Isolation and Characterization of β-Phenylethylamine-Producing Lactic Acid Bacteria from Dairy Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dairy Samples
2.2. Bacterial Strains and Culture Conditions
2.3. Isolation of PEA-Producing Microorganisms from Dairy Samples
2.4. DNA Manipulation
2.5. Identification of Isolates
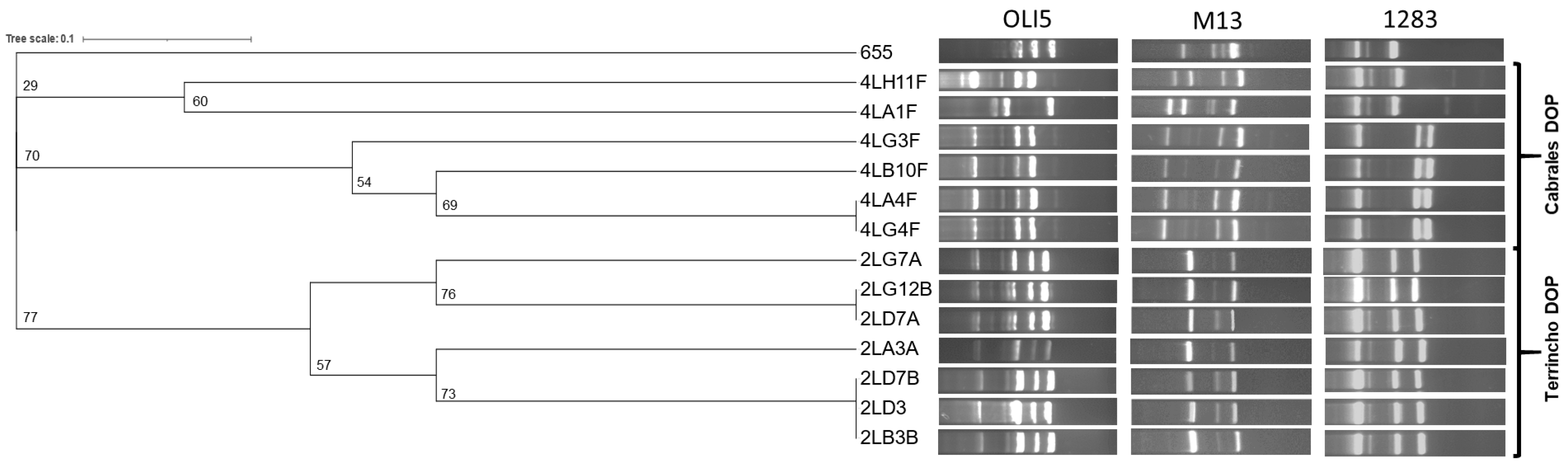
2.6. RAPD-PCR Typing
2.7. Technological Characterization
2.7.1. Determination of Milk Coagulating Capacity
2.7.2. Determination of Proteolytic Activity
2.7.3. Lactose and Citrate Utilization
2.7.4. Production of Volatile Compounds
2.7.5. Production of Organic Acids
2.8. Safety Evaluation
2.8.1. Antimicrobial Resistance
2.8.2. Presence of Virulence-Related Genes
2.8.3. Production of Biogenic Amines
2.9. Construction of a tdcA Mutant
3. Results
3.1. Isolation of PEA-Producing Strains
3.2. Verification of PEA-Producing Capacity in Culture Broth
3.3. PEA-Producing Isolates: Identification at the Species Level by 16S rRNA Gene Sequencing
3.4. RAPD-PCR Typing
3.5. Technological Characterization of PEA-Producing E. durans Isolates
3.5.1. Lactose and Citrate Utilization
3.5.2. Proteolytic Activity
3.5.3. Milk Clotting Capacity
3.5.4. Production of Volatile Compounds
3.5.5. Production of Organic Acids
3.6. Safety Assessment of PEA-Producing E. durans Isolates
3.6.1. Antibiotic Susceptibility
3.6.2. Virulence Factors
3.6.3. Production of Biogenic Amines
3.7. Typing of PEA-Producing E. durans Isolates
3.8. The Tyrosine Decarboxylase Gene (tdcA) Is Responsible for PEA Production in E. durans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace amines and their receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
- Shemiakova, T.S.; Efimova, E.V.; Gainetdinov, R.R. TAARs as novel therapeutic targets for the treatment of depression: A narrative review of the interconnection with monoamines and adult neurogenesis. Biomedicines 2024, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol. Ther. 2017, 180, 161–180. [Google Scholar] [CrossRef]
- Khan, M.Z.; Nawaz, W. The emerging roles of human trace amines and human trace amine-associated receptors (hTAARs) in central nervous system. Biomed. Pharmacother. 2016, 83, 439–449. [Google Scholar] [CrossRef]
- Zsilla, G.; Hegyi, D.E.; Baranyi, M.; Vizi, E.S. 3,4-Methylenedioxymethamphetamine, mephedrone, and β-phenylethylamine release dopamine from the cytoplasm by means of transporters and keep the concentration high and constant by blocking reuptake. Eur. J. Pharmacol. 2018, 837, 72–80. [Google Scholar] [CrossRef]
- Yilmaz, C.; Gökmen, V. Neuroactive compounds in foods: Occurrence, mechanism and potential health effects. Food Res. Int. 2020, 128, 108744. [Google Scholar] [CrossRef]
- Batista-Lima, F.J.; Rodrigues, F.M.D.S.; Gadelha, K.K.L.; de Oliveira, D.M.N.; Carvalho, E.F.; Oliveira, T.L.; Nóbrega, F.C.; Brito, T.S.; Magalhães, P.J.C. Dual excitatory and smooth muscle-relaxant effect of β-phenylethylamine on gastric fundus strips in rats. Clin. Exp. Pharmacol. Physiol. 2019, 46, 40–47. [Google Scholar] [CrossRef]
- Pae, C.-U.; Wang, S.-M. Trace amine receptors and mood disorders. In Trace Amines and Neurological Disorders; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–338. ISBN 9780128036167. [Google Scholar]
- Freyberg, Z.; Saavedra, J.M. Trace amines and trace amine-associated receptors: A new frontier in cell signaling. Cell. Mol. Neurobiol. 2020, 40, 189–190. [Google Scholar] [CrossRef]
- Halff, E.F.; Rutigliano, G.; Garcia-Hidalgo, A.; Howes, O.D. Trace amine-associated receptor 1 (TAAR1) agonism as a new treatment strategy for schizophrenia and related disorders. Trends Neurosci. 2023, 46, 60–74. [Google Scholar] [CrossRef]
- Merino del Portillo, M.; Clemente-Suárez, V.J.; Ruisoto, P.; Jimenez, M.; Ramos-Campo, D.J.; Beltran-Velasco, A.I.; Martínez-Guardado, I.; Rubio-Zarapuz, A.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional modulation of the gut–brain axis: A comprehensive review of dietary interventions in depression and anxiety management. Metabolites 2024, 14, 549. [Google Scholar] [CrossRef]
- Gutman, D.A.; Owens, M.J. Serotonin and norepinephrine transporter binding profile of SSRIs. Essent. Psychopharmacol. 2006, 7, 35–41. [Google Scholar] [PubMed]
- Lee, Y.-J.; Kim, H.R.; Lee, C.Y.; Hyun, S.-A.; Ko, M.Y.; Lee, B.-S.; Hwang, D.Y.; Ka, M. 2-Phenylethylamine (PEA) ameliorates corticosterone-induced depression-like phenotype via the BDNF/TrkB/CREB signaling pathway. Int. J. Mol. Sci. 2020, 21, 9103. [Google Scholar] [CrossRef] [PubMed]
- Irsfeld, M.; Spadafore, M.; Prüß, B.M. β-phenylethylamine, a small molecule with a large impact. Webmedcentral 2013, 4, 4409. [Google Scholar] [PubMed]
- Aucoin, M.; LaChance, L.; Naidoo, U.; Remy, D.; Shekdar, T.; Sayar, N.; Cardozo, V.; Rawana, T.; Chan, I.; Cooley, K. Diet and anxiety: A scoping review. Nutrients 2021, 13, 4418. [Google Scholar] [CrossRef]
- Kunugi, H. Depression and lifestyle: Focusing on nutrition, exercise, and their possible relevance to molecular mechanisms. Psychiatry Clin. Neurosci. 2023, 77, 420–433. [Google Scholar] [CrossRef]
- Latif, R. Chocolate/cocoa and human health: A review. Neth. J. Med. 2013, 71, 63–68. [Google Scholar]
- Garbarino, S.; Garbarino, E.; Lanteri, P. Cyrcadian rhythm, mood, and temporal patterns of eating chocolate: A scoping review of physiology, findings, and future directions. Nutrients 2022, 14, 3113. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Deus, V.L.; Tavano, O.L.; Gloria, M.B.A. In vitro bioaccessibility of amino acids and bioactive amines in 70% cocoa dark chocolate: What you eat and what you get. Food Chem. 2021, 343, 128397. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of compounds in preworkout supplements (“boosters”). Eur. J. Pharmacol. 2019, 859, 172515. [Google Scholar] [CrossRef]
- Wu, T.; Liu, R.; Zhang, L.; Rifky, M.; Sui, W.; Zhu, Q.; Zhang, J.; Yin, J.; Zhang, M. Dietary intervention in depression—A review. Food Funct. 2022, 13, 12475–12486. [Google Scholar] [CrossRef] [PubMed]
- Pinckaers, N.E.T.; Blankesteijn, W.M.; Mircheva, A.; Shi, X.; Opperhuizen, A.; Van Schooten, F.; Vrolijk, M.F. In vitro activation of human adrenergic receptors and trace amine-associated receptor 1 by Phenethylamine analogues present in food supplements. Nutrients 2024, 16, 1567. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA Panel on Biological Hazards (BIOHAZ). EFSA J. 2011, 9, 2393–2486. [Google Scholar]
- del Rio, B.; Fernandez, M.; Redruello, B.; Ladero, V.; Alvarez, M.A. New insights into the toxicological effects of dietary biogenic amines. Food Chem. 2024, 435, 137558. [Google Scholar] [CrossRef]
- Şanlı, T.; Şenel, E. Formation of biogenic amines in cheese. In Processing and Impact on Active Components in Food; Elsevier: Amsterdam, The Netherlands, 2015; pp. 223–230. ISBN 9780124047099. [Google Scholar]
- Bogdanović, T.; Petričević, S.; Brkljača, M.; Listeš, I.; Pleadin, J. Biogenic amines in selected foods of animal origin obtained from the Croatian retail market. Food Addit. Contam. Part A 2020, 37, 815–830. [Google Scholar] [CrossRef]
- Redruello, B.; Ladero, V.; Cuesta, I.; Alvarez-Buylla, J.R.; Martin, M.C.; Fernandez, M.; Alvarez, M.A. A fast, reliable, ultra high performance liquid chromatography method for the simultaneous determination of amino acids, biogenic amines and ammonium ions in cheese, using diethyl ethoxymethylenemalonate as a derivatising agent. Food Chem. 2013, 139, 1029–1035. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Fernandez, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. The biogenic amine tryptamine, unlike β-phenylethylamine, shows in vitro cytotoxicity at concentrations that have been found in foods. Food Chem. 2020, 331, 127303. [Google Scholar] [CrossRef]
- Manca, G.; Ru, A.; Siddi, G.; Mocci, A.M.; Murittu, G.; De Santis, E.P.L. Biogenic amines content in Fiore Sardo cheese in relation to free amino acids and physicochemical characteristics. Ital. J. Food Saf. 2020, 9, 8457. [Google Scholar] [CrossRef]
- Marcobal, A.; De Las Rivas, B.; Landete, J.M.; Tabera, L.; Munoz, R. Tyramine and phenylethylamine biosynthesis by food bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef]
- Landete, J.M.; Pardo, I.; Ferrer, S. Tyramine and phenylethylamine production among lactic acid bacteria isolated from wine. Int. J. Food Microbiol. 2007, 115, 364–368. [Google Scholar] [CrossRef]
- Marcobal, A.; de las Rivas, B.; Munoz, R. First genetic characterization of a bacterial beta-phenylethylamine biosynthetic enzyme in Enterococcus faecium RM58. FEMS Microbiol. Lett. 2006, 258, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Beutling, D.; Walter, D. 2-Phenylethylamine formation by enterococci in vitro. Eur. Food Res. Technol. 2002, 215, 240–242. [Google Scholar] [CrossRef]
- Bonetta, S.; Carraro, E.; Coisson, J.D.; Travaglia, F.; Arlorio, M. Detection of biogenic amine producer bacteria in a typical Italian goat cheese. J. Food Prot. 2008, 71, 205–209. [Google Scholar] [CrossRef]
- Gaglio, R.; Couto, N.; Marques, C.; de Fatima Silva Lopes, M.; Moschetti, G.; Pomba, C.; Settanni, L. Evaluation of antimicrobial resistance and virulence of enterococci from equipment surfaces, raw materials, and traditional cheeses. Int. J. Food Microbiol. 2016, 236, 107–114. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef]
- Pöntinen, A.K.; Top, J.; Arredondo-Alonso, S.; Tonkin-Hill, G.; Freitas, A.R.; Novais, C.; Gladstone, R.A.; Pesonen, M.; Meneses, R.; Pesonen, H.; et al. Apparent nosocomial adaptation of Enterococcus faecalis predates the modern hospital era. Nat. Commun. 2021, 12, 1523. [Google Scholar] [CrossRef]
- Durfee, T.; Nelson, R.; Baldwin, S.; Plunkett, G.; Burland, V.; Mau, B.; Petrosino, J.F.; Qin, X.; Muzny, D.M.; Ayele, M.; et al. The complete genome sequence of Escherichia coli DH10B: Insights into the biology of a laboratory workhorse. J. Bacteriol. 2008, 190, 2597–2606. [Google Scholar] [CrossRef]
- Redruello, B.; Saidi, Y.; Sampedro, L.; Ladero, V.; del Rio, B.; Alvarez, M.A. GABA-producing Lactococcus lactis strains isolated from camel’s milk as starters for the production of GABA-enriched cheese. Foods 2021, 10, 633. [Google Scholar] [CrossRef]
- Rodríguez, J.; Vázquez, L.; Flórez, A.B.; Mayo, B. Phenotype testing, genome analysis, and metabolic interactions of three lactic acid bacteria strains existing as a consortium in a naturally fermented milk. Front. Microbiol. 2022, 13, 1000683. [Google Scholar] [CrossRef]
- Sarquis, A.; Villarreal, L.A.; Ladero, V.; Maqueda, M.; del Rio, B.; Alvarez, M.A. Enterocin AS-48 inhibits the growth of -and biofilm formation by- lactic acid bacteria responsible for the accumulation of biogenic amines in cheese. Int. J. Food Sci. Technol. 2023, 58, 5865–5873. [Google Scholar] [CrossRef]
- Sarquis, A.; Ladero, V.; Díaz, M.; Sánchez-Llana, E.; Fernández, M.; Alvarez, M.A. The gene cluster associated with strong biofilm-formation capacity by histamine-producing Lentilactobacillus parabuchneri encodes a sortase-mediated pilus and is located on a plasmid. Food Res. Int. 2024, 175, 113777. [Google Scholar] [CrossRef] [PubMed]
- del Rio, B.; Alvarez-Sieiro, P.; Redruello, B.; Martin, M.C.; Fernandez, M.; Ladero, V.; Alvarez, M.A. Lactobacillus rossiae strain isolated from sourdough produces putrescine from arginine. Sci. Rep. 2018, 8, 3989. [Google Scholar] [CrossRef] [PubMed]
- del Rio, B.; Sánchez-Llana, E.; Martínez, N.; Fernández, M.; Ladero, V.; Alvarez, M.A. Isolation and characterization of Enterococcus faecalis-infecting bacteriophages from different cheese types. Front. Microbiol. 2021, 11, 592172. [Google Scholar] [CrossRef]
- Ladero, V.; Linares, D.M.; del Rio, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Draft genome sequence of the tyramine producer Enterococcus durans strain IPLA 655. Genome Announc 2013, 1, e00265-13. [Google Scholar] [CrossRef]
- Hevia, A.; Martinez, N.; Ladero, V.; Alvarez, M.A.; Margolles, A.; Sanchez, B. An extracellular serine/threonine-rich protein from Lactobacillus plantarum NCIMB 8826 is a novel aggregation-promoting factor with affinity to mucin. Appl. Environ. Microbiol. 2013, 79, 6059–6066. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Poyart, C.; Quesnes, G.; Trieu-Cuot, P. Sequencing the gene encoding manganese-dependent superoxide dismutase for rapid species identification of enterococci. J. Clin. Microbiol. 2000, 38, 415–418. [Google Scholar] [CrossRef]
- Ladero, V.; Fernandez, M.; Alvarez, M.A. Isolation and identification of tyramine-producing enterococci from human fecal samples. Can. J. Microbiol. 2009, 55, 215–218. [Google Scholar] [CrossRef]
- Akopyanz, N.; Bukanov, N.O.; Westblom, T.U.; Kresovich, S.; Berg, D.E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992, 20, 5137–5142. [Google Scholar] [CrossRef]
- Rodríguez, J.; González-Guerra, A.; Vázquez, L.; Fernández-López, R.; Flórez, A.B.; de la Cruz, F.; Mayo, B. Isolation and phenotypic and genomic characterization of Tetragenococcus spp. from two Spanish traditional blue-veined cheeses made of raw milk. Int. J. Food Microbiol. 2022, 371, 109670. [Google Scholar] [CrossRef]
- Fernandez, M.; Linares, D.M.; Alvarez, M.A. Sequencing of the tyrosine decarboxylase cluster of Lactococcus lactis IPLA 655 and the development of a PCR method for detecting tyrosine decarboxylating lactic acid bacteria. J. Food Prot. 2004, 67, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Ladero, V.; Redruello, B.; Sanchez-Llana, E.; del Rio, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. A PCR-DGGE method for the identification of histamine-producing bacteria in cheese. Food Control 2016, 63, 216–223. [Google Scholar] [CrossRef]
- Ladero, V.; Rattray, F.P.; Mayo, B.; Martin, M.C.; Fernandez, M.; Alvarez, M.A.; Martín, M.C.; Fernández, M.; Alvarez, M.A. Sequencing and transcriptional analysis of the biosynthesis gene cluster of putrescine-producing Lactococcus lactis. Appl. Environ. Microbiol. 2011, 77, 6409–6418. [Google Scholar] [CrossRef]
- Coton, M.; Romano, A.; Spano, G.; Ziegler, K.; Vetrana, C.; Desmarais, C.; Lonvaud-Funel, A.; Lucas, P.; Coton, E. Occurrence of biogenic amine-forming lactic acid bacteria in wine and cider. Food Microbiol. 2010, 27, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Reviriego, C.; Eaton, T.; Martin, R.; Jimenez, E.; Fernandez, L.; Gasson, M.J.; Rodriguez, J.M. Screening of virulence determinants in Enterococcus faecium strains isolated from breast milk. J. Hum. Lact. 2005, 21, 131–137. [Google Scholar] [CrossRef]
- Werner, G.; Fleige, C.; Geringer, U.; van Schaik, W.; Klare, I.; Witte, W. IS element IS16 as a molecular screening tool to identify hospital-associated strains of Enterococcus faecium. BMC Infect. Dis. 2011, 11, 80. [Google Scholar] [CrossRef]
- Rice, L.B.; Carias, L.; Rudin, S.; Vael, C.; Goossens, H.; Konstabel, C.; Klare, I.; Nallapareddy, S.R.; Huang, W.; Murray, B.E. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 2003, 187, 508–512. [Google Scholar] [CrossRef]
- Yu, K.; Hu, S.; Huang, J.; Mei, L.-H. A high-throughput colorimetric assay to measure the activity of glutamate decarboxylase. Enzyme Microb. Technol. 2011, 49, 272–276. [Google Scholar] [CrossRef]
- Saidi, Y.; del Rio, B.; Senouci, D.E.; Redruello, B.; Martinez, B.; Ladero, V.; Kihal, M.; Alvarez, M.A. Polyphasic characterisation of non-starter lactic acid bacteria from Algerian raw Camel’s milk and their technological aptitudes. Food Technol. Biotechnol. 2020, 58, 260–272. [Google Scholar] [CrossRef]
- Garcia-Vallve, S.; Palau, J.; Romeu, A. Horizontal gene transfer in glycosyl hydrolases inferred from codon usage in Escherichia coli and Bacillus subtilis. Mol. Biol. Evol. 1999, 16, 1125–1134. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Kempler, G.M.; McKay, L.L. Improved medium for detection of citrate-fermenting Streptococcus lactis subsp. diacetylactis. Appl. Environ. Microbiol. 1980, 39, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.D.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [CrossRef] [PubMed]
- EFSA Guidance on the safety assessment of Enterococcus faecium in animal nutrition. EFSA J. 2012, 10, 2682. [CrossRef]
- Linares, D.M.; Fernandez, M.; del Rio, B.; Ladero, V.; Martin, M.C.; Alvarez, M.A. The tyrosyl-tRNA synthetase like gene located in the tyramine biosynthesis cluster of Enterococcus durans is transcriptionally regulated by tyrosine concentration and extracellular pH. BMC Microbiol. 2012, 12, 23. [Google Scholar] [CrossRef]
- Rodríguez-Lucas, C.; Ladero, V. Enterococcal Phages: Food and Health Applications. Antibiotics 2023, 12, 842. [Google Scholar] [CrossRef]
- Buhnik-Rosenblau, K.; Matsko-Efimov, V.; Danin-Poleg, Y.; Franz, C.M.A.P.; Klein, G.; Kashi, Y. Biodiversity of Enterococcus faecalis based on genomic typing. Int. J. Food Microbiol. 2013, 165, 27–34. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A. Trace amines in neuropsychiatric disorders. In Trace Amines and Neurological Disorders; Elsevier: Amsterdam, The Netherlands, 2016; pp. 269–284. ISBN 9780128036167. [Google Scholar]
- Guru, S.; Thanki, S.; Thakkar, J.J. Analysing factors driving purchase and consumption behaviour towards organic food. Vis. J. Bus. Perspect. 2024, 1–17. [Google Scholar] [CrossRef]
- Bunkova, L.; Bunka, F.; Drab, V.; Kracmar, S.; Kuban, V. Effects of NaCl, lactose and availability of oxygen on tyramine production by the Enterococcus durans CCDM 53. Eur. Food Res. Technol. 2012, 234, 973–979. [Google Scholar] [CrossRef]
- Diaz, M.; Ladero, V.; del Rio, B.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Biofilm-forming capacity in biogenic amine-producing bacteria isolated from dairy products. Front. Microbiol. 2016, 7, 591. [Google Scholar] [CrossRef]
- Natrella, G.; Vacca, M.; Minervini, F.; Faccia, M.; De Angelis, M. A comprehensive review on the biogenic amines in cheeses: Their origin, chemical characteristics, hazard and reduction strategies. Foods 2024, 13, 2583. [Google Scholar] [CrossRef] [PubMed]
- Gatto, V.; Tabanelli, G.; Montanari, C.; Prodomi, V.; Bargossi, E.; Torriani, S.; Gardini, F. Tyrosine decarboxylase activity of Enterococcus mundtii: New insights into phenotypic and genetic aspects. Microb. Biotechnol. 2016, 9, 801–813. [Google Scholar] [CrossRef]
- Abarquero, D.; Flórez, A.B.; Tornadijo, M.E.; Fresno, J.M. Advantages and disadvantages of autochthonous enterococci strains for their potential use in cheese ripening: A preliminary study. Int. J. Food Sci. Technol. 2024, 59, 5675–5689. [Google Scholar] [CrossRef]
- Rocha, R.; Couto, N.; Pinto, R.P.; Vaz-Velho, M.; Fernandes, P.; Santos, J. Microbiological characterization of protected designation of origin Serra da Estrela cheese. Foods 2023, 12, 2008. [Google Scholar] [CrossRef]
- Tsanasidou, C.; Asimakoula, S.; Sameli, N.; Fanitsios, C.; Vandera, E.; Bosnea, L.; Koukkou, A.-I.; Samelis, J. Safety evaluation, biogenic amine formation, and enzymatic activity profiles of autochthonous enterocin-producing greek cheese isolates of the Enterococcus faecium/durans group. Microorganisms 2021, 9, 777. [Google Scholar] [CrossRef]
- Martino, G.P.; Espariz, M.; Gallina Nizo, G.; Esteban, L.; Blancato, V.S.; Magni, C. Safety assessment and functional properties of four enterococci strains isolated from regional Argentinean cheese. Int. J. Food Microbiol. 2018, 277, 1–9. [Google Scholar] [CrossRef]
- Grispoldi, L.; Karama, M.; El-Ashram, S.; Saraiva, C.; García-Díez, J.; Chalias, A.; Cenci-Goga, B.T. Evolution and antimicrobial resistance of enterococci isolated from Pecorino and goat cheese manufactured on-farm in an area facing constraints as per EU Regulation 1305/2013 in Umbria, Italy. Ital. J. Food Saf. 2022, 11, 10070. [Google Scholar] [CrossRef]
- Tadesse, B.T.; Svetlicic, E.; Zhao, S.; Berhane, N.; Jers, C.; Solem, C.; Mijakovic, I. Bad to the bone?—Genomic analysis of Enterococcus isolates from diverse environments reveals that most are safe and display potential as food fermentation microorganisms. Microbiol. Res. 2024, 283, 127702. [Google Scholar] [CrossRef]
- Lauková, A.; Tomáška, M.; Kmeť, V.; Strompfová, V.; Pogány Simonová, M.; Dvorožňáková, E. Slovak local Ewe’s milk lump cheese, a source of beneficial Enterococcus durans strain. Foods 2021, 10, 3091. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, D.-H.; Lim, H.-W.; Seo, K.-H. High prevalence of non-faecalis and non-faecium Enterococcus spp. in farmstead cheesehouse and their applicability as hygiene indicators. LWT 2020, 126, 109271. [Google Scholar] [CrossRef]
- Rampanti, G.; Ferrocino, I.; Harasym, J.; Foligni, R.; Cardinali, F.; Orkusz, A.; Milanović, V.; Franciosa, I.; Garofalo, C.; Mannozzi, C.; et al. Queijo Serra da Estrela PDO cheese: Investigation into its morpho-textural traits, microbiota, and volatilome. Foods 2022, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; Casella, T.; Gomes, E.S.; Nogueira, M.C.L.; De Dea Lindner, J.; Penna, A.L.B. Diversity of lactic acid bacteria isolated from Brazilian water buffalo mozzarella cheese. J. Food Sci. 2015, 80, M411–M417. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; Rodríguez, J.; Vázquez, L.; Flórez, A.B. Microbial interactions within the cheese ecosystem and their application to improve quality and safety. Foods 2021, 10, 602. [Google Scholar] [CrossRef]
- Graham, K.; Stack, H.; Rea, R. Safety, beneficial and technological properties of enterococci for use in functional food applications—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3836–3861. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Casella, S.; Hue, I.; Dousset, X.; Dora Gombossy de Melo Franco, B.; Todorov, S.D. Bacteriocinogenic potential and safety evaluation of non-starter Enterococcus faecium strains isolated from home made white brine cheese. Food Microbiol. 2014, 38, 228–239. [Google Scholar] [CrossRef]
- Aspri, M.; Bozoudi, D.; Tsaltas, D.; Hill, C.; Papademas, P. Raw donkey milk as a source of Enterococcus diversity: Assessment of their technological properties and safety characteristics. Food Control 2017, 73, 81–90. [Google Scholar] [CrossRef]
- Özkan, E.R.; Demirci, T.; Akın, N. In vitro assessment of probiotic and virulence potential of Enterococcus faecium strains derived from artisanal goatskin casing Tulum cheeses produced in central Taurus Mountains of Turkey. LWT 2021, 141, 110908. [Google Scholar] [CrossRef]
- Terzić-Vidojević, A.; Veljović, K.; Begović, J.; Filipić, B.; Popović, D.; Tolinački, M.; Miljković, M.; Kojić, M.; Golić, N. Diversity and antibiotic susceptibility of autochthonous dairy enterococci isolates: Are they safe candidates for autochthonous starter cultures? Front. Microbiol. 2015, 6, 160882. [Google Scholar] [CrossRef]
- Heikens, E.; Bonten, M.J.M.; Willems, R.J.L. Enterococcal surface protein Esp is important for biofilm formation of Enterococcus faecium E1162. J. Bacteriol. 2007, 189, 8233–8240. [Google Scholar] [CrossRef]
- Leavis, H.; Top, J.; Shankar, N.; Borgen, K.; Bonten, M.; van Embden, J.; Willems, R.J.L. A novel putative Enterococcal pathogenicity island linked to the esp virulence gene of Enterococcus faecium and associated with epidemicity. J. Bacteriol. 2004, 186, 672–682. [Google Scholar] [CrossRef]
- van Schaik, W.; Top, J.; Riley, D.R.; Boekhorst, J.; Vrijenhoek, J.E.P.; Schapendonk, C.M.E.; Hendrickx, A.P.A.; Nijman, I.J.; Bonten, M.J.M.; Tettelin, H.; et al. Pyrosequencing-based comparative genome analysis of the nosocomial pathogen Enterococcus faecium and identification of a large transferable pathogenicity island. BMC Genom. 2010, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Dinić, M.; Tolinački, M.; Mihajlović, S.; Terzić-Vidojević, A.; Bojić, S.; Djokić, J.; Golić, N.; Veljović, K. New insight into biofilm formation ability, the presence of virulence genes and probiotic potential of Enterococcus sp. dairy isolates. Front. Microbiol. 2018, 9, 302393. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Pisano, M.B.; Corda, A.; Fadda, M.E.; Piras, C. Genotypic and technological characterization of enterococci isolated from artisanal Fiore Sardo cheese. J. Dairy Res. 2004, 71, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Komprda, T.; Burdychova, R.; Dohnal, V.; Cwikova, O.; Sladkova, P.; Dvorackova, H. Tyramine production in Dutch-type semi-hard cheese from two different producers. Food Microbiol. 2008, 25, 219–227. [Google Scholar] [CrossRef]
- Tittarelli, F.; Perpetuini, G.; Di Gianvito, P.; Tofalo, R. Biogenic amines producing and degrading bacteria: A snapshot from raw ewes’ cheese. LWT 2019, 101, 1–9. [Google Scholar] [CrossRef]
- Ladero, V.; Fernández, M.; Calles-Enríquez, M.; Sánchez-Llana, E.; Cañedo, E.; Martín, M.C.; Alvarez, M.A. Is the production of the biogenic amines tyramine and putrescine a species-level trait in enterococci? Food Microbiol. 2012, 30, 132–138. [Google Scholar] [CrossRef]
- Andersen, G.; Marcinek, P.; Sulzinger, N.; Schieberle, P.; Krautwurst, D. Food sources and biomolecular targets of tyramine. Nutr. Rev. 2019, 77, 107–115. [Google Scholar] [CrossRef]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef]
- Gardini, F.; Martuscelli, M.; Caruso, M.C.; Galgano, F.; Crudele, M.A.; Favati, F.; Guerzoni, M.E.; Suzzi, G. Effects of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis. Int. J. Food Microbiol. 2001, 64, 105–117. [Google Scholar] [CrossRef]
- Gardini, F.; Bover-Cid, S.; Tofalo, R.; Belletti, N.; Gatto, V.; Suzzi, G.; Torriani, S. Modeling the aminogenic potential of Enterococcus faecalis EF37 in dry fermented sausages through chemical and molecular approaches. Appl. Environ. Microbiol. 2008, 74, 2740–2750. [Google Scholar] [CrossRef]
- Food and Drug Administration, FDA. EAFUS: A food additive database. EAFUS 1998, 1–153.
- European Comission E.C. List of flavouring substances. Off. J. Eur. Union 2012, L267/1, 1–161. [Google Scholar]
- Horne, S.M.; Ugrinov, A.; Prüβ, B.M. The food anti-microbials β-Phenylethylamine (-HCl) and ethyl acetoacetate do not change during the heating process. Antibiotics 2021, 10, 418. [Google Scholar] [CrossRef] [PubMed]


| Sample | Milk Type | Milk Treatment | Ripening Period | Number of Colonies Picked Off | Positive Colour Change | ||
|---|---|---|---|---|---|---|---|
| MRS | LGM17 | PCA | |||||
| Terrincho DOP | Sheep | Raw | Medium | 94 | 94 | - | 23 |
| Roncal DOP | Sheep | Raw | Long | 94 | 94 | - | 9 |
| Cabrales DOP | Cow, Sheep, Goat | Raw | Long | 94 | 94 | - | 19 |
| Sheep Cheese | Sheep | Raw | Very long | 94 | 94 | - | 0 |
| Cow’s milk curd | Cow | Raw | Very short | 94 | 94 | 94 | 12 |
| Sheep’s milk curd | Sheep | Raw | Very short | 94 | 94 | 94 | 0 |
| Strains | Relevant Genotype, Description, or Properties | Reference or Source |
|---|---|---|
| E. coli DH10B | F–, mcrA Δ(mrr-hsdRMS-mcrBC), ϕ80lacZΔM15, ΔlacX74, recA1, endA1, araD139, Δ (ara-leu)7697, galU, galK, λ–rpsL(StrR), nupG | [39] |
| E. coli IPLA1305 | E. coli DH10B harbouring pIPLA1305; AmpR, EryR, CmR | This work |
| Lactococcus lactis LEY6 | Positive control for technological traits | [40] |
| L. lactis subsp. lactis biovar diacetylactis LA1 | Citrate utilization positive control | [41] |
| E. faecalis V583 | Tyramine- and putrescine-producing strain | [42] |
| L. parabuchneri IPLA11150 | Histamine-producing strain | [43] |
| F. rossiae D87 | Putrescine-producing strain | [44] |
| E. faecalis CECT 795 | Strain carrying gelE gene, indicator of antibiotic susceptibility | CECT |
| E. faecium VR1 | Strain carrying efaA, esp and IS 16 genes | [45] |
| E. faecium DAPTO R | Strain carrying hylEfam | [45] |
| E. durans IPLA655 | Tyramine- and PEA-producing strain | [46] |
| E. durans IPLA655-Δtdc | E. durans 655 derivative in which the tdc gene was replaced with the cat gene; CmR | This work |
| Plasmids | Relevant genotype, description or properties | Reference or source |
| pMN1 | Cloning vector designed to make knockout mutants in Gram-positive bacteria; AmpR, EryR, CmR | [47] |
| pIPLA1305 | pMN1 with upstream and downstream flanking regions of tdc gene; AmpR, EryR, CmR | This work |
| Primers | Sequence 5′-3′ | Function/reference |
| 27F | AGAGTTTGATCMTGGCTCAG | Species identification [48] |
| 1492R | TACGGYTACCTTGTTACGACTT | Species identification [48] |
| sodA1 | CCITAYICITAYGAYGCIYTIGARCC | Identification of enterococcal species [49] |
| sodA2 | ARRTARTAIGCRTGYTCCCAIACRTC | Identification of enterococcal species [49] |
| OLI5 | AACGCGCAAC | Typification of the isolates by RAPD [50] |
| 1283 | GCGATCCCCA | Typification of the isolates by RAPD [51] |
| M13 | GAGGGTGGCGGTTCT | Typing of the isolates by RAPD [52] |
| tdc1 | AACTATCGTATGGATATCAACG | Detection of the tdcA gene [53] |
| tdc2 | TAGTCAACCATATTGAAATCTGG | Detection of the tdcA gene [53] |
| hdcDG-F | CCTGGTCAAGGCTATGGTGTATGGTC | Detection of the hdcA gene [54] |
| hdcDG-R | GGTTTCATCATTGCGTGTGCAAA | Detection of the hdcA gene [54] |
| AgmSq1 | CAAGATTTDTTCTGGGCHTTYTTCTC | Detection of the AgdI cluster [55] |
| AgmSq1 | TTGGHCCACARTCACGAACCCT | Detection of the AgdI cluster [55] |
| ODC1 | NCAYAARCAACAAGYNGG | Detection of the odc gene [56] |
| ODC2 | GRTANGGNTNNGCACCTTC | Detection of the odc gene [56] |
| TE9 | ACCCCGTATCATTGGTTT | Detection of the gelE gene [57] |
| TE10 | ACGCATTGCTTTTCCATC | Detection of the gelE gene [57] |
| TE34 | TTGCTAATGCTAGTCCACGACC | Detection of the esp gene [57] |
| TE36 | GCGTCAACACTTGCATTGCCGAA | Detection of the esp gene [57] |
| TE37 | AACAGATCCGCATGAATA | Detection of the efa gene [57] |
| TE38 | CATTTCATCATCTGATAGTA | Detection of the efa gene [57] |
| IS16-F | CATGTTCCACGAACCAGAG | Detection of IS16 gene [58] |
| IS16-R | TCAAAAAGTGGGCTTGGC | Detection of the IS16 gene [58] |
| hylEfm-F | GAGTAGAGGAATATCTTAGC | Detection of the hylEfm gene [59] |
| hylEfm-R | AGGCTCCAATTCTGT | Detection of the hylEfm gene [59] |
| KOtdcUpEcoRI, | CCCCATTTTGAATTCTACCAATTC | Amplification of the upstream tdc flanking regions (this work) |
| KOtdcUpBamHI, | GGGGGATCCGTTCTCAGCTTTGTCCCCG | Amplification of the upstream tdc flanking regions (this work) |
| KOtdcDwBamHI | GGGGGATCCAAATCTACGCAGATCAATTATTAGC | Amplification of the downstream tdc flanking regions (this work) |
| KOtdcDwHindIII | CTGGAAAAGCTTTTGACCAAGAGAAGTCACC | Amplification of the downstream tdc flanking regions (this work) |
| 655DtdcUP | GGTTGTCGTTAATACAATCC | Verification of the E. durans 655 Δtdc genotype (this work) |
| 655DtdcDW | CAATAACCGAAAGCAAACAG | Verification of the E. durans 655 Δtdc genotype (this work) |
| Sample | Analyzed Wells | PEA Producers in Resting Cells (Well) | PEA Producers in Broth | Species Identification (Number) |
|---|---|---|---|---|
| Terrincho | 23 | 23 | 15 | L. brevis (2) |
| E. faecalis (2) | ||||
| E. faecium (4) | ||||
| E. durans (7) | ||||
| Roncal | 9 | 2 | 2 | E. faecalis (2) |
| Cabrales | 19 | 11 | 11 | L. brevis (2) |
| E. faecalis (1) | ||||
| E. faecium (2) | ||||
| E. durans (6) | ||||
| Cow’s milk curd | 12 | 5 | 5 | E. faecalis (3) |
| E. faecium (2) |
| Species | Origin | Isolate | PEA (mM) |
|---|---|---|---|
| Enterococcus faecalis | Terrincho DOP | 2LG2 | 1.0 ± 0.1 |
| Enterococcus faecalis | Terrincho DOP | 2LH4 | 1.2 ± 0.2 |
| Enterococcus faecalis | Roncal DOP | 3LB10 | 0.5 ± 0.1 |
| Enterococcus faecalis | Roncal DOP | 3LF6 | 0.3 ± 0.1 |
| Enterococcus faecalis | Cabrales DOP | 4LH12F | 1.4 ± 0.2 |
| Enterococcus faecalis | Cow’s milk curd | 8ME4AF | 0.4 ± 0.1 |
| Enterococcus faecalis | Cow’s milk curd | 8MD4F | 0.3 ± 0.1 |
| Enterococcus faecalis | Cow’s milk curd | 8MH2F | 0.2 ± 0.0 |
| Enterococcus faecium | Terrincho DOP | 2LC8 | 1.2 ± 0.2 |
| Enterococcus faecium | Terrincho DOP | 2LD4 | 1.2 ± 0.2 |
| Enterococcus faecium | Terrincho DOP | 2LF6 | 1.1 ± 0.2 |
| Enterococcus faecium | Terrincho DOP | 2LF9 | 0.9 ± 0.1 |
| Enterococcus faecium | Cabrales DOP | 4LA7F | 1.7 ± 0.2 |
| Enterococcus faecium | Cabrales DOP | 4LF10F | 1.4 ± 0.2 |
| Enterococcus faecium | Cow’s milk curd | 8MG2AF | 0.2 ± 0.0 |
| Enterococcus faecium | Cow’s milk curd | 8MC4F | 0.2 ± 0.1 |
| Enterococcus durans | Terrincho DOP | 2LA3A | 1.2 ± 0.2 |
| Enterococcus durans | Terrincho DOP | 2LB3B | 1.3 ± 0.3 |
| Enterococcus durans | Terrincho DOP | 2LD3 | 1.3 ± 0.3 |
| Enterococcus durans | Terrincho DOP | 2LD7A | 1.3 ± 0.3 |
| Enterococcus durans | Terrincho DOP | 2LD7B | 1.3 ± 0.3 |
| Enterococcus durans | Terrincho DOP | 2LG7A | 1.4 ± 0.3 |
| Enterococcus durans | Terrincho DOP | 2LG12B | 1.3 ± 0.3 |
| Enterococcus durans | Cabrales DOP | 4LA1F | 1.7 ± 0.1 |
| Enterococcus durans | Cabrales DOP | 4LA4F | 1.7 ± 0.3 |
| Enterococcus durans | Cabrales DOP | 4LB10F | 1.7 ± 0.3 |
| Enterococcus durans | Cabrales DOP | 4LG3F | 2.0 ± 0.1 |
| Enterococcus durans | Cabrales DOP | 4LG4F | 1.7 ± 0.3 |
| Enterococcus durans | Cabrales DOP | 4LH11F | 1.7 ± 0.1 |
| Leviactobacillus brevis | Terrincho DOP | 2MB9 | 0.9 ± 0.0 |
| Leviactobacillus brevis | Terrincho DOP | 2MH3 | 0.9 ± 0.1 |
| Leviactobacillus brevis | Cabrales DOP | 4ME9F | 0.5 ± 0.1 |
| Leviactobacillus brevis | Cabrales DOP | 4MF9F | 0.5 ± 0.1 |
| E. durans Isolate | Lactose Utilization (BCP Test) | Citrate Utilization | Proteolytic Activity | Milk Coagulating Capacity |
|---|---|---|---|---|
| 4LA1F | + | − | High | Moderate |
| 4LA4F | + | − | High | Null |
| 4LB10F | + | − | High | Null |
| 4LG3F | + | − | High | Null |
| 4LG4F | + | − | High | Null |
| 4LH11F | + | − | Low | Low |
| 2LA3A | + | − | Null | Null |
| 2LB3B | + | − | High | Null |
| 2LD3 | + | − | High | Null |
| 2LD7A | + | − | Low | Null |
| 2LD7B | + | − | Low | Null |
| 2LG7A | + | − | Null | Null |
| 2LG12B | + | − | High | Null |
| IPLA655 | + | − | Low | Null |
| E. durans Isolate | Lactose | Glucose | Galactose | Citric Acid | Pyruvic Acid | Lactic Acid | Acetic Acid |
|---|---|---|---|---|---|---|---|
| 4LA1F | 387.1 ± 28.4 | 0 | 0.8 ± 0.05 | 0 | 0.5 ± 0.03 | 40.5 ± 2.3 | 9 ± 0.7 |
| 4LA4F | 820.3 ± 67.7 | 0 | 2.3 ± 0.3 | 27.6 ± 5.5 | 0.3 ± 0.1 | 17.5 ± 1.9 | 2.6 ± 0.7 |
| 4LB10F | 993.4 ± 72 | 0 | 2.4 ± 0.2 | 35.3 ± 2.3 | 0.2 ± 0.02 | 20 ± 1.9 | 2.4 ± 0.4 |
| 4LG3F | 801.7 ± 92.2 | 0 | 1.9 ± 0.2 | 28.5 ± 3.4 | 0.1 ± 0.03 | 17.8 ± 1.7 | 2.1 ± 0.3 |
| 4LG4F | 1048.1 ± 84.8 | 0 | 2.4 ± 0.2 | 37.6 ± 2.8 | 0.2 ± 0.03 | 20.6 ± 3.2 | 3 ± 0.5 |
| 4LH11F | 946.5 ± 20.7 | 0 | 1.6 ± 0.06 | 35.6 ± 0.8 | 0.2 ± 0.009 | 35.2 ± 0.1 | 1.9 ± 0.03 |
| 2LA3A | 821.2 ± 21.3 | 0 | 1.4 ± 0.08 | 30 ± 0.5 | 0.2 ± 0.05 | 22.9 ± 0.5 | 2.5 ± 0.5 |
| 2LB3B | 913.6 ± 54.4 | 0 | 1.57 ± 0.2 | 33.7 ± 0.6 | 0.2 ± 0.06 | 27.8 ± 1.2 | 2.1 ± 0.4 |
| 2LD3 | 995.3 ± 80 | 0 | 1.4 ± 0.1 | 35.1 ± 0.4 | 0.2 ± 0.04 | 25.2 ± 2.8 | 3.4 ± 0.8 |
| 2LD7A | 841.1 ± 14.1 | 0 | 1.6 ± 0.01 | 31.7 ± 0.9 | 0.15 ± 0.01 | 24.8 ± 1 | 2.4 ± 0.04 |
| 2LD7B | 814.7 ± 63.3 | 0 | 1.1 ± 0.1 | 28.4 ± 0.1 | 0.1 ± 0.01 | 19.4 ± 0.7 | 2.2 ± 0.2 |
| 2LG7A | 1112.5 ± 14.6 | 0 | 1.9 ± 0.2 | 41.2 ± 0.8 | 0.2 ± 0.01 | 17.6 ± 0.6 | 3.2 ± 0.2 |
| 2LG12B | 815.9 ± 83.6 | 0 | 1.1 ± 0.1 | 27.9 ± 0.1 | 0.1 ± 0.05 | 15.5 ± 5.8 | 1.9 ± 0.7 |
| IPLA655 | 1058.5 ± 97.7 | 0 | 1.6 ± 0.2 | 37 ± 0.02 | 0.1 ± 0.02 | 19.1 ± 1.7 | 2.2 ± 0.3 |
| Milk | 1075.8 ± 149.3 | 1.5 ± 0.2 | 2.71 ± 0.4 | 0 | 0 | 0 | 0 |
| Compound | 4LA1F | 4LA4F | 4LB10F | 4LG3F | 4LG4F | 4LH11F | 2LA3A | 2LB3B | 2LD3 | 2LD7A | 2LD7B | 2LG7A | 2LG12B | IPLA655 | MILK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||||||||||
| Acetaldehyde | 0.9 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.03 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.9 ± 0.4 | 2.1 ± 0.01 | 1.7 ± 0.4 | 1 ± 0.9 | 1.5 ± 1.3 | 2.9 ± 0.5 | 3.1 ± 0.2 | 3.1 ± 0.1 | 4.1 ± 0.5 |
| Benzaldehyde | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.6 ± 0.5 |
| Ketones | |||||||||||||||
| 2-Tridecanone | 1.1 ± 0.1 | 1.7 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 1 ± 0.04 | 1 ± 0.2 | 0.9 ± 0.02 | 1 ± 0.07 | 1 ± 0.2 | 1.1 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.03 | 1.6 ± 0.1 |
| 2-Pentadecanone | 0.4 ± 0.05 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.6 ± 0.02 |
| 2-Heptanone | 1.1 ± 0.2 | 2.5 ± 0.4 | 2.7 ± 0.6 | 2.9 ± 0.4 | 3.2 ± 0.3 | 3 ± 0.1 | 3.1 ± 0.5 | 2.7 ± 0.03 | 3 ± 0.5 | 3.3 ± 0.1 | 5.3 ± 3.8 | 5.5 ± 6.1 | 10.4 ± 0.7 | 10.2 ± 0.6 | 14.7 ± 0.6 |
| 2-Nonanone | 4.3 ± 0.3 | 8.1 ± 0.5 | 7.4 ± 0.6 | 7.9 ± 0.2 | 7.7 ± 0.1 | 8.5 ± 0.4 | 8.3 ± 1.6 | 8.3 ± 0.4 | 7.9 ± 0.3 | 8 ± 0.3 | 7.9 ± 0.1 | 7.1 ± 0.6 | 8.1 ± 0.3 | 8 ± 0.6 | 9.5 ± 1.3 |
| 2-Undecanone | 3.2 ± 0.2 | 4.4 ± 0.1 | 3.9 ± 0.4 | 3.9 ± 0.2 | 3.4 ± 0.2 | 3.8 ± 0.1 | 3.7 ± 0.6 | 3.7 ± 0.4 | 3.4 ± 0.1 | 3.6 ± 0.2 | 3.6 ± 0.3 | 3 ± 0.2 | 4.2 ± 0.2 | 4 ± 0.1 | 3.7 ± 0.1 |
| Lactones | |||||||||||||||
| Acetoin | 21.2 ± 3.2 | ND | 12.5 ± 13.5 | ND | ND | 2.6 ± 2.3 | ND | 4.1 ± 0.3 | 1.4 ± 1.4 | 2.2 ± 0.8 | ND | 9.7 ± 0.5 | ND | ND | ND |
| γ-Dodecalactone | 1.2 ± 0.05 | 1.9 ± 0.1 | 1.9 ± 0.3 | 1.9 ± 0.1 | 1.9 ± 0.2 | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.03 | 1.8 ± 0.1 | 1.6 ± 0.02 | 1.8 ± 0.1 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.01 |
| δ-Dodecalactone | 1.2 ± 0.02 | 1.6 ± 0.05 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.06 | ND | 1 ± 0.9 | 1.5 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.1 |
| δ-Decalactone | 1.2 ± 0.04 | 1.6 ± 0.1 | 1.5 ± 0.2 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.2 | 1.4 ± 0.03 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.05 | 1.5 ± 0.1 | 1.3 ± 0.03 |
| Acids | |||||||||||||||
| Acetic acid | 96 ± 11.4 | 37.8 ± 1.6 | 45.7 ± 7.1 | 32.2 ± 3.4 | 29.6 ± 0.8 | 28.8 ± 2.7 | 31.9 ± 2.5 | 29.8 ± 4.2 | 28.3 ± 2.3 | 26.2 ± 4.8 | 22 ± 4.7 | 18.8 ± 2.6 | 13.8 ± 1.9 | 14.9 ± 2.2 | ND |
| Butanoic acid | 6 ± 0.6 | 8 ± 0.3 | 6.5 ± 1 | 6.9 ± 0.3 | 6.5 ± 0.1 | 7.9 ± 0.5 | 6.8 ± 1.5 | 6.7 ± 0.2 | 6.4 ± 0.5 | 5.9 ± 0.2 | 6.1 ± 0.6 | 4 ± 0.2 | 6.2 ± 0.4 | 5.9 ± 0.3 | 1.3 ± 1.1 |
| Benzoic acid | 4.3 ± 3.7 | ND | ND | ND | ND | 3.2 ± 2.8 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Hexanoic acid | 58.8 ± 3 | 59.6 ± 3.3 | 60 ± 2.3 | 60.4 ± 7 | 60.6 ± 1.6 | 75.6 ± 3.7 | 66.6 ± 13.9 | 70 ± 1.6 | 67.2 ± 4.2 | 65.9 ± 2.4 | 65.1 ± 4.4 | 36.1 ± 1.7 | 65.3 ± 3.9 | 64.3 ± 2.4 | 5.8 ± 0.7 |
| Heptanoic acid | 1 ± 0.3 | 1 ± 0.1 | ND | ND | ND | ND | ND | ND | ND | ND | 0.5 ± 0.5 | ND | 1 ± 0.04 | 1 ± 0.1 | ND |
| Octanoic acid | 81.9 ± 2.9 | 97.6 ± 4.5 | 97.9 ± 7.5 | 101.4 ± 11.3 | 102.1 ± 3 | 110.5 ± 3.6 | 104 ± 18 | 108.2 ± 9.1 | 103.8 ± 4.7 | 104.4 ± 6.8 | 104.9 ± 5.3 | 66.6 ± 4.7 | 105.8 ± 4.6 | 105.1 ± 3.5 | 9.1 ± 1.9 |
| Nonanoic acid | 1.6 ± 0.3 | 2.1 ± 0.5 | 1.7 ± 0.3 | 2.1 ± 0.5 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.5 | 1.7 ± 0.9 | 1.3 ± 0.2 | 1 ± 0.2 | 1.4 ± 0.06 | 2.2 ± 1.3 | 1.7 ± 0.5 | 1.8 ± 0.3 | ND |
| n-Decanoic acid | 42 ± 2 | 59 ± 4.2 | 58.5 ± 6 | 54.1 ± 1.7 | 56 ± 2.4 | 58.5 ± 0.7 | 56.9 ± 9.8 | 54 ± 2.2 | 53.9 ± 2.5 | 62.8 ± 6.8 | 61.5 ± 4.2 | 50.5 ± 1.6 | 62.4 ± 1.5 | 58.3 ± 2 | 9.9 ± 3 |
| 9-Decenoic acid | 4.3 ± 0.1 | 5.4 ± 0.6 | 5.1 ± 0.6 | 4.1 ± 0.4 | 4.4 ± 0.2 | 5.3 ± 0.2 | 4.6 ± 1.1 | 4.6 ± 0.7 | 4.5 ± 0.3 | 5.5 ± 0.4 | 5.6 ± 0.9 | 3.2 ± 0.5 | 5.8 ± 0.3 | 5.3 ± 0.3 | ND |
| Dodecanoic acid | 4.3 ± 0.1 | 6.6 ± 0.3 | 7 ± 1 | 5.8 ± 1.6 | 6.2 ± 0.5 | 6.9 ± 0.3 | 6.2 ± 1.5 | 4.7 ± 0.1 | 4.2 ± 0.3 | 8.3 ± 1.5 | 7.1 ± 2.6 | ND | 6.5 ± 0.9 | 5.9 ± 1.2 | ND |
| 9-Octadecenoic acid | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.4 ± 1.2 | ND | ND | ND | ND |
| Cyclohexanecarboxylic acid | 1.5 ± 0.2 | ND | ND | ND | ND | 2.2 ± 0.9 | 6.7 ± 2.9 | 7.7 ± 3 | 9 ± 0.6 | 10.6 ± 0.8 | 9.5 ± 1.2 | ND | 11.6 ± 2 | 14.6 ± 0.9 | ND |
| Alcohols | |||||||||||||||
| 2-Nonanol | 1 ± 0.1 | ND | 0.6 ± 0.04 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 2-methyl-1-Hexadecanol | 1 ± 0.1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Sulphur compounds | |||||||||||||||
| Dimethyl sulphone | ND | ND | 0.7 ± 0.1 | ND | ND | ND | ND | ND | ND | ND | 0.4 ± 0.4 | 1.2 ± 0.3 | ND | 0.4 ± 0.3 | 1.7 ± 0.3 |
| E. durans Isolate | AMP10 | CN10 | K30 | S10 | C30 | TE30 | VA30 | E15 | DA2 |
|---|---|---|---|---|---|---|---|---|---|
| 4LA1F | S | S | S | S | S | S | S | S | S |
| 4LA4F | S | S | S | S | S | S | S | S | R |
| 4LB10F | S | S | S | R | S | S | S | S | R |
| 4LG3F | S | S | S | R | S | S | S | R | R |
| 4LG4F | S | S | S | S | S | S | S | R | R |
| 4LH11F | S | S | S | S | S | S | S | S | S |
| 2LA3A | S | S | S | S | S | S | S | S | S |
| 2LB3B | S | S | S | S | S | S | S | S | S |
| 2LD3 | S | S | S | S | S | S | S | S | S |
| 2LD7A | S | S | S | S | S | S | S | S | S |
| 2LD7B | S | S | S | S | S | S | S | S | S |
| 2LG7A | S | S | S | S | S | S | S | S | S |
| 2LG12B | S | S | S | R | S | S | S | S | R |
| IPLA655 | S | S | S | R | S | S | S | S | R |
| E. durans Isolate | MIC (mg/L) |
|---|---|
| 4LA1F | 0.5 |
| 4LA4F | 0.25 |
| 4LB10F | 0.25 |
| 4LG3F | 0.25 |
| 4LG4F | 0.25 |
| 4LH11F | 1 |
| 2LA3A | 1 |
| 2LB3B | 0.5 |
| 2LD3 | 1 |
| 2LD7A | 1 |
| 2LD7B | 1 |
| 2LG7A | 1 |
| 2LG12B | 1 |
| IPLA655 | 0.5 |
| E. durans Isolate | gelE | efaA | esp | hylEfam | IS16 |
|---|---|---|---|---|---|
| 4LA1F | - | - | - | - | - |
| 4LA4F | - | - | - | - | - |
| 4LB10F | - | - | - | - | - |
| 4LG3F | - | - | - | - | - |
| 4LG4F | - | - | - | - | - |
| 4LH11F | - | - | - | - | - |
| 2LA3A | - | - | - | - | - |
| 2LB3B | - | - | - | - | - |
| 2LD3 | - | - | - | - | - |
| 2LD7A | - | - | + | - | - |
| 2LD7B | - | - | - | - | - |
| 2LG7A | - | - | - | - | - |
| 2LG12B | - | - | - | - | - |
| IPLA655 | - | - | - | - | - |
| E. durans Isolate | Tyramine | Histamine | Putrescine (AGDI) | Putrescine (ODC) |
|---|---|---|---|---|
| 4LA1F | + | - | - | - |
| 4LA4F | + | - | - | - |
| 4LB10F | + | - | - | - |
| 4LG3F | + | - | - | - |
| 4LG4F | + | - | - | - |
| 4LH11F | + | - | - | - |
| 2LA3A | + | - | - | - |
| 2LB3B | + | - | - | - |
| 2LD3 | + | - | - | - |
| 2LD7A | + | - | - | - |
| 2LD7B | + | - | - | - |
| 2LG7A | + | - | - | - |
| 2LG12B | + | - | - | - |
| IPLA655 | + | - | - | - |
| Strain | Tyramine (mM) | PEA (mM) |
|---|---|---|
| IPLA655 | 2.5 | 2.1 |
| IPLA655-Δtdc | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado, A.; Fernández, E.; González, H.; Fernández, M.; Alvarez, M.A.; Ladero, V. Isolation and Characterization of β-Phenylethylamine-Producing Lactic Acid Bacteria from Dairy Products. Microorganisms 2025, 13, 966. https://doi.org/10.3390/microorganisms13050966
Casado A, Fernández E, González H, Fernández M, Alvarez MA, Ladero V. Isolation and Characterization of β-Phenylethylamine-Producing Lactic Acid Bacteria from Dairy Products. Microorganisms. 2025; 13(5):966. https://doi.org/10.3390/microorganisms13050966
Chicago/Turabian StyleCasado, Angel, Eva Fernández, Héctor González, María Fernández, Miguel A. Alvarez, and Victor Ladero. 2025. "Isolation and Characterization of β-Phenylethylamine-Producing Lactic Acid Bacteria from Dairy Products" Microorganisms 13, no. 5: 966. https://doi.org/10.3390/microorganisms13050966
APA StyleCasado, A., Fernández, E., González, H., Fernández, M., Alvarez, M. A., & Ladero, V. (2025). Isolation and Characterization of β-Phenylethylamine-Producing Lactic Acid Bacteria from Dairy Products. Microorganisms, 13(5), 966. https://doi.org/10.3390/microorganisms13050966








