Biofilms from Klebsiella pneumoniae: Matrix Polysaccharide Structure and Interactions with Antimicrobial Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Antimicrobial Peptides
2.3. Evaluation of the Antimicrobial Activity of Peptides against Planktonic Cells
2.4. Biofilm Assays
2.5. Confocal Microscopy (CLSM) Experiments
2.6. Flow Cytometric Analysis
2.7. Biofilm Production and Extraction and Purification of Exopolysaccharides
2.8. Exopolysaccharides Composition and Glycosidic Linkages Determination
2.9. Circular Dichroism Experiments
3. Results
3.1. Strains Description
3.2. Growth Kinetic Assays
3.3. Evaluation of Bac7(1–35) Uptake in K. pneumoniae Strains
3.4. Effect of BMAP-27 on Bacterial Membrane Integrity
3.5. Biofilm Formation at Sub-Inhibitory Concentrations of AMPs
3.6. Characterisation of the Biofilm Matrix Polysaccharide Produced by KpTs101 and KpTs113
3.7. Evaluation of the Protective Effect of the Matrix
3.8. Circular Dichroism (CD) Spectroscopic Study
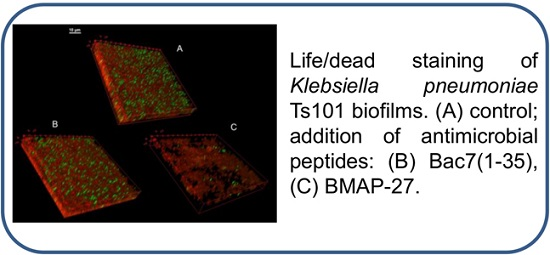
3.9. Confocal Microscopy of KpTs101 Biofilms
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2014. Antimicrobial Resistance and Healthcare-Associated Infections. ECDC: Stockholm, 2015. Available online: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-annual-epidemiological-report.pdf (accessed on 1 April 2016).
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [PubMed]
- Campos, M.A.; Vargas, M.A.; Regueiro, V.; Llompart, C.M.; Albertí, S.; Bengoechea, J.A. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 2004, 72, 7107–7114. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Fu, C.-I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Phil. Trans. R. Soc. B 2016, 371, 20150292. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Scocchi, M.; Podda, E.; Skerlavaj, B.; Dolzani, L.; Gennaro, R. Antimicrobial activity of Bac7 fragments against drug-resistant clinical isolates. Peptides 2004, 25, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.M.; de la Fuente-Núñez, C.; Baquir, B.; Faria-Junior, C.; Franco, O.L.; Hancock, R.E. Antibiofilm peptides increase the susceptibility of carbapenemase-producing Klebsiella pneumoniae clinical isolates to β-lactam antibiotics. Antimicrob. Agents Chemother. 2015, 59, 3906–3912. [Google Scholar] [CrossRef] [PubMed]
- Tomasinsig, L.; Benincasa, M.; Scocchi, M.; Skerlavaj, B.; Tossi, A.; Zanetti, M.; Gennaro, R. Role of cathelicidin peptides in bovine host defense and healing. Probiotics Antimicrob. Proteins 2010, 2, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Scocchi, M.; Tossi, A.; Gennaro, R. Proline-rich antimicrobial peptides: Converging to a non-lytic mechanism of action. Cell Mol. Life Sci. 2011, 68, 2317–2330. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Crocetta, V.; Scocchi, M.; Pomponio, S.; di Vincenzo, V.; Mardirossian, M.; Gherardi, G.; Fiscarelli, E.; Dicuonzo, G.; Gennaro, R.; et al. Potential novel therapeutic strategies in cystic fibrosis: Antimicrobial and anti-biofilm activity of natural and designed α-helical peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol. 2012, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Benincasa, M.; Zahariev, S.; Scocchi, M.; Berti, F.; Gennaro, R.; Tossi, A. Effect of size and N-terminal residue characteristics on bacterial cell penetration and antibacterial activity of the proline-rich peptide Bac7. J. Med. Chem. 2015, 58, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Tomasinsig, L.; Skerlavaj, B.; Scarsini, M.; Guida, F.; Piccinini, R.; Tossi, A.; Zanetti, M. Comparative activity and mechanism of action of three types of bovine antimicrobial peptides against pathogenic Prototheca spp. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2012, 18, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Skerlavaj, B.; Gennaro, R.; Bagella, L.; Merluzzi, L.; Risso, A.; Zanetti, M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 1996, 271, 28375–28381. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, S.M.; Foster, J.M.; Emerson, J.; Burns, J.L. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 2004, 42, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Toftgaard Nielsen, A.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Pacor, S.; Gennaro, R.; Scocchi, M. Rapid and reliable detection of antimicrobial peptide penetration into Gram-negative bacteria based on fluorescence quenching. Antimicrob. Agents Chemother. 2009, 53, 3501–3504. [Google Scholar] [CrossRef] [PubMed]
- Podda, E.; Benincasa, M.; Pacor, S.; Micali, F.; Mattiuzzo, M.; Gennaro, R.; Scocchi, M. Dual mode of action of Bac7, a proline-rich antibacterial peptide. Biochim. Biophys. Acta 2006, 1760, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2011. [Google Scholar] [CrossRef]
- Albersheim, P.; Nevins, D.J.; English, P.D.; Karr, A. A method for the analysis of sugars in plant cell wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 1967, 5, 340–345. [Google Scholar] [CrossRef]
- Kakehi, K.; Honda, S. Silyl ethers of carbohydrates. In Analysis of Carbohydrates by GLC and MS; Biermann, C.J., McGinnis, G.D., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 43–85. [Google Scholar]
- Harris, P.J.; Henry, R.J.; Blakeney, A.B.; Stone, B.A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr. Res. 1984, 127, 59–73. [Google Scholar] [CrossRef]
- Sweet, D.P.; Shapiro, R.H.; Albersheim, P. Quantitative analysis by various GLC response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydr. Res. 1975, 40, 217–225. [Google Scholar] [CrossRef]
- Kawamura, H.; Nishi, J.; Imuta, N.; Tokuda, K.; Miyanohara, H.; Hashiguchi, T.; Zenmyo, M.; Yamamoto, T.; Ijiri, K.; Kawano, Y.; et al. Quantitative analysis of biofilm formation of methicillin-resistant Staphylococcus aureus (MRSA) strains from patients with orthopaedic device-related infections. FEMS Immunol. Med. Microbiol. 2011, 63, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Richards, J.C.; Perry, M.B.; Clarke, B.R.; MacLean, L.L. Expression of two structurally distinct d-galactan O antigens in the lipopolysaccharide of Klebsiella pneumoniae serotype O1. J. Bacteriol. 1991, 173, 1420–1431. [Google Scholar] [PubMed]
- Cescutti, P.; de Benedetto, G.; Rizzo, R. Structural determination of the polysaccharide isolated from biofilms produced by a clinical strain of Klebsiella pneumoniae. Carbohydr. Res. 2016, 430, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Choy, Y.-M.; Dutton, G.G.S.; Zanlungo, A.M. The structure of the capsular polysaccharide of Klebsiella K-type 24. Can. J. Chem. 1973, 51, 1819–1825. [Google Scholar]
- Ahmad, A.; Azmi, S.; Srivastava, R.M.; Srivastava, S.; Pandey, B.K.; Saxena, R.; Bajpai, V.K.; Ghosh, J.K. Design of nontoxic analogues of cathelicidin-derived bovine antimicrobial peptide BMAP-27: The role of leucine as well as phenylalanine zipper sequences in determining its toxicity. Biochemistry 2009, 48, 10905–10917. [Google Scholar] [CrossRef] [PubMed]
- Tani, A.; Lee, S.; Oishi, O.; Aoyagi, H.; Ohno, M. Interaction of the fragments characteristic of bactenecin 7 with phospholipid bilayers and their antimicrobial activity. J. Biochem. 1995, 117, 560–565. [Google Scholar] [PubMed]
- Tomasinsig, L.; Morgera, F.; Antcheva, N.; Pacor, S.; Skerlavaj, B.; Zanetti, M.; Tossi, A. Structure dependence of biological activities for primate cathelicidins. J. Pept. Sci. 2009, 15, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Pacor, S.; Guida, F.; Antcheva, N.; Tossi, A. Native oligomerization determines the mode of action and biological activities of human cathelicidin LL-37. Biochem. J. 2014, 457, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Foschiatti, M.; Cescutti, P.; Tossi, A.; Rizzo, R. Inhibition of cathelicidin activity by bacterial exopolysaccharides. Mol. Microbiol. 2009, 72, 1137–1146. [Google Scholar]
- Chen, Y.H.; Yang, J.T.; Chau, K.T. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry 1974, 13, 3350–3359. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Herasimenka, Y.; Benincasa, M.; Mattiuzzo, M.; Cescutti, P.; Gennaro, R.; Rizzo, R. Interaction of antimicrobial peptides with bacterial polysaccharides from lung pathogens. Peptides 2005, 26, 1127–1132. [Google Scholar] [CrossRef] [PubMed]








| BF 1 Production | No. of Strains | Representative Isolate | BF Index | Bac7(1–35) | BMAP-27 | ||
|---|---|---|---|---|---|---|---|
| MIC | BIC | MIC | BIC | ||||
| Excellent | 2 | KpTs101 | 145 | 2 | 64 | 2 | >64 |
| KpMn7 | 101 | 4 | 32 | 4 | 64 | ||
| Good | 15 | ATCC 700603 | 76 | 4 | 32 | 4 | 64 |
| KpTs107 | 67 | 4 | 16 | 4 | >64 | ||
| Scarce | 13 | KpTs12 | 13 | 4 | 8 | 4 | 16 |
| KpTs109 | 13 | 4 | 32 | 4 | 32 | ||
| Flocs | 1 | KpTs113 | - | 2 | 64 | 2 | >64 |
| Strain | Bac7(1–35) | BMAP-27 | ||||
|---|---|---|---|---|---|---|
| MIC 1 | BIC 1 | Ps 2 | MIC 1 | BIC 1 | Ps 2 | |
| KpTs101 | 2 | 64 | 32 | 2 | >64 | 64 |
| KpTs113 | 2 | 64 | 32 | 2 | >64 | 64 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benincasa, M.; Lagatolla, C.; Dolzani, L.; Milan, A.; Pacor, S.; Liut, G.; Tossi, A.; Cescutti, P.; Rizzo, R. Biofilms from Klebsiella pneumoniae: Matrix Polysaccharide Structure and Interactions with Antimicrobial Peptides. Microorganisms 2016, 4, 26. https://doi.org/10.3390/microorganisms4030026
Benincasa M, Lagatolla C, Dolzani L, Milan A, Pacor S, Liut G, Tossi A, Cescutti P, Rizzo R. Biofilms from Klebsiella pneumoniae: Matrix Polysaccharide Structure and Interactions with Antimicrobial Peptides. Microorganisms. 2016; 4(3):26. https://doi.org/10.3390/microorganisms4030026
Chicago/Turabian StyleBenincasa, Monica, Cristina Lagatolla, Lucilla Dolzani, Annalisa Milan, Sabrina Pacor, Gianfranco Liut, Alessandro Tossi, Paola Cescutti, and Roberto Rizzo. 2016. "Biofilms from Klebsiella pneumoniae: Matrix Polysaccharide Structure and Interactions with Antimicrobial Peptides" Microorganisms 4, no. 3: 26. https://doi.org/10.3390/microorganisms4030026
APA StyleBenincasa, M., Lagatolla, C., Dolzani, L., Milan, A., Pacor, S., Liut, G., Tossi, A., Cescutti, P., & Rizzo, R. (2016). Biofilms from Klebsiella pneumoniae: Matrix Polysaccharide Structure and Interactions with Antimicrobial Peptides. Microorganisms, 4(3), 26. https://doi.org/10.3390/microorganisms4030026