Multicenter Evaluation of a Sequence-Based Protocol for Subtyping Shiga Toxins and Standardizing Stx Nomenclature. J. Clin. Microbiol. 2012, 50, 2951–2963. [Google Scholar] [CrossRef]
- Yang, X.; Bai, X.; Zhang, J.; Sun, H.; Fu, S.; Fan, R.; He, X.; Scheutz, F.; Matussek, A.; Xiong, Y. Escherichia coli strains producing a novel Shiga toxin 2 subtype circulate in China. Int. J. Med. Microbiol. 2020, 310, 151377. [Google Scholar] [CrossRef]
- Gill, A.; Dussault, F.; McMahon, T.; Petronella, N.; Wang, X.; Cebelinski, E.; Scheutz, F.; Weedmark, K.; Blais, B.; Carrillo, C. Characterization of Atypical Shiga Toxin Gene Sequences and Description of Stx2j, a New Subtype. J. Clin. Microbiol. 2022, 60, e0222921. [Google Scholar] [CrossRef]
- Bai, X.; Scheutz, F.; Dahlgren, H.M.; Hedenström, I.; Jernberg, C. Characterization of Clinical Escherichia coli Strains Producing a Novel Shiga Toxin 2 Subtype in Sweden and Denmark. Microorganisms 2021, 9, 2374. [Google Scholar] [CrossRef]
- Scheutz, F. Taxonomy Meets Public Health: The Case of Shiga Toxin-Producing Escherichia coli. Microbiol. Spectr. 2014, 2, EHEC-0019-2013. [Google Scholar] [CrossRef]
- Lindqvist, R.; Flink, C.; Lindblad, M. Classification and ranking of shigatoxin-producing Escherichia coli (STEC) genotypes detected in food based on potential public health impact using clinical data. Microb. Risk Anal. 2023, 23, 100246. [Google Scholar] [CrossRef]
- Projahn, M.; Schumann, S.; Müller, S.; Ferl, M.; Scholz, H.; Göhler, A.; Schuh, E.; Borowiak, M. Draft genome sequence of a Shiga toxin-producing Escherichia coli strain from deer meat showing an IS-element integration in the B-subunit of the Shiga toxin Stx2b gene. Genome Announc. 2024, 13, e0109323. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Liu, Q.; Sun, H.; Luo, M.; Xiong, Y.; Matussek, A.; Hu, B.; Bai, X. Genomic Characteristics of Stx2e-Producing Escherichia coli Strains Derived from Humans, Animals, and Meats. Pathogens 2021, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Nishiya, Y.; Kawamura, Y. Reactivation of insertionally inactivated Shiga toxin 2 genes of Escherichia coli O157:H7 caused by nonreplicative transposition of the insertion sequence. Appl. Environ. Microbiol. 2000, 66, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- DIN CEN ISO/TS 13136:2013-04; Microbiology of Food and Animal Feed—Real-Time Polymerase Chain Reaction (PCR)-Based Method for the Detection of Food-Borne Pathogens—Horizontal Method for the Detection of Shiga Toxin Producing Escherichia coli (STEC) and the Determination of O157, O111, O26, O103 and O145 Serogroups. ISO: Geneva, Switzerland, 2023.
- De Coster, W.; D’hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Joensen, K.G.; Tetzschner, A.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and Easy In Silico Serotyping of Escherichia coli Isolates by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef]
- Ingle, D.J.; Valcanis, M.; Kuzevski, A.; Tauschek, M.; Inouye, M.; Stinear, T.; Levine, M.M.; Robins-Browne, R.M.; Holt, K.E. In silico serotyping of E. coli from short read data identifies limited novel O-loci but extensive diversity of O:H serotype combinations within and between pathogenic lineages. Microb. Genom. 2016, 2, e000064. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 2021, 11, 12728. [Google Scholar] [CrossRef]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [PubMed]
- Cowley, L.A.; Dallman, T.J.; Fitzgerald, S.; Irvine, N.; Rooney, P.J.; McAteer, S.P.; Day, M.; Perry, N.T.; Bono, J.L.; Jenkins, C.; et al. Short-term evolution of Shiga toxin-producing Escherichia coli O157:H7 between two food-borne outbreaks. Microb. Genom. 2016, 2, e000084. [Google Scholar] [CrossRef]
- Patel, P.N.; Lindsey, R.L.; Garcia-Toledo, L.; Rowe, L.A.; Batra, D.; Whitley, S.W.; Drapeau, D.; Stoneburg, D.; Martin, H.; Juieng, P.; et al. High-Quality Whole-Genome Sequences for 77 Shiga Toxin-Producing Escherichia coli Strains Generated with PacBio Sequencing. Genome Announc. 2018, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Yano, B.; Taniguchi, I.; Gotoh, Y.; Hayashi, T.; Nakamura, K. Dynamic changes in Shiga toxin (Stx) 1 transducing phage throughout the evolution of O26:H11 Stx-producing Escherichia coli. Sci. Rep. 2023, 13, 4935. [Google Scholar] [CrossRef] [PubMed]
- Osborn, B.; Hatfield, J.; Lanier, W.; Wagner, J.; Oakeson, K.; Casey, R.; Bullough, J.; Kache, P.; Miko, S.; Kunz, J.; et al. Shiga Toxin-Producing Escherichia coli O157:H7 Illness Outbreak Associated with Untreated, Pressurized, Municipal Irrigation Water—Utah, 2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 411–416. [Google Scholar] [CrossRef]
- Lindsey, R.L.; Prasad, A.; Feldgarden, M.; Gonzalez-Escalona, N.; Kapsak, C.; Klimke, W.; Melton-Celsa, A.; Smith, P.; Souvorov, A.; Truong, J.; et al. Identification and Characterization of ten Escherichia coli Strains Encoding Novel Shiga Toxin 2 Subtypes, Stx2n as Well as Stx2j, Stx2m, and Stx2o, in the United States. Microorganisms 2023, 11, 2561. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).