The Role of Salivary Diagnostic Techniques in Screening for Active Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Protocol
2.2. Search String
2.2.1. MeSH
2.2.2. Non-MeSH
2.3. Selection of Studies
2.4. Protocol for Data Extraction
2.5. Data Analysis
3. Results
3.1. Article Screening Process
3.2. Characteristics of Selected Studies
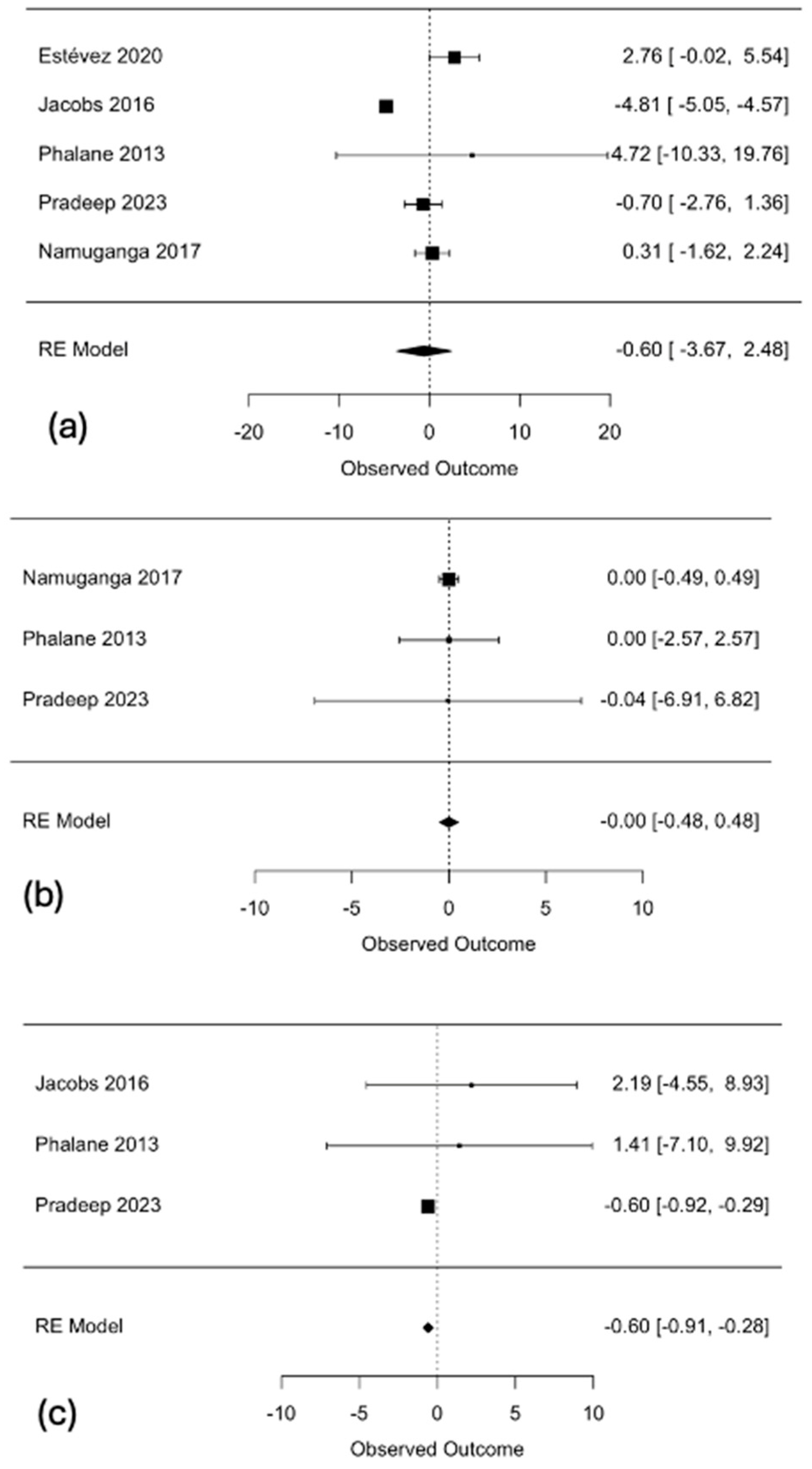
3.3. Primary Meta-Analysis
3.4. Subgroup Analyses
3.5. Salivary Molecular Diagnostic Assays
4. Discussion
4.1. Interleukins and Tuberculosis
4.1.1. IL-6
4.1.2. IL-5
4.1.3. IL-17
4.1.4. The Role of Interleukins in Latent Tuberculosis
4.2. Molecular Diagnostic Assays
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Deck, F.; Guld, J. The WHO tuberculin test. Bull. Int. Union. Tuberc. 1964, 34, 53–70. [Google Scholar]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.; Greenaway, C.; Pai, M.; Menzies, D. False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? Int. J. Tuberc. Lung Dis. 2006, 10, 1192–1204. [Google Scholar]
- Pai, M.; Denkinger, C.M.; Kik, S.V.; Rangaka, M.X.; Zwerling, A.; Oxlade, O.; Metcalfe, J.Z.; Cattamanchi, A.; Dowdy, D.W.; Dheda, K.; et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin. Microbiol. Rev. 2014, 27, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Mahairas, G.G.; Sabo, P.J.; Hickey, M.J.; Singh, D.C.; Stover, C.K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 1996, 178, 1274–1282. [Google Scholar] [CrossRef]
- Sørensen, A.L.; Nagai, S.; Houen, G.; Andersen, P.; Andersen, A.B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 1995, 63, 1710–1717. [Google Scholar] [CrossRef]
- Saluzzo, F.; Mantegani, P.; Poletti de Chaurand, V.; Cirillo, D.M. QIAreach QuantiFERON-TB for the diagnosis of Mycobacterium tuberculosis infection. Eur. Respir. J. 2022, 59, 2102563. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis—Rapid Diagnostics for Tuberculosis Detection; World Health Organization© World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Pande, T.; Pai, M.; Khan, F.A.; Denkinger, C.M. Use of chest radiography in the 22 highest tuberculosis burden countries. Eur. Respir. J. 2015, 46, 1816–1819. [Google Scholar] [CrossRef]
- Kik, S.V.; Denkinger, C.M.; Chedore, P.; Pai, M. Replacing smear microscopy for the diagnosis of tuberculosis: What is the market potential? Eur. Respir. J. 2014, 43, 1793–1796. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update; World Health Organization Copyright © World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. High Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting, 28–29 April 2014; World Health Organization: Geneva, Switzerland, 2014.
- Jones-López, E.C.; Namugga, O.; Mumbowa, F.; Ssebidandi, M.; Mbabazi, O.; Moine, S.; Mboowa, G.; Fox, M.P.; Reilly, N.; Ayakaka, I.; et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: A household contact study. Am. J. Respir. Crit. Care Med. 2013, 187, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Menezes, G.B.L.; Pereira, F.A.; Duarte, C.A.B.; Carmo, T.M.A.; Silva Filho, H.P.D.; Zarife, M.A.; Krieger, M.A.; Reis, E.A.G.; Reis, M.G. Hepatitis C virus quantification in serum and saliva of HCV-infected patients. Mem. Inst. Oswaldo Cruz 2012, 107, 680–683. [Google Scholar] [CrossRef]
- Malamud, D. Saliva as a diagnostic fluid. Dent. Clin. North. Am. 2011, 55, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.P.; Diniz, D.G.; Moimaz, S.A.; Sumida, D.H.; Okamoto, A.C. Saliva: Reflection of the body. Int. J. Infect. Dis. 2010, 14, e184–e188. [Google Scholar] [CrossRef]
- Malathi, N.; Mythili, S.; Vasanthi, H.R. Salivary diagnostics: A brief review. ISRN Dent. 2014, 2014, 158786. [Google Scholar] [CrossRef]
- Meyer, A.J.; Atuheire, C.; Worodria, W.; Kizito, S.; Katamba, A.; Sanyu, I.; Andama, A.; Ayakaka, I.; Cattamanchi, A.; Bwanga, F.; et al. Sputum quality and diagnostic performance of GeneXpert MTB/RIF among smear-negative adults with presumed tuberculosis in Uganda. PLoS ONE 2017, 12, e0180572. [Google Scholar] [CrossRef]
- Jacobs, R.; Maasdorp, E.; Malherbe, S.; Loxton, A.G.; Stanley, K.; Van Der Spuy, G.; Walzl, G.; Chegou, N.N. Diagnostic Potential of Novel Salivary Host Biomarkers as Candidates for the Immunological Diagnosis of Tuberculosis Disease and Monitoring of Tuberculosis Treatment Response. PLoS ONE 2016, 11, e0160546. [Google Scholar] [CrossRef]
- Estévez, O.; Anibarro, L.; Garet, E.; Pallares, Á.; Pena, A.; Villaverde, C.; Del Campo, V.; González-Fernández, Á. Identification of candidate host serum and saliva biomarkers for a better diagnosis of active and latent tuberculosis infection. PLoS ONE 2020, 15, e0235859. [Google Scholar] [CrossRef]
- Pradeep, M.; Sinha, N.; Goyal, P.; Kumar, S.; Singh, A.; Singh, I. Role of Biomarkers (Interleukins (IL)-2,5,6,16,17,1b) in Saliva and Serum for Diagnosis of Pulmonary Tuberculosis and for Monitoring Response to Intensive Phase Treatment. J. Clin. Diagn. Res. 2023, 17, OC09–OC12. [Google Scholar]
- Phalane, K.G.; Kriel, M.; Loxton, A.G.; Menezes, A.; Stanley, K.; Van der Spuy, G.D.; Walzl, G.; Chegou, N.N. Differential expression of host biomarkers in saliva and serum samples from individuals with suspected pulmonary tuberculosis. Mediat. Inflamm. 2013, 2013, 981984. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Tshehla, E.; Malherbe, S.; Kriel, M.; Loxton, A.G.; Stanley, K.; van der Spuy, G.; Walzl, G.; Chegou, N.N. Host biomarkers detected in saliva show promise as markers for the diagnosis of pulmonary tuberculosis disease and monitoring of the response to tuberculosis treatment. Cytokine 2016, 81, 50–56. [Google Scholar] [CrossRef]
- Namuganga, A.R.; Chegou, N.N.; Mubiri, P.; Walzl, G.; Mayanja-Kizza, H. Suitability of saliva for Tuberculosis diagnosis: Comparing with serum. BMC Infect. Dis. 2017, 17, 600. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, T.S.; Prins, B.P.; Abdollahi, M.; Armstrong, N.J.; Aslibekyan, S.; Bain, L.; Jefferis, B.; Baumert, J.; Beekman, M.; Ben-Shlomo, Y.; et al. Genome-wide association study of circulating interleukin 6 levels identifies novel loci. Hum. Mol. Genet. 2021, 30, 393–409. [Google Scholar] [CrossRef]
- Shi, J.; Dong, W.; Ma, Y.; Liang, Q.; Shang, Y.; Wang, F.; Huang, H.; Pang, Y. GeneXpert MTB/RIF Outperforms Mycobacterial Culture in Detecting Mycobacterium tuberculosis from Salivary Sputum. Biomed. Res. Int. 2018, 2018, 1514381. [Google Scholar] [CrossRef] [PubMed]
- Mesman, A.W.; Calderon, R.; Soto, M.; Coit, J.; Aliaga, J.; Mendoza, M.; Franke, M.F. Mycobacterium tuberculosis detection from oral swabs with Xpert MTB/RIF ULTRA: A pilot study. BMC Res. Notes 2019, 12, 349. [Google Scholar] [CrossRef]
- Byanyima, P.; Kaswabuli, S.; Musisi, E.; Nabakiibi, C.; Zawedde, J.; Sanyu, I.; Sessolo, A.; Andama, A.; Worodria, W.; Huang, L.; et al. Feasibility and Sensitivity of Saliva GeneXpert MTB/RIF Ultra for Tuberculosis Diagnosis in Adults in Uganda. Microbiol. Spectr. 2022, 10, e0086022. [Google Scholar] [CrossRef]
- van Soolingen, D.; Wisselink, H.J.; Lumb, R.; Anthony, R.; van der Zanden, A.; Gilpin, C. Practical biosafety in the tuberculosis laboratory: Containment at the source is what truly counts. Int. J. Tuberc. Lung Dis. 2014, 18, 885–889. [Google Scholar] [CrossRef]
- Zaporojan, N.; Negrean, R.A.; Hodisan, R.; Zaporojan, C.; Csep, A.; Zaha, D.C. Evolution of Laboratory Diagnosis of Tuberculosis. Clin. Pract. 2024, 14, 388–416. [Google Scholar] [CrossRef]
- Martinez, A.N.; Mehra, S.; Kaushal, D. Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. J. Infect. Dis. 2013, 207, 1253–1261. [Google Scholar] [CrossRef]
- Rottenberg, M.E.; Carow, B. SOCS3 and STAT3, major controllers of the outcome of infection with Mycobacterium tuberculosis. Semin. Immunol. 2014, 26, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, M.; Repsilber, D.; Kleinsteuber, K.; Gutschmidt, A.; Schommer-Leitner, S.; Black, G.; Walzl, G.; Kaufmann, S.H. Suppressor of cytokine signaling-3 is affected in T-cells from tuberculosisTB patients. Clin. Microbiol. Infect. 2011, 17, 1323–1331. [Google Scholar] [CrossRef]
- Croker, B.A.; Krebs, D.L.; Zhang, J.G.; Wormald, S.; Willson, T.A.; Stanley, E.G.; Robb, L.; Greenhalgh, C.J.; Förster, I.; Clausen, B.E.; et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 2003, 4, 540–545. [Google Scholar] [CrossRef]
- Vivekanandan, M.M.; Adankwah, E.; Aniagyei, W.; Acheampong, I.; Yeboah, A.; Arthur, J.F.; Lamptey, M.N.; Abass, M.K.; Gawusu, A.; Kumbel, F.; et al. Plasma cytokine levels characterize disease pathogenesis and treatment response in tuberculosis patients. Infection 2023, 51, 169–179. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Ahmed, A.M.; Choudhuri, S.; Sen, A.; Hazra, A.; Pal, N.K.; Bhattacharya, B.; Bahar, B. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Mol. Immunol. 2014, 62, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Harling, K.; Adankwah, E.; Güler, A.; Afum-Adjei Awuah, A.; Adu-Amoah, L.; Mayatepek, E.; Owusu-Dabo, E.; Nausch, N.; Jacobsen, M. Constitutive STAT3 phosphorylation and IL-6/IL-10 co-expression are associated with impaired T-cell function in tuberculosis patients. Cell. Mol. Immunol. 2019, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Zambuzi, F.A.; Cardoso-Silva, P.M.; Espindola, M.S.; Soares, L.S.; Galvão-Lima, L.J.; Brauer, V.S.; Gomes, M.S.; Amaral, L.R.; Schaller, M.; Bollela, V.R.; et al. Identification of promising plasma immune biomarkers to differentiate active pulmonary tuberculosis. Cytokine 2016, 88, 99–107. [Google Scholar] [CrossRef]
- Kumar, N.P.; Moideen, K.; Banurekha, V.V.; Nair, D.; Babu, S. Plasma Proinflammatory Cytokines Are Markers of Disease Severity and Bacterial Burden in Pulmonary Tuberculosis. Open Forum Infect. Dis. 2019, 6, ofz257. [Google Scholar] [CrossRef]
- Bai, R.; Tao, L.; Li, B.; Liu, A.; Dai, X.; Ji, Z.; Jian, M.; Ding, Z.; Luo, L.; Chen, T.; et al. Using cytometric bead arrays to detect cytokines in the serum of patients with different types of pulmonary tuberculosis. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419845176. [Google Scholar] [CrossRef]
- Xiong, W.; Dong, H.; Wang, J.; Zou, X.; Wen, Q.; Luo, W.; Liu, S.; He, J.; Cai, S.; Ma, L. Analysis of Plasma Cytokine and Chemokine Profiles in Patients with and without Tuberculosis by Liquid Array-Based Multiplexed Immunoassays. PLoS ONE 2016, 11, e0148885. [Google Scholar] [CrossRef]
- Verbon, A.; Juffermans, N.; Van Deventer, S.J.H.; Speelman, P.; Van Deutekom, H.; Van Der Poll, T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin. Exp. Immunol. 1999, 115, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef]
- Morimoto, A.; Nakazawa, Y.; Ishii, E. Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr. Int. 2016, 58, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. S2), S3. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Boyd, M.B.; Street, N.E.; Lipscomb, M.F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 1998, 160, 2393–2400. [Google Scholar] [CrossRef]
- Morikawa, K.; Oseko, F.; Morikawa, S.; Imai, K.; Sawada, M. Recombinant human IL-5 augments immunoglobulin generation by human B lymphocytes in the presence of IL-2. Cell. Immunol. 1993, 149, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Antas, P.R.; Sales, J.S.; Pereira, K.C.; Oliveira, E.B.; Cunha, K.S.; Sarno, E.N.; Sampaio, E.P. Patterns of intracellular cytokines in CD4 and CD8 T cells from patients with mycobacterial infections. Braz. J. Med. Biol. Res. 2004, 37, 1119–1129. [Google Scholar] [CrossRef]
- Diedrich, C.R.; Mattila, J.T.; Flynn, J.L. Monocyte-derived IL-5 reduces TNF production by Mycobacterium tuberculosis-specific CD4 T cells during SIV/M. tuberculosis coinfection. J. Immunol. 2013, 190, 6320–6328. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Barron, L.; Thompson, R.W.; Madala, S.K.; Wilson, M.S.; Cheever, A.W.; Ramalingam, T. Quantitative assessment of macrophage functions in repair and fibrosis. Curr. Protoc. Immunol. 2011, 14, Unit14.22. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Holtappels, G.; Zhang, N.; Kubica, M.; Deswarte, K.; Derycke, L.; Claeys, S.; Hammad, H.; Brusselle, G.G.; Vandenabeele, P.; et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy 2011, 66, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Kahnert, A.; Seiler, P.; Stein, M.; Bandermann, S.; Hahnke, K.; Mollenkopf, H.; Kaufmann, S.H. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur. J. Immunol. 2006, 36, 631–647. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, R.C.; Dietlin, T.A.; Dixon Gray, J.; Gilmore, W. Macrophage-derived nitric oxide inhibits the proliferation of activated T helper cells and is induced during antigenic stimulation of resting T cells. Cell. Immunol. 2000, 199, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liva, S.M.; de Vellis, J. IL-5 induces proliferation and activation of microglia via an unknown receptor. Neurochem. Res. 2001, 26, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Shames, B.D.; Meng, X.; Fullerton, D.A.; McIntyre, R.C., Jr.; Grover, F.L.; Harken, A.H. Nitric oxide downregulates lung macrophage inflammatory cytokine production. Ann. Thorac. Surg. 1998, 66, 313–317. [Google Scholar] [CrossRef]
- Sergejeva, S.; Ivanov, S.; Lötvall, J.; Lindén, A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2005, 33, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Kolls, J.K.; Lindén, A. Interleukin-17 family members and inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef]
- Miyamoto, M.; Prause, O.; Sjöstrand, M.; Laan, M.; Lötvall, J.; Lindén, A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 2003, 170, 4665–4672. [Google Scholar] [CrossRef]
- Kumar, R.; Theiss, A.L.; Venuprasad, K. RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol. 2021, 42, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Umemura, M.; Yahagi, A.; Hamada, S.; Begum, M.D.; Watanabe, H.; Kawakami, K.; Suda, T.; Sudo, K.; Nakae, S.; Iwakura, Y.; et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007, 178, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Dragon, S.; Saffar, A.S.; Shan, L.; Gounni, A.S. IL-17 attenuates the anti-apoptotic effects of GM-CSF in human neutrophils. Mol. Immunol. 2008, 45, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; De Luca, A.; Bonifazi, P.; Montagnoli, C.; Bozza, S.; Moretti, S.; Belladonna, M.L.; Vacca, C.; Conte, C.; Mosci, P.; et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 2007, 37, 2695–2706. [Google Scholar] [CrossRef]
- Holscher, C.; Holscher, A.; Ruckerl, D.; Yoshimoto, T.; Yoshida, H.; Mak, T.; Saris, C.; Ehlers, S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J. Immunol. 2005, 174, 3534–3544. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J.E.; Khader, S.A.; Solache, A.; Gilmartin, L.; Ghilardi, N.; deSauvage, F.; Cooper, A.M. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J. Immunol. 2004, 173, 7490–7496. [Google Scholar] [CrossRef]
- Torrado, E.; Fountain, J.J.; Liao, M.; Tighe, M.; Reiley, W.W.; Lai, R.P.; Meintjes, G.; Pearl, J.E.; Chen, X.; Zak, D.E.; et al. Interleukin 27R regulates CD4+ T cell phenotype and impacts protective immunity during Mycobacterium tuberculosis infection. J. Exp. Med. 2015, 212, 1449–1463. [Google Scholar] [CrossRef]
- Khader, S.A.; Cooper, A.M. IL-23 and IL-17 in tuberculosis. Cytokine 2008, 41, 79–83. [Google Scholar] [CrossRef]
- Gopal, R.; Monin, L.; Slight, S.; Uche, U.; Blanchard, E.; AFallert Junecko, B.; Ramos-Payan, R.; Stallings, C.L.; Reinhart, T.A.; Kolls, J.K.; et al. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014, 10, e1004099. [Google Scholar] [CrossRef]
- Khader, S.A.; Gopal, R. IL-17 in protective immunity to intracellular pathogens. Virulence 2010, 1, 423–427. [Google Scholar] [CrossRef]
- Shen, H.; Chen, Z.W. The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell. Mol. Immunol. 2018, 15, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, N.; Kolls, J.K. Updates on T helper type 17 immunity in respiratory disease. Immunology 2019, 156, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Won, E.J.; Choi, J.H.; Cho, Y.N.; Jin, H.M.; Kee, H.J.; Park, Y.W.; Kwon, Y.S.; Kee, S.J. Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease. J. Infect. 2017, 74, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Moideen, K.; Kumar, N.P.; Bethunaickan, R.; Banurekha, V.V.; Nair, D.; Babu, S. Heightened systemic levels of anti-inflammatory cytokines in pulmonary tuberculosis and alterations following anti-tuberculosis treatment. Cytokine 2020, 127, 154929. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Vascular endothelial growth factor levels in tuberculosis: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268543. [Google Scholar] [CrossRef]
- Fiorenza, G.; Rateni, L.; Farroni, M.A.; Bogué, C.; Dlugovitzky, D.G. TNF-alpha, TGF-beta and NO relationship in sera from tuberculosis (TB) patients of different severity. Immunol. Lett. 2005, 98, 45–48. [Google Scholar] [CrossRef]
- Bennett, A.; Kojima, K.; Heisz, M.; Booth, C.; McKinney, M.; Summermatter, K.; Blacksell, S. Laboratory Biosafety Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Xu, Y.; Wang, G.; Xu, M. Biohazard levels and biosafety protection for Mycobacterium tuberculosis strains with different virulence. Biosaf. Health 2020, 2, 135–141. [Google Scholar] [CrossRef]
- Tang, P.; Liu, R.; Qin, L.; Xu, P.; Xiong, Y.; Deng, Y.; Lv, Z.; Shang, Y.; Gao, X.; Yao, L.; et al. Accuracy of Xpert® MTB/RIF Ultra test for posterior oropharyngeal saliva for the diagnosis of paucibacillary pulmonary tuberculosis: A prospective multicenter study. Emerg. Microbes Infect. 2023, 12, 2148564. [Google Scholar] [CrossRef]
- Shenai, S.; Amisano, D.; Ronacher, K.; Kriel, M.; Banada, P.P.; Song, T.; Lee, M.; Joh, J.S.; Winter, J.; Thayer, R.; et al. Exploring alternative biomaterials for diagnosis of pulmonary tuberculosis in HIV-negative patients by use of the GeneXpert MTB/RIF assay. J. Clin. Microbiol. 2013, 51, 4161–4166. [Google Scholar] [CrossRef]
- Holani, A.G.; Ganvir, S.M.; Shah, N.N.; Bansode, S.C.; Shende, I.; Jawade, R.; Bijjargi, S.C. Demonstration of mycobacterium tuberculosis in sputum and saliva smears of tuberculosis patients using ziehl neelsen and flurochrome staining- a comparative study. J. Clin. Diagn. Res. 2014, 8, Zc42. [Google Scholar]
- Raras, T.Y.; Sholeh, G.; Lyrawati, D. Salivary sIg-A response against the recombinant Ag38 antigen of Mycobacterium tuberculosis Indonesian strain. Int. J. Clin. Exp. Med. 2014, 7, 129–135. [Google Scholar] [PubMed]
- Mardining Raras, T.Y.; Rahmadani, N.; Arthamin, M.Z.; Rizki, M. Preliminary Test of Candidate Rapid Diagnostic Test for the Detection of 38 kDa Mycobacterium Tuberculosis Antigen in Saliva. Open Biotechnol. J. 2024, 18, e18740707277154. [Google Scholar] [CrossRef]
- Hansen, J.; Kolbe, K.; König, I.R.; Scherliess, R.; Hellfritzsch, M.; Malm, S.; Müller-Loennies, S.; Zallet, J.; Hillemann, D.; Wiesmüller, K.H.; et al. Lipobiotin-capture magnetic bead assay for isolation, enrichment and detection of Mycobacterium tuberculosis from saliva. PLoS ONE 2022, 17, e0265554. [Google Scholar] [CrossRef]

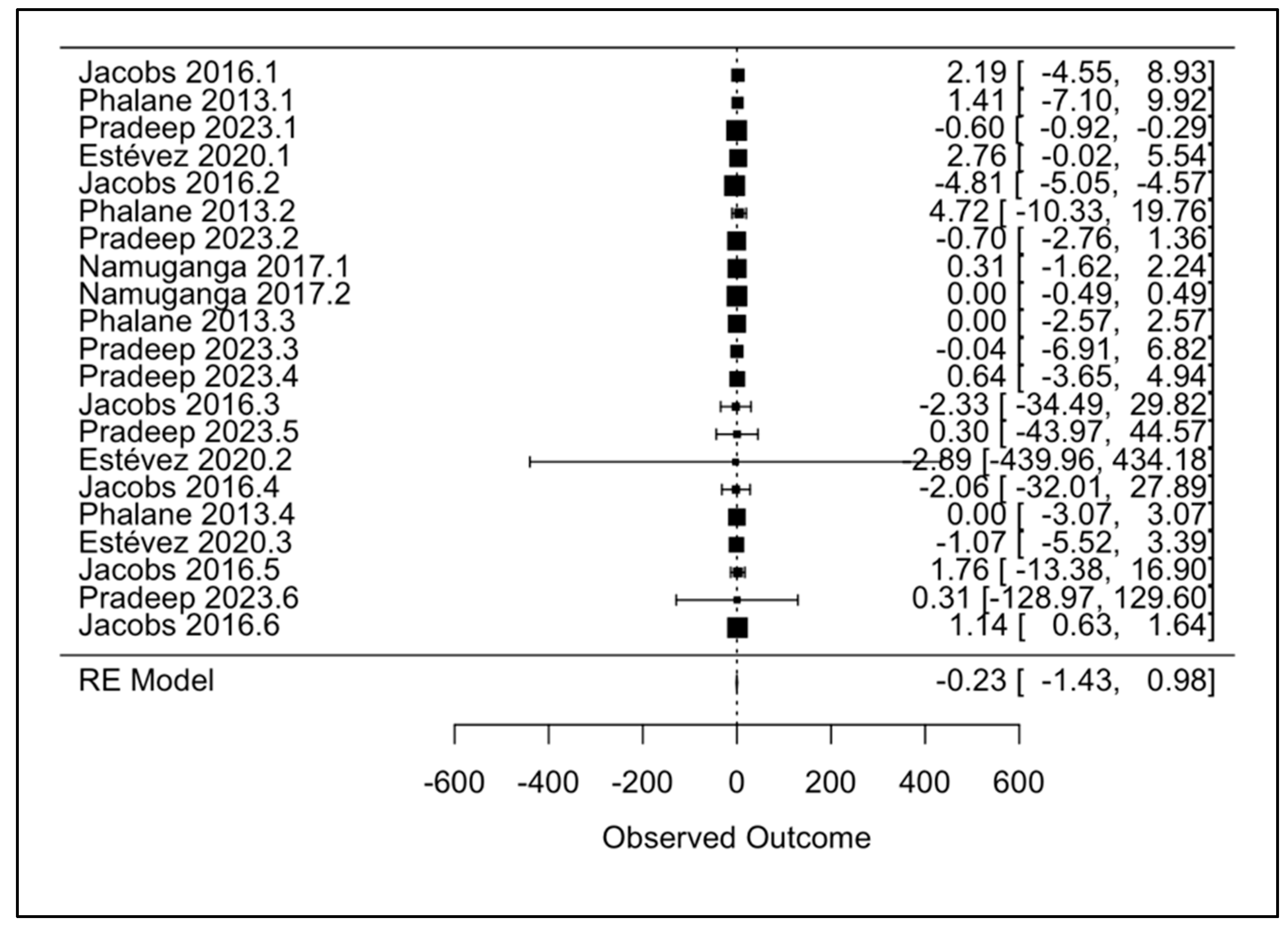

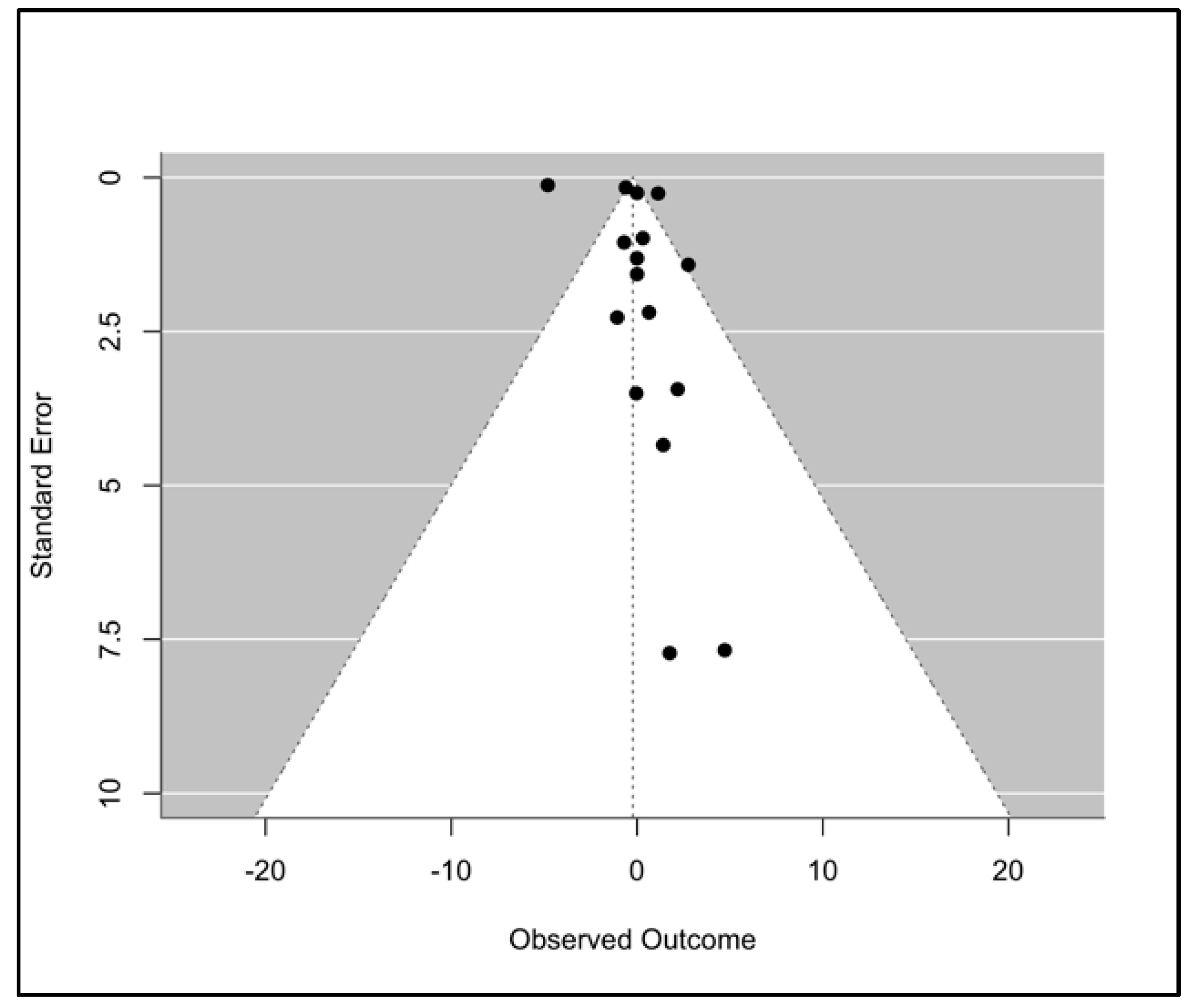
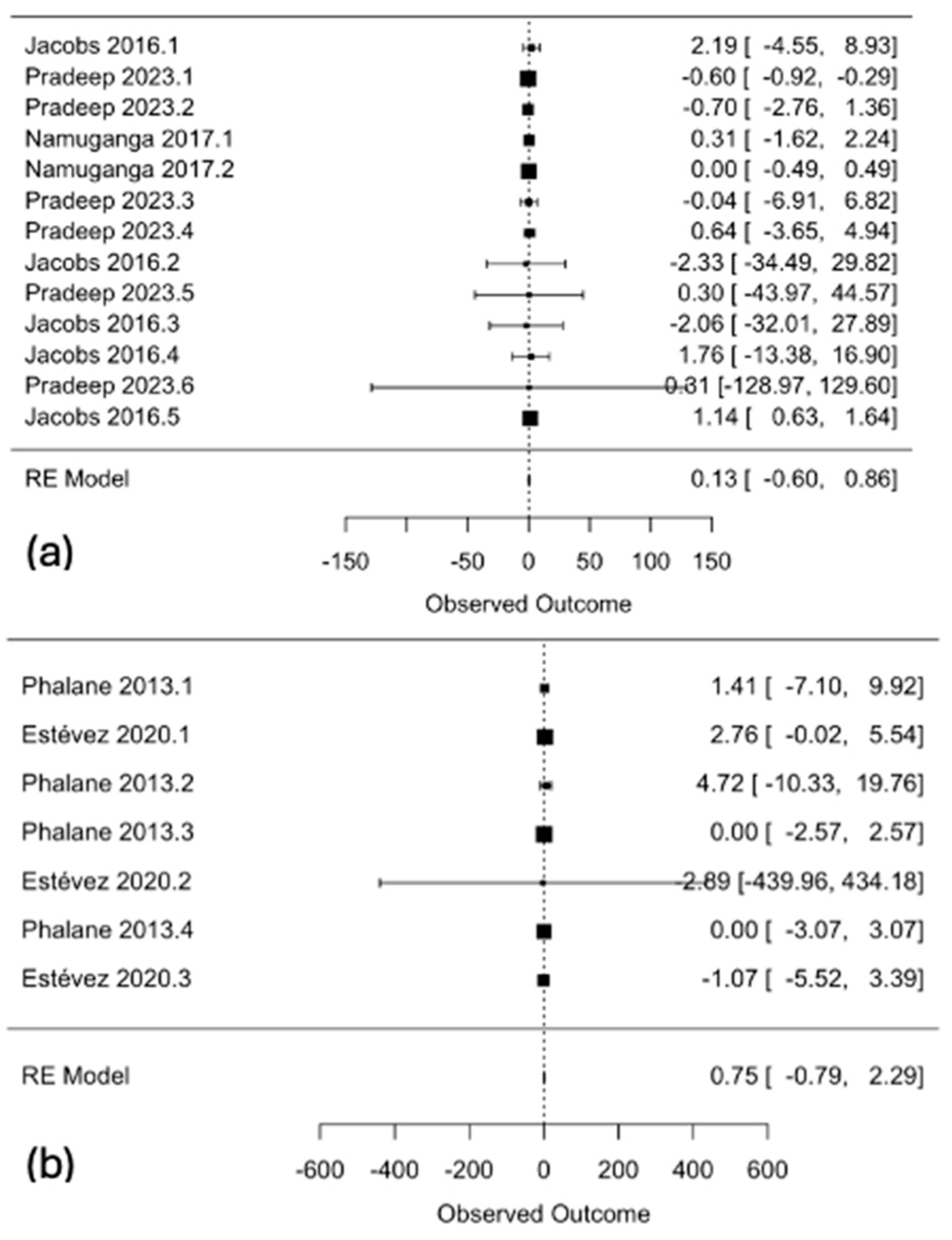
| (a) | ||
| PICO Component | Inclusion | Exclusion |
| Population (P) | Adult humans (≥18 years old) with active TB or latent TB | Pediatric populations, animal populations, non-TB populations |
| Intervention (I) | Measurement of salivary interleukin levels | Studies without data on salivary interleukins |
| Comparison (C) | Measurement of salivary interleukin levels in controls (healthy/ORD) | Studies without comparable groups |
| Outcome (O) | Quantitative interleukin data (e.g., pg/mL) with diagnostic relevance (e.g., sensitivity, specificity) | Outcomes unrelated to diagnostic accuracy |
| Other Criteria | Diagnostic accuracy studies, including cross-sectional, prospective, or retrospective study design | Reviews, letters, personal opinions, book chapters, theses, conference posters or papers, patents, meta-analyses |
| Studies in English | Non-English studies | |
| Moderate or low bias (QUADAS-2) | High risk of bias (QUADAS-2) | |
| (b) | ||
| PICO Component | Inclusion | Exclusion |
| Population (P) | Adult humans (≥18 years old) with active TB or latent TB | Pediatric populations, animal populations, non-TB populations |
| Intervention (I) | Salivary Mtb levels from molecular assays (e.g., GeneXpert MTB/RIF Ultra, GeneXpert MTB/RIF (Xpert), etc.) in active or latent TB patients | Unrelated interventions not using molecular assays |
| Comparison (C) | Salivary Mtb levels from molecular assays (e.g., GeneXpert MTB/RIF Ultra, GeneXpert MTB/RIF (Xpert), etc.) in controls | Studies without comparable groups |
| Outcome (O) | Diagnostic accuracy metrics (e.g., sensitivity, specificity, AUC, cost per test) | Lack of diagnostic performance metrics |
| Other Criteria | Diagnostic accuracy studies, including cross-sectional, prospective, or retrospective study design | Reviews, letters, personal opinions, book chapters, theses, conference posters or papers, patents, meta-analyses |
| Studies in English | Non-English studies | |
| Moderate or low bias (QUADAS-2) | High risk of bias (QUADAS-2) |
| Study | Intervention Group | Control Group | Active TB Sample Size | Latent TB Sample Size | Control Sample Size | Total Sample Size |
|---|---|---|---|---|---|---|
| Jacobs 2016 [21] | Active TB patients | ORD | 32 | - | 72 | 104 |
| Jacobs 2016.2 [25] | Active (confirmed or probable) TB patients | ORD | 18 | - | 33 | 51 |
| Phalane 2013 [24] | Active TB patients | No TB Disease (not specified as ORD or healthy) | 11 | - | 27 | 38 |
| Pradeep 2023 [23] | Active TB patients | ORD | 40 | - | 40 | 80 |
| Namuganga 2017 [26] | Active and latent TB patients | ORD | 39 | 21 | 18 | 78 |
| Estévez 2020 [22] | Active and latent TB patients | Healthy | 28 | 27 | 42 | 97 |
| Study | Interleukins | Active TB Levels (pg/mL) | Latent TB Levels (pg/mL) | Control Levels (pg/mL) |
|---|---|---|---|---|
| Jacobs 2016 [21] | IL-6 | 0.8 | - | 1.4 |
| IL-8 | 36.5 | - | 68.3 | |
| Jacobs 2016.2 [25] | IL-1β | 16.9 | - | 36.4 |
| IL-16 | 20.01 | - | 56.1 | |
| IL-17A | 13.8 | - | 6.1 | |
| IL-23 | 0.3 | - | 0.0 | |
| Phalane 2013 [24] | IL-5 | 0.9 | - | 0.0 |
| IL-6 | 37.3 | - | 0.0 | |
| IL-9 | 0.0 | - | 0.0 | |
| IL-17 | 18.9 | - | 12.6 | |
| Pradeep 2023 [23] | IL-1β | 12.44 | - | 8.95 |
| IL-2 | 55.43 | - | 39.04 | |
| IL-5 | 75.75 | - | 95.28 | |
| IL-6 | 14.46 | - | 12.28 | |
| IL-16 | 1253.91 | - | 1382.62 | |
| IL-17 | 11.7 | - | 10.76 | |
| Namuganga 2017 [26] | IL-2 | 0.0 | - | 0.0 |
| IL-5 | 1.6 | - | 1.6 | |
| IL-6 | 5.11 | - | 4.8 | |
| Estévez 2020 [22] | IL-1α | 1331 | 965 | 670.5 |
| IL-6 | 2.1 | 2.0 | 6.1 | |
| IL-12p40 | 4.807 | 0 | 2.326 |
| Parameter | Value |
|---|---|
| Model | Weighted regression with multiplicative dispersion |
| Predictor | Standard error |
| Test statistic (t) | 1.2252 |
| Degrees of freedom (df) | 19 |
| p-value | 0.2335 |
| Intercept (Limit as SE at 0, b) | −2.7160 |
| 95% Confidence Interval (CI) | −4.0350, −1.3969 |
| Criterion | Minimal Requirements |
|---|---|
| Goal and potential market | A test used during a patient’s first encounter with the health-care system to identify patients with any symptoms or risk factors for active pulmonary TB, including patients coinfected with HIV, those who do not have TB, and those in need of referral for further confirmatory testing |
| Pricing (of individual tests) | <USD 2.00 |
| Diagnostic sensitivity | Overall sensitivity should be >90% when compared with the confirmatory test for pulmonary TB |
| Diagnostic specificity | Overall specificity should be >70% when compared with the confirmatory test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, R.; Tama, M.; Sharief, S.; Zeidan, O.; Rady, S.M.A.; Chacko, K.S.; Nair, B.; Bhojaraja, V.S.; Shetty, J.K. The Role of Salivary Diagnostic Techniques in Screening for Active Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis. Microorganisms 2025, 13, 973. https://doi.org/10.3390/microorganisms13050973
Darwish R, Tama M, Sharief S, Zeidan O, Rady SMA, Chacko KS, Nair B, Bhojaraja VS, Shetty JK. The Role of Salivary Diagnostic Techniques in Screening for Active Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis. Microorganisms. 2025; 13(5):973. https://doi.org/10.3390/microorganisms13050973
Chicago/Turabian StyleDarwish, Radwan, Maya Tama, Sidra Sharief, Osama Zeidan, Sara Mohammed Ahmed Rady, Kareeza Selby Chacko, Bindhu Nair, Vijayalakshmi S. Bhojaraja, and Jeevan K. Shetty. 2025. "The Role of Salivary Diagnostic Techniques in Screening for Active Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis" Microorganisms 13, no. 5: 973. https://doi.org/10.3390/microorganisms13050973
APA StyleDarwish, R., Tama, M., Sharief, S., Zeidan, O., Rady, S. M. A., Chacko, K. S., Nair, B., Bhojaraja, V. S., & Shetty, J. K. (2025). The Role of Salivary Diagnostic Techniques in Screening for Active Pulmonary Tuberculosis: A Systematic Review and Meta-Analysis. Microorganisms, 13(5), 973. https://doi.org/10.3390/microorganisms13050973






