Microbial Population Changes and Their Relationship with Human Health and Disease
Abstract
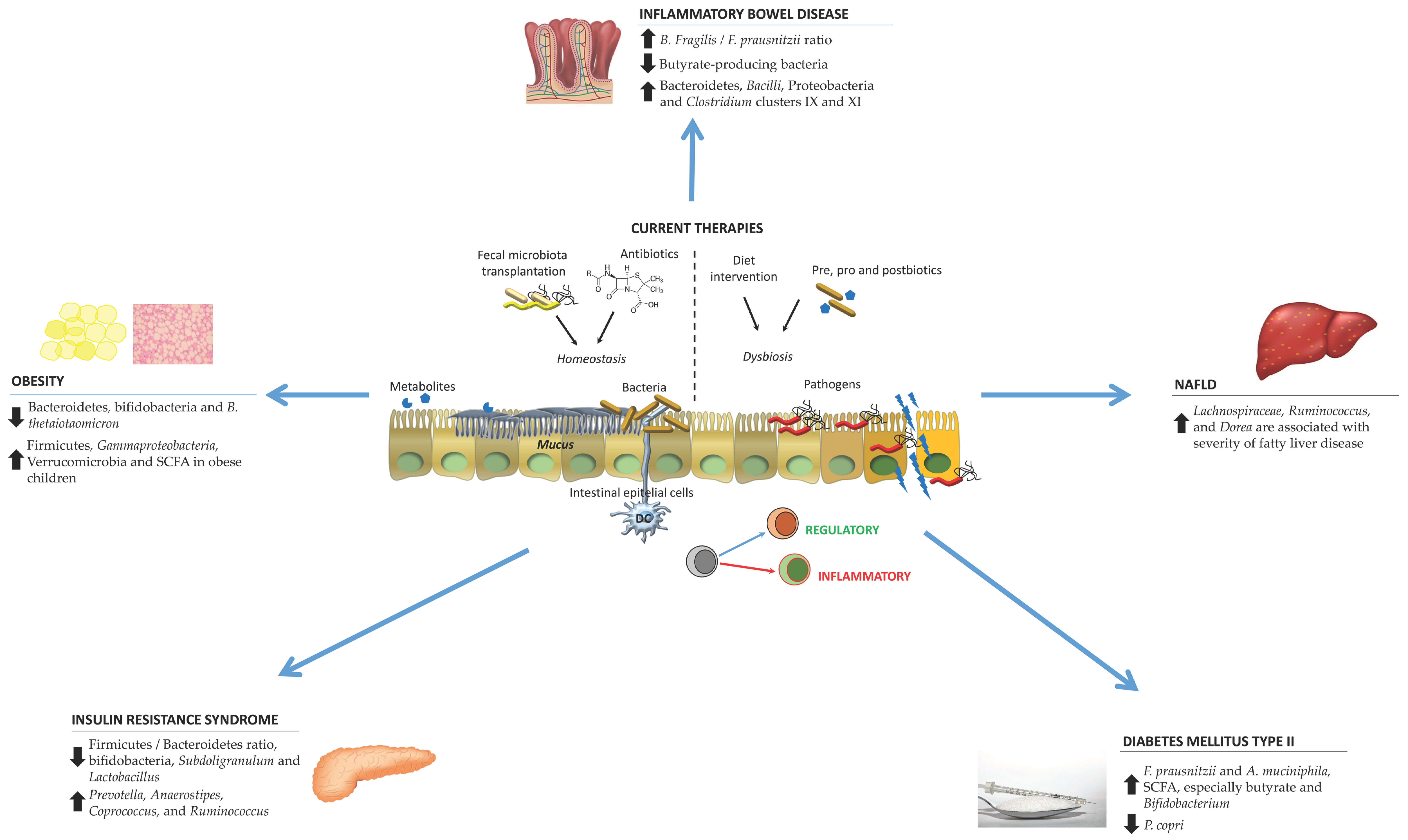
:1. Introduction
2. Gut Microbiota Changes in Particular Pathologies
2.1. Obesity
2.1.1. Antibiotics and Obesity
2.1.2. Gut Microbiota and Childhood Obesity
2.1.3. Bariatric Surgery and the Gut Microbiota
2.1.4. Potential Gut Microbiota Biomarkers of Obesity
2.1.5. Clinical Trials
2.2. Inflammatory Bowel Disease
2.2.1. Ulcerative Colitis
2.2.2. Crohn’s Disease
2.3. Non-Alcoholic Fatty Liver Disease
2.4. Insulin Resistance Syndrome
2.5. Diabetes Mellitus Type II
3. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilbert, J.A.; Krajmalnik-Brown, R.; Porazinska, D.L.; Weiss, S.J.; Knight, R. Toward effective probiotics for autism and other neurodevelopmental disorders. Cell 2013, 155, 1446–1448. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Immune-Mediated Mechanisms of Action of Probiotics and Synbiotics in Treating Pediatric Intestinal Diseases. Nutrients 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ashida, H.; Ogawa, M.; Yoshikawa, Y.; Mimuro, H.; Sasakawa, C. Bacterial interactions with the host epithelium. Cell Host Microbe 2010, 8, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, D.; Vrese, M.; Heller, K.J.; Schrezenmeir, J. Effect of natural commensal-origin DNA on toll-like receptor 9 (TLR9) signaling cascade, chemokine IL-8 expression, and barrier integritiy of polarized intestinal epithelial cells. Inflamm. Bowel Dis. 2010, 16, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008, 8, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Charbonnier, L.M.; Noval Rivas, M.; Georgiev, P.; Li, N.; Gerber, G.; Bry, L.; Chatila, T.A. MyD88 Adaptor-Dependent Microbial Sensing by Regulatory T Cells Promotes Mucosal Tolerance and Enforces Commensalism. Immunity 2015, 43, 289–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methe, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.; Ahmed, A.M.; Subramanian, S.; Griffin, N.W.; Drewry, L.L.; Petri, W.A., Jr.; Haque, R.; Ahmed, T.; Gordon, J.I. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 2014, 515, 423–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Demoor, T.; Rauch, M.; Faruqi, A.A.; Jang, S.; Johnson, C.C.; Boushey, H.A.; Zoratti, E.; Ownby, D.; Lukacs, N.W.; et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Dao, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.L.; Chevallier, J.M.; et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Robles-Sanchez, C.; Abadia-Molina, F.; Moron-Calvente, V.; Saez-Lara, M.J.; Ruiz-Bravo, A.; Jimenez-Valera, M.; Gil, A.; Gomez-Llorente, C.; Fontana, L. Adamdec1, Ednrb and Ptgs1/Cox1, inflammation genes upregulated in the intestinal mucosa of obese rats, are downregulated by three probiotic strains. Sci. Rep. 2017, 7, 1939. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42 Pt 2 (Suppl. 3), S156–S159. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Boirie, Y.; Cederholm, T.; Chourdakis, M.; Cuerda, C.; Delzenne, N.M.; Deutz, N.E.; Fouque, D.; Genton, L.; Gil, C.; et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin. Nutr. 2017, 36, 917–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gerard, P. Gut microbiota and obesity. Cell Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of Gut Microbiota in the Aetiology of Obesity: Proposed Mechanisms and Review of the Literature. J. Obes. 2016, 2016, 7353642. [Google Scholar] [CrossRef] [PubMed]
- Turta, O.; Rautava, S. Antibiotics, obesity and the link to microbes—What are we doing to our children? BMC Med. 2016, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Blustein, J.; Liu, M.; Corwin, E.; Cox, L.M.; Blaser, M.J. Infant antibiotic exposures and early-life body mass. Int. J. Obes. 2013, 37, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ajslev, T.A.; Andersen, C.S.; Gamborg, M.; Sorensen, T.I.; Jess, T. Childhood overweight after establishment of the gut microbiota: The role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int. J. Obes. 2011, 35, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Yallapragada, S.G.; Nash, C.B.; Robinson, D.T. Early-Life Exposure to Antibiotics, Alterations in the Intestinal Microbiome, and Risk of Metabolic Disease in Children and Adults. Pediatr. Ann. 2015, 44, e265–e269. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.; Reigstad, C.S.; Bäckhed, F. Intestinal microbiota during infancy and its implications for obesity. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, A.; Fernandes, M.; Rodrigues, V.; Groppo, F.; Cardoso, A.; Avila-Campos, M.; Nakano, V. Correlation between body mass index and faecal microbiota from children. Clin. Microbiol. Infect. 2016, 22, 258. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.S.; Pollak, J.; Bailey-Davis, L.; Hirsch, A.G.; Cosgrove, S.E.; Nau, C.; Kress, A.M.; Glass, T.A.; Bandeen-Roche, K. Antibiotic use and childhood body mass index trajectory. Int. J. Obes. 2016, 40, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.C.; Forrest, C.B.; Zhang, P.; Richards, T.M.; Livshits, A.; DeRusso, P.A. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014, 168, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Stewart, A.W.; Braithwaite, I.; Beasley, R.; Hancox, R.J.; Mitchell, E.A.; Group, I.P.T.S. Antibiotic treatment during infancy and increased body mass index in boys: An international cross-sectional study. Int. J. Obes. 2014, 38, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; Stefanis, C.; Gnani, D.; Furlanello, C.; Zandona, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Nirmalkar, K.; Hoyo-Vadillo, C.; Garcia-Espitia, M.; Ramirez-Sanchez, D.; Garcia-Mena, J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.C.; Hume, M.P.; Martinez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E. Gut Microbiota, Prebiotics, Probiotics, and Synbiotics in Management of Obesity and Prediabetes: Review of Randomized Controlled Trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, C.; Petrof, E.O.; Allen-Vercoe, E. Fecal Microbiota-based Therapeutics for Recurrent Clostridium difficile Infection, Ulcerative Colitis and Obesity. EBioMedicine 2016, 13, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marotz, C.A.; Zarrinpar, A. Treating Obesity and Metabolic Syndrome with Fecal Microbiota Transplantation. Yale J. Biol. Med. 2016, 89, 383–388. [Google Scholar] [PubMed]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Hu, Y.; Bruner, D.W. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7-18 years old children from the American Gut Project. Pediatr. Obes. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rampelli, S.; Guenther, K.; Turroni, S.; Wolters, M.; Veidebaum, T.; Kourides, Y.; Molnar, D.; Lissner, L.; Benitez-Paez, A.; Sanz, Y.; et al. Pre-obese children’s dysbiotic gut microbiome and unhealthy diets may predict the development of obesity. Commun. Biol. 2018, 1, 222. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.F.; Cortes-Oliveira, C.; Pinhel, M.A.S.; Nonino, C.B. Bariatric Surgery and Precision Nutrition. Nutrients 2017, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Sioka, E.; Chatedaki, C.; Zacharoulis, D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: A Systematic Review and Meta-analysis. Obes. Surg. 2017, 27, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Varin, T.V.; Schertzer, J.D.; Marette, A. The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery. Can. J. Diabetes 2017, 41, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Shi, J.; Wu, X.; Peng, Z.; Xin, C.; Zhang, L.; Liu, Y.; Gao, M.; Xu, S.; Han, H.; et al. Presence of Torque teno sus virus 1 and 2 in porcine circovirus 3-positive pigs. Transbound. Emerg. Dis. 2018, 65, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Stahlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fandriks, L.; le Roux, C.W.; Nielsen, J.; Backhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, A.P.; Paziuk, M.; Luevano, J.M., Jr.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra141. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jorgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Del Chierico, F.; Abbatini, F.; Russo, A.; Quagliariello, A.; Reddel, S.; Capoccia, D.; Caccamo, R.; Ginanni Corradini, S.; Nobili, V.; De Peppo, F.; et al. Gut Microbiota Markers in Obese Adolescent and Adult Patients: Age-Dependent Differential Patterns. Front. Microbiol. 2018, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.R.; Cantarel, B.L.; Lamendella, R.; Darzi, Y.; Mongodin, E.F.; Pan, C.; Shah, M.; Halfvarson, J.; Tysk, C.; Henrissat, B.; et al. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn’s disease. PLoS ONE 2012, 7, e49138. [Google Scholar] [CrossRef] [PubMed]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- De Caro, G.; Gaiani, F.; Duranti, S.; Fugazza, A.; Madia, C.; Milani, C.; Mancabelli, L.; Turroni, F.; de’ Angelis, G.L.; Carra, M.C.; et al. Inflammatory Bowel Disease. Am. J. Gastroenterol. 2016, 111, S260–S336. [Google Scholar] [CrossRef] [Green Version]
- Suskind, D.L.; Brittnacher, M.J.; Wahbeh, G.; Shaffer, M.L.; Hayden, H.S.; Qin, X.; Singh, N.; Damman, C.J.; Hager, K.R.; Nielson, H.; et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitkin, S.; Pokrotnieks, J. Clinical Potential of Anti-inflammatory Effects of Faecalibacterium prausnitzii and Butyrate in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Christophersen, C.T.; Bird, A.R.; Conlon, M.A.; Rosella, O.; Gibson, P.R.; Muir, J.G. Abnormal fibre usage in UC in remission. Gut 2015, 64, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Lowenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015, 149, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017, 66, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Rossen, N.G.; van der Spek, M.J.; Hartman, J.H.; Huuskonen, L.; Korpela, K.; Salojarvi, J.; Aalvink, S.; de Vos, W.M.; D’Haens, G.R.; et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017, 11, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, D.; Sasaki, T.; Osada, T.; Kuwahara-Arai, K.; Haga, K.; Shibuya, T.; Hiramatsu, K.; Watanabe, S. Changes in Intestinal Microbiota Following Combination Therapy with Fecal Microbial Transplantation and Antibiotics for Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.; Connor, S.; Ng, W.; Paramsothy, R. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Lo, B.; Prosberg, M.V.; Gluud, L.L.; Chan, W.; Leong, R.W.; van der List, E.; van der Have, M.; Sarter, H.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; et al. Systematic review and meta-analysis: Assessment of factors affecting disability in inflammatory bowel disease and the reliability of the inflammatory bowel disease disability index. Aliment. Pharmacol. Ther. 2018, 47, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Dobrolyubova, E.; Ruchkina, I.; Parfenov, A.; Knyazev, O. Ulcerative colitis (UC) with IBS-like disorders: Particular qualities of clinical manifestations and medical therapy. United Eur. Gastroenterol. J. 2017, 5, A293. [Google Scholar] [CrossRef]
- LaMere, B.; Wendt, E.R.; Kanwar, B.; Lynch, S.V. Investigating the Microbiome in a Phase 1b Study of Andecaliximab in Ulcerative Colitis. In Proceedings of the XXV UEG Week 2017, Barcelona, Spain, 28 October–1 November 2017; p. 103. [Google Scholar]
- Ananthakrishnan, A.N.; Luo, C.; Yajnik, V.; Khalili, H.; Garber, J.J.; Stevens, B.W.; Cleland, T.; Xavier, R.J. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe 2017, 21, 603–610. [Google Scholar] [CrossRef] [PubMed]
- World Congress of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2016, 63 (Suppl. 2), S14–S15. [CrossRef]
- Matsuoka, K.; Uemura, Y.; Kanai, T.; Kunisaki, R.; Suzuki, Y.; Yokoyama, K.; Yoshimura, N.; Hibi, T. Efficacy of Bifidobacterium breve Fermented Milk in Maintaining Remission of Ulcerative Colitis. Dig. Dis. Sci. 2018, 63, 1910–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, M.C.; Quintero, M.A.; Martinez, A.; Kerman, D.; Deshpande, A.R.; Damas, O.; Pignac-Kobinger, J.; Santaolalla, R.; Knight, K.; Rodriguez, V. P069 Low fat diet improves quality of life and changes the microbiome in a catered, cross-over design intervention of uc patients with quiescent disease: results of a pilot study. Gastroenterology 2018, 154, S36. [Google Scholar] [CrossRef]
- Rajca, S.; Grondin, V.; Louis, E.; Vernier-Massouille, G.; Grimaud, J.C.; Bouhnik, Y.; Laharie, D.; Dupas, J.L.; Pillant, H.; Picon, L.; et al. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L. Reply to can fecal microbial transplant effectively treat Crohn’s disease? Inflamm. Bowel Dis. 2015, 21, E8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.X.; Bu, C. Fecal Microbiota Transplant For Crohn’s Disease: A Prospective, Randomized Study In Chinese Population. In Proceedings of the XXV UEG Week Barcelona, Barcelona, Spain, 28 October–1 November 2017; p. 112. [Google Scholar]
- Zhou, Y.; Xu, Z.Z.; He, Y.; Yang, Y.; Liu, L.; Lin, Q.; Nie, Y.; Li, M.; Zhi, F.; Liu, S.; et al. Gut Microbiota Offers Universal Biomarkers across Ethnicity in Inflammatory Bowel Disease Diagnosis and Infliximab Response Prediction. mSystems 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.K.; Koumpouras, C.; Telesco, S.; Monast, C.S.; Brodmerkel, C.; Schloss, P.D. The Fecal Microbiome as a Tool for Monitoring and Predicting Response Outcomes in Ustekinumab-Treated, Anti-TNFî ‘Refractory Crohn’s Disease Patients: Results from the Certifi Study. Gastroenterology 2017, 152, S191. [Google Scholar] [CrossRef]
- Halmos, E.; Christophersen, C.; Bird, A.; Shepherd, S.; Muir, J.; Gibson, P. prebiotic effect of Fodmaps in patients with Crohn’s disease: A randomised controlled trial. J. Gastroenterol. Hepatol. 2015, 30, 157–158. [Google Scholar]
- Brunt, E.M.; Wong, V.W.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers 2015, 1, 15080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anstee, Q.M.; Day, C.P. The genetics of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Fotbolcu, H.; Zorlu, E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J. Gastroenterol. 2016, 22, 4079–4090. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstler, A.J.; Aumiller, T.; Degen, C.; Durr, M.; Weiss, E.; Maier, I.B.; Schattenberg, J.M.; Jin, C.J.; Sellmann, C.; Bergheim, I. Insulin resistance alters hepatic ethanol metabolism: Studies in mice and children with non-alcoholic fatty liver disease. Gut 2016, 65, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, Q.; Li, H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanisms and Therapy. Nutrients 2017, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Li, L.; Yu, C.H.; Shen, Z.; Chen, L.H.; Li, Y.M. Effects of prebiotics on non-alcoholic fatty liver disease. In Proceedings of the XXXVIIth National Congress of Gastroenterology, Hepatology and Digestive Endoscopy, Bucharest, Romania, 22–24 June 2017. [Google Scholar]
- Lambert, J.E.; Parnell, J.A.; Eksteen, B.; Raman, M.; Bomhof, M.R.; Rioux, K.P.; Madsen, K.L.; Reimer, R.A. Gut microbiota manipulation with prebiotics in patients with non-alcoholic fatty liver disease: A randomized controlled trial protocol. BMC Gastroenterol. 2015, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Poeta, M.; Pierri, L.; Vajro, P. Gut–liver axis derangement in non-alcoholic fatty liver disease. Children 2017, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Properzi, C.; Liddle, C.; Melton, P.; Ariff, A.; O’Sullivan, T.; Sherriff, J.; Coulter, S.; Christophersen, C.; Morrison, M. Bile Acids, Hepatic Steatosis and Gut Microbiome in Patients Undergoing Dietary Intervention for Non-Alcoholic Fatty Liver Disease. In Hepatology; Wiley: Hoboken, NJ, USA, 2018; p. 972A. [Google Scholar]
- Sherf-Dagan, S.; Zelber-Sagi, S.; Zilberman-Schapira, G.; Webb, M.; Buch, A.; Keidar, A.; Raziel, A.; Sakran, N.; Goitein, D.; Goldenberg, N.; et al. Probiotics administration following sleeve gastrectomy surgery: A randomized double-blind trial. Int. J. Obes. 2018, 42, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Orr, D.W.; Murphy, R. prebiotic supplementation with inulin following metronidazole therapy achieves greater Alt reduction in Non-alcoholic Fatty Liver Disease (NAFLD): A randomised double-blind placebo controlled trial: 2176. Hepatology 2015, 62, 1268A–1269A. [Google Scholar]
- Kobyliak, N.; Bosak, N.; Falalyeyeva, T.; Beregova, T.; Bodnar, P. Effect of a probiotic on fatty liver index and liver stiffness in NAFLD patients: Randomized clinical trial. J. Hepatol. 2017, 66, S426–S427. [Google Scholar] [CrossRef]
- Ahn, S.; Jun, D.; Kim, E.; Oh, H.; Jeong, J.; Sohn, J.; Jang, E. Change of microbiota in patients with improved fatty liver and obesity. J. Hepatol. 2018, 68, S838–S839. [Google Scholar] [CrossRef]
- Bomhof, M.R.; Parnell, J.A.; Ramay, H.R.; Crotty, P.; Rioux, K.P.; Probert, C.S.; Jayakumar, S.; Raman, M.; Reimer, R.A. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: A pilot clinical trial. Eur. J. Nutr. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Manzhalii, E.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; Stremmel, W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J. Dig. Dis. 2017, 18, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014, 39, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, S.M.; Couto, C.A.; Costa-Silva, L.; Armiliato, G.N.; Pereira, C.A.; Martins, F.S.; Ferrari Mde, L.; Vilela, E.G.; Torres, H.O.; Cunha, A.S.; et al. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients 2016, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Asghari-Jafarabadi Rad PhD, M. The Effect of Probiotic and Conventional Yogurt Consumptions on Anthropometric Parameters in Individuals with Non Alcoholic Fatty Liver Disease. J. Babol Univ. Med. Sci. 2014, 16, 55–62. [Google Scholar]
- Kessoku, T.; Imajo, K.; Honda, Y.; Kato, T.; Ogawa, Y.; Tomeno, W.; Higurashi, T.; Yoneda, M.; Shimakawa, M.; Tanaka, Y. Characteristics of Fecal Microbiota in Japanese Patients with Nonalcoholic Fatty Liver Disease: A Connection among Gut-Permeability, Endotoxin and NAFLD. Gastroenterology 2017, 152, S1200. [Google Scholar] [CrossRef]
- Lelouvier, B.; Servant, F.; Paisse, S.; Brunet, A.C.; Benyahya, S.; Serino, M.; Valle, C.; Ortiz, M.R.; Puig, J.; Courtney, M.; et al. Changes in blood microbiota profiles associated with liver fibrosis in obese patients: A pilot analysis. Hepatology 2016, 64, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Rao, G. Insulin resistance syndrome. Am. Fam. Physician 2001, 63, 1159–1163, 1165–1166. [Google Scholar] [PubMed]
- Mazidi, M.; Rezaie, P.; Kengne, A.P.; Mobarhan, M.G.; Ferns, G.A. Gut microbiome and metabolic syndrome. Diabetes Metab. Syndr. 2016, 10, S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Mutie, P.M.; Giordano, G.N.; Franks, P.W. Lifestyle precision medicine: The next generation in type 2 diabetes prevention? BMC Med. 2017, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Sarno, R.I.; Giorgio, V.; Miele, L. Gut microbiota, obesity and metabolic disorders. Minerva Gastroenterol. Dietol. 2017, 63, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef] [PubMed]
- Caricilli, A.M.; Saad, M.J. Gut microbiota composition and its effects on obesity and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushugulova, A.; Forslund, S.K.; Costea, P.I.; Kozhakhmetov, S.; Khassenbekova, Z.; Urazova, M.; Nurgozhin, T.; Zhumadilov, Z.; Benberin, V.; Driessen, M.; et al. Metagenomic analysis of gut microbial communities from a Central Asian population. BMJ Open 2018, 8, e021682. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, K.H.; Frost, M.; Bahl, M.I.; Licht, T.R.; Jensen, U.S.; Rosenberg, J.; Pedersen, O.; Hansen, T.; Rehfeld, J.F.; Holst, J.J.; et al. Effect of Antibiotics on Gut Microbiota, Gut Hormones and Glucose Metabolism. PLoS ONE 2015, 10, e0142352. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Cleary, S.; Bahrami, B.; Reynolds, N.; Macfarlane, G.T. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebo-controlled crossover study. Aliment. Pharmacol. Ther. 2013, 38, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, E.; Callegari, M.L.; Raia, V.; Viscovo, S.; Scotto, R.; Ferrari, S.; Morelli, L.; Buccigrossi, V.; Lo Vecchio, A.; Ruberto, E.; et al. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: A randomised clinical trial. PLoS ONE 2014, 9, e87796. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Fernandez-Caballero, J.A.; Chueca, N.; Garcia, F.; Gomez-Llorente, C.; Saez-Lara, M.J.; Fontana, L.; Gil, A. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients 2015, 7, 3999–4015. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J. Am. Heart Assoc. 2018, 7, e008342. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Garcia-Carpintero, S.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Delgado-Lista, J.; Perez-Martinez, P.; Rangel Zuniga, O.A.; Quintana-Navarro, G.M.; Landa, B.B.; Clemente, J.C.; et al. The gut microbial community in metabolic syndrome patients is modified by diet. J. Nutr. Biochem. 2016, 27, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haro, C.; Garcia-Carpintero, S.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Landa, B.B.; Clemente, J.C.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61, 1700300. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Lahti, L.; Salojarvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuno, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Y.; Mu, C.; He, X.; Zheng, K.; Guo, H.; Zhu, W. Characteristics of gut microbiota and its response to a Chinese Herbal Formula in elder patients with metabolic syndrome. Drug Discov. Ther. 2018, 12, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrugger, S.; Maerkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frokiaer, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Velikonja, A.; Lipoglavsek, L.; Zorec, M.; Orel, R.; Avgustin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2018, 55, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Leber, B.; Lemesch, S.; Trajanoski, S.; Bashir, M.; Horvath, A.; Tawdrous, M.; Stojakovic, T.; Fauler, G.; Fickert, P.; et al. Lactobacillus casei Shirota Supplementation Does Not Restore Gut Microbiota Composition and Gut Barrier in Metabolic Syndrome: A Randomized Pilot Study. PLoS ONE 2015, 10, e0141399. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Burch, E.; Williams, L.T.; Makepeace, H.; Alston-Knox, C.; Ball, L. How Does Diet Change with A Diagnosis of Diabetes? Protocol of the 3D Longitudinal Study. Nutrients 2019, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Sato, J.; Kanazawa, A.; Azuma, K.; Ikeda, F.; Goto, H.; Komiya, K.; Kanno, R.; Tamura, Y.; Asahara, T.; Takahashi, T.; et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci. Rep. 2017, 7, 12115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-Lopez, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-Lopez, A.; Avila-Nava, A.; Fernandez, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Zohar, Y.; Hill, J.O.; Astrup, A. Personalized Dietary Management of Overweight and Obesity Based on Measures of Insulin and Glucose. Annu. Rev. Nutr. 2018, 38, 245–272. [Google Scholar] [CrossRef] [PubMed]
- Chavanelle, V.; Otero, Y.F.; Sirvent, P.; Cani, P.D.; Peltier, S. Pleiotropic Effects of Totum-63—Simultaneous Targeting of Multiple Diabetes Mediators. Am. Diabetes Assoc. 2018. [Google Scholar] [CrossRef]
- Elbere, I.; Kalnina, I.; Silamikelis, I.; Konrade, I.; Zaharenko, L.; Sekace, K.; Radovica-Spalvina, I.; Fridmanis, D.; Gudra, D.; Pirags, V.; et al. Association of metformin administration with gut microbiome dysbiosis in healthy volunteers. PLoS ONE 2018, 13, e0204317. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; van der Beek, C.M.; Hermes, G.D.A.; Goossens, G.H.; Jocken, J.W.E.; Holst, J.J.; van Eijk, H.M.; Venema, K.; Smidt, H.; Zoetendal, E.G.; et al. Supplementation of Diet With Galacto-oligosaccharides Increases Bifidobacteria, but Not Insulin Sensitivity, in Obese Prediabetic Individuals. Gastroenterology 2017, 153, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; Alshathry, A.; Moyses, H.E.; Clough, G.F.; Wright, M.; Patel, J.; et al. Design and rationale of the INSYTE study: A randomised, placebo controlled study to test the efficacy of a synbiotic on liver fat, disease biomarkers and intestinal microbiota in non-alcoholic fatty liver disease. Contemp. Clin. Trials 2018, 71, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.; Munoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Ghavami, A.; Rahbar Saadat, Y.; Mesri Alamdari, N.; Alipour, S.; Dastouri, M.R.; Ostadrahimi, A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. J. Cardiovasc. Thorac. Res. 2017, 9, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Jafarabadi, M.A.; Alizadeh, E.; Ghavami, A.; Saadat, Y.R.; Alamdari, N.M.; Dastouri, M.R.; Alipour, S.; Ostadrahimi, A. The effects of sodium butyrate and high-performance inulin supplementation on the promotion of gut bacterium Akkermansia muciniphila growth and alterations in miR-375 and KLF5 expression in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Eur. J. Integr. Med. 2018, 18, 1–7. [Google Scholar]
- Mitchell, C.M.; Davy, B.M.; Halliday, T.M.; Hulver, M.W.; Neilson, A.P.; Ponder, M.A.; Davy, K.P. The effect of prebiotic supplementation with inulin on cardiometabolic health: Rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes. Contemp. Clin. Trials 2015, 45, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Yang, L.; Ma, M.; Liu, Y. Improving the metabolism of glucose and lipids in patients with prediabetes by affecting the gut microbiota. In Diabetes-Metabolism Research and Reviews; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Martinez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE 2010, 5, e15046. [Google Scholar] [CrossRef] [PubMed]
- Shimozato, A.; Sasaki, M.; Ogasawara, N.; Funaki, Y.; Ebi, M.; Goto, C.; Koikeda, S.; Joh, T.; Kasugai, K. Transglucosidase improves the bowel movements in type 2 diabetes mellitus patients: A preliminary randomized double-blind, placebo-controlled study. United Eur. Gastroenterol. J. 2017, 5, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Mobini, R.; Tremaroli, V.; Stahlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Berteus Forslund, H.; Perkins, R.; Backhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Bacopoulou, F.; Michos, A. The impact of probiotics’ administration on glycemic control, body composition, gut microbiome, mitochondria, and other hormonal signals in adolescents with prediabetes—A randomized, controlled trial study protocol. Contemp. Clin. Trials Commun. 2018, 11, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Javadi, A.; Ejtahed, H.S.; Mirmiran, P.; Javadi, M.; Yousefinejad, A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Samah, S.; Ramasamy, K.; Lim, S.M.; Neoh, C.F. Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2016, 118, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, S.M.; Barra, N.G.; Ashkar, A.A.; Schertzer, J.D. Immunometabolism of T cells and NK cells: Metabolic control of effector and regulatory function. Inflamm. Res. 2018, 67, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Soergel, D.A.; Dey, N.; Knight, R.; Brenner, S.E. Selection of primers for optimal taxonomic classification of environmental 16S rRNA gene sequences. ISME J. 2012, 6, 1440–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzosa, E.A.; Hsu, T.; Sirota-Madi, A.; Shafquat, A.; Abu-Ali, G.; Morgan, X.C.; Huttenhower, C. Sequencing and beyond: Integrating molecular ‘omics’ for microbial community profiling. Nat. Rev. Microbiol. 2015, 13, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef] [PubMed]
| Reference | Characteristics | Disease | Method | Primary Results |
|---|---|---|---|---|
| Turnbaugh et al., 2009 [34] | 154 adult female monozygotic and dizygotic twin pairs concordant for leanness or obesity | Obesity | Sequencing (16S rRNA) | Gut microbiomes are shared among family members, but the gut microbial community varies in each individual. |
| Ignacio et al., 2016 [40] | Correlation between BMI and fecal microbiota in 84 children | Obesity | qRT-PCR | Significant association between the number of Lactobacillus spp. and B. fragilis group members and BMI. |
| Riva et al., 2017 [44] | Characterization of the gut microbiota in 78 obese and normal-weight children aged 6 to 16 | Obesity | Sequencing (16S rRNA) | Elevated levels of Firmicutes and depleted levels of Bacteroidetes. |
| Nicolucci et al., 2017 [48] | 42 obese children who received either oligofructose-enriched inulin or placebo | Obesity | Sequencing (16S rRNA) | Significant increases in species of the genus Bifidobacterium and decreases in B. vulgatus within the group that consumed oligofructose-enriched inulin. |
| Zhang et al., 2015 [54] | Intervention trial in 38 Prader-Willi syndrome and simple obesity children. | Prader-Willi syndrome and obesity | Analysis of prevalent bacterial draft genomes assembled directly from metagenomic datasets | Non-digestible carbohydrates induced significant weight loss and concomitant structural changes in the gut microbiota. |
| Bai et al., 2018 [55] | 267 children (7–18 years old) analyzed according to their lifestyles | Obesity | Sequencing (16S rRNA) | Lower BMI and exercise frequency were associated with depleted Actinobacteria; Proteobacteria was significantly enriched in individuals with higher BMI levels; and Firmicutes was significantly enriched in individuals participating in frequent exercise. |
| Rampelli et al., 2018 [56] | 70 children analyzed in a two-time point 4-year prospective study | Pre-obese | Sequencing (16S rRNA) | Pre-obese dysbiosis and unhealthy diets were correlated and suggested to be predictors of obesity. |
| Tremaroli et al., 2015 [61] | Gut microbiome analysis of 14 women 9.4 years after bariatric surgery was performed | Obesity | High-quality Illumina reads alignment analysis | Bariatric surgery induces long-term alterations in the human gut microbiome. Surgically altered microbiomes contribute to fat mass regulation. |
| Palleja et al., 2016 [63] | Gut microbiome analysis 1 and 3 months after bariatric surgery in 13 patients | Obesity | Shotgun metagenomic sequencing | 31 microbial species showed altered relative abundances within the first 3 months, 16 of which maintained their altered relative abundances 1 year after surgery. F. prausnitzii was the only species that decreased in relative abundance. |
| Liu et al., 2017 [64] | Gut microbiome analysis of obese and post-bariatric intervention individuals in a cohort of 257 lean and obese young individuals | Obesity | Metagenome-wide association | Abundance of B. thetaiotaomicron was markedly decreased in obese individuals. Bariatric surgery intervention reversed obesity associated microbial alterations, including the decreased abundance of B. thetaiotaomicron. |
| Aron-Wisnewsky et al. [22] | 61 severely obese subjects of whom 24 were followed 1, 3, and 12 months post-bariatric surgery | Obesity | Shotgun metagenomics | Although bariatric surgery increased MGR one year after surgery, most RYGB patients remained with low MGR one year postsurgery. |
| Del Chierico et al., 2018 [67] | Gut microbiome analysis of 69 adolescent and adult patients | Obesity | Sequencing (16S rRNA) | Microbial markers, F. prausnitzii and Actinomyces assigned to the microbiota of obese adolescents. Parabacteroides, Rikenellaceae, Bacteroides caccae, Barnesiellaceae and Oscillospira were assigned to the microbiota of normal weight adolescents. |
| Le Chatelier et al., 2013 [68] | Gut microbiome analysis of 292 adult patients | Obesity | Sequencing (16S rRNA) | Individuals with low bacterial richness are characterized by increased overall adiposity compared to high bacterial richness individuals. |
| Reference | Disease | Intervention | Primary Results | Method |
|---|---|---|---|---|
| Sitkin et al., 2018 [76] | 40 UC patients | - | High B. fragilis/F. prausnitzii, depleted BPB, low Bifidobacterium. | qRT-PCR |
| Ishikawa et al., 2018 [81] | 36 UC mild–severe patients | FMT+AFM pretreatment | Bacteroidetes were recovered. | Sequencing (16S rRNA) |
| Matsuoka et al., 2018 [88] | 43 UC remission patients, 20–70 y/o | Bifidobacterium breve Yakult | Increase in C. leptum. | qRT-PCR |
| Phillips et al., 2018 [89] | UC quiescent | Low fat diet | High Bacteroidetes. | - |
| Ananthakrishnan et al., 2017 [86] | 43 UC patients | Vedolizumab | In non-remission, high S. salivarius. | Sequencing (V4 16S rRNA) |
| Lamere et al. 2017 [85] | 59 UC patients | Andecaliximab | High Clostridia and Akkermansia. | Sequencing |
| Fuentes et al., 2017 [80] | 33 UC mild–moderate patients | FMT | Low Clostridium cluster XIVa, non-responders had high Bacteroidetes. | Sequencing (16S rRNA) |
| Dobrolyubova et al., 2017 [84] | 162 UC patients, 35–41 y/o | 5-ASA | In remission, low Bifidobacilles and Lactobacillus, high Klebsiella, Proteus, Citrobacter, and hemolytic E. coli. | - |
| Lee et al., 2016 [79] | 22 UC active and remission patients, >18 y/o | - | Bacteroidetes absent in patients with active UC. | Sequencing (16S rRNA) |
| De Caro et al., 2016 [73] | 14 UC active and remission patients, mean 39 y/o | Infliximab, adalimumab, azathioprine or 5-ASA | Low bifidobacteria. | Metagenomic |
| Paramsothy et al., 2016 [82] | 81 UC patients | FMT | Barnesiella was associated with remission; Fusobacterium and Sutterella were associated with a lack of remission. | Sequencing (16S rRNA) |
| Hart et al., 2016 [87] | 7 UC patients, 5–18 y/o | CS | High bifidobacteria and Clostridium and low Faecalibacterium. | Sequencing (16S rRNA) |
| Rossen et al., 2015 [78] | 58 mild–moderate UC patients | - | Low Clostridium clusters IV, XIVa and XVIII and high in Bacteroidetes, Bacilli, Proteobacteria and Clostridium cluster IX and XI. | qRT-PCR |
| James et al., 2014 [77] | 37 UC patients, >18 y/o | - | UC patients have more Clostridium cluster XIVa. Lower A. muciniphila. | qRT-PCR |
| Doherty et al., 2017 [94] | 350 moderate–severe CD patients, 18–76 y/o | Ustekinumab | High Faecalibacterium in responders and remission patients. | Sequencing (V4 16S rRNA) |
| Zhou et al., 2017 [93] | 16 CD patients | Infliximab | Incremental change in Clostridiales. | Sequencing (V4 16S rRNA) |
| Yang et al., 2017 [92] | 31 active CD patients | FMT | Low Bacteroides, Roseburia, and Phascolarctobacterium, Eubacterium; high Bilophila, Streptococcus, Clostridium and Paraprevotella. | Sequencing (V4 16S rRNA) |
| Hart et al., 2016 [87] | 22 CD patients, 5–18 y/o | EEN or CS | Decreased in Prevotella, Bifidobacteria and Enterobacteriaceae. | Sequencing (16S rRNA) |
| Halmos et al., 2015 [95] | 8 quiescent CD patients | FODMAP diet | Increased Clostridium cluster XIVa and A. muciniphila. F. prausnitzii was unaltered. | - |
| Suskind et al., 2015 [74] | 9 mild–moderate CD patients, 12–19 y/o | FMT | High E. coli in response to inflammation. | Sequencing (V4 16S rRNA) |
| Rajca et al., 2015 [90] | 19 relapser and 14 non-relapser patients | - | Low C. coccoides, C. leptum and F. prausnitzii; high E. coli. | Sequencing (V4 16S rRNA) |
| Reference | Characteristics | Intervention | Time (weeks) | Methodological Procedure | Primary Results |
|---|---|---|---|---|---|
| Del Chierico et al., 2017 [45] | 61 children and adolescents (7–16 y/o). NAFLD (n = 27), NASH (n = 26), or obesity (n = 58) | NA | NA | Metagenomics and metabolomics analyses | Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria were the principal differences. |
| Kessouku et al., 2017 [115] | 201 adults. NAFLD = 143 (77 mild fibrosis and 56 severe fibrosis) | NA | NA | 16S rRNA gene sequencing and blood endotoxin activity assay | F. prausnitzii decreased in NAFLD patients and elevated blood-endotoxin in NAFLD. |
| Lelouvier et al. 2016 [116] | 44 adults (40–60 y/o) BMI > 40. Fibrosis 71 blood and fecal sample from Italy, 37 blood and 44 fecal samples from Spain | NA | NA | 16S rRNA gene quantitation by qRT-PCR and 16S metagenomic sequencing | Changes in Sphingomonas and Bosea correlated significantly with fibrosis. Ruminococcaceae, Lachnospiraceae, Coriobacteriaceae, and Fusobacteriaceae are modified in LF. |
| Anh et al., 2018 [109] | 34 adults Obesity plus NAFLD | Mixture of lactobacilli and bifidobacteria | 12 | qRT-PCR and 16S rRNA gene microbiome sequencing | Fatty liver improvement related to increases in Ruminococcaceae-2, Lachnospiraceae-2, Coprococcus, Lachnospiraceae-1, Ruminococcus, and Dorea. |
| Bomhof et al., 2018 [110] | Adults NASH | Oligofructose | 24 | Not indicated | Increase in Bifidobacterium. Decreased Clostridium clusters XI and I from prebiotic supplementation in patients with NASH. |
| Manzhalii et al., 2017 [111] | 38 adults NASH | Probiotic cocktail: lactobacilli, bifidobacteria, and S. thermophilus | 12 | Not indicated | Increased abundances of bifidobacteria, Lactobacillus, E. coli, and E. faecalis, among others. |
| Reference | Characteristics | Procedure | Main Results |
|---|---|---|---|
| Kushugulova et al., 2018 [126] | 58 IRS patients | 16S rRNA gene sequencing | IRS patient showed reduced Firmicutes/Bacteroidetes ratio, Bifidobacteria and Subdoligranulum and increased Prevotella. |
| Haro et al., 2017 [136] | 33 adult obese patients with severe IRS vs. 32 non IRS obese patients and 41 normal-weight subjects | 16S rRNA gene sequencing | After administration of MD and LF diet for 2 years, MD decreased the F/B ratio, Bacteroidetes, Bacteroides and Prevotella, and increased Faecalibacterium in the IRS group. |
| Haro et al., 2016 | 138 IRS patients | 16S rRNA gene sequencing | At time 0, increased Bacteroides, Eubacterium. and Lactobacillus genera and reduced the fragilis group, P. distasonis, B. thetaiotaomicron, F. prausnitzii, F. nucleatum, B. longum, B. adolescentis, the R. flavefaciens subgroup, and E. rectale in IRS patients at time 0. In a two-year intervention, Mediterranean diet and low-fat high-carbohydrate diet partly restored P. distasonis, F. prausnitzii, B. thetaiotaomicron, B. adolescentis, and B. longum levels. |
| Salonen et al., 2014 [137] | 12 adult IRS patients | HITChip phyloge Neticmicroarray and q-PCR | Dietary intervention: 1 week M diet, 3 weeks RS diet, 3 weeks NSP diet, and 3 weeks WL diet. Multiple Ruminococcaceae phylotypes increased with the RS diet, and Lachnospiraceae phylotypes were primarily increased by the NSP diet. |
| Moreno-Indias et al., 2015 [138] | 10 adult IRS patients | RT-PCR | Intake of wine and de-alcoholized red wine for 30 days/each increased bifidobacteria and Lactobacillus and Faecalibacterium prausnitzii and Roseburia and decreased Escherichia coli and Enterobacter cloacae. |
| Ni Y el al., 2018 [139] | 12 elderly IRS patients (60–90 y/o) | 16S rRNA gene sequencing | YDT supplementation for 4 days reduced Bacteroidales Incertae Sedi, Enterobacteriaceae Incertae Sedis and circulating lipoprotein(a) in correlation with Acinetobacter species. |
| Roager et al., 2019 [140] | 50 IRS patients | 16S rRNA gene sequencing | Administration of whole vs. refined grains for 8 weeks increased F. prausnitzii, P. copri, and Clostridiales but decreased B. thetaiotaomicron. |
| Smits et al., 2018 [134] | 10 adults IRS | 16S rRNA gene sequencing | Vegan FMT increased the levels of Lachnospiraceae, especially B. formatexigens and M. hypermegale, as well as L. bovis. |
| Velikonja et al., 2018 [141] | 27 adults IRS patients | qRT-PCR, and 16S rRNA gene sequencing | β-Glucans induced an increase in A. rectalis and decreased the levels of Coriobacteriales and Clostridiales associated with a reduction in total plasma cholesterol. |
| Stadlbauer et al., 2015 [142] | 13 adults IRS patients | 16S rRNA gene sequencing | Intake of LcS for 12 weeks increased Parabacteroides but did not restore the gut microbiota composition, gut barrier, or Bacteroidetes/Firmicutes ratio. |
| Vrieze et al., 2014 [143] | 100 adults IRS patients | qRT-PCR and Human Intestinal Tract Chip microarray | Administration of 500 mg/day of vancomycin for 1 week reduced Gram-positive bacteria (especially Firmicutes), secondary bile acids and peripheral insulin sensitivity while increasing Gram-negative bacteria (especially Proteobacteria) and reducing peripheral insulin sensitivity. |
| Reference | Characteristics | Procedure | Primary Results |
|---|---|---|---|
| Stefanaki et al., 2018 [163] | RCT with 50 adolescents. Probiotics and healthier lifestyle interventions | Body composition, glycemic and gut microbiota measurements | Probiotic administration was safe and useful for preventing the onset of pre-diabetes. |
| Tong et al., 2018 [151] | RCT in T2D and hyperlipidemia patients for 12 weeks with metformin and Chinese medicine treatment in 450 patients | 16S rRNA gene (V3 and V4 regions) sequencing | Significantly decreased hyperglycemia and hyperlipidemia, enrichment in Blautia and Faecalibacterium spp. |
| Zhao et al., 2018 [155] | 43 Chinese patients administered a high-fiber diet/prebiotics and a control. Both groups were treated with acarbose | Identification of SCFA-producing bacterial strains by metagenomic sequencing | Increased SCFA levels in the human bowel of the dietary fibers/prebiotics group. Improvement in hemoglobin A1c levels by elevating glucagon-like petide-1 production. |
| Roshanravan et al., 2018 [164] | 59 overweight and obese patients with T2D received sodium butyrate, inulin powder or both or a placebo | 16S rRNA gene analysis of A. muciniphila by quantitative real-time PCR | Increased A. muciniphila and decreased TNF-α mRNA expression. |
| Medina-Vera et al., 2018 [147] | 81 patients with T2D divided into placebo and functional food-based diet (high fiber, polyphenol rich and vegetable protein) groups | Determination of fecal microbiota | Increased F. prausnitzii and A. muciniphila and decreased P. copri. Improvement in glucose, insulin, HOMA-IR, and LPS levels. |
| Elbere et al., 2018 [150] | 18 healthy subjects were treated with metformin for 7 days | 16S rRNA gene (V3 region) | Diversity of gut microbiota decreased (reduction of Peptostreptococcaceae and Clostridiacea_1) after metformin treatment. |
| Shimozato et al., 2017 [161] | 66 T2D patients with and without chronic bowel movement disorder treated with placebo or transglucosidase | Analysis of fecal microbiota (amplification of 16S rRNA gene with T-RFLP) | Transglucosidase treatment modified the fecal microbiota (Prevotella, Bacteroides, Bifidobacterium, and the Clostridium sub-cluster XIVa) and the fecal SCFA and significantly improved bowel movements. |
| Canfora et al., 2017 [152] | Supplementation with galacto-oligosaccharides in 44 prediabetic patients | Fecal microbiota composition | Galacto-oligosaccharide supplementation increased Bifidobacterium. |
| Sato et al., 2017 [146] | Supplementation with L. casei in 68 T2D patients | Analysis of fecal microbiota | Probiotic administration increased C. coccoides, C. leptum, and Lactobacillus. |
| Mobini et al., 2016 [162] | 46 patients with T2D on insulin therapy and L. reuteri DSM 17938 supplementation | Fecal microbiota composition | No changes in microbiota were observed. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Mercado, A.I.; Navarro-Oliveros, M.; Robles-Sánchez, C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Muñoz-Quezada, S.; Fontana, L.; Abadía-Molina, F. Microbial Population Changes and Their Relationship with Human Health and Disease. Microorganisms 2019, 7, 68. https://doi.org/10.3390/microorganisms7030068
Álvarez-Mercado AI, Navarro-Oliveros M, Robles-Sánchez C, Plaza-Díaz J, Sáez-Lara MJ, Muñoz-Quezada S, Fontana L, Abadía-Molina F. Microbial Population Changes and Their Relationship with Human Health and Disease. Microorganisms. 2019; 7(3):68. https://doi.org/10.3390/microorganisms7030068
Chicago/Turabian StyleÁlvarez-Mercado, Ana Isabel, Miguel Navarro-Oliveros, Cándido Robles-Sánchez, Julio Plaza-Díaz, María José Sáez-Lara, Sergio Muñoz-Quezada, Luis Fontana, and Francisco Abadía-Molina. 2019. "Microbial Population Changes and Their Relationship with Human Health and Disease" Microorganisms 7, no. 3: 68. https://doi.org/10.3390/microorganisms7030068







