Virological Passive Surveillance of Avian Influenza and Arboviruses in Wild Birds: A Two-Year Study (2023–2024) in Lombardy, Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Samples Collection
2.3. Detection of Viral Genomes
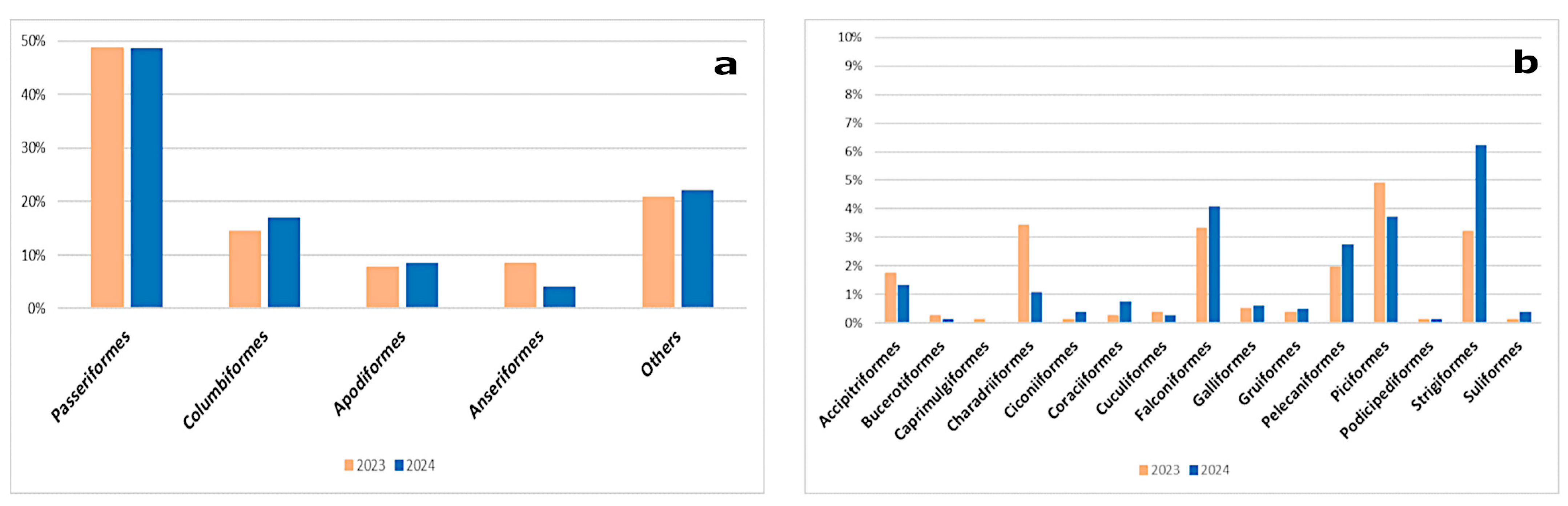
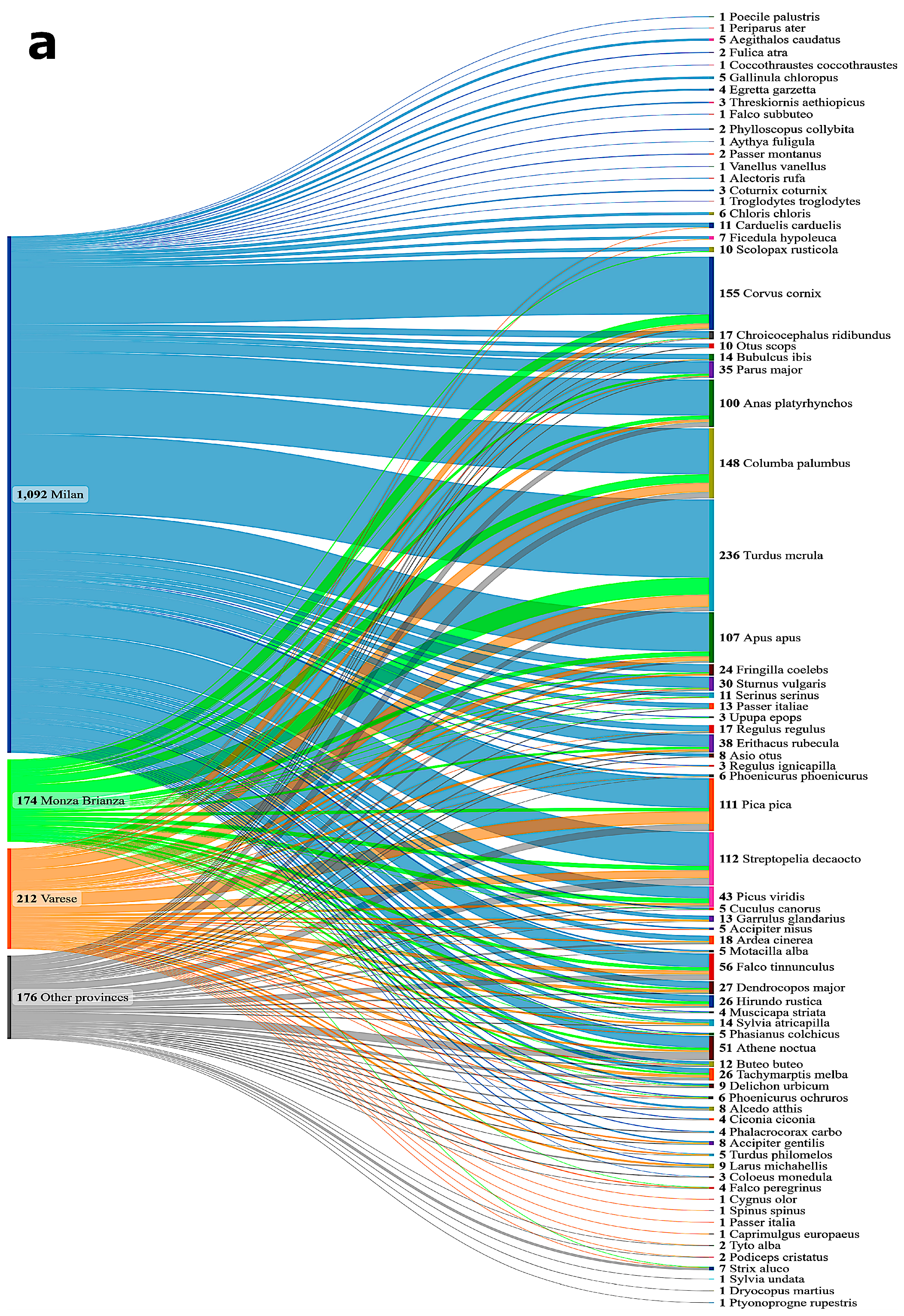
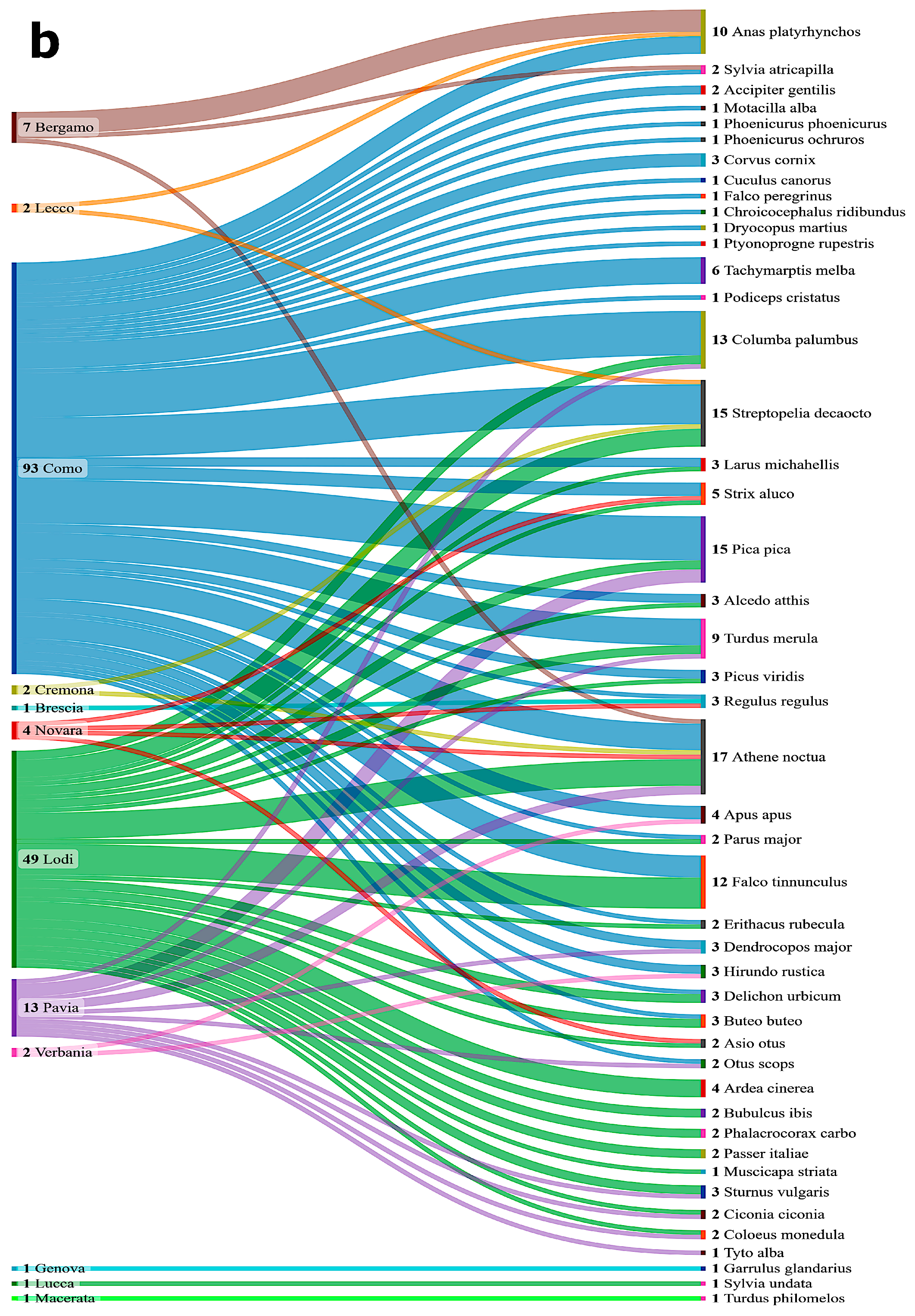
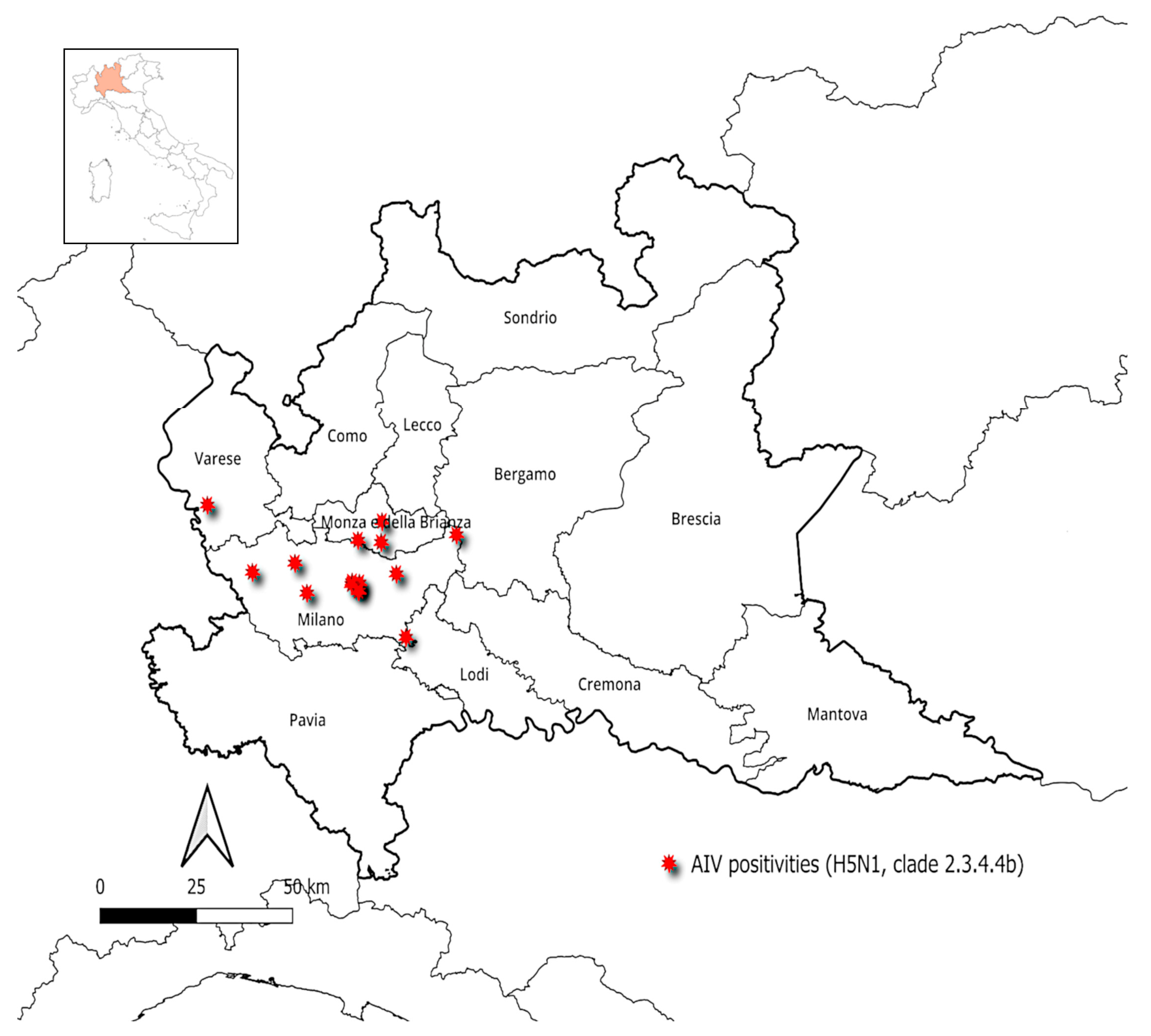
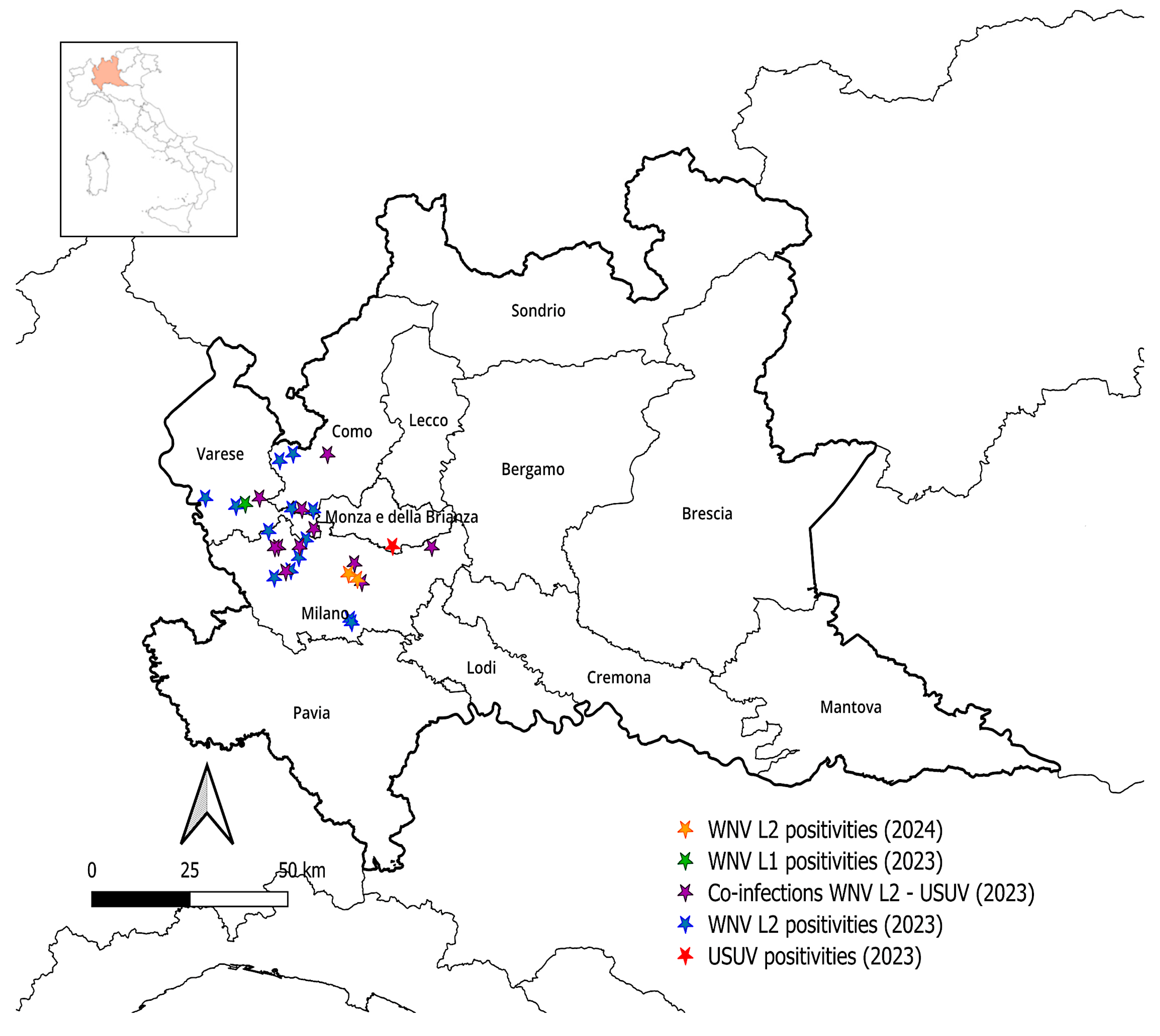
3. Results
3.1. Prevalence of AIVs
3.2. Prevalence of WNV and USUV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.A. Wild birds and zoonotic pathogens. In Zoonoses: Infections Affecting Humans and Animals; Sing, A., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–31. [Google Scholar]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian influenza in wild birds and poultry: Dissemination pathways, monitoring methods, and virus ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- ENETWILD Consortium; Flavia, O.; Sascha, K.; Carola, S.-L.; Christoph, S.; Valerie, A.; Alina, A.; Sophia, B.; Hannes, B.; Caroline, B.; et al. The role of mammals in Avian Influenza: A review. EFSA Support. Publ. 2024, 21, 8692E. [Google Scholar] [CrossRef]
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses: ICTV. Available online: https://ictv.global/taxonomy/taxondetails?taxnode_id=202403956&taxon_name=Alphainfluenzavirus%20influenzae (accessed on 11 April 2025).
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Global patterns of influenza A virus in wild birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef]
- Fouchier, R.A.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef]
- Yoon, S.-W.; Webby, R.J.; Webster, R.G. Evolution and ecology of influenza A viruses. In Influenza Pathogenesis and Control–Volume I; Compans, R.W., Oldstone, M.B.A., Eds.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2014; pp. 359–375. [Google Scholar]
- Swayne, D.E.; Sims, L.D. Avian Influenza. In Veterinary Vaccines: Principles and Applications, 1st ed.; Metwally, S., El Idrissi, A., Viljoen, G., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2021; pp. 229–251. [Google Scholar]
- Franҫa, M.S.; Brown, J.D. Influenza pathobiology and pathogenesis in avian species. In Influenza Pathogenesis and Control–Volume I; Compans, R.W., Oldstone, M.B.A., Eds.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2014; Volume 385, pp. 221–242. [Google Scholar]
- Artois, M.; Bicout, D.; Doctrinal, D.; Fouchier, R.; Gavier-Widen, D.; Globig, A.; Hagemeijer, W.; Mundkur, T.; Munster, V.; Olsen, B. Outbreaks of highly pathogenic avian influenza in Europe: The risks associated with wild birds. Rev. Sci. Tech. 2009, 28, 69–92. [Google Scholar] [CrossRef]
- Krauss, S.; Webster, R.G. Avian influenza virus surveillance and wild birds: Past and present. Avian Dis. 2010, 54, 394–398. [Google Scholar] [CrossRef]
- Duan, C.; Li, C.; Ren, R.; Bai, W.; Zhou, L. An overview of avian influenza surveillance strategies and modes. Sci. One Health 2023, 2, 100043. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.; Hinrichs, J. The economics of animal influenza. In Animal Influenza, 2nd ed.; Swayne, D.E., Ed.; John Wiley and Sons, Inc.: Ames, IA, USA, 2016; pp. 45–73. [Google Scholar]
- Swayne, D.E. Avian influenza control strategies. In Avian Influenza; Swayne, D.E., Ed.; Blackwell Publishing Professional: Ames, IA, USA, 2008; pp. 287–299. [Google Scholar]
- Feare, C.J.; Yasué, M. Asymptomatic infection with highly pathogenic avian influenza H5N1 in wild birds: How sound is the evidence? Virol. J. 2006, 3, 96. [Google Scholar] [CrossRef]
- Spackman, E. The ecology of avian influenza virus in wild birds: What does this mean for poultry? Poult. Sci. 2009, 88, 847–850. [Google Scholar] [CrossRef]
- Zannoli, S.; Sambri, V. West Nile virus and Usutu virus co-circulation in Europe: Epidemiology and implications. Microorganisms 2019, 7, 184. [Google Scholar] [CrossRef]
- Fros, J.J.; Miesen, P.; Vogels, C.B.; Gaibani, P.; Sambri, V.; Martina, B.E.; Koenraadt, C.J.; van Rij, R.P.; Vlak, J.M.; Takken, W.; et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health 2015, 1, 31–36. [Google Scholar] [CrossRef]
- Pfeffer, M.; Dobler, G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit. Vectors 2010, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile virus: From the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Roesch, F.; Fajardo, A.; Moratorio, G.; Vignuzzi, M. Usutu virus: An arbovirus on the rise. Viruses 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Dauphin, G.; Zientara, S.; Zeller, H.; Murgue, B. West Nile: Worldwide current situation in animals and humans. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 343–355. [Google Scholar] [CrossRef]
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef]
- Bowen, R.A.; Nemeth, N.M. Experimental infections with West Nile virus. Curr. Opin. Infect. Dis. 2007, 20, 293–297. [Google Scholar] [CrossRef]
- Faggioni, G.; De Santis, R.; Pomponi, A.; Grottola, A.; Serpini, G.F.; Meacci, M.; Gennari, W.; Tagliazucchi, S.; Pecorari, M.; Monaco, F.; et al. Prevalence of Usutu and West Nile virus antibodies in human sera, Modena, Italy, 2012. J. Med. Virol. 2018, 90, 1666–1668. [Google Scholar] [CrossRef] [PubMed]
- Grottola, A.; Marcacci, M.; Tagliazucchi, S.; Gennari, W.; Di Gennaro, A.; Orsini, M.; Monaco, F.; Marchegiano, P.; Marini, V.; Meacci, M.; et al. Usutu virus infections in humans: A retrospective analysis in the municipality of Modena, Italy. Clin. Microbiol. Infect. 2017, 23, 33–37. [Google Scholar] [CrossRef]
- Ulbert, S. West Nile virus vaccines–current situation and future directions. Hum. Vaccin. Immunother. 2019, 15, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.-C.; Blázquez, A.-B. Usutu virus: Current knowledge and future perspectives. Virus Adapt. Treat. 2017, 9, 27–40. [Google Scholar] [CrossRef]
- Winston, D.J.; Vikram, H.R.; Rabe, I.B.; Dhillon, G.; Mulligan, D.; Hong, J.C.; Busuttil, R.W.; Nowicki, M.J.; Mone, T.; Civen, R.; et al. Donor-derived West Nile virus infection in solid organ transplant recipients: Report of four additional cases and review of clinical, diagnostic, and therapeutic features. Transplantation 2014, 97, 881–889. [Google Scholar] [CrossRef]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Santini, M.; Barbic, L.; Kosuta, I.; Savic, V.; Tabain, I.; Vilibic-Cavlek, T. West Nile virus: An emerging threat in transplant population. Vector Borne Zoonotic Dis. 2020, 20, 613–618. [Google Scholar] [CrossRef]
- Cadar, D.; Maier, P.; Müller, S.; Kress, J.; Chudy, M.; Bialonski, A.; Schlaphof, A.; Jansen, S.; Jöst, H.; Tannich, E.; et al. Blood donor screening for West Nile virus (WNV) revealed acute Usutu virus (USUV) infection, Germany, September 2016. Eurosurveillance 2017, 22, 30501. [Google Scholar] [CrossRef]
- Aberle, S.W.; Kolodziejek, J.; Jungbauer, C.; Stiasny, K.; Aberle, J.H.; Zoufaly, A.; Hourfar, M.K.; Weidner, L.; Nowotny, N. Increase in human West Nile and Usutu virus infections, Austria, 2018. Eurosurveillance 2018, 23, 1800545. [Google Scholar] [CrossRef]
- Trogu, T.; Canziani, S.; Salvato, S.; Tolini, C.; Grilli, G.; Chiari, M.; Farioli, M.; Alborali, L.; Gaffuri, A.; Sala, G.; et al. Survey on the presence of viruses of economic and zoonotic importance in avifauna in northern Italy. Microorganisms 2021, 9, 1957. [Google Scholar] [CrossRef]
- National Surveillance Plan for Avian Influenza—2023. Available online: https://www.izsvenezie.it/documenti/temi/influenza-aviaria//piani-sorveglianza/piano-nazionale-influenza-aviaria-2023.pdf (accessed on 16 December 2024).
- National Arbovirosis Plan-2020–2025. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2947_allegato.pdf (accessed on 16 December 2024).
- Spackman, E.; Senne, D.A.; Myers, T.J.; Bulaga, L.L.; Garber, L.P.; Perdue, M.L.; Lohman, K.; Daum, L.T.; Suarez, D.L. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 2002, 40, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Pavlidis, T.; Coward, V.J.; Voermans, J.; Koch, G.; Hanna, A.; Banks, J.; Brown, I.H. Validated RealTime reverse transcriptase PCR methods for the diagnosis and pathotyping of Eurasian H7 avian influenza viruses. Influenza Other Respir. Viruses 2009, 3, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic Protocols. Available online: https://www.izsvenezie.com/reference-laboratories/avian-influenza-newcastle-disease/diagnostic-protocols/ (accessed on 14 April 2025).
- Hassan, K.E.; Ahrens, A.K.; Ali, A.; El-Kady, M.F.; Hafez, H.M.; Mettenleiter, T.C.; Beer, M.; Harder, T. Improved Subtyping of Avian Influenza Viruses Using an RT-qPCR-Based Low Density Array: ‘Riems Influenza a Typing Array’, Version 2 (RITA-2). Viruses 2022, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Slomka, M.J.; Reid, S.M.; Thomas, S.S.; Mahmood, S.; Byrne, A.M.P.; Cooper, J.; Russell, C.; Mollett, B.C.; Agyeman-Dua, E.; et al. Development and Application of Real-Time PCR Assays for Specific Detection of Contemporary Avian Influenza Virus Subtypes N5, N6, N7, N8, and N9. Avian Dis. 2018, 63, 209–218. [Google Scholar] [CrossRef]
- Hoffmann, B.; Hoffmann, D.; Henritzi, D.; Beer, M.; Harder, T.C. Riems influenza a typing array (RITA): An RT-qPCR-based low density array for subtyping avian and mammalian influenza a viruses. Sci. Rep. 2016, 6, 27211. [Google Scholar] [CrossRef]
- Slomka, M.J.; Coward, V.J.; Banks, J.; Löndt, B.Z.; Brown, I.H.; Voermans, J.; Koch, G.; Handberg, K.J.; Jørgensen, P.H.; Cherbonnel-Pansart, M.; et al. Identification of Sensitive and Specific Avian Influenza Polymerase Chain Reaction Methods Through Blind Ring Trials Organized in the European Union. Avian Dis. 2007, 51, 227–234. [Google Scholar] [CrossRef]
- Tang, Y.; Anne Hapip, C.; Liu, B.; Fang, C.T. Highly sensitive TaqMan RT-PCR assay for detection and quantification of both lineages of West Nile virus RNA. J. Clin. Virol. 2006, 36, 177–182. [Google Scholar] [CrossRef]
- Cavrini, F.; Della Pepa, M.E.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. A rapid and specific real-time RT-PCR assay to identify Usutu virus in human plasma, serum, and cerebrospinal fluid. J. Clin. Virol. 2011, 50, 221–223. [Google Scholar] [CrossRef]
- Del Amo, J.; Sotelo, E.; Fernández-Pinero, J.; Gallardo, C.; Llorente, F.; Agüero, M.; Jiménez-Clavero, M.A. A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- EFSA; ECDC; EURL; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Mirinaviciute, G.; Niqueux, É.; et al. Scientific report: Avian influenza overview December 2022–March 2023. EFSA J. 2023, 21, 43. [Google Scholar] [CrossRef]
- EFSA; ECDC; EURL; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinaviciute, G.; Niqueux, É.; Stahl, K.; et al. Scientific report: Avian influenza overview March–April 2023. EFSA J. 2023, 21, 45. [Google Scholar] [CrossRef]
- EFSA. Annual report of the scientific network on animal health 2023. EFSA Support. Publ. 2023, 20, 8550E. [Google Scholar] [CrossRef]
- Trogu, T.; Bellini, S.; Canziani, S.; Carrera, M.; Chiapponi, C.; Chiari, M.; Farioli, M.; Fusaro, A.; Savegnago, E.; Nucci, A.; et al. Surveillance for avian influenza in wild birds in the Lombardy region (Italy) in the period 2022–2024. Viruses 2024, 16, 1668. [Google Scholar] [CrossRef]
- Zenatello, M.; Baccetti, N.; Luchetta, A. International Waterbird Census Report Italy 2009–2018; Technical Report; Institute for Environmental Protection and Research (ISPRA): Ozzano Emilia (BO), Italy, 2021. [Google Scholar] [CrossRef]
- Brambilla, M.; Longoni, V.; Calvi, G.; Ambrosini, R.; Rubolini, D. Il Censimento International Waterbird Census (IWC) in Lombardia nel 2023; Regione Lombardia: Milano, Italy, 2023. [Google Scholar]
- Pellitteri Rosa, D.; Longoni, V.; Gazzola, A.; Sotta, A. Il Censimento International Waterbird Census (IWC) in Lombardia nel 2024; Regione Lombardia: Milano, Italy, 2024. [Google Scholar]
- EFSA; ECDC; EURL; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Ståhl, K.; Staubach, C.; et al. Scientific report: Avian influenza overview December 2023–March 2024. EFSA J. 2024, 22, 69. [Google Scholar] [CrossRef]
- EFSA; ECDC; EURL; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Ståhl, K.; et al. Scientific report: Avian influenza overview September–December 2023. EFSA J. 2023, 21, 62. [Google Scholar] [CrossRef]
- Webby, R.J.; Uyeki, T.M. An update on highly pathogenic avian influenza A(H5N1) virus, clade 2.3.4.4b. J. Infect. Dis. 2024, 230, 533–542. [Google Scholar] [CrossRef]
- EFSA; ECDC; EURL; Brown, I.; Kuiken, T.; Mulatti, P.; Smietanka, K.; Staubach, C.; Stroud, D.; Therkildsen, O.R.; et al. Scientific report: Avian influenza overview September–November 2017. EFSA J. 2017, 15, 70. [Google Scholar] [CrossRef]
- Reinartz, R.; Slaterus, R.; Foppen, R.; Stahl, J. Update of the target list of wild bird species for passive surveillance of H5 HPAI viruses in the EU. EFSA Support. Publ. 2024, 21, 8807E. [Google Scholar] [CrossRef]
- Webster, R.G.; Guan, Y.; Peiris, M.; Walker, D.; Krauss, S.; Zhou, N.N.; Govorkova, E.A.; Ellis, T.M.; Dyrting, K.C.; Sit, T.; et al. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 2002, 76, 118–126. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, P.; Jia, B.; Xu, C.; Wei, L.; Shan, F.; Luo, K.; Xin, C.; Zhang, K.; Liao, M. Pathogenicity in quails and mice of H5N1 highly pathogenic avian influenza viruses isolated from ducks. Vet. Microbiol. 2011, 152, 258–265. [Google Scholar] [CrossRef]
- Bertran, K.; Dolz, R.; Busquets, N.; Gamino, V.; Vergara-Alert, J.; Chaves, A.J.; Ramis, A.; Abad, X.F.; Höfle, U.; Majó, N. Pathobiology and transmission of highly and low pathogenic avian influenza viruses in European quail (Coturnix c. coturnix). Vet. Res. 2013, 44, 23. [Google Scholar] [CrossRef] [PubMed]
- Perkins, L.E.; Swayne, D.E. Pathogenicity of a Hong Kong–origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeons. Avian Dis. 2002, 46, 53–63. [Google Scholar] [CrossRef]
- Cardona, C.J.; Xing, Z.; Sandrock, C.E.; Davis, C.E. Avian influenza in birds and mammals. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 255–273. [Google Scholar] [CrossRef]
- Abolnik, C. A current review of avian influenza in pigeons and doves (Columbidae). Vet. Microbiol. 2014, 170, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, H.; Hubálek, Z.; Bakonyi, T.; Nowotny, N. Zoonotic mosquito-borne flaviviruses: Worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet. Microbiol. 2010, 140, 271–280. [Google Scholar] [CrossRef]
- Komar, N.; Panella, N.A.; Burns, J.E.; Dusza, S.W.; Mascarenhas, T.M.; Talbot, T.O. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg. Infect. Dis. 2001, 7, 621–623. [Google Scholar] [CrossRef] [PubMed]
- ECDC. West Nile virus infection. In ECDC. Annual epidemiological Report for 2018; European Centre for Disease Prevention and Control, ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Epidemiological Bulletins of WND n. 18 of 9 November 2023—National Results. Available online: https://www.epicentro.iss.it/westnile/bollettino/Bollettino_WND_2023_18.pdf (accessed on 27 December 2024).
- Epidemiological Bulletins of WND n. 18 of 31 October 2024—National Results. Available online: https://www.epicentro.iss.it/westnile/bollettino/Bollettino_WND_2024_18.pdf (accessed on 27 December 2024).
- Weissenböck, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef]
- Pérez-Ramírez, E.; Llorente, F.; Jiménez-Clavero, M.Á. Experimental infections of wild birds with West Nile virus. Viruses 2014, 6, 752–781. [Google Scholar] [CrossRef]
- Jourdain, E.; Gauthier-Clerc, M.; Bicout, D.J.; Sabatier, P. Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg. Infect. Dis. 2007, 13, 365–372. [Google Scholar] [CrossRef]
- McLean, R.G.; Ubico, S.R.; Bourne, D.; Komar, N. West Nile virus in livestock and wildlife. In Japanese Encephalitis and West Nile Viruses; Current Topics in Microbiology and Immunology; Mackenzie, J.S., Barrett, A.D.T., Deubel, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 267, pp. 271–308. [Google Scholar]
- Fall, G.; Di Paola, N.; Faye, M.; Dia, M.; Freire, C.C.M.; Loucoubar, C.; Zanotto, P.M.A.; Faye, O.; Sall, A.A. Biological and phylogenetic characteristics of West African lineages of West Nile virus. PLoS Negl. Trop. Dis. 2017, 11, e0006078. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Iapaolo, F.; Monaco, F.; Fusco, G.; de Martinis, C.; Portanti, O.; Di Gennaro, A.; Curini, V.; Polci, A.; Berjaoui, S.; et al. West Nile virus Lineage 1 in Italy: Newly introduced or a re-occurrence of a previously circulating strain? Viruses 2022, 14, 64. [Google Scholar] [CrossRef]
- Mencattelli, G.; Iapaolo, F.; Polci, A.; Marcacci, M.; Di Gennaro, A.; Teodori, L.; Curini, V.; Di Lollo, V.; Secondini, B.; Scialabba, S.; et al. West Nile virus lineage 2 overwintering in Italy. Trop. Med. Infect. Dis. 2022, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Johnson, N. The role of birds of prey in West Nile Virus epidemiology. Vaccines 2020, 8, 550. [Google Scholar] [CrossRef]
- Rizzoli, A.; Bolzoni, L.; Chadwick, E.A.; Capelli, G.; Montarsi, F.; Grisenti, M.; de la Puente, J.M.; Muñoz, J.; Figuerola, J.; Soriguer, R.; et al. Understanding West Nile virus ecology in Europe: Culex pipiens host feeding preference in a hotspot of virus emergence. Parasit. Vectors 2015, 8, 213. [Google Scholar] [CrossRef]
- Jourdain, E.; Toussaint, Y.; Leblond, A.; Bicout, D.; Sabatier, P.; Gauthier-Clerc, M. Bird species potentially involved in introduction, amplification, and spread of West Nile virus in a mediterranean wetland, the Camargue (southern France). Vector Borne Zoonotic Dis. 2007, 7, 15–33. [Google Scholar] [CrossRef]
- Körsten, C.; Al-Hosary, A.A.; Holicki, C.M.; Schäfer, M.; Tews, B.A.; Vasić, A.; Ziegler, U.; Groschup, M.H.; Silaghi, C. Simultaneous coinfections with West Nile virus and Usutu virus in Culex pipiens and Aedes vexans mosquitoes. Transbound. Emerg. Dis. 2023, 2023, 6305484. [Google Scholar] [CrossRef]
- Santos, P.D.; Michel, F.; Wylezich, C.; Höper, D.; Keller, M.; Holicki, C.M.; Szentiks, C.A.; Eiden, M.; Muluneh, A.; Neubauer-Juric, A.; et al. Co-infections: Simultaneous detections of West Nile virus and Usutu virus in birds from Germany. Transbound. Emerg. Dis. 2022, 69, 776–792. [Google Scholar] [CrossRef]
- Longoni, V.; Rubolini, D.; Pinoli, G.; Fasola, M. Population trends of wintering waterbirds in Lombardy between 2002 and 2013. Riv. Ital. Di Ornitol. 2015, 84, 3–66. [Google Scholar] [CrossRef]
- D'Antoni, S.; Battisti, C.; Cenni, M.; Rossi, G.L. Contributi per la tutela della biodiversità delle zone umide. Rapp. ISPRA 2011, 153, 461. [Google Scholar] [CrossRef]
- Simonin, Y. Circulation of West Nile virus and Usutu virus in Europe: Overview and challenges. Viruses 2024, 16, 599. [Google Scholar] [CrossRef]
- National Zootechnical Database Italy. Italian National Zootechnical Registry, Poultry. Available online: https://www.vetinfo.it/j6_statistiche/#/report-pbi/41 (accessed on 2 January 2025).
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly pathogenic avian influenza viruses at the wild–domestic bird interface in Europe: Future directions for research and surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Bertran, K.; Kwon, J.-H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union One Health 2022 zoonoses report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Rose, K.; Newman, S.; Uhart, M.; Lubroth, J. Wild Bird Highly Pathogenic Avian Influenza Surveillance: Sample Collection from Healthy, Sick and Dead Birds; Food & Agriculture Organization (FAO): Rome, Italy, 2006; Volume 4, pp. 1–56. [Google Scholar]
- Giglia, G.; Mencattelli, G.; Lepri, E.; Agliani, G.; Gobbi, M.; Gröne, A.; van den Brand, J.M.A.; Savini, G.; Mandara, M.T. West Nile virus and Usutu virus: A post-mortem monitoring study in wild birds from rescue centers, central Italy. Viruses 2022, 14, 1994. [Google Scholar] [CrossRef]
- Mancuso, E.; Cecere, J.G.; Iapaolo, F.; Di Gennaro, A.; Sacchi, M.; Savini, G.; Spina, F.; Monaco, F. West Nile and Usutu virus introduction via migratory birds: A retrospective analysis in Italy. Viruses 2022, 14, 416. [Google Scholar] [CrossRef]
- Hoye, B.J.; Munster, V.J.; Nishiura, H.; Klaassen, M.; Fouchier, R.A. Surveillance of wild birds for avian influenza virus. Emerg. Infect. Dis. 2010, 16, 1827–1834. [Google Scholar] [CrossRef]
- Poulson, R.L.; Brown, J.D. Wild bird surveillance for avian influenza virus. In Animal Influenza Virus: Methods and Protocols, 3rd ed.; Spackman, E., Ed.; Humana Press: New York, NY, USA, 2020; pp. 93–112. [Google Scholar]
| Order | Species | Total (2023) | Positivities for AIV | Type |
|---|---|---|---|---|
| Charadriiformes | Black-headed Gull (Chroicocephalus ridibundus) | 17 | 13 pos | H5N1 HPAIV, clade 2.3.4.4b |
| Galliformes | Common Quail (Coturnix coturnix) | 1 | 1 pos | H5N1 HPAIV, clade 2.3.4.4b |
| Columbiformes | Common Woodpigeon (Columba palumbus) | 69 | 1 pos | H5N1 HPAIV, clade 2.3.4.4b |
| Total | 15 |
| Order | Species | Total (2023) | Positivities for WNV Only | Positivities for USUV Only | Co-Infection Condition |
|---|---|---|---|---|---|
| Accipitriformes | Northern Goshawk (Accipiter gentilis) | 7 | 4 pos WNV-L2 | - | - |
| Eurasian Buzzard (Buteo buteo) | 4 | - | - | 1 pos WNV-L2 and USUV | |
| Apodiformes | Alpine Swift (Tachymarptis melba) | 9 | 1 pos WNV-L2 | - | 1 pos WNV-L2 and USUV |
| Columbiformes | Common Woodpigeon (Columba palumbus) | 69 | - | - | 1 pos WNV-L2 and USUV |
| Falconiformes | Common Kestrel (Falco tinnunculus) | 25 | 1 pos WNV-L2 1 pos WNV-L1 | - | 1 pos WNV-L2 and USUV |
| Passeriformes | European Goldfinch (Carduelis carduelis) | 6 | 1 pos WNV-L2 | - | - |
| Hooded Crow (Corvus cornix) | 72 | 4 pos WNV-L2 | - | 3 pos WNV-L2 and USUV | |
| Eurasian Magpie (Pica pica) | 51 | 2 pos WNV-L2 | - | 1 pos WNV-L2 and USUV | |
| Common Blackbird (Turdus merula) | 130 | - | 1 pos | 3 pos WNV-L2 and USUV | |
| Total | 14 | 1 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapi, M.C.; Martin, A.M.M.; Lelli, D.; Lavazza, A.; Raimondi, S.; Farioli, M.; Chiari, M.; Grilli, G. Virological Passive Surveillance of Avian Influenza and Arboviruses in Wild Birds: A Two-Year Study (2023–2024) in Lombardy, Italy. Microorganisms 2025, 13, 958. https://doi.org/10.3390/microorganisms13050958
Rapi MC, Martin AMM, Lelli D, Lavazza A, Raimondi S, Farioli M, Chiari M, Grilli G. Virological Passive Surveillance of Avian Influenza and Arboviruses in Wild Birds: A Two-Year Study (2023–2024) in Lombardy, Italy. Microorganisms. 2025; 13(5):958. https://doi.org/10.3390/microorganisms13050958
Chicago/Turabian StyleRapi, Maria Cristina, Ana Maria Moreno Martin, Davide Lelli, Antonio Lavazza, Stefano Raimondi, Marco Farioli, Mario Chiari, and Guido Grilli. 2025. "Virological Passive Surveillance of Avian Influenza and Arboviruses in Wild Birds: A Two-Year Study (2023–2024) in Lombardy, Italy" Microorganisms 13, no. 5: 958. https://doi.org/10.3390/microorganisms13050958
APA StyleRapi, M. C., Martin, A. M. M., Lelli, D., Lavazza, A., Raimondi, S., Farioli, M., Chiari, M., & Grilli, G. (2025). Virological Passive Surveillance of Avian Influenza and Arboviruses in Wild Birds: A Two-Year Study (2023–2024) in Lombardy, Italy. Microorganisms, 13(5), 958. https://doi.org/10.3390/microorganisms13050958









