The Role of Grass in the Epidemiology of a Phytoplasma Disease Affecting Trees and Other Plants of the Sabana de Bogotá, Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant DNA Extractions
2.3. Phytoplasma Detection and Sequence Analysis
3. Results
3.1. Symptom Observation
3.2. Phytoplasma Detection
3.3. Sequence Comparisons
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCR | Polymerase chain reaction |
| RFLP | Restriction fragment length polymorphism |
References
- Marcone, C. Molecular biology and pathogenicity of phytoplasmas. Ann. Appl. Biol. 2014, 165, 199–221. [Google Scholar] [CrossRef]
- Bertaccini, A.; Duduk, B.; Paltrinieri, S.; Contaldo, N. Phytoplasmas and phytoplasma diseases: A severe threat to agriculture. Am. J. Plant Sci. 2014, 5, 1763–1788. [Google Scholar] [CrossRef]
- Bertaccini, A.; Lee, I.M. Phytoplasmas: An Update. In Plant Pathogenic Bacteria-I: Characterisation and Epidemiology of Phytoplasma-Associated Diseases, 1st ed.; Rao, G., Bertaccini, A., Fiore, N., Liefting, L., Eds.; Springer: Singapore, 2018; pp. 1–29. [Google Scholar] [CrossRef]
- IRPCM Phytoplasma/Spiroplasma Working Team–Phytoplasma Taxonomy Group. ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int. J. Syst. Evol. Microbiol. 2004, 54, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Arocha-Rosete, Y.; Contaldo, N.; Duduk, B.; Fiore, N.; Montano, H.G.; Kube, M.; Kuo, C.H.; Martini, M.; Oshima, K. Revision of the ‘Candidatus Phytoplasma’ species description guidelines. Int. J. Syst. Evol. Microbiol. 2022, 72, 005353. [Google Scholar] [CrossRef]
- Perilla-Henao, L.M.; Dickinson, M.; Franco-Lara, L. First report of ‘Candidatus Phytoplasma asteris’ affecting woody hosts (Fraxinus uhdei, Populus nigra, Pittosporum undulatum, and Croton spp.) in Colombia. Plant Dis. 2012, 96, 1372. [Google Scholar] [CrossRef]
- Perilla, L.M.; Franco-Lara, L. Phytoplasmas of group 16SrI associated with strawberry (Fragaria x ananassa) in Colombia. In Proceedings of the 22nd International Conference on Virus and Other Graft Transmission Diseases of Fruit Crops, Rome, Italy, 3–8 June 2012; Volume 22, p. 342. [Google Scholar]
- Franco-Lara, L.; Perilla-Henao, L.M. Phytoplasma diseases in trees of Bogotá, Colombia: A serious risk for urban trees and Crops. In Phytoplasmas and Phytoplasma Disease Management: How to Reduce Their Economic Impact, 1st ed.; Bertaccini, A., Ed.; IPWG—International Phytoplasmologist Working Group: Bologna, Italy, 2014; pp. 90–100. [Google Scholar]
- Franco-Lara, L.; Contaldo, N.; Mejia, J.F.; Paltrinieri, S.; Duduk, B.; Bertaccini, A. Detection and identification of phytoplasmas associated with declining Liquidambar styraciflua trees in Colombia. Trop. Plant Pathol. 2017, 42, 352–361. [Google Scholar] [CrossRef]
- Franco-Lara, L.; García, J.A.; Bernal, Y.E.; Rodríguez, R.A. Diversity of the ‘Candidatus Phytoplasma asteris’ and ‘Candidatus Phytoplasma fraxini’ isolates that infect urban trees in Bogotá, Colombia. Int. J. Syst. Evol. Microbiol. 2020, 70, 6508–6517. [Google Scholar] [CrossRef]
- Lamilla, J.; Solano, C.J.; Franco-Lara, L. Epidemiological characterization of a disease associated with phytoplasmas in Andean oak, Quercus humboldtii Bonpland, in Bogotá—Colombia. For. Pathol. 2022, 52, e12730. [Google Scholar] [CrossRef]
- Franco-Lara, L.; Varela-Correa, C.A.; Guerrero-Carranza, G.P.; Quintero-Vargas, J.C. Association of phytoplasmas with a new disease of potato crops in Cundinamarca, Colombia. Crop Prot. 2023, 163, 106123. [Google Scholar] [CrossRef]
- Bartholomäus, A.; De la Rosa Cortes, A.; Santos Gutiérrez, J.O.; Acero Duarte, L.E.; Moosbrugger, W. El Manto de la Tierra: Flora de Los Andes; Corporación Autónoma Regional de las Cuencas de los Ríos Bogotá, Ubaté y Suárez: Bogotá, Colombia, 2008. [Google Scholar]
- Bardi, J.; Borzone, H.; Scaramuzzino, R.; Villamil, C.; Laddaga, J.; Millione, G. Obtención de barbados de Salix humboldtiana (sauce criollo) destinados a su reintroducción en arroyos del centro de la provincia de Buenos Aires. Quebracho 2016, 24, 41–42. [Google Scholar]
- Giraldo-Cañas, D. Las Gramíneas en Colombia: Riqueza, Distribución, Endemismo, Invasión, Migración, Usos y Taxonomías Populares; Instituto de Ciencias Naturales, Universidad Nacional de Colombia: Bogotá, Colombia, 2013; pp. 38–43. [Google Scholar]
- Silva-Castaño, A.; Ariza-Molina, N.; Rincón-Romero, A.; Villegas-Botero, N.; Ospina-Corredor, N.; Sánchez, F. Composición, abundancia y riqueza de Cicadellidae (Insecta: Hemiptera) en un campus en la Sabana de Bogotá, Colombia. Rev. Fac. Cienc. Básicas 2019, 15, 7–17. [Google Scholar] [CrossRef]
- Silva-Castaño, A.F.; Wilson, M.R.; Brochero, H.L.; Franco-Lara, L. Biodiversity, bugs, and barcodes: The Cicadellidae associated with grassland and phytoplasmas in the Sabana de Bogotá, Colombia. Fla. Entomol. 2019, 102, 755–762. [Google Scholar] [CrossRef]
- Perilla-Henao, L.; Wilson, M.R.; Franco-Lara, L. Leafhoppers Exitianus atratus and Amplicephalus funzaensis transmit phytoplasmas of groups 16SrI and 16Sr VII in Colombia. Plant Pathol. 2016, 65, 1200–1209. [Google Scholar] [CrossRef]
- Tripplehorn, C.A.; Johnson, N.F. Borror and DeLong’s Introduction to the Study of Insects; Thomson Brooks/Cole: Belmont, CA, USA, 2005. [Google Scholar]
- Prince, J.P.; Davis, R.E.; Wolf, T.K.; Lee, I.M.; Mogen, B.D.; Dally, E.L.; Bertaccini, A.; Credi, R.; Barba, M. Molecular detection of diverse mycoplasmalike organisms (MLOs) associated with grapevine yellows and their classification with aster yellows, X-disease, and elm yellows MLOs. Phytopathology 1993, 83, 1130–1137. [Google Scholar] [CrossRef]
- Harrison, N.; Davis, R.; Helmick, E. DNA extraction from arborescent monocots and how to deal with other challenging hosts. In Phytoplasmas, 1st ed.; Dickinson, M., Hodgetts, J., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 123–138. [Google Scholar] [CrossRef]
- Oxelman, B.; Lidén, M.; Berglund, D. Chloroplast rps 16 intron phylogeny of the tribe Sileneae (Caryophyllaceae). Plant Syst. Evol. 1997, 206, 393–410. [Google Scholar] [CrossRef]
- Lee, I.M.; Gundersen-Rindal, D.; Davis, R.; Bottner, K. ‘Candidatus Phytoplasma asteris’, a novel phytoplasma taxon associated with aster yellows and related diseases. Int. J. Syst. Evol. Microbiol. 2004, 54, 1037–1048. [Google Scholar] [CrossRef]
- Gundersen, D.E.; Lee, I.M. Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathol. Mediterr. 1996, 35, 144–151. [Google Scholar]
- Seemüller, E.; Schneider, B.; Mäurer, R.; Ahrens, U.; Daire, X.; Kison, H.; Lorenz, K.H.; Firrao, G.; Avinent, L.; Sears, B.B. Phylogenetic classification of phytopathogenic mollicutes by sequence analysis of 16S ribosomal DNA. Int. J. Syst. Evol. Microbiol. 1994, 44, 440–446. [Google Scholar] [CrossRef]
- Lee, I.M.; Gundersen-Rindal, D.E.; Davis, R.E.; Bartoszyk, I.M. Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. Int. J. Syst. Evol. Microbiol. 1998, 48, 1153–1169. [Google Scholar] [CrossRef]
- Abeysinghe, S.; Abeysinghe, P.D.; Kanatiwela-de Silva, C.; Udagama, P.; Warawichanee, K.; Aljafar, N.; Kawicha, P.; Dickinson, M. Refinement of the taxonomic structure of 16SrXI and 16SrXIV phytoplasmas of gramineous plants using multilocus sequence typing. Plant Dis. 2016, 100, 2001–2010. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, W.; Lee, I.M.; Shao, J.; Suo, X.; Davis, R.E. The iPhyClassifier, an Interactive Online Tool for Phytoplasma Classification and Taxonomic Assignment. In Phytoplasma. Methods and Protocols, 1st ed.; Dickinson, M., Hodgetts, J., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 329–338. [Google Scholar] [CrossRef]
- Ustun, N.; Zamharir, M.G.; Al-Sadi, A.M. Updates on phytoplasma diseases management. In Characterization, Epidemiology, and Management, 1st ed.; Tiwari, A.K., Caglayan, K., M Al-Sadi, A., Azadvar, M., Abeysinghe, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 97–123. [Google Scholar] [CrossRef]
- Burckhardt, D.; Pinzón, O.P. A second Bactericera species from South America: The willow feeding B. minuta in Colombia with description of the previously unknown immatures (Hemiptera, Sternorrhyncha, Psylloidea, Triozidae). Bull. Soc. Entomol. Fr. 2024, 129, 319–330. [Google Scholar] [CrossRef]
- Jarausch, B.; Tedeschi, R.; Sauvion, N.; Gross, J.; Jarausch, W. Psyllid vectors. In Phytoplasmas Plant Pathogenic Bacteria-II: Transmission and Management of Phytoplasma-associated Diseases, 1st ed.; Bertaccini, A., Weintraub, P., Rao, G.P., Fiore, N., Eds.; Springer: Singapore, 2019; pp. 53–78. [Google Scholar] [CrossRef]
- Luis-Pantoja, M.; Paredes-Tomás, C.; Uneau, Y.; Myrie, W.; Morillon, R.; Satta, E.; Contaldo, N.; Pacini, F.; Bertaccini, A. Identification of ‘Candidatus Phytoplasma’ species in “huanglongbing” infected citrus orchards in the Caribbean. Eur. J. Plant Pathol. 2021, 160, 185–198. [Google Scholar] [CrossRef]
- Marcone, C.; Ragozzino, A.; Cousin, M.T.; Berges, R.; Seemüller, E. Phytoplasma diseases of trees and shrubs of urban areas in Europe. Int. Symp. Urban Tree Health 1997, 496, 69–76. [Google Scholar] [CrossRef]
- Mou, H.Q.; Xu, X.; Wang, R.R.; Tian, Q.; Wei, Y.; Zhu, S.F.; Liao, X.L.; Zhao, W.J. Salix tetradenia H and.-Mazz: A new natural plant host of ‘Candidatus phytoplasma’. For. Pathol. 2014, 44, 56–61. [Google Scholar] [CrossRef]
- Pavlović, Đ.; Stojanović, D.; Jošić, L.D.; Starović, S. Phytoplasma disease of medicinal plants in Serbia. CMAPSEEC 2014, 8, 321. [Google Scholar]
- Marcone, C.; Bellardi, M.G.; Bertaccini, A. Phytoplasma diseases of medicinal and aromatic plants. J. Plant Pathol. 2016, 98, 379–404. [Google Scholar]
- Christensen, N.M.; Nicolaisen, M.; Hansen, M.; Schulz, A. Distribution of phytoplasmas in infected plants as revealed by real-time PCR and bioimaging. Mol. Plant-Microbe Interact. 2004, 17, 1175–1184. [Google Scholar] [CrossRef]
- Siddique, A.B.M.; Guthrie, J.N.; Walsh, K.B.; White, D.T.; Scott, P.T. Histopathology and within-plant distribution of the phytoplasma associated with Australian papaya dieback. Plant Dis. 1998, 82, 1112–1120. [Google Scholar] [CrossRef]
- Herath, P.; Hoover, G.A.; Angelini, E.; Moorman, G.W. Detection of elm yellows phytoplasma in elms and insects using real-time PCR. Plant Dis. 2010, 94, 1355–1360. [Google Scholar] [CrossRef]
- Osman, F.; Rowhani, A. Application of a spotting sample preparation technique for the detection of pathogens in woody plants by RT-PCR and real-time PCR (TaqMan). J. Virol. Methods 2006, 133, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Donkersley, P.; Silva, F.W.; Alves, M.S. Asymptomatic Phytoplasma Reveal a New Troublesome infection. In Plant Diseases: Current Threats and Management Trends; Topolovec-Pintaric, S., Ed.; BoD—Books on Demand: Hamburg, Germany, 2020; pp. 97–116. [Google Scholar] [CrossRef]
- Bertaccini, A.; Bellardi, M.G.; Botti, S.; Paltrinieri, S.; Restuccia, P. Phytoplasma infection in Asclepias physocarpa. Acta Hortic. 2004, 722, 349–354. [Google Scholar] [CrossRef]
- Zwolińska, A.; Krawczyk, K.; Borodynko-Filas, N.; Pospieszny, H. Non-crop sources of Rapeseed Phyllody phytoplasma (‘Candidatus Phytoplasma asteris’: 16SrI-B and 16SrI-(B/L) L), and closely related strains. Crop Prot. 2019, 119, 59–68. [Google Scholar] [CrossRef]
- Mall, S.; Rao, G.P.; Marcone, C. Phytoplasma diseases of weeds: Detection, taxonomy and diversity. In Recent Trends in Biotechnology and Microbiology; Gaur, R.K., Sharma, P., Pratap, R., Sharma, K.P., Sharma, M., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 87–108. [Google Scholar]
- Duduk, B.; Stepanović, J.; Yadav, A.; Rao, G.P. Phytoplasmas in weeds and wild plants. In Phytoplasmas: Plant Pathogenic Bacteria—I: Characterisation and Epidemiology of Phytoplasma-Associated Diseases; Rao, G., Bertaccini, A., Fiore, N., Liefting, L., Eds.; Springer: Singapore, 2018; pp. 313–345. [Google Scholar] [CrossRef]
- Bedendo, I.P.; Lopes, J.R.S. Impact and management of major phytoplasma diseases in Brazil. In Sustainable Management of Phytoplasma Diseases in Crops Grown in the Tropical Belt: Biology and Detection; Springer: Cham, Switzerland, 2019; pp. 251–268. [Google Scholar] [CrossRef]
- Berger, J.; Schweigkofler, W.; Kerschbamer, C.; Roschatt, C.; Dalla Vía, J.; Baric, S. Occurrence of Stolbur phytoplasma in the vector Hyalesthes obsoletus, herbaceous host plants and grapevine in South Tyrol (Northern Italy). Vitis 2009, 48, 185–192. [Google Scholar]
- Panassiti, B.; Hartig, F.; Fahrentrapp, J.; Breuer, M.; Biedermann, R. Identifying local drivers of a vector-pathogen-disease system using Bayesian modeling. Basic Appl. Ecol. 2017, 18, 75–85. [Google Scholar] [CrossRef]
- Thorat, V.; Bhale, U.; Sawant, V.; More, V.; Jadhav, P.; Mane, S.S.; Nandanwar, R.S.; Tripathi, S.; Yadav, A. Alternative weed hosts harbors 16SrII group phytoplasmas associated with little leaf and witches’ broom diseases of various crops in India. Phytopathogenic Mollicutes 2016, 6, 50–55. [Google Scholar] [CrossRef]
- Alhudaib, K.; Arocha, Y.; Wilson, M.; Jones, P. Molecular identification, potential vectors and alternative hosts of the phytoplasma associated with a lime decline disease in Saudi Arabia. Crop Prot. 2009, 28, 13–18. [Google Scholar] [CrossRef]
- Blanche, K.R.; Tran-Nguyen, L.T.T.; Gibb, K.S. Detection, identification and significance of phytoplasmas in grasses in northern Australia. Plant Pathol. 2003, 52, 505–512. [Google Scholar] [CrossRef]
- Asudi, G.O.; Van den Berg, J.; Midega, C.A.; Schneider, B.; Seemüller, E.; Pickett, J.A.; Khan, Z.R. Detection, identification, and significance of phytoplasmas in wild grasses in East Africa. Plant Dis. 2016, 100, 108–115. [Google Scholar] [CrossRef]
- Jurga, M.; Zwolińska, A. Phytoplasmas in Poaceae species: A threat to the most important cereal crops in Europe. J. Plant Pathol. 2020, 102, 287–297. [Google Scholar] [CrossRef]
- Silva-Castaño, A.F.; Brochero, H.; Franco-Lara, L. Insects as potential vectors of phytoplasmas in urban trees in a mega-city: A case study in Bogotá, Colombia. Urban Ecosyst. 2024, 27, 1509–1525. [Google Scholar] [CrossRef]

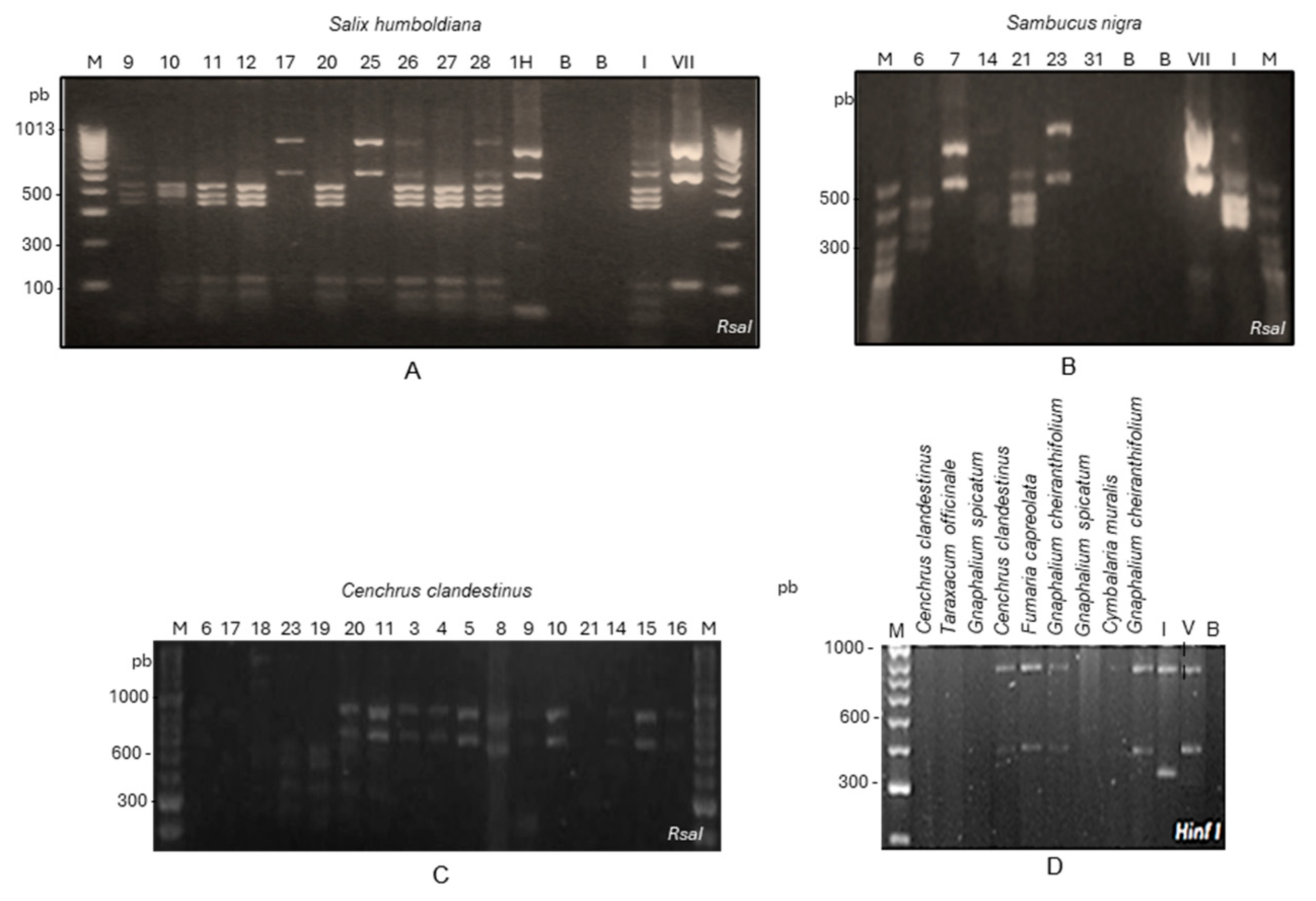
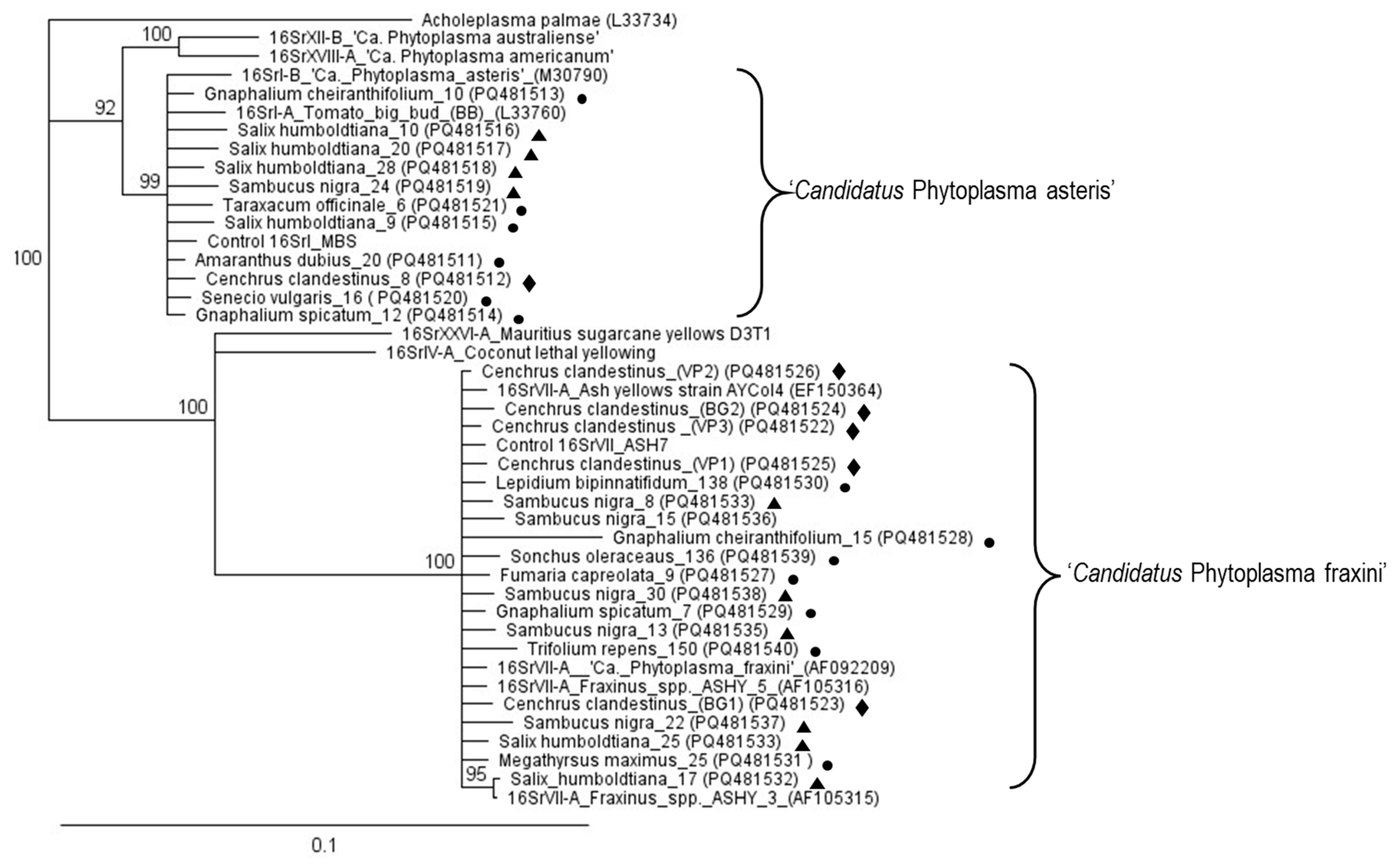

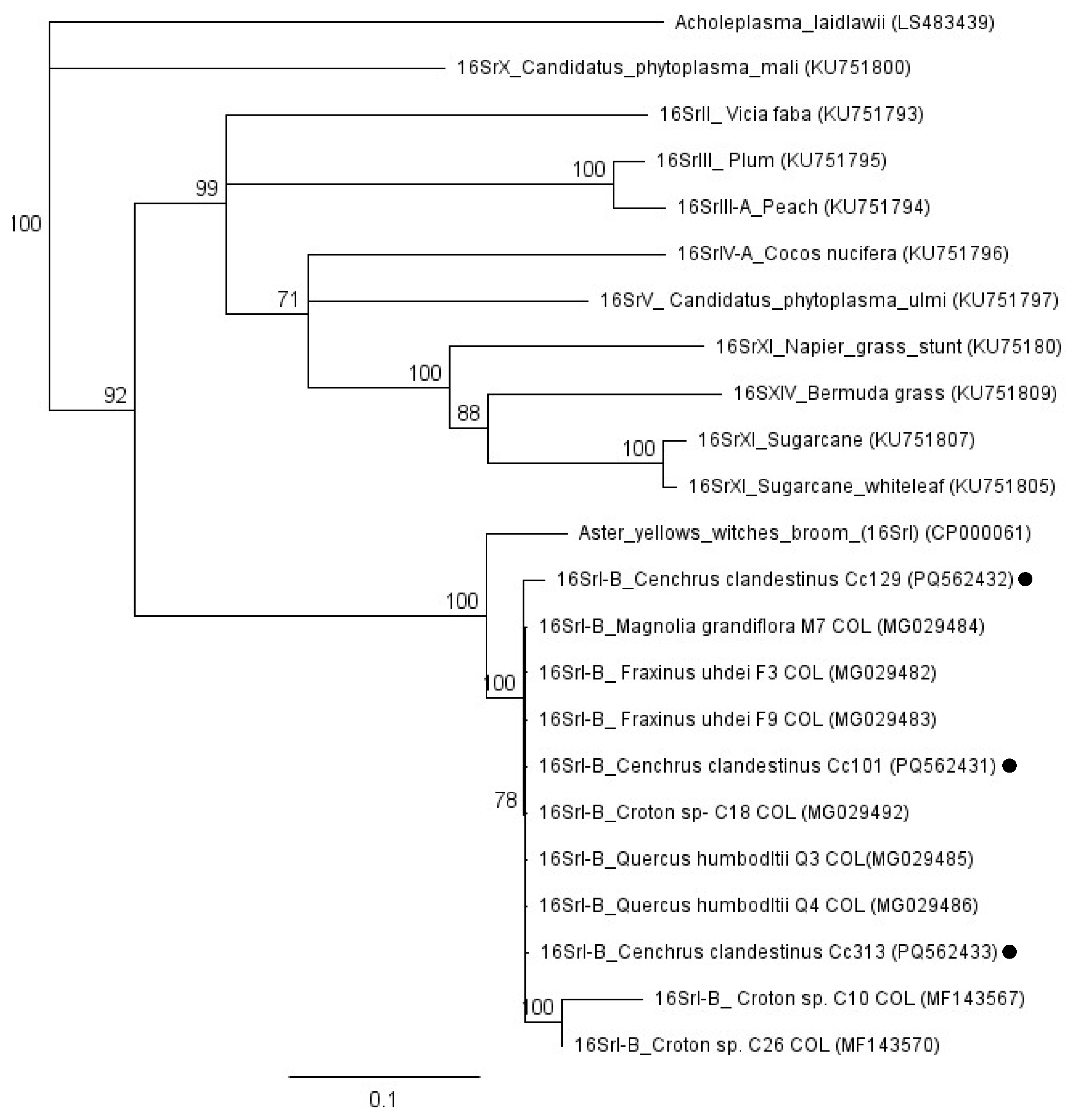
| Symptoms | % Prevalence (n = 40) | ||||
|---|---|---|---|---|---|
| Timiza 1 | Median Strip 200 St 1 | Funza 2 | Cajicá 2 | Average | |
| Epicormic shoots | 100 | 100 | 100 | 100 | 100 |
| Crown deformation | 100 | 100 | 100 | 100 | 100 |
| Abnormal elongation of apical shoots | 100 | 80 | 80 | 90 | 87.5 |
| Vertical branches | 70 | 60 | 60 | 80 | 67.5 |
| Atypical elongation of internodes | 60 | 80 | 70 | 60 | 67.5 |
| Dead branches | 10 | 40 | 30 | 0 | 20 |
| Small leaves | 0 | 0 | 0 | 10 | 2.5 |
| Symptoms | % Prevalence (n = 40) | ||||
|---|---|---|---|---|---|
| Timiza 1 | Santa Helena 1 | Funza 2 | Cajicá 2 | Average | |
| Defoliation | 100 | 100 | 100 | 100 | 100 |
| Dead branches | 80 | 80 | 80 | 90 | 82.5 |
| Crown deformation | 70 | 70 | 90 | 100 | 82.5 |
| Epicormic shoots | 70 | 60 | 80 | 80 | 72.5 |
| Vertical branches | 10 | 40 | 30 | 40 | 30 |
| Abnormal elongation of apical shoots | 20 | 30 | 20 | 30 | 25 |
| Tufted foliage | 30 | 0 | 20 | 10 | 15 |
| Small leaves | 0 | 0 | 0 | 10 | 2.5 |
| Species | Locations | Positive/Total Samples | Primers P1A/P7A R16mF2/R16mR1 | Primers P1A/P7A R16F2n/ R16R2 | Primers P1A7P7 AfU5/rU3 | RFLP | Sequencing | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 16SrI | 16SrVII | 16SrI + 16SrVII | 16SrI | 16SrVII | ||||||
| Salix humboldtiana | Timiza Park | 1/5 | 1 | 1 | ||||||
| 200 St | 4/5 | 4 | 4 | 2 | ||||||
| Cajicá | 3/6 | 2 | 1 | 1 | 1 | 1 | 1 | |||
| Funza | 5/6 | 5 | 2 | 2 | 1 | 1 | 1 | |||
| Sambucus nigra | Timiza Park | 3/4 | 2 | 2 | 1 | 1 | 1 | 1 | ||
| Santa Helena Park | 3/4 | 1 | 2 | 1 | 2 | |||||
| Cajicá | 4/4 | 2 | 2 | 1 | 1 | 1 | 1 | |||
| Funza | 3/4 | 1 | 2 | 2 | ||||||
| Amaranthus dubius | Bogotá—N | 1/11 | 1 * | 1 | ||||||
| Cymbalaria muralis | Bogotá—E | 1/5 | 1 * | 1 | ||||||
| Fumaria capreolata | Bogotá—E | 1/3 | 1 * | 1 | ||||||
| Gnaphalium cheiranthifolium | Bogotá—E | 2/3 | 2 * | 1 | 1 | |||||
| Gnaphalium spicatum | Bogotá—E, N | 2/5 | 2 * | 1 | 1 | |||||
| Lepidium bipinnatifidum | Bogotá—W, N | 2/7 | 2 * | 1 | 1 | |||||
| Megathyrsus maximus | Bogotá—E | 1/2 | 1 * | 1 | ||||||
| Myosotis sylvatica | Bogotá—E | 1/2 | 1 * | 1 | ||||||
| Plantago major | Bogotá—S | 1/3 | 1 * | 1 | ||||||
| Senecio vulgaris | Bogotá—W | 2/10 | 2 * | 1 | 1 | |||||
| Sonchus oleraceus | Bogotá—N | 1/5 | 1 * | 1 | ||||||
| Spergula arvensis | Bogotá—E | 1/1 | 1* | 1 | ||||||
| Taraxacum officinale | Bogotá—E, N, S | 2/14 | 2 * | 1 | 1 | |||||
| Cenchrus clandestinus | Bogotá—E | 2/6 | 2 * | 1 | 1 | |||||
| Bogotá—BG | 16/24 | 16 | 1 | 3 | 1 | 2 | ||||
| Bogotá-—PV | 16/24 | 16 | 1 | 4 | 1 | 4 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Lara, L.; Campo-Garnica, A.C.; French, I.C.; Solano, C.J.; Vargas, M.N. The Role of Grass in the Epidemiology of a Phytoplasma Disease Affecting Trees and Other Plants of the Sabana de Bogotá, Colombia. Microorganisms 2025, 13, 967. https://doi.org/10.3390/microorganisms13050967
Franco-Lara L, Campo-Garnica AC, French IC, Solano CJ, Vargas MN. The Role of Grass in the Epidemiology of a Phytoplasma Disease Affecting Trees and Other Plants of the Sabana de Bogotá, Colombia. Microorganisms. 2025; 13(5):967. https://doi.org/10.3390/microorganisms13050967
Chicago/Turabian StyleFranco-Lara, Liliana, Aura Cristina Campo-Garnica, Iris Calanit French, Cindy Julieth Solano, and Maria Nathalia Vargas. 2025. "The Role of Grass in the Epidemiology of a Phytoplasma Disease Affecting Trees and Other Plants of the Sabana de Bogotá, Colombia" Microorganisms 13, no. 5: 967. https://doi.org/10.3390/microorganisms13050967
APA StyleFranco-Lara, L., Campo-Garnica, A. C., French, I. C., Solano, C. J., & Vargas, M. N. (2025). The Role of Grass in the Epidemiology of a Phytoplasma Disease Affecting Trees and Other Plants of the Sabana de Bogotá, Colombia. Microorganisms, 13(5), 967. https://doi.org/10.3390/microorganisms13050967






