Green Myco-Synthesis of Zinc Oxide Nanoparticles Using Cortinarius sp.: Hepatoprotective, Antimicrobial, and Antioxidant Potential for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
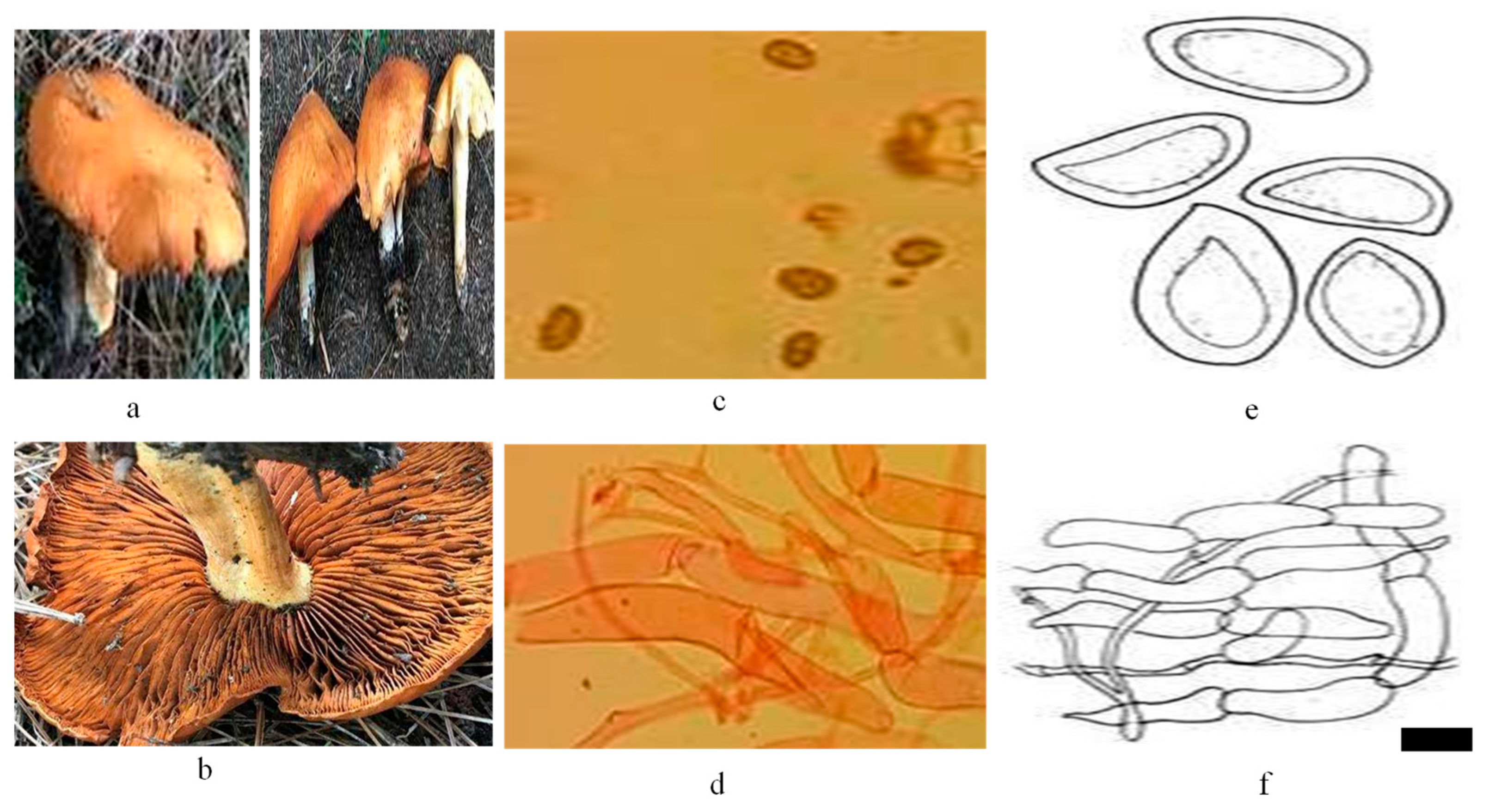
2.1. Sample Collection and Processing
2.2. Chemicals
2.3. Preparation of Mushroom Extract and Myco-Synthesis of ZnO Myco-Nanoparticles
2.4. Structural Analysis of ZnO MNPs
2.5. Biomedical Applications of ZnO-MNPs
2.5.1. Evaluation of Anti-Cancer Potential of ZnO-MNPs
Ethical Approval
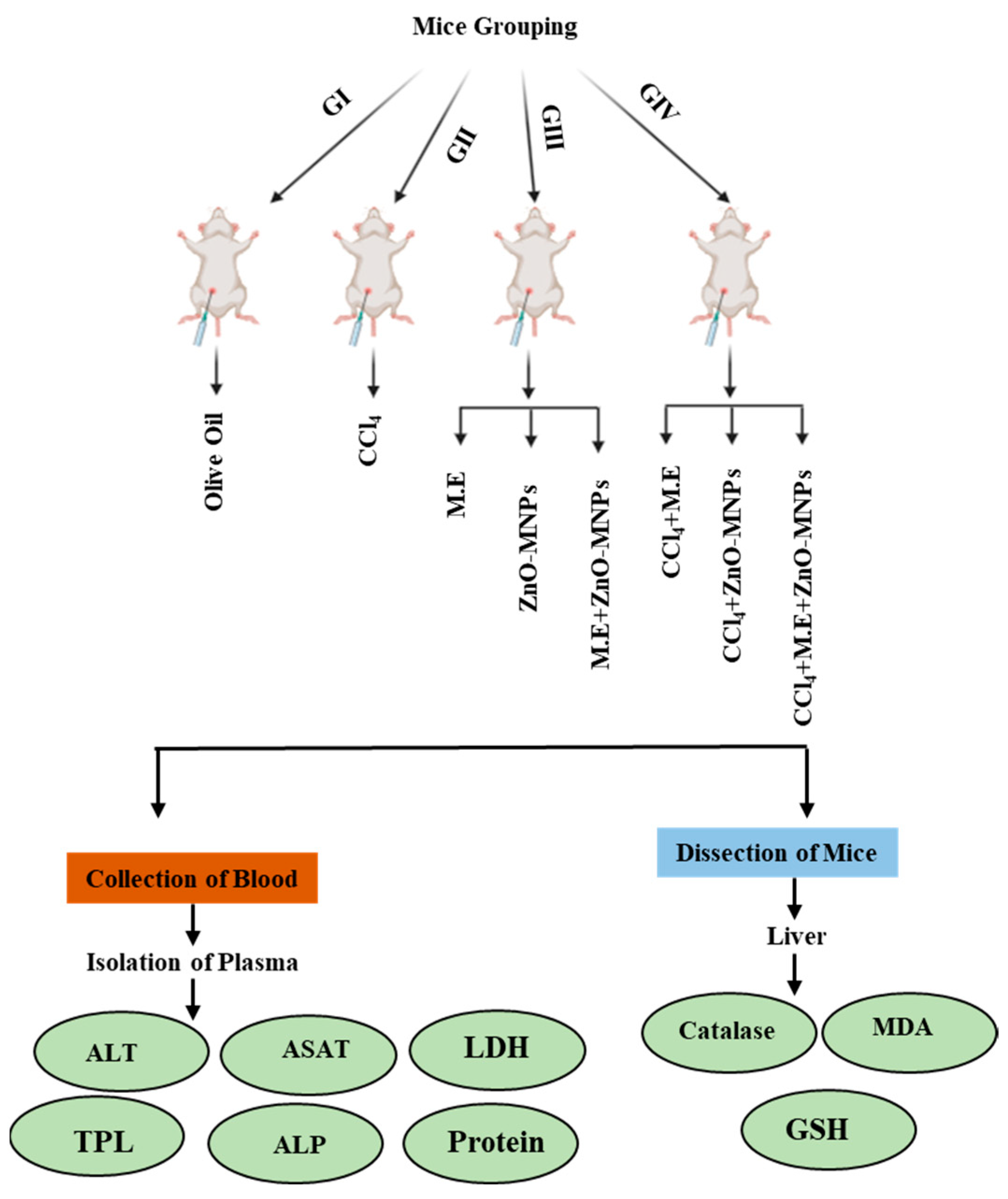
Experimental Animals and Dose Preparation
Biochemical Analysis
2.5.2. Evaluation of Antimicrobial Potential of ZnO-MNPs
Antibacterial Activity
Antifungal Activity
2.5.3. Evaluation of Antioxidant Potential of ZnO-MNPs
2.6. Statistical Analysis
3. Results
3.1. Characterization
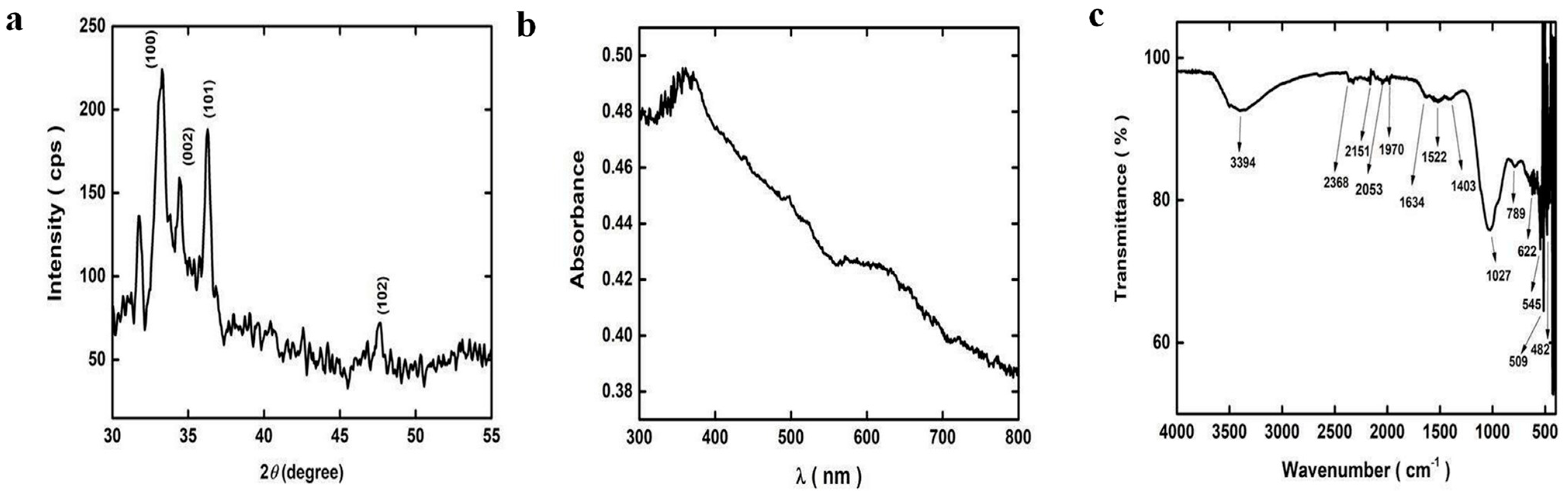
3.1.1. XRD (X-Ray Diffraction) Analysis of ZnO-MNPs
3.1.2. Ultraviolet Visible Spectroscopy Analysis of ZnO-MNPs
3.1.3. FTIR Analysis of ZnO-MNPs
3.2. Biomedical Applications of ZnO-MNPs
3.2.1. Anti-Cancer Activity (Effects on Biochemical Components)
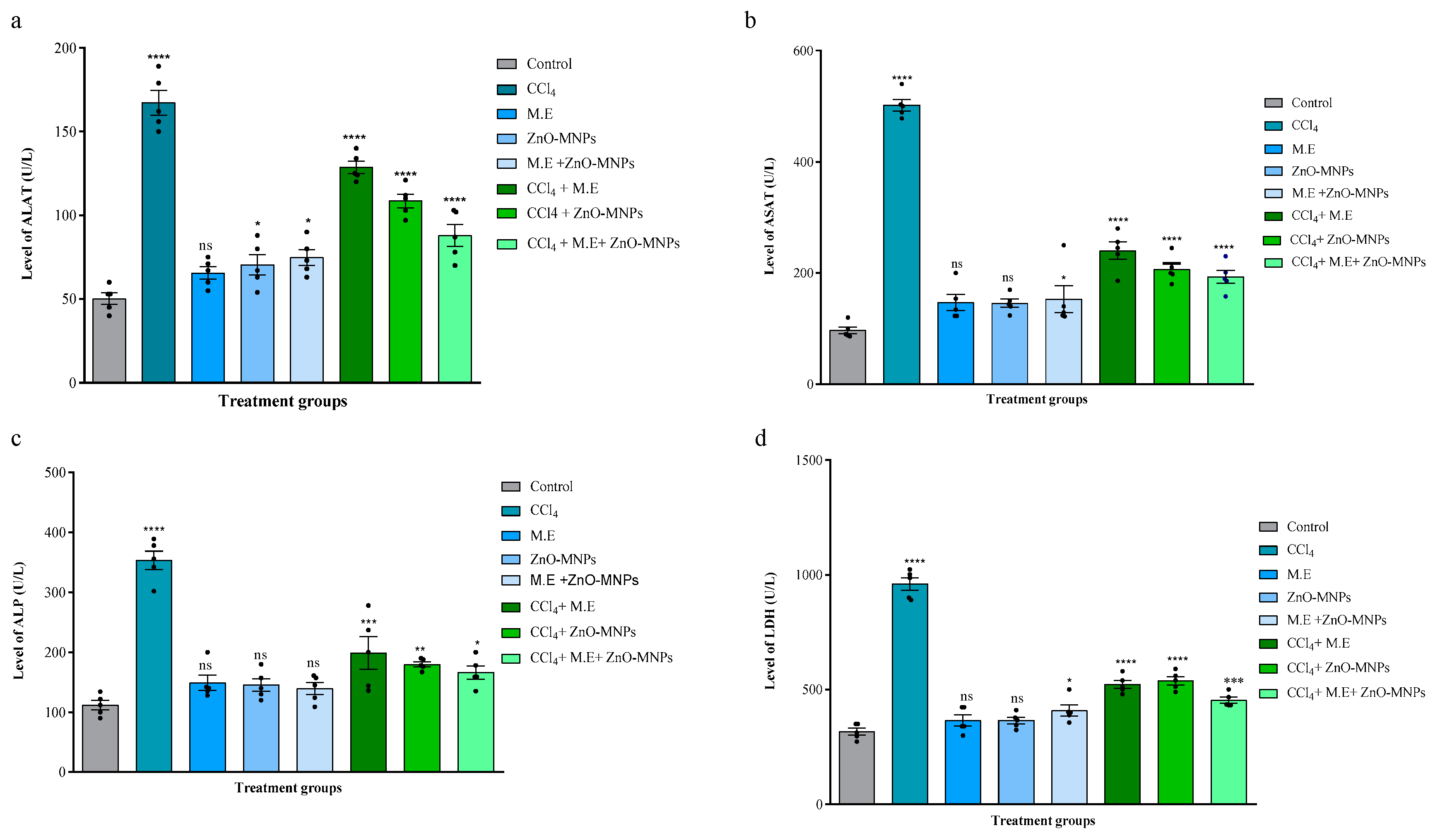
Effect on ALAT
Effect on ASAT
Effect on ALP
Effect on LDH
Effect on GSH
Effect on MDA
Effect on Catalase
Effect on Total Bilirubin
Effect on Total Protein
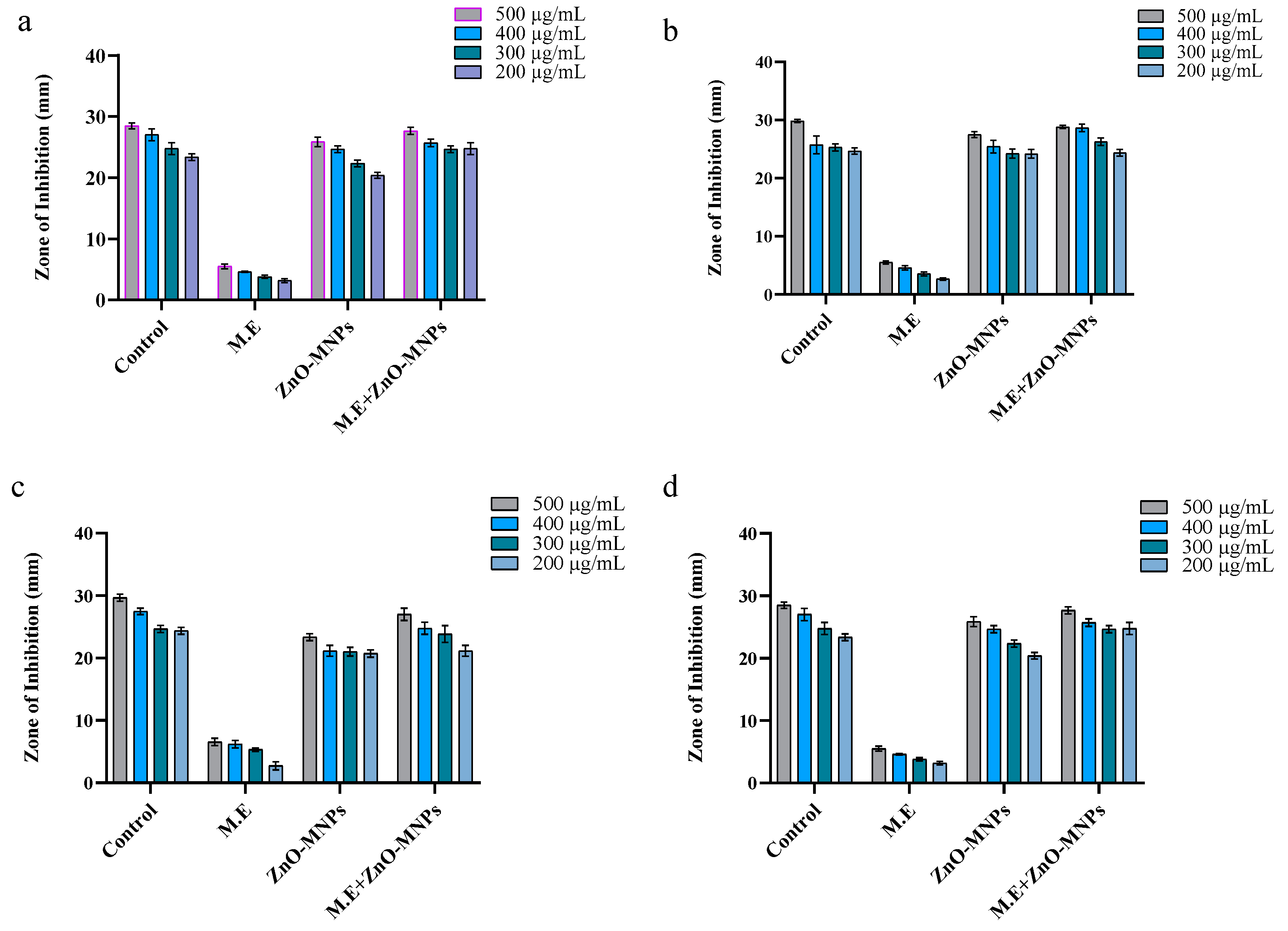
3.3. Antimicrobial assays
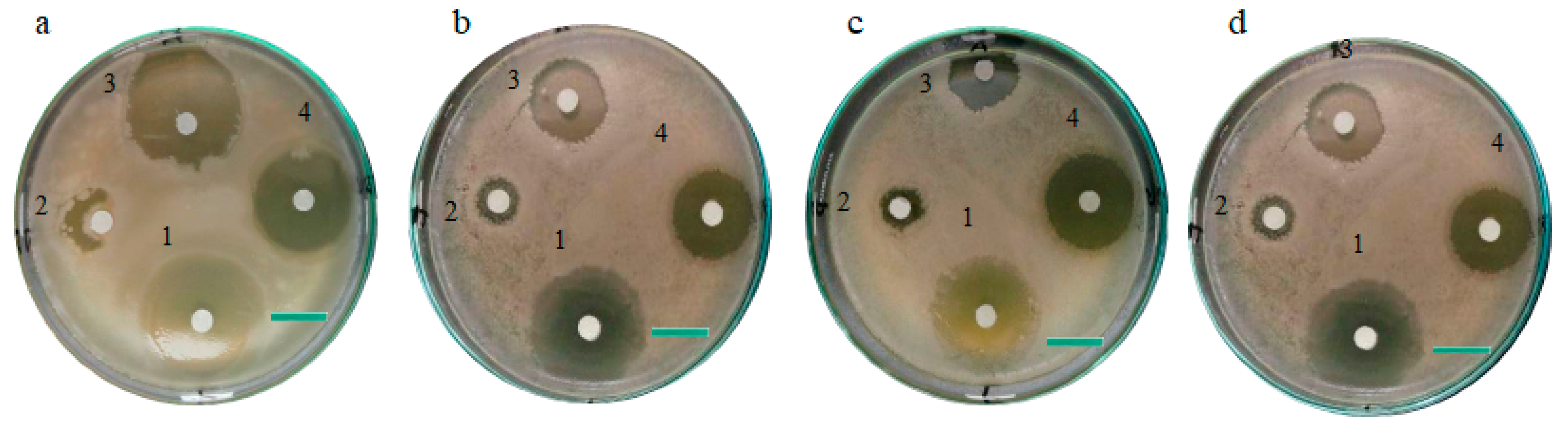
3.3.1. Antibacterial Activity
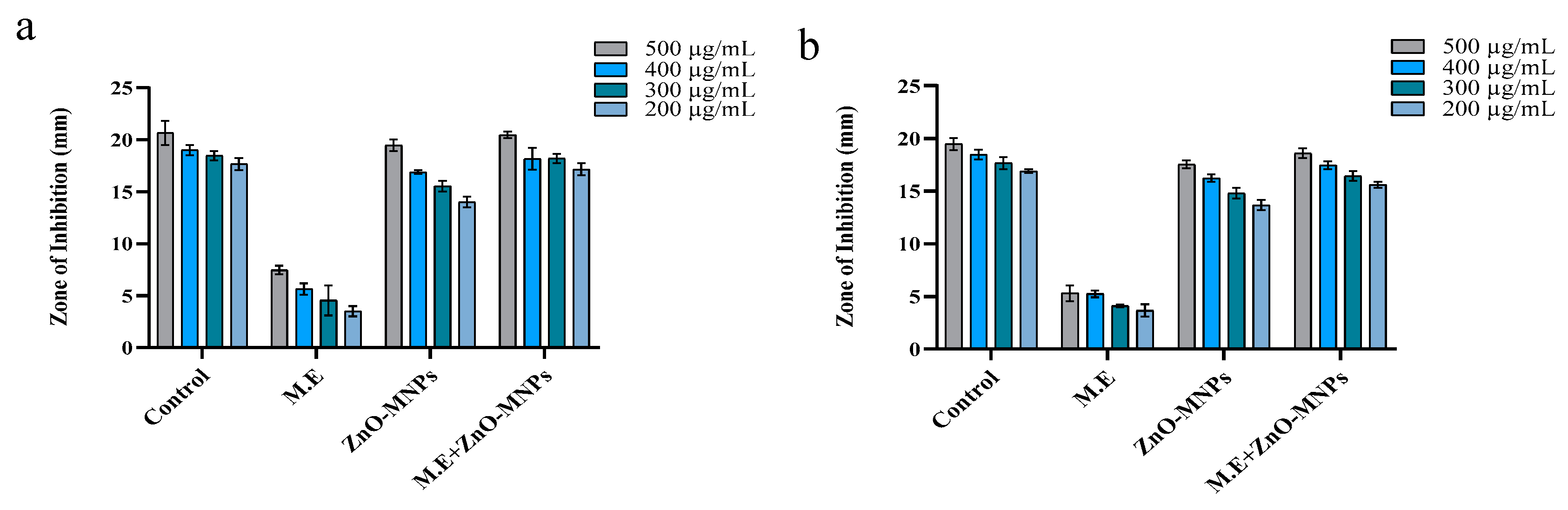
3.3.2. Antifungal Activity
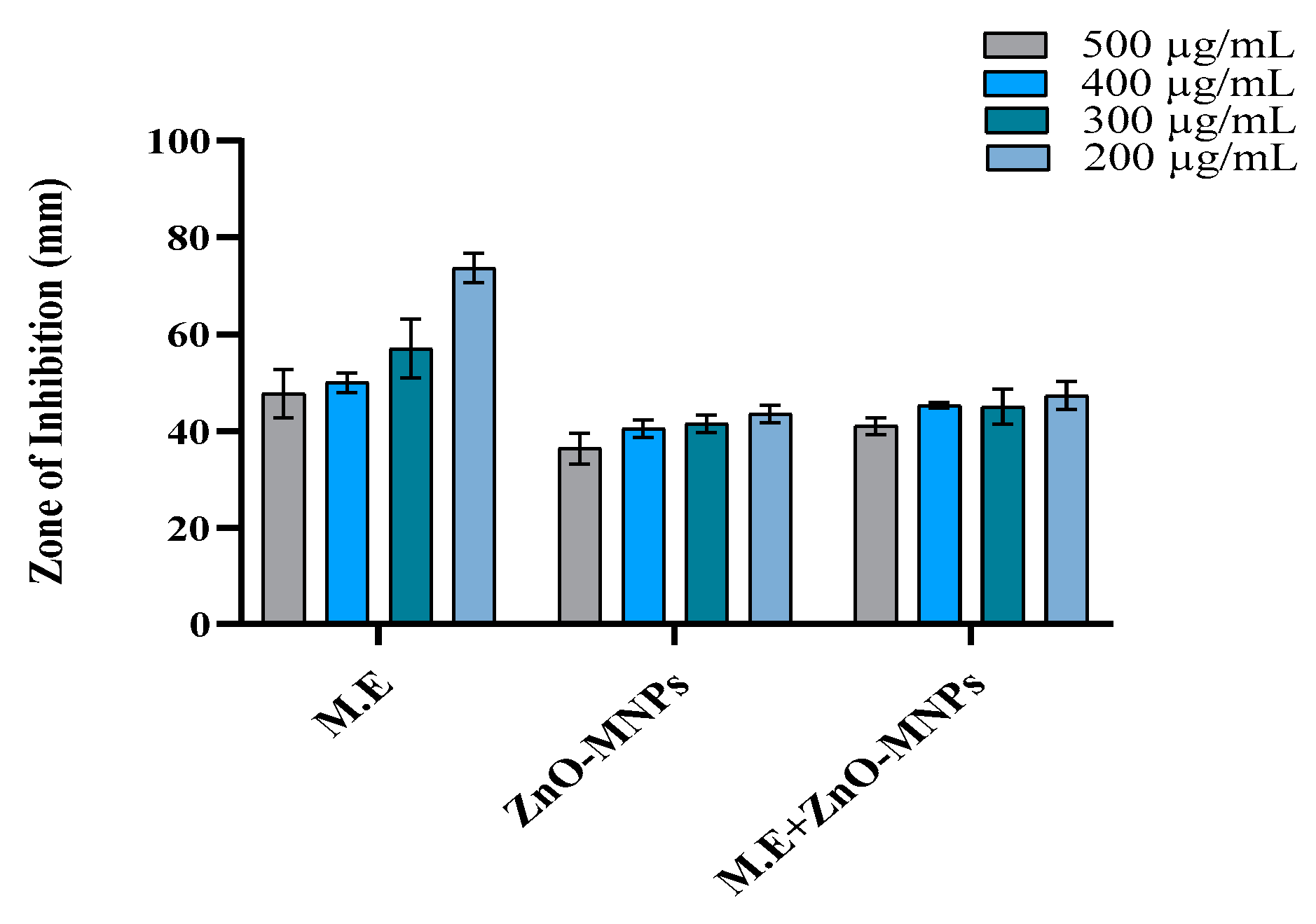
3.3.3. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZnO-MNPs | Zinc oxide myco-nanoparticles |
| M.E | Mushroom extract |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| CCl4 | Carbon tetrachloride |
| ALT | Alanine aminotransferase |
| ASAT | Aspartate aminotransferase |
| LDH | Lactate dehydrogenase |
| MDA | Melanin dialdehyde |
References
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-Friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef]
- Sharaf, O.M.; Al-Gamal, M.S.; Ibrahim, G.A.; Dabiza, N.M.; Salem, S.S.; El-Ssayad, M.F.; Youssef, A.M. Evaluation and Characterization of Some Protective Culture Metabolites in Free and Nano-Chitosan-Loaded Forms against Common Contaminants of Egyptian Cheese. Carbohydr. Polym. 2019, 223, 115094. [Google Scholar] [CrossRef]
- Shamim, A.; Mahmood, T.; Abid, M.B. Biogenic Synthesis of Zinc Oxide (ZnO) Nanoparticles Using a Fungus (Aspergillus niger) and Their Characterization. Int. J. Chem. 2019, 11, 119. [Google Scholar] [CrossRef]
- Rao, K.M.; Suneetha, M.; Park, G.T.; Babu, A.G.; Han, S.S. Hemostatic, Biocompatible, and Antibacterial Non-Animal Fungal Mushroom-Based Carboxymethyl Chitosan-ZnO Nanocomposite for Wound-Healing Applications. Int. J. Biol. Macromol. 2020, 155, 71–80. [Google Scholar] [CrossRef]
- Xiong, H.M. ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef] [PubMed]
- Fu, X. Oxidative Stress Induced by CuO Nanoparticles (CuO NPs) to Human Hepatocarcinoma (HepG2) Cells. J. Cancer Ther. 2015, 6, 889. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Green Synthesis of Copper Nanoparticles Using Ginkgo biloba L. Leaf Extract and Their Catalytic Activity for the Huisgen [3+2] Cycloaddition of Azides and Alkynes at Room Temperature. J. Colloid Interface Sci. 2015, 457, 141–147. [Google Scholar] [CrossRef]
- Bai, X.; Li, L.; Liu, H.; Tan, L.; Liu, T.; Meng, X. Solvothermal Synthesis of ZnO Nanoparticles and Anti-Infection Application In Vivo. ACS Appl. Mater. Interfaces 2015, 7, 1308–1317. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X.; Wei, L.; Chen, L.; Song, B.; Shao, L. The Toxicology of Ion-Shedding Zinc Oxide Nanoparticles. Crit. Rev. Toxicol. 2016, 46, 348–384. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour. -Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Sajjad, S.; Leghari, S.A.K.; Ryma, N.U.A.; Farooqi, S.A. Green Synthesis of Metal-Based Nanoparticles and Their Applications. In Green Metal Nanoparticles: Synthesis, Characterization and Their Applications; Wiley: Hoboken, NJ, USA, 2018; pp. 23–77. [Google Scholar]
- Abd El Aty, A.A.; Mohamed, A.A.; Zohair, M.M.; Soliman, A.A. Statistically Controlled Biogenesis of Silver Nano-Size by Penicillium chrysogenum MF318506 for Biomedical Application. Biocatal. Agric. Biotechnol. 2020, 25, 101592. [Google Scholar] [CrossRef]
- Govindappa, M.; Farheen, H.; Chandrappa, C.; Rai, R.V.; Raghavendra, V.B. Mycosynthesis of Silver Nanoparticles Using Extract of Endophytic Fungi, Penicillium Species of Glycosmis mauritiana, and Its Antioxidant, Antimicrobial, Anti-Inflammatory and Tyrokinase Inhibitory Activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035014. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.-D.; El-Sadany, M.A.-H. Eco-Friendly Approach Utilizing Green Synthesized Nanoparticles for Paper Conservation against Microbes Involved in Biodeterioration of Archaeological Manuscript. Int. Biodeterior. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Mosallam, F.M.; Fathy, R.M. Penicillium chrysogenum-Mediated Mycogenic Synthesis of Copper Oxide Nanoparticles Using Gamma Rays for In Vitro Antimicrobial Activity against Some Plant Pathogens. J. Clust. Sci. 2020, 31, 79–90. [Google Scholar] [CrossRef]
- Zhong, Q.; Tian, J.; Liu, T.; Guo, Z.; Ding, S.; Li, H. Preparation and Antibacterial Properties of Carboxymethyl Chitosan/ZnO Nanocomposite Microspheres with Enhanced Biocompatibility. Mater. Lett. 2018, 212, 58–61. [Google Scholar] [CrossRef]
- Cruz, D.M.; Mostafavi, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.L.; Webster, T.J. Green Nanotechnology-Based Zinc Oxide (ZnO) Nanomaterials for Biomedical Applications: A Review. J. Phys. Mater. 2020, 3, 034005. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc Oxide Nanoparticles: Biological Synthesis and Biomedical Applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Cahú, T.B.; Silva, R.A.; Silva, R.P.; Silva, M.M.; Arruda, I.R.; Silva, J.F.; Bezerra, R.S. Evaluation of Chitosan-Based Films Containing Gelatin, Chondroitin 4-Sulfate and ZnO for Wound Healing. Appl. Biochem. Biotechnol. 2017, 183, 765–777. [Google Scholar] [CrossRef]
- Le, T.C.; Yin, H.; Chen, R.; Chen, Y.; Zhao, L.; Casey, P.S.; Winkler, D.A. An Experimental and Computational Approach to the Development of ZnO Nanoparticles That Are Safe by Design. Small 2016, 12, 3568–3577. [Google Scholar] [CrossRef]
- Gao, Y.; Anand, M.A.V.; Ramachandran, V.; Karthikkumar, V.; Shalini, V.; Vijayalakshmi, S.; Ernest, D. Biofabrication of Zinc Oxide Nanoparticles from Aspergillus niger, Their Antioxidant, Antimicrobial and Anti-Cancer Activity. J. Clust. Sci. 2019, 30, 937–946. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Chaurasia, V.; Pal, S.; Tiwari, B. Prediction of Benign and Malignant Breast Cancer Using Data Mining Techniques. J. Algorithms Comput. Technol. 2018, 12, 119–126. [Google Scholar] [CrossRef]
- Rane, J.; Jadhao, R.; Bakal, R. Liver Diseases and Herbal Drugs: A Review. J. Innov. Pharm. Biol. Sci. 2016, 3, 24–36. [Google Scholar]
- Oyinloye, B.E.; Osunsanmi, F.O.; Ajiboye, B.O.; Ojo, O.A.; Kappo, A.P. Modulatory Effect of Methanol Extract of Piper guineense in CCl4-Induced Hepatotoxicity in Male Rats. Int. J. Environ. Res. Public Health 2017, 14, 955. [Google Scholar] [CrossRef]
- Selvinsimpson, S.; Gnanamozhi, P.; Pandiyan, V.; Govindasamy, M.; Habila, M.A.; AlMasoud, N.; Chen, Y. Synergetic Effect of Sn Doped ZnO Nanoparticles Synthesized via Ultrasonication Technique and Its Photocatalytic and Antibacterial Activity. Environ. Res. 2021, 197, 111115. [Google Scholar] [CrossRef]
- Naveed Ul Haq, A.; Nadhman, A.; Ullah, I.; Mustafa, G.; Yasinzai, M.; Khan, I. Synthesis Approaches of Zinc Oxide Nanoparticles: The Dilemma of Ecotoxicity. J. Nanomater. 2017, 2017, 8510342. [Google Scholar] [CrossRef]
- Preethi, P.S.; Narenkumar, J.; Prakash, A.A.; Abilaji, S.; Prakash, C.; Rajasekar, A.; Valli, G. Myco-Synthesis of Zinc Oxide Nanoparticles as Potent Anti-Corrosion of Copper in Cooling Towers. J. Clust. Sci. 2019, 30, 1583–1590. [Google Scholar] [CrossRef]
- Mughal, T.A.; Ali, S.; Hassan, A.; Saleem, M.Z.; Mumtaz, S.; Mumtaz, S. Tetrachloride-Induced Hepatocellular Damage in Balb C Mice and Pharmacological Intervention by Extract of Daucus carota. RADS J. Pharm. Pharm. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Bowers Jr, G.N.; McComb, R.B. A Continuous Spectrophotometric Method for Measuring the Activity of Serum Alkaline Phosphatase. Clin. Chem. 1966, 12, 70–89. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Dangerfield, W.; Finlayson, R. Estimation of Bilirubin in Serum. J. Clin. Pathol. 1953, 6, 173. [Google Scholar] [CrossRef]
- Wilkinson, J.H.; Moss, D. Serum Enzymes. CRC Crit. Rev. Clin. Lab. Sci. 1970, 1, 599–637. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lück, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1965; pp. 885–894. [Google Scholar]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-Assisted Silver Nanoparticle Synthesis and Their Applications. Bioprocess Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Mycogenic Nanoparticles and Their Bio-Prospective Applications: Current Status and Future Challenges. J. Nanostructure Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Singdevsachan, S.K.; Parida, U.K.; Panda, S.K.; Mohanta, T.K.; Bae, H. Green Synthesis and Antimicrobial Activity of Silver Nanoparticles Using Wild Medicinal Mushroom Ganoderma applanatum (Pers.) Pat. from Similipal Biosphere Reserve, Odisha, India. IET Nanobiotechnol. 2016, 10, 184–189. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Nayak, D.; Biswas, K.; Singdevsachan, S.K.; Abd_Allah, E.F.; Hashem, A.; Mohanta, T.K. Silver Nanoparticles Synthesized Using Wild Mushroom Show Potential Antimicrobial Activities against Food Borne Pathogens. Molecules 2018, 23, 655. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, T.; Liimatainen, K.; Nuytinck, J.; Kirk, P.; Ibarguren, I.O.; Garibay-Orijel, R.; Ruotsalainen, J. Identifying and Naming the Currently Known Diversity of the Genus Hydnum, with an Emphasis on European and North American Taxa. Mycologia 2018, 110, 890–918. [Google Scholar] [CrossRef] [PubMed]
- Thukkaram Sudhakar, T.S.; Anima Nanda, A.N.; Babu, S.; Sreenivasan Janani, S.J.; Evans, M.; Markose, T. Synthesis of Silver Nanoparticles from Edible Mushroom and Its Antimicrobial Activity against Human Pathogens. Int. J. Pharm. Sci. Res. 2014, 5, 1718–1723. [Google Scholar]
- Nadhim Owaid, M.; Al-Saeedi, S.S.S.; Abed, I.A. Study on UV-Visible for Detection of Biosynthesis of Silver Nanoparticles by Oyster Mushroom’s Extracts. J. Water Environ. Nanotechnol. 2017, 2, 66–70. [Google Scholar]
- Arun, G.; Eyini, M.; Gunasekaran, P. Green Synthesis of Silver Nanoparticles Using the Mushroom Fungus Schizophyllum commune and Its Biomedical Applications. Biotechnol. Bioprocess Eng. 2014, 19, 1083–1090. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal Strain Impacts the Shape, Bioactivity and Multifunctional Properties of Green Synthesized Zinc Oxide Nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of Zinc Oxide Nanoparticles Using Plant Leaf Extract against Urinary Tract Infection Pathogen. Resour. -Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Abdelhakim, H.K.; El-Sayed, E.; Rashidi, F.B. Biosynthesis of Zinc Oxide Nanoparticles with Antimicrobial, Anticancer, Antioxidant, and Photocatalytic Activities by the Endophytic Alternaria tenuissima. J. Appl. Microbiol. 2020, 128, 1634–1646. [Google Scholar] [CrossRef]
- Raja, A.; Ashokkumar, S.; Marthandam, R.P.; Jayachandiran, J.; Khatiwada, C.P.; Kaviyarasu, K.; Swaminathan, M. Eco-Friendly Preparation of Zinc Oxide Nanoparticles Using Tabernaemontana divaricata and Its Photocatalytic and Antimicrobial Activity. J. Photochem. Photobiol. B Biol. 2018, 181, 53–58. [Google Scholar] [CrossRef]
- Marcellin, P.; Kutala, B.K. Liver Diseases: A Major, Neglected Global Public Health Problem Requiring Urgent Actions and Large-Scale Screening. Liver Int. 2018, 38, 2–6. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Imam, F.; Nadeem, A.; Al-Harbi, M.M.; Iqbal, M.; Ahmad, S.F. Carbon Tetrachloride-Induced Hepatotoxicity in Rat Is Reversed by Treatment with Riboflavin. Int. Immunopharmacol. 2014, 21, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Meligi, N.M.; Ismail, S.A.; Tawfik, N.S. Protective Effects of Honey and Bee Venom against Lipopolysaccharide and Carbon Tetrachloride-Induced Hepatoxicity and Lipid Peroxidation in Rats. Toxicol. Res. 2020, 9, 693–705. [Google Scholar] [CrossRef]
- Al-Snai, A.; Mousa, H.; Majid, W.J. Medicinal Plants Possessed Hepatoprotective Activity. IOSR J. Pharm. 2019, 9, 26–56. [Google Scholar]
- Mughal, T.A.; Ali, S.; Khatoon, S.; Khalil, S.; Mumtaz, S. Protective Effect of Helianthus annuus Seeds Extract against CCl4-Induced Hepatocellular Damage. Pak. J. Biochem. Biotechnol. 2023, 4, 37–45. [Google Scholar] [CrossRef]
- Ebaid, H.; Al-Tamimi, J.; Hassan, I.; Habila, M.A.; Rady, A.M.; Alhazza, I.M.; Ahmed, A.M. Effect of Selenium Nanoparticles on Carbon Tetrachloride-Induced Hepatotoxicity in the Swiss Albino Rats. Appl. Sci. 2021, 11, 3044. [Google Scholar] [CrossRef]
- Selvam, K.; Sudhakar, C.; Senthilkumar, B.; Sakthivel, V.; Ragu Prasath, A.; Sangameshwaran, V. Green Synthesis and Characterisation of Iron Oxide Nanoparticle Using Ziziphus oenoplia Fruit Extract: A Biomedical and Environmental Potential. Waste Biomass Valorization 2024, 15, 6415–6429. [Google Scholar] [CrossRef]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Esteve, M.A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef]
- Sell, M.; Lopes, A.R.; Escudeiro, M.; Esteves, B.; Monteiro, A.R.; Trindade, T.; Cruz-Lopes, L. Application of nanoparticles in cancer treatment: A concise review. Nanomaterials 2023, 13, 2887. [Google Scholar] [CrossRef]
- Danas, A.Y.; Labulo, A.H.; Usman, A.; Hassan, I.; Terna, A.D.; Ogungbemiro, F.O.; Isah, M. Green Synthesis of Zinc Oxide Nanoparticles: A Trifecta of Antioxidant, Antifungal, and Catalytic Excellence. Recent Adv. Nat. Sci. 2024, 127, 127. [Google Scholar] [CrossRef]
- Abbas, M.; Hanif, U.; Nasir, A.; Zahid, F.; Liaqat, I.; Ali, S.; Khan, S. Green Synthesis and Application of Zinc Oxide (ZnO) Nanoparticles from Cenchrus setigerus Vahl in Herbal Handwash as Antimicrobial Reducer. ChemistrySelect 2025, 10, e202404733. [Google Scholar] [CrossRef]
- Hayat, K.; Din, I.U.; Alam, K.; Khan, F.U.; Khan, M.; Mohamed, H.I. Green Synthesis of Zinc Oxide Nanoparticles Using Plant Extracts of Fumaria officinalis and Peganum harmala and Their Antioxidant and Antibacterial Activities. Biomass Convers. Biorefinery 2024, 15, 9565–9579. [Google Scholar] [CrossRef]
- Saravanan, S.; Dubey, R. Synthesis of SiO2 Nanoparticles by Sol-Gel Method and Their Optical and Structural Properties. Rom. J. Inf. Sci. Technol. 2020, 23, 105–112. [Google Scholar]
- Dianati, E.; Hojati, V.; Khayatzadeh, J.; Balanezhad, S.Z. The Green-Synthesized Curcumin-Mediated Zinc Oxide Nanoparticles (CmZnO-NP) as the Exclusive Antioxidant and Efficient Wound Healing Agent Compared with Curcumin, Methanol, Phenytoin, and ZnO. Inorg. Nano-Met. Chem. 2023, 53, 985–994. [Google Scholar] [CrossRef]
- Ihsan, M.; Din, I.U.; Alam, K.; Munir, I.; Mohamed, H.I.; Khan, F. Green Fabrication, Characterization of Zinc Oxide Nanoparticles Using Plant Extract of Momordica charantia and Curcuma zedoaria and Their Antibacterial and Antioxidant Activities. Appl. Biochem. Biotechnol. 2023, 195, 3546–3565. [Google Scholar] [CrossRef]
- Aydin Acar, C.; Gencer, M.A.; Pehlivanoglu, S.; Yesilot, S.; Donmez, S. Green and Eco-Friendly Biosynthesis of Zinc Oxide Nanoparticles Using Calendula officinalis Flower Extract: Wound Healing Potential and Antioxidant Activity. Int. Wound J. 2024, 21, e14413. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazal, U.; Zada, A.; Hanif, M.; Lee, S.Y.; Faisal, M.; Alatar, A.A.; Sultana, T.; Sohail. Green Myco-Synthesis of Zinc Oxide Nanoparticles Using Cortinarius sp.: Hepatoprotective, Antimicrobial, and Antioxidant Potential for Biomedical Applications. Microorganisms 2025, 13, 956. https://doi.org/10.3390/microorganisms13050956
Fazal U, Zada A, Hanif M, Lee SY, Faisal M, Alatar AA, Sultana T, Sohail. Green Myco-Synthesis of Zinc Oxide Nanoparticles Using Cortinarius sp.: Hepatoprotective, Antimicrobial, and Antioxidant Potential for Biomedical Applications. Microorganisms. 2025; 13(5):956. https://doi.org/10.3390/microorganisms13050956
Chicago/Turabian StyleFazal, Uzma, Ahmad Zada, Muhammad Hanif, Shiou Yih Lee, Mohammad Faisal, Abdulrahman A. Alatar, Tahira Sultana, and Sohail. 2025. "Green Myco-Synthesis of Zinc Oxide Nanoparticles Using Cortinarius sp.: Hepatoprotective, Antimicrobial, and Antioxidant Potential for Biomedical Applications" Microorganisms 13, no. 5: 956. https://doi.org/10.3390/microorganisms13050956
APA StyleFazal, U., Zada, A., Hanif, M., Lee, S. Y., Faisal, M., Alatar, A. A., Sultana, T., & Sohail. (2025). Green Myco-Synthesis of Zinc Oxide Nanoparticles Using Cortinarius sp.: Hepatoprotective, Antimicrobial, and Antioxidant Potential for Biomedical Applications. Microorganisms, 13(5), 956. https://doi.org/10.3390/microorganisms13050956








