Prevalence of Colistin-Resistant Klebsiella pneumoniae Isolates in Turkey over a 20-Year Period: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
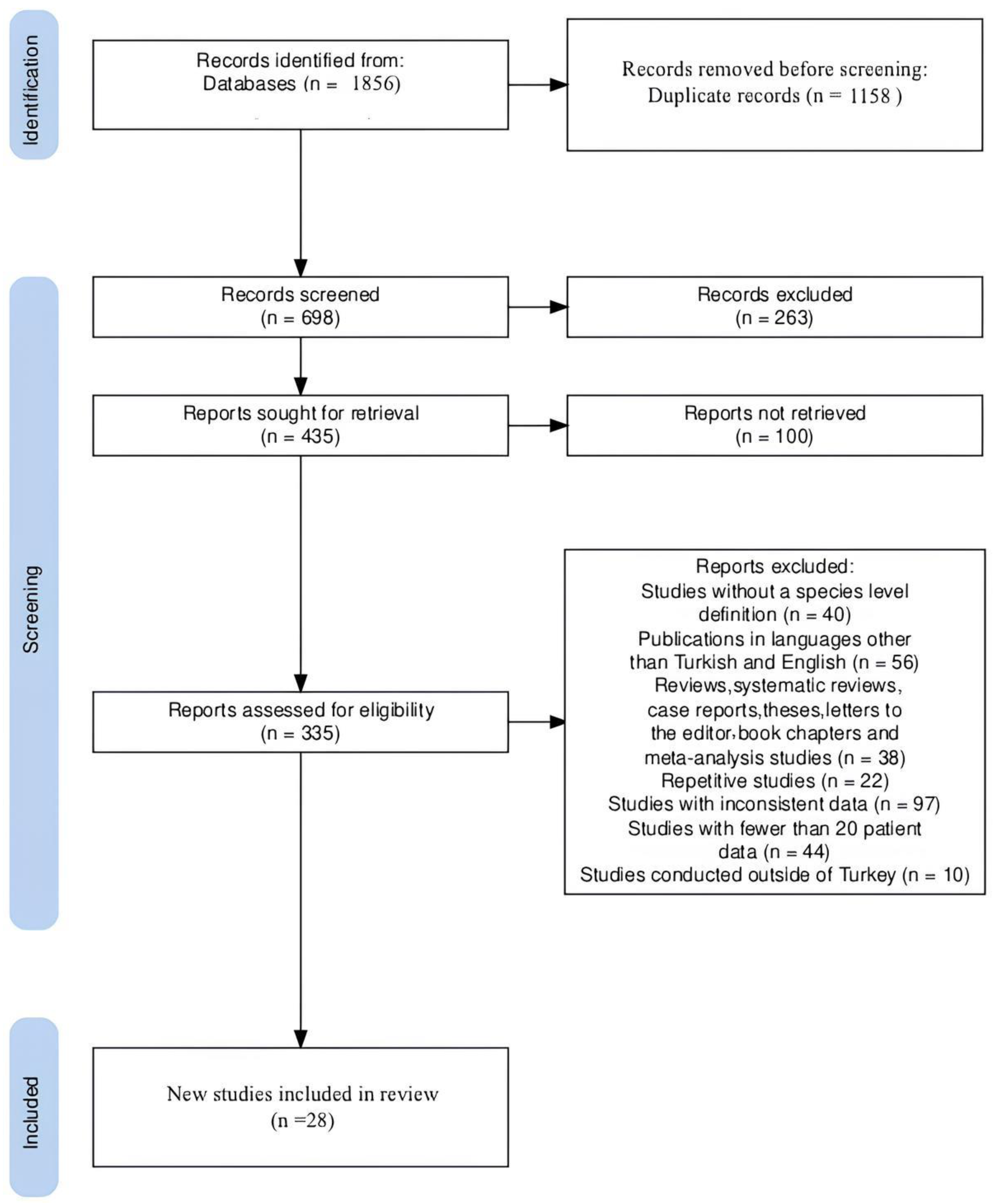
2.2. Literature Search
2.3. Exclusion Criteria
2.4. Inclusion Criteria
2.5. Data Evaluation and Statistical Analysis
3. Results
Publication Bias and Heterogeneity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiology and molecular biology reviews. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Narimisa, N.; Goodarzi, F.; Bavari, S. Prevalence of colistin resistance of Klebsiella pneumoniae isolates in Iran: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 29. [Google Scholar] [CrossRef]
- Aydemir, Ö.; Ormanoğlu, G.; Ayhancı, T.; Zengin, M.; Köroğlu, M. Investigation of in vitro efficacy of quercetin-meropenem combination in carbapenemase-producing Klebsiella pneumoniae isolates. J. Infect. Dev. Ctries 2023, 17, 1325–1329. [Google Scholar] [CrossRef]
- Kahraman, E.P.; Çiftci, İ.H. The Antibiotic Resistance Patterns of Klebsiella pneumoniae Clinic Isolates: A Comprehensive Meta-Analysis. Open J. Bacteriol. 2017, 1, 021–026. [Google Scholar] [CrossRef]
- Pitout, J.D.; Nordmann, P.; Poirel, L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Cannatelli, A.; D’Andrea, M.M.; Giani, T.; Di Pilato, V.; Arena, F.; Ambretti, S.; Gaibani, P.; Rossolini, G.M. In Vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob. Agents Chemother. 2013, 57, 5521–5526. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Ahmed, M.A.E.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Aydemir, Ö.; Şahin, E.Ö.; Ayhancı, T.; Ormanoğlu, G.; Aydemir, Y.; Köroğlu, M.; Altındiş, M. Investigation of in-vitro efficacy of intravenous fosfomycin in extensively drug-resistant Klebsiella pneumoniae isolates and effect of glucose 6-phosphate on sensitivity results. Int. J. Antimicrob. Agents 2022, 59, 106489. [Google Scholar] [CrossRef]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance 2017, 22, 30589. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Nation, R.L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob. Agents Chemother. 2017, 61, e02319-16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, Y.; Wang, Z.; Hu, N.; Liu, Q.; Zhou, W.; Li, X.; Hu, L.; Guo, J.; Huang, X.; et al. Molecular mechanisms of colistin resistance in Klebsiella pneumoniae in a tertiary care teaching hospital. Front. Cell. Infect. Microbiol. 2021, 11, 673503. [Google Scholar] [CrossRef]
- Alizadeh, H.; Alizadeh, H.; Alireza, K.; Fahimeh, A.; Nima, B. Molecular characteristics of carbapenem-resistant Klebsiella pneumoniae isolates producing blaVIM, blaNDM, and blaIMP in clinical centers in Isfahan, Iran. Jundishapur J. Microbiol. 2021, 14, e114473. [Google Scholar] [CrossRef]
- Uzairue, L.I.; Rabaan, A.A.; Adewumi, F.A.; Okolie, O.J.; Folorunso, J.B.; Bakhrebah, M.A.; Garout, M.; Alfouzan, W.A.; Halwani, M.A.; Alamri, A.A.; et al. Global prevalence of colistin resistance in Klebsiella pneumoniae from bloodstream infection: A systematic review and meta-analysis. Pathogens 2022, 11, 1092. [Google Scholar] [CrossRef]
- Jayol, A.; Nordmann, P.; Desroches, M.; Decousser, J.W.; Poirel, L. Acquisition of broad-spectrum cephalosporin resistance leading to colistin resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2016, 60, 3199–3201. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumar, S.; Zhang, L.; Wu, H. Klebsiella pneumonia and Its Antibiotic Resistance: A Bibliometric Analysis. BioMed Res. Int. 2022, 2022, 1668789. [Google Scholar] [CrossRef] [PubMed]
- Caniaux, I.; Van Belkum, A.; Zambardi, G.; Poirel, L.; Gros, M.F. MCR: Modern colistin resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 415–420. [Google Scholar] [CrossRef]
- Aghapour, Z.; Gholizadeh, P.; Ganbarov, K.; Bialvaei, A.Z.; Mahmood, S.S.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect. Drug Resist. 2019, 12, 965–975. [Google Scholar] [CrossRef]
- Talat, A.; Khan, F.; Khan, A.U. Genome analyses of colistin-resistant high-risk blaNDM-5 producing Klebsiella pneumoniae ST147 and Pseudomonas aeruginosa ST235 and ST357 in clinical settings. BMC Microbiol. 2024, 24, 174. [Google Scholar] [CrossRef]
- Skov, R.L.; Monnet, D.L. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Euro surveillance: Bulletin Europeen sur les maladies transmissibles. Eur. Commun. Dis. Bull. 2016, 21, 30155. [Google Scholar]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Arabacı, Ç.; Dal, T.; Başyiğit, T.; Genişel, N.; Durmaz, R. Investigation of carbapenemase and mcr-1 genes in carbapenem-resistant Klebsiella pneumoniae isolates. J. Infect. Dev. Ctries. 2019, 13, 504–509. [Google Scholar] [CrossRef]
- Özkaya, E.; Buruk, C.K.; Tosun, İ.; Toraman, B.; Kaklıkkaya, N.; Aydın, F. Klinik Enterobacterales izolatlarında plazmit aracılı mcr kolistin direnç geninin araştırılması. Mikrobiyol. Bul. 2020, 54, 191–202. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Porritt, K.; Gomersall, J.; Lockwood, C. Study selection and critical appraisal. Am. J. Nurs. 2014, 114, 47–52. [Google Scholar] [CrossRef]
- Dizbay, M.; Guzel Tunccan, O.; Karasahin, O.; Aktas, F. Emergence of carbapenem-resistant Klebsiella spp. infections in a Turkish university hospital: Epidemiology and risk factors. J. Infect. Dev. Ctries. 2014, 8, 44–49. [Google Scholar] [CrossRef]
- Zarakolu, P.; Eser, O.K.; Aladag, E.; Al-Zahrani, I.A.; Day, K.M.; Atmaca, O.; Boral, B.; Cakir, B.; Perry, J.D.; Akova, M. Epidemiology of carbapenem-resistant Klebsiella pneumoniae colonization: A surveillance study at a Turkish university hospital from 2009 to 2013. Diagn. Microbiol. Infect. Dis. 2016, 85, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Ergönül, Ö.; Aydin, M.; Azap, A.; Başaran, S.; Tekin, S.; Kaya, Ş.; Gülsün, S.; Yörük, G.; Kurşun, E.; Yeşilkaya, A.; et al. Healthcare-associated Gram-negative bloodstream infections: Antibiotic resistance and predictors of mortality. J. Hosp. Infect. 2016, 94, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Iraz, M.; Düzgün, A.; Sandallı, C.; Doymaz, M.Z.; Akkoyunlu, Y.; Saral, A.; Peleg, A.Y.; Özgümüş, O.B.; Beriş, F.; Karaoğlu, H.; et al. Distribution of β-lactamase genes among carbapenem-resistant Klebsiella pneumoniae strains isolated from patients in Turkey. Ann. Lab. Med. 2015, 35, 595–601. [Google Scholar] [CrossRef]
- Cizmeci, Z.; Aktas, E.; Otlu, B.; Acikgoz, O.; Ordekci, S. Molecular characterization of carbapenem-resistant Enterobacteriaceae yields increasing rates of NDM-1 carbapenemases and colistin resistance in an OXA-48-endemic area. J. Chemother. 2017, 29, 344–350. [Google Scholar] [CrossRef]
- Aydın, M.; Ergönül, Ö.; Azap, A.; Bilgin, H.; Aydın, G.; Çavuş, S.A.; Akalın, H. Rapid emergence of colistin resistance and its impact on fatality among healthcare-associated infections. J. Hosp. Infect. 2018, 98, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Ece, G.; Tunc, E.; Otlu, B.; Aslan, D.; Ece, C. Detection of blaOXA-48 and clonal relationship in carbapenem-resistant Klebsiella pneumoniae isolates at a tertiary care center in Western Turkey. J. Infect. Public Health 2018, 11, 640–642. [Google Scholar] [CrossRef]
- Koyuncu Özyurt, Ö.; Özhak, B.; Öğünç, D.; Yıldız, E.; Çolak, D.; Günseren, F.; Öngüt, G. Kolistinin Gram-negatif Bakterilere İn Vitro Etkinliğinin Belirlenmesinde BD Phoenix100 Sistemi ve Kolistin Sıvı Disk Elüsyon Yöntemlerinin Değerlendirilmesi. [Evaluation of the BD Phoenix100 System and Colistin Broth Disk Elution Method for Antimicrobial Susceptibility Testing of Colistin Against Gram-negative Bacteria]. Mikrobiyol. Bul. 2019, 53, 254–261. [Google Scholar]
- Davarci, İ.; Şenbayrak, S.; Aksaray, S.; Koçoğlu, M.E.; Kuşkucu, M.A.; Samasti, M. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae isolates. Anatolian Clin. 2019, 24, 1–7. [Google Scholar] [CrossRef]
- Özkul Koçak, C.; Hazırolan, G. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae clinical isolates. Turk. Mikrobiyol. Cemiy. Derg. 2019, 49, 17–23. [Google Scholar]
- Kansak, N.; Aksaray, S.; Aslan, M.; Adaleti, R.; Gönüllü, N. Detection of colistin resistance among multidrug-resistant Klebsiella pneumoniae and Escherichia coli clinical isolates in Turkey. Acta Microbiol. Immunol. Hung. 2021, 68, 99–106. [Google Scholar] [CrossRef]
- Aygar, İ.S. Karbapenem dirençli Klebsiella pneumoniae kökenlerinde yıllar içerisinde kolistin MİK değerindeki artışın In Vitro değerlendirilmesi. Turk. Mikrobiyol. Cemiy. Derg. 2020, 50, 164–171. [Google Scholar]
- Kilic, U.; Koroglu, M.; Olmez, M.; Altindis, M. Investigation of the In Vitro Effectiveness of Aztreonam/Avibactam, Colistin/Apramycin, and Meropenem/Apramycin Combinations Against Carbapenemase-Producing, Extensively Drug-Resistant Klebsiella pneumoniae Strains. Microb. Drug Resist. 2020, 26, 1–7. [Google Scholar] [CrossRef]
- Çolak, M.; Çakmaklıoğulları, E.K. Prevalence and antibiotic resistance of bacterial pathogens in respiratory tract samples of geriatric patients. J. Surg. Med. 2021, 5, 472–477. [Google Scholar] [CrossRef]
- Koçer, I.; Zer, Y.; Büyüktas, A. The detection of colistin resistance in carbapenem-resistant Klebsiella pneumoniae isolates. Int. J. Adv. Med. 2021, 8, 357–361. [Google Scholar] [CrossRef]
- Genişel, N.; Özcan, N.; Gül, K.; Akpolat, N.; Atmaca, S.; Kenar, L.; Altanlar, N.; Dal, T. Molecular investigation of carbapenem and colistin resistance mechanisms in Klebsiella pneumoniae bloodstream isolates. FABAD J. Pharm. Sci. 2021, 46, 289–298. [Google Scholar]
- Eren, E.; Ulu-Kılıç, A.; Türe, Z.; Cevahir, F.; Kılıç, H.; Alp-Meşe, E. Risk factors of mortality in patients with bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae. Klimik Derg. 2021, 34, 56–60. [Google Scholar] [CrossRef]
- Unlu, O.; Ersoz, B.R.; Istanbullu Tosun, A.; Demirci, M. Epidemic Klebsiella pneumoniae ST258 incidence in ICU patients admitted to a university hospital in Istanbul. J. Infect. Dev. Ctries. 2021, 15, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Süzük Yıldız, S.; Şimşek, H.; Bakkaloğlu, Z.; Numanoğlu Çevik, Y.; Hekimoğlu, C.H.; Kılıç, S.; Alp Meşe, E.; Ulusal Karbapenemaz Sürveyans Çalışma Grubu. Türkiye’de 2019 yılı içinde izole edilen Escherichia coli ve Klebsiella pneumoniae izolatlarında karbapenemaz epidemiyolojisi [The Epidemiology of Carbapenemases in Escherichia coli and Klebsiella pneumoniae Isolated in 2019 in Turkey]. Mikrobiyol. Bul. 2021, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Özmen, C.; Şimşek-Yavuz, S.; Başaran, S.; Çağatay, A.; Özsüt, H.; Eraksoy, H. Comparison of classical methods and chromogen media for detection of stool colonisation by carbapenem-resistant Enterobacteriaceae. Klimik Derg. 2022, 35, 171–178. (In Turkish) [Google Scholar] [CrossRef]
- Baykara, B.; Cimentepe, M.; Kandemir, T.; Koksal, F. Investigation of the relationship between colistin resistance and capsule serotypes in carbapenem-resistant Klebsiella pneumoniae strains. New Microbiol. 2022, 45, 124–129. [Google Scholar]
- Beşli, Y.; Liste, Ü.; Kırbaş, E.; Sancak, B. Escherichia coli ve Klebsiella pneumoniae izolatlarında kolistin duyarlılığının belirlenmesinde resapolymyxin NP testinin kullanımı. Mikrobiyol Bul. 2022, 56, 349–356. [Google Scholar] [CrossRef]
- Hoşbul, T.; Aydoğan, C.N.; Kaya, S.; Bedir, O.; Gümral, R.; Albay, A. Karbapenem Dirençli Klebsiella pneumoniae Klinik İzolatlarına Karşı Seftazidim-avibaktam ve Kolistinin In Vitro Etkinliği. [In Vitro Activity of Ceftazidime-avibactam and Colistin Against Carbapenem-Resistant Klebsiella pneumoniae Clinical Isolates]. Mikrobiyol. Bul. 2022, 56, 218–229. [Google Scholar] [CrossRef]
- Mermutluoğlu, Ç.; Çiftçi, E.Z.; Özcan, N.; Dayan, S. Investigation of antibiotic susceptibilities in carbapenem-resistant Klebsiella pneumoniae and Escherichia coli strains isolated from clinical samples: A four-year analysis in a comprehensive healthcare facility. Van Tıp. Derg. 2023, 30, 374–381. [Google Scholar] [CrossRef]
- Köse, Ş.; Dal, T.; Çetinkaya, R.A.; Arı, O.; Yenilmez, E.; Temel, E.N.; Çetin, E.S.; Arabacı, Ç.; Büyüktuna, S.A.; Hasbek, M.; et al. Molecular epidemiological investigation of carbapenem-resistant Klebsiella pneumoniae isolated from intensive care unit patients of six geographical regions of Turkey. J. Infect. Dev. Ctries. 2023, 17, 1446–1451. [Google Scholar] [CrossRef]
- Ibik, Y.E.; Ejder, N.; Sevim, E.; Rakici, E.; Tanriverdi, E.S.; Copur Cicek, A. Evaluating Molecular Epidemiology of Carbapenem Non-Susceptible Klebsiella pneumoniae Isolates with MLST, MALDI-TOF MS, PFGE. Ann. Clin. Microbiol. Antimicrob. 2023, 27, 93. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Lawrence, M.; Keith, M. Research Methods in Education, 6th ed.; Routledge: London, UK, 2007. [Google Scholar]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ebrahimi, E.; Sholeh, M.; Dousti, R.; Kouhsari, E. A systematic review and meta-analysis: Rising prevalence of colistin resistance in ICU-acquired Gram-negative bacteria. APMIS 2025, 133, e13508. [Google Scholar] [CrossRef]
- Petrosillo, N.; Taglietti, F.; Granata, G. Treatment options for colistin-resistant Kebsiella pneumoniae: Present and future. J. Clin. Med. 2019, 8, 934. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-resistant Klebsiella pneumoniae: Virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, E.; De Gaetano, S.; Midiri, A.; Mancuso, G.; Giovanna, P.; Giuliana, D.; Zummo, S.; Biondo, C. The Antimicrobial Resistance Pandemic Is Here: Implementation Challenges and the Need for the One Health Approach. Hygiene 2024, 4, 297–316. [Google Scholar] [CrossRef]
- Turlej-Rogacka, A.; Xavier, B.B.; Janssens, L.; Lammens, C.; Zarkotou, O.; Pournaras, S.; Goossens, H.; Malhotra-Kumar, S. Evaluation of colistin stability in agar and comparison of four methods for MIC testing of colistin. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 37, 345–353. [Google Scholar] [CrossRef]
- Poonam, A.R.; Bilolikar, A.K.; Sahu, S. A comparative study among different methods of detection of colistin resistant gram-negative bacilli in a tertiary care hospital. J. Med. Sci. Res. 2020, 8, 47–56. [Google Scholar]
- Tansarli, G.S.; Papaparaskevas, J.; Balaska, M.; Samarkos, M.; Pantazatou, A.; Markogiannakis, A.; Mantzourani, M.; Polonyfi, K.; Daikos, G.L. Colistin resistance in carbapenemase-producing Klebsiella pneumoniae bloodstream isolates: Evolution over 15 years and temporal association with colistin use by time series analysis. Int. J. Antimicrob. Agents 2018, 52, 397–403. [Google Scholar] [CrossRef]
- Hamel, M.; Chatzipanagiotou, S.; Hadjadj, L.; Petinaki, E.; Papagianni, S.; Charalampaki, N.; Tsiplakou, S.; Papaioannou, V.; Skarmoutsou, N.; Spiliopoulou, I.; et al. Inactivation of mgrB gene regulator and resistance to colistin is becoming endemic in carbapenem-resistant Klebsiella pneumoniae in Greece: A nationwide study from 2014 to 2017. Int. J. Antimicrob. Agents 2020, 55, 105930. [Google Scholar] [CrossRef]
- Markovska, R.; Marteva-Proevska, Y.; Velinov, T.; Pavlov, I.; Kaneva, R.; Boyanova, L. Detection of different colistin resistance mechanisms among multidrug resistant Klebsiella pneumoniae isolates in Bulgaria. Acta Microbiol. Immunol. Hung. 2022, 69, 220–227. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 5.0. 2015. Available online: www.eucast.org (accessed on 29 January 2025).
- Hombach, M.; Bloemberg, G.V.; Böttger, E.C.J. Effects of clinical breakpoint changes in CLSI guidelines 2010/2011 and EUCAST guidelines 2011 on antibiotic susceptibility test reporting of Gram-negative bacilli. Antimicrob. Chemother. 2012, 67, 622–632. [Google Scholar] [CrossRef]
- Aydemir, Ö.; Çetin, S.; Can, N.; Ormanoğlu, G.; Köroğlu, M. Comparison of six different methods used in detection of colistin resistance in extensively drug-resistant K. pneumoniae, A. baumannii and P. aeruginosa isolates with the reference method broth microdilution method. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2024, 44, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.F.; Tanrıverdi, E.S.; Yalçın, M.; Bayramlar, O.F.; Yıldız Kaya, S.; Karaali, R.; Kuşkucu, M.A.; Köksal Çakırlar, F.; Otlu, B.; Mete, B.; et al. Resistance genes and mortality in carbapenem-resistant Klebsiella pneumoniae bacteremias: Effects of the COVID-19 pandemic. Balkan Med. J. 2024, 41, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.; Lee, M.; Kim, H.J.; Kim, M.; Kwon, R.; Lee, S.W.; Koyanagi, A.; Smith, L.; Kim, M.S.; et al. National Trends in Allergic Rhinitis and Chronic Rhinosinusitis and COVID-19 Pandemic-Related Factors in South Korea, from 1998 to 2021. Int. Arch. Allergy Immunol. 2024, 185, 355–361. [Google Scholar] [CrossRef]
- Xu, T.; Fang, D.; Li, F.; Wang, Z.; Liu, Y. Vitamin B6 resensitizes mcr-carrying Gram-negative bacteria to colistin. Commun. Biol. 2025, 8, 459. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Naqvi, R.A.; Alizadehsani, R.; Hussain, S.; Moqurrab, S.A.; Lee, S.W. An adaptive ensemble deep learning framework for reliable detection of pandemic patients. Comput. Biol. Med. 2024, 168, 107836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahraman Kilbas, E.P.; Kilbas, I.; Ciftci, I.H. Prevalence of Colistin-Resistant Klebsiella pneumoniae Isolates in Turkey over a 20-Year Period: A Systematic Review and Meta-Analysis. Microorganisms 2025, 13, 974. https://doi.org/10.3390/microorganisms13050974
Kahraman Kilbas EP, Kilbas I, Ciftci IH. Prevalence of Colistin-Resistant Klebsiella pneumoniae Isolates in Turkey over a 20-Year Period: A Systematic Review and Meta-Analysis. Microorganisms. 2025; 13(5):974. https://doi.org/10.3390/microorganisms13050974
Chicago/Turabian StyleKahraman Kilbas, Elmas Pinar, Imdat Kilbas, and Ihsan Hakki Ciftci. 2025. "Prevalence of Colistin-Resistant Klebsiella pneumoniae Isolates in Turkey over a 20-Year Period: A Systematic Review and Meta-Analysis" Microorganisms 13, no. 5: 974. https://doi.org/10.3390/microorganisms13050974
APA StyleKahraman Kilbas, E. P., Kilbas, I., & Ciftci, I. H. (2025). Prevalence of Colistin-Resistant Klebsiella pneumoniae Isolates in Turkey over a 20-Year Period: A Systematic Review and Meta-Analysis. Microorganisms, 13(5), 974. https://doi.org/10.3390/microorganisms13050974








