Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells
Abstract
:1. Introduction
2. Immune Health-Promoting Benefits of EPS from LAB
3. Porcine Intestinal Epithelial Cells and Inflammation
4. Anti-Inflammatory Effects of EPS from LAB in PIE Cells
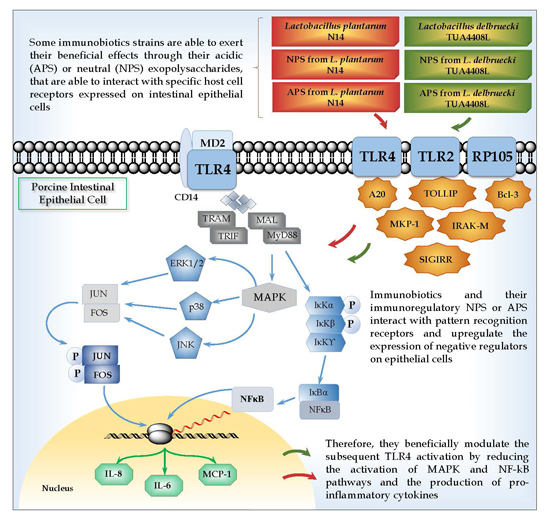
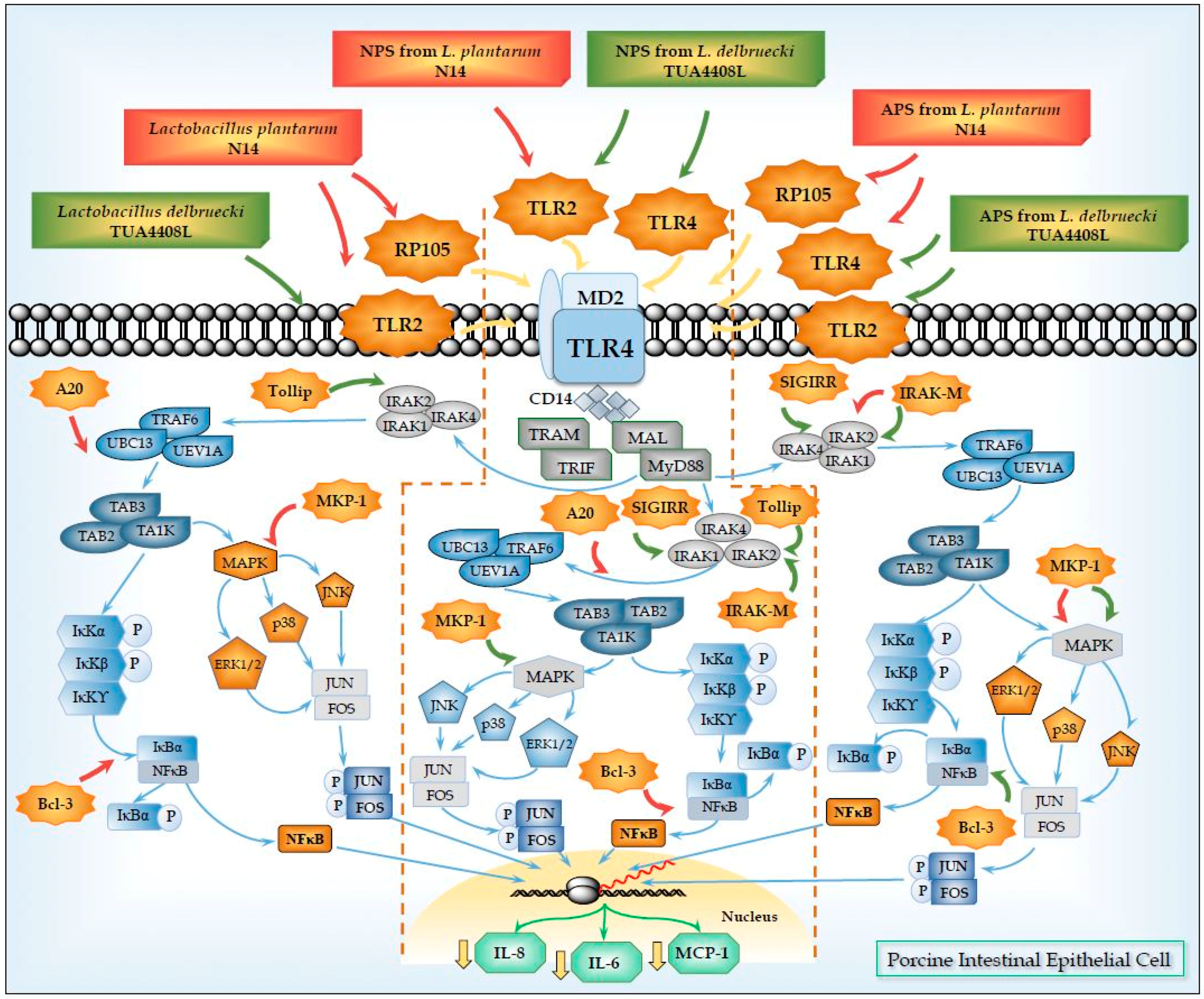
5. Role of TLR2 in the Immunomodulatory Effects of NPS from Lactobacilli
6. Role of TLR4 and RP105 in the Immunomodulatory Effects of APS from Lactobacilli
7. Conclusions
Conflicts of Interest
References
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- De Vuyst, L.; de Vin, F.; Vaningelgem, F.; Degeest, B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 687–707. [Google Scholar] [CrossRef]
- Matsuguchi, T.; Takagi, A.; Matsuzaki, T.; Nagaoka, M.; Ishikawa, K.; Yokokura, T.; Yoshikai, Y. Lipoteichoic Acids from Lactobacillus Strains Elicit Strong Tumor Necrosis Factor Alpha-Inducing Activities in Macrophages through Toll-Like Receptor 2. Clin. Diagn. Lab. Immunol. 2003, 10, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Dalpke, A.H.; Frey, M.; Morath, S.; Hartung, T.; Heeg, K. Interaction of Lipoteichoic Acid and CpG-DNA During Activation of Innate Immune Cells. Immunobiology 2002, 206, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D.; Karmeli, F.; Takabayashi, K.; Hayashi, T.; Leider-Trejo, L.; Lee, J.; Leoni, L.M.; Raz, E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 2002, 122, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Suzuki, Y.; Hirota, T. Cholesterol Lowering Activity of Ropy Fermented Milk. J. Food Sci. 1992, 57, 1327–1329. [Google Scholar] [CrossRef]
- Hosono, A.; Lee, J.; Ametani, A.; Natsume, M.; Hirayama, M.; Adachi, T.; Kaminogawa, S. Characterization of a Water-soluble Polysaccharide Fraction with Immunopotentiating Activity from Bifidobacterium adolescentis M101–4. Biosci. Biotechnol. Biochem. 1997, 61, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Chabot, S.; Yu, H.-L.; Léséleuc, L.D.; Cloutier, D.; Calsteren, M.-R.V.; Lessard, M.; Roy, D.; Lacroix, M.; Oth, D. Exopolysaccharides from Lactobacillus rhamnosus RW-9595M stimulate TNF. Lait 2001, 81, 683–697. [Google Scholar] [CrossRef]
- Kitazawa, H.; Harata, T.; Uemura, J.; Saito, T.; Kaneko, T.; Itoh, T. Phosphate group requirement for mitogenic activation of lymphocytes by an extracellular phosphopolysaccharide from Lactobacillus delbrueckii ssp. bulgaricus. Int. J. Food Microbiol. 1998, 40, 169–175. [Google Scholar] [CrossRef]
- Torino, M.I.; Font de Valdez, G.; Mozzi, F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front. Microbiol. 2015, 6, 834. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Exopolysaccharides Produced by Lactic Acid Bacteria and Bifidobacteria as Fermentable Substrates by the Intestinal Microbiota. Crit. Rev. Food Sci. Nutr. 2016, 56, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-Like Receptor 2 Pathway Establishes Colonization by a Commensal of the Human Microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; López, P.; Gueimonde, M.; los Reyes-Gavilán, C.G.; Suárez, A.; Margolles, A.; Ruas-Madiedo, P. Immune Modulation Capability of Exopolysaccharides Synthesised by Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Protein 2012, 4, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Hall, L.J.; van Sinderen, D. Bifidobacterium breve UCC2003 surface exopolysaccharide production is a beneficial trait mediating commensal-host interaction through immune modulation and pathogen protection. Gut Microbes 2012, 3, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; López, P.; Strahinic, I.; Suárez, A.; Kojic, M.; Fernández-García, M.; Topisirovic, L.; Golic, N.; Ruas-Madiedo, P. Characterisation of the exopolysaccharide (EPS)-producing Lactobacillus paraplantarum BGCG11 and its non-EPS producing derivative strains as potential probiotics. Int. J. Food Microbiol. 2012, 158, 155–162. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Monteserín, D.C.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Margolles, A.; Suárez, A.; Ruas-Madiedo, P. Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro responses upon interaction with human cells. Food Res. Int. 2012, 46, 99–107. [Google Scholar] [CrossRef]
- Bleau, C.; Monges, A.; Rashidan, K.; Laverdure, J.P.; Lacroix, M.; Van Calsteren, M.R.; Millette, M.; Savard, R.; Lamontagne, L. Intermediate chains of exopolysaccharides from Lactobacillus rhamnosus RW-9595M increase IL-10 production by macrophages. J. Appl. Microbiol. 2010, 108, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Lehner, M.D.; Morath, S.; Michelsen, K.S.; Schumann, R.R.; Hartung, T. Induction of Cross-Tolerance by Lipopolysaccharide and Highly Purified Lipoteichoic Acid via Different Toll-Like Receptors Independent of Paracrine Mediators. J. Immunol. 2001, 166, 5161–5167. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Tseng, K.-C.; Chiang, S.-S.; Lee, B.-H.; Hsu, W.-H.; Pan, T.-M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, H.; Itoh, T.; Tomioka, Y.; Mizugaki, M.; Yamaguchi, T. Induction of IFN-γ and IL-1α production in macrophages stimulated with phosphopolysaccharide produced by Lactococcus lactis ssp. cremoris. Int. J. Food Microbiol. 1996, 31, 99–106. [Google Scholar] [CrossRef]
- Uemura, J.; Itoh, T.; Kasneko, T.; Noda, K. Chemical characterization of extracellular polysaccharide from Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1. Milchwissenschaft 1998, 53, 443–446. [Google Scholar]
- Nishimura-Uemura, J.; Kitazawa, H.; Kawai, Y.; Itoh, T.; Oda, M.; Saito, T. Functional alteration of murine macrophages stimulated with extracellular polysaccharides from Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Food Microbiol. 2003, 20, 267–273. [Google Scholar] [CrossRef]
- Patten, D.A.; Leivers, S.; Chadha, M.J.; Maqsood, M.; Humphreys, P.N.; Laws, A.P.; Collett, A. The structure and immunomodulatory activity on intestinal epithelial cells of the EPSs isolated from Lactobacillus helveticus sp. rosyjski and Lactobacillus acidophilus sp. 5e2. Carbohydr. Res. 2014, 384, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.H.; Sedlak, C.; Käser, T.; Pasternak, A.; Levast, B.; Gerner, W.; Saalmüller, A.; Summerfield, A.; Gerdts, V.; Wilson, H.L.; et al. The porcine innate immune system: An update. Dev. Comp. Immunol. 2014, 45, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Schroyen, M.; Tuggle, C.K. Current transcriptomics in pig immunity research. Mamm. Genome 2015, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K. Advances in Swine Biomedical Model Genomics. Int. J. Biol. Sci. 2007, 3, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, L.; Kapetanovic, R.; Sester, D.P.; Hume, D.A. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J. Leukoc. Biol. 2011, 89, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, R.; Fairbairn, L.; Beraldi, D.; Sester, D.P.; Archibald, A.L.; Tuggle, C.K.; Hume, D.A. Pig Bone Marrow-Derived Macrophages Resemble Human Macrophages in Their Response to Bacterial Lipopolysaccharide. J. Immunol. 2012, 188, 3382–3394. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Moue, M.; Tohno, M.; Shimazu, T.; Kido, T.; Aso, H.; Saito, T.; Kitazawa, H. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochim. Biophys. Acta (BBA) Gen. Subj. 2008, 1780, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Fujie, H.; Villena, J.; Tohno, M.; Morie, K.; Shimazu, T.; Aso, H.; Suda, Y.; Shimosato, T.; Iwabuchi, N.; Xiao, J.-Z.; et al. Toll-like receptor-2-activating bifidobacteria strains differentially regulate inflammatory cytokines in the porcine intestinal epithelial cell culture system: Finding new anti-inflammatory immunobiotics. FEMS Immunol. Med. Microbiol. 2011, 63, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wachi, S.; Kanmani, P.; Tomosada, Y.; Kobayashi, H.; Yuri, T.; Egusa, S.; Shimazu, T.; Suda, Y.; Aso, H.; Sugawara, M.; et al. Lactobacillus delbrueckii TUA4408L and its extracellular polysaccharides attenuate enterotoxigenic Escherichia coli-induced inflammatory response in porcine intestinal epitheliocytes via Toll-like receptor-2 and 4. Mol. Nutr. Food Res. 2014, 58, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Villena, J.; Tohno, M.; Fujie, H.; Hosoya, S.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; Saito, T.; et al. Immunobiotic Lactobacillus jensenii Elicits Anti-Inflammatory Activity in Porcine Intestinal Epithelial Cells by Modulating Negative Regulators of the Toll-Like Receptor Signaling Pathway. Infect. Immun. 2012, 80, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, A.; Gómez-Hurtado, I.; Moya-Pérez, Á.; Zapater, P.; Peiró, G.; González-Navajas, J.M.; Gómez Del Pulgar, E.M.; Such, J.; Sanz, Y.; Francés, R. Bifidobacterium pseudocatenulatum CECT7765 promotes a TLR2-dependent anti-inflammatory response in intestinal lymphocytes from mice with cirrhosis. Eur. J. Nutr. 2016, 55, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Xu, D.; Brint, E.K.; O’Neill, L.A.J. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, S.; Villena, J.; Shimazu, T.; Tohno, M.; Fujie, H.; Chiba, E.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; et al. Immunobiotic lactic acid bacteria beneficially regulate immune response triggered by poly(I:C) in porcine intestinal epithelial cells. Vet. Res. 2011, 42, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tomosada, Y.; Villena, J.; Murata, K.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.-Z.; Saito, T.; Kitazawa, H. Immunoregulatory Effect of Bifidobacteria Strains in Porcine Intestinal Epithelial Cells through Modulation of Ubiquitin-Editing Enzyme A20 Expression. PLoS ONE 2013, 8, e59259. [Google Scholar]
- Murofushi, Y.; Villena, J.; Morie, K.; Kanmani, P.; Tohno, M.; Shimazu, T.; Aso, H.; Suda, Y.; Hashiguchi, K.; Saito, T.; et al. The Toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum N14. Mol. Immunol. 2015, 64, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Yoshida, M.; Kitazawa, H.; Araki, E.; Gomyo, T. Improvements in Seasonal Allergic Disease with Lactobacillus plantarum No. 14. Biosci. Biotechnol. Biochem. 2010, 74, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, K.; Nagata, Y.; Yoshida, M.; Murohushi, Y.; Kitazawa, H. Chemical and immunological characterization of extracellular polysaccharides produced by Lactobacillus plantarum No. 14. Jpn. J. Lactic Acid Bact. 2011, 22, 100–105. [Google Scholar] [CrossRef]
- Suda, Y.; (Miyagi University, Sendai, Japan); Masumizu, Y.; (Tohoku University, Sendai, Japan); Iida, H.; (Tohoku University, Sendai, Japan); Komatsu, R.; (Tohoku University, Sendai, Japan); Kanmani, P.; (Tohoku University, Sendai, Japan); Kober, A.H.; (Tohoku University, Sendai, Japan); Egusa, S.; (MARUSAN-AI Co., Ltd., Aichi, Japan); Villena, J.; (CERELA-CONICET, Tucuman, Argentina); Kitazawa, H.; (Tohoku University, Sendai, Japan). Personal communication, 2016.
- Ciszek-Lenda, M.; Nowak, B.; Śróttek, M.; Gamian, A.; Marcinkiewicz, J. Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37. Effects on the production of inflammatory mediators by mouse macrophages. Int. J. Exp. Pathol. 2011, 92, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, H.; Itoh, T.; Yamaguchi, T. Induction of macrophage cytotoxicity by slime products produced by Lactococcus lactis ssp. cremoris. Anim. Sci. Technol. 1991, 62, 861–866. [Google Scholar]
- Kitazawa, H.; Yamaguchi, T.; Itoh, T. B-cell mitogenic activity of slime product(s) produced from slime-forming, encapsulated Lactococcus lactis ssp. cremoris. J. Dairy Sci. 1992, 75, 2946–2951. [Google Scholar] [CrossRef]
- Kitazawa, H.; Yamaguchi, T.; Fujimoto, Y.; Itoh, T. Comparative activity of B-cell mitogen, a phosphopolysaccharide, produced by L. lactis ssp. cremoris on various lymphocytes. Anim. Sci. Technol. 1993, 64, 605–607. [Google Scholar]
- Kitazawa, H.; Yamaguchi, T.; Fujimoto, Y.; Itoh, T. An analysis of mitogenic response of phosphopolysaccharide, a B-cell mitogen produced by Lactococcus lactis ssp. cremoris, to spleen cells. Anim. Sci. Technol. 1993, 64, 807–812. [Google Scholar]
- Kitazawa, H.; Ishii, Y.; Uemura, J.; Kawai, Y.; Saito, T.; Kaneko, T.; Noda, K.; Itoh, T. Augmentation of macrophage functions by an extracellular phosphopoly saccharide from Lactobacillus delbrueckii ssp. bulgaricus. Food Microbiol. 2000, 17, 109–118. [Google Scholar] [CrossRef]
- Yasuda, E.; Serata, M.; Sako, T. Suppressive Effect on Activation of Macrophages by Lactobacillus casei Strain Shirota Genes Determining the Synthesis of Cell Wall-Associated Polysaccharides. Appl. Environ. Microbiol. 2008, 74, 4746–4755. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, C.; Kamishima, K.; Matsumoto, K.; Koga, H.; Katayama, T.; Yamamoto, K.; Hisa, K. Immunomodulating activity of exopolysaccharide-producing Leuconostoc mesenteroides strain NTM048 from green peas. J. Appl. Microbiol. 2014, 116, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.; Qin, J.; Zhao, Z.; Qian, Y.; Naramura, M.; Tian, L.; Towne, J.; Sims, J.E.; Stark, G.R.; Li, X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 2003, 4, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Gulen, M.F.; Qin, J.; Yao, J.; Bulek, K.; Kish, D.; Altuntas, C.Z.; Wald, D.; Ma, C.; Zhou, H.; et al. The Toll–Interleukin-1 Receptor Member SIGIRR Regulates Colonic Epithelial Homeostasis, Inflammation, and Tumorigenesis. Immunity 2007, 26, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Gulen, M.F.; Kang, Z.; Bulek, K.; Youzhong, W.; Kim, T.W.; Chen, Y.; Altuntas, C.Z.; Sass Bak-Jensen, K.; McGeachy, M.J.; Do, J.-S.; et al. The Receptor SIGIRR Suppresses Th17 Cell Proliferation via Inhibition of the Interleukin-1 Receptor Pathway and mTOR Kinase Activation. Immunity 2010, 32, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Qian, Y.; Yao, J.; Grace, C.; Li, X. SIGIRR Inhibits Interleukin-1 Receptor- and Toll-like Receptor 4-mediated Signaling through Different Mechanisms. J. Biol. Chem. 2005, 280, 25233–25241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ghosh, S. Negative Regulation of Toll-like Receptor-mediated Signaling by Tollip. J. Biol. Chem. 2002, 277, 7059–7065. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.L.; Turer, E.E.; Lee, E.G.; Ahmad, R.-C.; Wheeler, M.T.; Tsui, C.; Hurley, P.; Chien, M.; Chai, S.; Hitotsumatsu, O. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 2004, 5, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.M.; Green, S.; Sarma, V.; Holzman, L.B.; Wolf, F.W.; O’Rourke, K.; Ward, P.A.; Prochownik, E.V.; Marks, R.M. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J. Biol. Chem. 1990, 265, 2973–2978. [Google Scholar] [PubMed]
- Opipari, A.W.; Boguski, M.S.; Dixit, V.M. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J. Biol. Chem. 1990, 265, 14705–14708. [Google Scholar] [PubMed]
- Opipari, A.W.; Hu, H.M.; Yabkowitz, R.; Dixit, V.M. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J. Biol. Chem. 1992, 267, 12424–12427. [Google Scholar] [PubMed]
- Beyaert, R.; Heyninck, K.; van Huffel, S. A20 and A20-binding proteins as cellular inhibitors of nuclear factor-κB-dependent gene expression and apoptosis. Biochem. Pharmacol. 2000, 60, 1143–1151. [Google Scholar] [CrossRef]
- Ma, A.; Malynn, B.A. A20: Linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 2012, 12, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; Kaptein, T.M.; Wevers, B.A.; Mesman, A.W.; Geijtenbeek, T.B.H. Fucose-specific DC-SIGN signalling directs T helper cell type-2 responses via IKKε- and CYLD-dependent Bcl3 activation. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M.S. New regulators of NF-[kappa]B in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Mühlbauer, M.; Chilton, P.M.; Mitchell, T.C.; Jobin, C. Impaired Bcl3 Up-regulation Leads to Enhanced Lipopolysaccharide-induced Interleukin (IL)-23P19 Gene Expression in IL-10−/− Mice. J. Biol. Chem. 2008, 283, 14182–14189. [Google Scholar] [CrossRef] [PubMed]
- Wessells, J.; Baer, M.; Young, H.A.; Claudio, E.; Brown, K.; Siebenlist, U.; Johnson, P.F. BCL-3 and NF-κB p50 Attenuate Lipopolysaccharide-induced Inflammatory Responses in Macrophages. J. Biol. Chem. 2004, 279, 49995–50003. [Google Scholar] [CrossRef] [PubMed]
- Boutros, T.; Chevet, E.; Metrakos, P. Mitogen-Activated Protein (MAP) Kinase/MAP Kinase Phosphatase Regulation: Roles in Cell Growth, Death, and Cancer. Pharmacol. Rev. 2008, 60, 261–310. [Google Scholar] [CrossRef] [PubMed]
- Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008, 27, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.L.; Camps, M.; Rommel, C.; Mackay, C.R. Targeting dual-specificity phosphatases: Manipulating MAP kinase signalling and immune responses. Nat. Rev. Drug Discov. 2007, 6, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Hammer, M.; Mages, J. DUSP Meet Immunology: Dual Specificity MAPK Phosphatases in Control of the Inflammatory Response. J. Immunol. 2006, 177, 7497–7504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, M.; Fukuda, K.; Im, J.; Yao, P.; Cui, W.; Bulek, K.; Zepp, J.; Wan, Y.; Whan Kim, T.; et al. IRAK-M mediates Toll-like receptor/IL-1R-induced NF-κB activation and cytokine production. EMBO J. 2013, 32, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Julian, M.W.; Strange, H.R.; Ballinger, M.N.; Hotchkiss, R.S.; Papenfuss, T.L.; Crouser, E.D. Tolerance and Cross-Tolerance Following Toll-Like Receptor (TLR)-4 and -9 Activation Are Mediated by IRAK-M and Modulated by IL-7 in Murine Splenocytes. PLoS ONE 2015, 10, e0132921. [Google Scholar] [CrossRef] [PubMed]
- Zeuthen, L.H.; Fink, L.N.; Frøkiær, H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 2008, 124, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Roselli, M.; Imbinto, A.; Seeboth, J.; Oswald, I.P.; Mengheri, E. Lactobacillus amylovorus Inhibits the TLR4 Inflammatory Signaling Triggered by Enterotoxigenic Escherichia coli via Modulation of the Negative Regulators and Involvement of TLR2 in Intestinal Caco-2 Cells and Pig Explants. PLoS ONE 2014, 9, e94891. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Saha, P.; Banik, G.; Saha, D.R.; Grover, S.; Batish, V.K.; Das, S. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int. Immunopharmacol. 2016, 36, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Suzuki, R.; Fujie, H.; Chiba, E.; Takahashi, T.; Tomosada, Y.; Shimazu, T.; Aso, H.; Ohwada, S.; Suda, Y.; et al. Immunobiotic Lactobacillus jensenii Modulates the Toll-Like Receptor 4-Induced Inflammatory Response via Negative Regulation in Porcine Antigen-Presenting Cells. Clin. Vaccine Immunol. 2012, 19, 1038–1053. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Tomosada, Y.; Villena, J.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.-Z.; Saito, T.; Kitazawa, H. Bifidobacterium breve MCC-117 Induces Tolerance in Porcine Intestinal Epithelial Cells: Study of the Mechanisms Involved in the Immunoregulatory Effect. Biosci. Microb. Food Health 2014, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Yang, Y.-L.; Chen, Y.-P.; Hua, K.-F.; Lu, C.-P.; Sheu, F.; Lin, G.-H.; Tsay, S.-S.; Liang, S.-M.; Wu, S.-H. A Novel Exopolysaccharide from the Biofilm of Thermus aquaticus YT-1 Induces the Immune Response through Toll-like Receptor 2. J. Biol. Chem. 2011, 286, 17736–17745. [Google Scholar] [CrossRef] [PubMed]
- Graveline, R.; Segura, M.; Radzioch, D.; Gottschalk, M. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 2007, 19, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Yamashita, Y.; Ogata, M.; Sudo, T.; Kimoto, M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J. Immunol. 1995, 154, 3333–3340. [Google Scholar] [PubMed]
- Nagai, Y.; Kobayashi, T.; Motoi, Y.; Ishiguro, K.; Akashi, S.; Saitoh, S.-I.; Kusumoto, Y.; Kaisho, T.; Akira, S.; Matsumoto, M.; et al. The Radioprotective 105/MD-1 Complex Links TLR2 and TLR4/MD-2 in Antibody Response to Microbial Membranes. J. Immunol. 2005, 174, 7043–7049. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-I.; Hong, M.; Wilson, I.A. An unusual dimeric structure and assembly for TLR4 regulator RP105–MD-1. Nat. Struct. Mol. Biol. 2011, 18, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Shimazu, R.; Kondo, J.; Niki, T.; Akashi, S.; Ogata, H.; Yamashita, Y.; Miura, Y.; Kimoto, M. Mouse MD-1, a Molecule That Is Physically Associated with RP105 and Positively Regulates Its Expression. J. Immunol. 1998, 161, 1348–1353. [Google Scholar] [PubMed]
- Divanovic, S.; Trompette, A.; Atabani, S.F.; Madan, R.; Golenbock, D.T.; Visintin, A.; Finberg, R.W.; Tarakhovsky, A.; Vogel, S.N.; Belkaid, Y.; et al. Inhibition of TLR-4/MD-2 signaling by RP105/MD-1. J. Endotoxin Res. 2005, 11, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Divanovic, S.; Trompette, A.; Atabani, S.F.; Madan, R.; Golenbock, D.T.; Visintin, A.; Finberg, R.W.; Tarakhovsky, A.; Vogel, S.N.; Belkaid, Y.; et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat. Immunol. 2005, 6, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Wezel, A.; de Vries, M.R.; Maassen, J.M.; Kip, P.; Peters, E.A.; Karper, J.C.; Kuiper, J.; Bot, I.; Quax, P.H.A. Deficiency of the TLR4 analogue RP105 aggravates vein graft disease by inducing a pro-inflammatory response. Sci. Rep. 2016, 6, 24248. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.E.; Blumenthal, A. The RP105/MD-1 complex: Molecular signaling mechanisms and pathophysiological implications. J. Leukoc. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tohno, M.; Shimazu, T.; Ueda, W.; Anzawa, D.; Aso, H.; Nishimura, J.; Kawai, Y.; Saito, Y.; Saito, T.; Kitazawa, H. Molecular cloning of porcine RP105/MD-1 involved in recognition of extracellular phosphopolysaccharides from Lactococcus lactis ssp. cremoris. Mol. Immunol. 2007, 44, 2566–2577. [Google Scholar] [CrossRef] [PubMed]
- Capitán-Cañadas, F.; Ortega-González, M.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; de Medina, F.S.; Martínez-Augustin, O. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol. Nutr. Food Res. 2014, 58, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Kanatani, H.; Nakamura, A.; Ibuki, M.; Mine, Y. β-1,4-Mannobiose Stimulates Innate Immune Responses and Induces TLR4-Dependent Activation of Mouse Macrophages but Reduces Severity of Inflammation during Endotoxemia in Mice. J. Nutr. 2013, 143, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Zenhom, M.; Hyder, A.; de Vrese, M.; Heller, K.J.; Roeder, T.; Schrezenmeir, J. Prebiotic Oligosaccharides Reduce Proinflammatory Cytokines in Intestinal Caco-2 Cells via Activation of PPARγ and Peptidoglycan Recognition Protein 3. J. Nutr. 2011, 141, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Ortega-González, M.; Ocón, B.; Romero-Calvo, I.; Anzola, A.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; Sánchez de Medina, F.; Martínez-Augustin, O. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol. Nutr. Food Res. 2014, 58, 384–393. [Google Scholar] [CrossRef] [PubMed]

| Immunological Effects | Host | Strain | References |
|---|---|---|---|
| Induction of cytokine production by macrophages, especially TNF-α, IL-6, and IL-12. Desensitization of macrophages. Decrease of TNF-α production after re-stimulation with EPS. | Murine peritoneal macrophages | Lactobacillus rhamnosus KL37 | [43] |
| Modulation of immune cell recruitment and cytokine production. Reduction of Citrobacter rodentium colonization. | Mice | Bifidobacterium breve UCC2003 | [15] |
| Down-regulation of inflammatory response. | Human peripheral blood mononuclear cells | Lactobacillus paraplantarum BGCG11 | [16] |
| Immunostimulatory effect on macrophages and lymphocytes. Increase of pro-inflammatory cytokines expression, mainly IL-8. | HT29-19A cell line | Lactobacillus helveticus sp. Rosyjski Lactobacillus acidophilus sp. 5e2 | [24] |
| Stimulation of immune response. Mitogenic activity. Cytotoxicity. Induction of INF-γ, and IL-1α synthesis on spleen macrophages. | B lymphocytes and murine macrophages | Lactococcus lactis subsp. cremoris KVS20 | [21,44,45,46,47] |
| Increase of macrophage phagocytic activity. Increase of murine splenocytes mitogenic activity. Enhancement of macrophages cytotoxicity against tumour cells. Induction of cytokine production in macrophages. | Murine lymphocytes and murine macrophages including cell line J774.1 | Lactobacillus bulgaricus OLL1073-R1 | [9,23,48] |
| Reduction of immune cells reaction against LPS. Decrease in the production of TNF-α, IL-12, IL-10, and IL-6. | Murine spleen cells and murine RAW macrophages | Lactobacillus casei Shirota | [49] |
| Reduction of TNF-α, IL-6, and IL-12. Induction of high levels of IL-10. | Murine macrophages and splenic lymphocytes | Lactobacillus rhamnosus RW-9595M Lactobacillus rhamnosus ATCC9595 | [18] |
| Induction of tolerogenic dendritic cells. Improvement in the production of immunosuppressor cytokines. Expansion of regulatory Foxp3+CD25hi Treg cells. Control of Th17 cells differentiation. | Mice | Bifidobacterium animalis subsp. lactis IPLA-R1 | [13] |
| Induction of IL-6, IL-1β, and TNF-α. Promotion of phagocytosis, and increase of NO. | Murine RAW macrophages | Lactobacillus paracasei NTU101 Lactobacillus plantarum NTU102 | [20] |
| Stimulation of IgA production. | Mice | Leuconostoc mesenteroides NTM048 | [50] |
| Regulator | Name | Described Effects | References |
|---|---|---|---|
| SIGIRR | Single immunoglobulin interleukin-1 related receptor | SIGIRR acts as a negative regulator of IL-1 and TLR signaling. High expression of SIGIRR in epithelial cells indicates that SIGIRR may serve mainly to decrease the immune response in cells that are continually exposed to microorganisms, such as colon and lung epithelial cells. SIGIRR is an important modulator of intestinal epithelial homeostasis and a key regulator of mucosal immunity, maintaining microbial tolerance of the intestinal epithelial layer. | [51,52,53,54,55] |
| Tollip | Toll interacting protein | Tollip was associated with TLR2 and TLR4 and play an inhibitory role in TLR-mediated cell activation. The primary role of Tollip-mediated pathway may be to maintain immune cells in a quiescent state in the absence of infection and facilitate the termination of TLR-induced cell signaling during inflammation and infection. | [56] |
| A20 (TNFAIP3) | Tumor necrosis factor alpha-induced protein-3 | A20 is a zinc finger protein that functions via its two ubiquitin-editing activities. These two activities cooperatively down-regulate TRAF6 and terminate NF-kB signaling. A20 plays an essential role in the response to TNF-α and microbial products such as LPS. Inhibitor of NF-κB signaling induced by TNF-α, IL-1, CD40, PRRs, and T cell and B cell antigen receptor activation. | [57,58,59,60,61,62,63] |
| Bcl-3 | B-cell lymphoma-3 | Bcl-3 functions as an inhibitor of NF-κB activity by stabilizing repressive NF-κB homodimers in a DNA-bound state and preventing the binding of transcriptionally active dimers. Repressive complexes through the induction of Bcl-3 expression has been proposed to function during the processes of LPS tolerance. Bcl-3 has been reported to be involved in restricting inflammation by both suppressing IL-23 and inducing IL-10. | [64,65,66,67] |
| MKP-1 | Mitogen-activated protein kinase phosphatase-1 | MKP-1 plays a role in the inhibition of pro-inflammatory mRNA expression by inactivating MAPK. MKP-1 desensitizes cells to TLR ligands by inactivating p38 signaling pathway in enterocytes. MKP-1 desactivates MAPK (ERK, JNK, p38) by dephosphorilation. | [68,69,70] |
| IRAK-M | Interleukin-1 receptor-associated kinase M | IRAK-M is thought to bind MyD88/IRAK-4 and inhibit IRAK-4 phosphorylation of IRAK-1. This prevents formation of TRAF6/IRAK-1 complexes, which initiate IκB kinase and MAPK signaling pathways. IRAK-M-dependent pathway only induces expression of genes that are not regulated at the post-transcriptional levels (including inhibitory molecules SOCS-1, SHIP-1, A20 and IkBa), exerting an overall inhibitory effect on inflammatory response. The interaction of IRAK-M with IRAK-2 also suppresses inflammation, by suppressing cytokine and chemokine production. | [71,72] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells. Microorganisms 2016, 4, 27. https://doi.org/10.3390/microorganisms4030027
Laiño J, Villena J, Kanmani P, Kitazawa H. Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells. Microorganisms. 2016; 4(3):27. https://doi.org/10.3390/microorganisms4030027
Chicago/Turabian StyleLaiño, Jonathan, Julio Villena, Paulraj Kanmani, and Haruki Kitazawa. 2016. "Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells" Microorganisms 4, no. 3: 27. https://doi.org/10.3390/microorganisms4030027
APA StyleLaiño, J., Villena, J., Kanmani, P., & Kitazawa, H. (2016). Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells. Microorganisms, 4(3), 27. https://doi.org/10.3390/microorganisms4030027