Industrial Validation of a Promising Functional Strain of Lactobacillus plantarum to Improve the Quality of Italian Sausages
Abstract
:1. Introduction
2. Materials and Methods
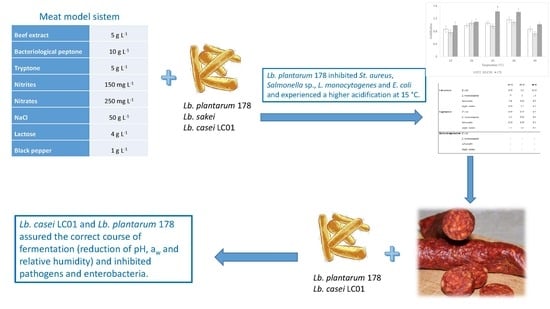
2.1. Microorganisms
2.2. Acidification
2.3. Bioactivity toward Foodborne Pathogens
2.4. Industrial Fermentation
- (a)
- The pH was measured on sausage homogenate through a pH meter Crison 2001 (Crison Instruments, Barcelona, Spain).
- (b)
- Color was monitored by colorimetric measurements using a Tristimulus Colorimeter Chromameter-2 Reflectance (Minolta, Osaka, Japan), equipped with a CR-300 measuring head. The instrument was standardized against a white tile before each determination. The color of the sausages was determined by a Hunter scale as L * (brightness), a * and b * (hue and saturation of the color). Data were the average of at least five repetitions.
- (c)
- Water activity measurements were performed by using a hygrometer AQUA LAB CX-2 (Decagon Device, Pullman, WA, USA).
- (d)
- Moisture content was measured by using Sartorius Thermal Balance (Antela, Florence, Italy), at 130 °C until the samples reached a constant weight.
2.5. Statistic
3. Results and Discussion
3.1. Preliminary Validation
3.2. Validation at Industrial Level
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bevilacqua, A.; Corbo, M.R.; Speranza, B.; Di Maggio, B.; Gallo, M.; Sinigaglia, M. Functional Starter Cultures for Meat: A Case Study on Technological and Probiotic Characterization. Food Nutr. Sci. 2015, 6, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Krockel, L. Chapter 5—The role of lactic acid bacteria. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, M., Ed.; InTech Open Publishing: Rijeka, Croatia, 2013; pp. 129–152. [Google Scholar]
- Zeng, H.; Yan, Y.; Liberti, F.; Bartocci, P.; Fantozzi, F. Technical and economic feasibility analysis of an anerobic digestion plant fed with canteen food waste. Energy Convers. Manag. 2019, 180, 938–948. [Google Scholar]
- Landeta, G.; Curiel, J.A.; Carrascosa, A.V.; Muñoz, R.; de las Rivas, B. Technological and safety properties of lactic acid bacteria isolated from Spanish dry-cured sausages. Meat Sci. 2013, 95, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammor, M.S.; Mayo, B. Selection criteria for lactic acid bacteria to be used as functional starter cultures in dry sausage production: An update. Meat Sci. 2007, 76, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.R.; Lima, I.A.; Martins, M.L.; Junior, A.A.B.; Filho, R.A.T.; Ramos, A.L.S.; Ramos, E.M. Application of Lactobacillus paracasei LPC02 and lactulose as a potential symbiotic system in the manufacture of dry-fermented sausage. LWT Food Sci. Technol. 2019, 102, 254–259. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, S.; Liu, R.; Chen, L.; Yang, B.; Yu, H.; Wu, M.; Zhang, W.; Zhou, G. Effects of Lactobacillus plantarum NJAU-01 on the protein oxidation of fermented sausage. Food Chem. 2019, 295, 361–367. [Google Scholar] [CrossRef]
- Macedo, R.E.F.; Pflanzer, S.B., Jr.; Terra, N.N.; Freitas, R.J.S. Desenvolvimento de um embutido fermentado por Lactobacillus probióticos: Características de qualidade. Ciência Tecnol. Alime. 2008, 28, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Rebucci, R.; Sangalli, L.; Fava, M.; Bersani, C.; Cantoni, C.; Baldi, A. Evaluation of functional aspects in Lactobacillus strains isolated from dry fermented sausages. J. Food Qual. 2007, 30, 187–201. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Speranza, B.; Di Maggio, B.; Gallo, M.; Sinigaglia, M. Use of alginate beads as carriers for lactic acid bacteria in a structured system and preliminary validation in a meat product. Meat Sci. 2016, 111, 198–203. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Speranza, B.; Gallo, M.; Campaniello, D.; Sinigaglia, M. Selection of lactic acid bacteria for sausages: Design of a selection protocol combining statistic tools, technological and functional properties. LWT Food Sci. Technol. 2017, 81, 144–152. [Google Scholar] [CrossRef]
- Zambonelli, C.; Tini, V.; Giudici, P.; Grazia, L. Microbiologia Degli Alimenti Fermentati, 1st ed.; Edagricole: Bologna, Italy, 2001. [Google Scholar]
- Benito, M.J.; Martìn, A.; Arandam, E.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Còrdoba, M.G. Characterization and selection of autochthonous lactic acid bacteria isolated from traditional Iberian dry-fermented salchichòn and chorizo sausages. J. Food Sci. 2007, 72, M193–M201. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.G.; Ricciardi, A.; Zotta, T.; Sico, M.A.; Salzano, G. Technological and safety characterization of coagulase-negative staphylococci from traditionally fermented sausages of Basilicata Region (Southern Italy). Meat Sci. 2009, 83, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Talon, R.; Leroy, S.; Lebert, I. Microbial ecosystems of traditional fermented meat products: The importance of indigenous starters. Meat Sci. 2007, 77, 55–62. [Google Scholar] [CrossRef]
- Cocolin, L.; Dolci, P.; Rantsiou, K. Biodiversity and dynamics of meat fermentations: The contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci. 2011, 89, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Monfort, J.M. Bacterial starter cultures for meat fermentation. Food Chem. 1997, 59, 547–554. [Google Scholar] [CrossRef]
- Carnevali, P.; Ciati, R.; Leporati, A.; Paese, A. Liquid sourdough fermentation: Industrial application perspectives. Food Microbiol. 2007, 24, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Mataragas, M.; Bellio, A.; Rovetto, F.; Astegiano, S.; Decastelli, L.; Cocolin, L. Risk-based control of food-borne pathogens Listeria monocytogenes and Salmonella enterica in the Italian fermented sausages Cacciatore and Felino. Meat Sci. 2015, 103, 39–45. [Google Scholar] [CrossRef]
- Cenci-Coga, B.T.; Karama, M.; Sechi, P.; Iuletto, M.F.; Grispoldi, L.; Selvaggini, R.; Ceccarelli, M.; Barbera, S. Fate of selected pathogens in spiked «SALAME NOSTRANO» produced without added nitrates following the application of NONIT™ technology. Meat Sci. 2008, 139, 247–254. [Google Scholar] [CrossRef]
- Fieira, C.; Marchi, J.F.; Marafão, D.; da Trindade, A.A. The impact of the partial replacement of sodium chloride in the development of starter cultures during Italian salami production. Braz. J. Food Technol. 2018, 21, e2015036. [Google Scholar] [CrossRef] [Green Version]
- Babić, I.; Markov, K.; Kovačević, D.; Trontel, A.; Slavica, A.; Đugum, J.; Čvek, D.; Svetec, I.K.; Posavec, S.; Frece, J. Identification and characterization of potential autochthonous starter cultures from a Croatian “brand” product “Slavonski kulen”. Meat Sci. 2011, 88, 517–524. [Google Scholar] [CrossRef]
- Di Luccia, A.; Tremonte, P.; Trani, A.; Loizzo, P.; La Gatta, B.; Succi, M.; Sorrentino, E.; Coppola, R. Influence of starter cultures and KCl on some biochemical, microbiological and sensory features of soppressata molisana, an Italian fermented sausage. Eur. Food Res. Technol. 2016, 242, 855–867. [Google Scholar] [CrossRef]
- Zambonelli, C.; Papa, F.; Romano, P.; Suzzi, G.; Grazia, L. Microbiologia dei Salumi; Edagricole: Bologna, Italy, 1992. [Google Scholar]
- Lebert, I.; Leroy, S.; Giammarinaro, P.; Lebert, A.; Chacornac, J.P.; Bover-Cid, S.; Vidal-Carou, M.C.; Talon, R. Diversity of microorganisms in the environment and dry fermented sausages of small traditional French processing units. Meat Sci. 2007, 76, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, G.; Coloretti, F.; Chiavari, C.; Grazia, L.; Lanciotti, R.; Gardini, F. Effects of starter cultures and fermentation climate on the properties of two types of typical Italian dry fermented sausages produced under industrial conditions. Food Control 2012, 26, 416–426. [Google Scholar] [CrossRef]
- Comi, G.; Urso, R.; Iacumin, L.; Rantsiou, K.; Cattaneo, P.; Cantoni, C.; Cocolin, L. Characterisation of naturally fermented sausages produced in the North East of Italy. Meat Sci. 2005, 69, 381–392. [Google Scholar] [CrossRef]
- Holko, I.; Hrabě, J.; Šalaková, A.; Rada, V. The substitution of a traditional starter culture in mutton fermented sausages by Lactobacillus acidophilus and Bifidobacterium animalis. Meat Sci. 2013, 94, 275–279. [Google Scholar] [CrossRef]
- Dalla Santa, O.R.; de Macedo, R.E.F.; Dalla Santa, H.S.; Zanette, C.M.; de Freitas, R.J.S.; Terra, N.N. Use of starter cultures isolated from native microbiota of artisanal sausage in the production of Italian sausage. Food Sci. Technol. 2014, 34, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Gounadaki, A.; Skandamis, P.; Drosinos, E.H.; Nychas, G.J.E. Survival of Listeria monocytogenes during the fermentation and ripening process of sausages. In Proceedings of the 1st Hellenic Symposium in Food Biotechnology and Technology; Association of Greek Chemists and Hellenic Association of Chemical Engineers: Athens, Greece, 2005; pp. 436–441. [Google Scholar]
- Speranza, B.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. A possible approach to assess acidification of meat starter cultures: A case study from some wild strains of Lactobacillus plantarum. J. Sci. Food Agric. 2017, 97, 2961–2968. [Google Scholar] [CrossRef]






| 6 h | 24 h | |
|---|---|---|
| Temperature | ** | ns |
| Strains | ns | ns |
| Temperature/strains | ns | * |
| 15 °C | 25 °C | 30 °C | ||
|---|---|---|---|---|
| Cell Culture | E. coli | 0.55 | 0.5 | 0.45 |
| L. monocytogenes | - | 1 | 1.4 | |
| Salmonella | 0.8 | 0.35 | 0.3 | |
| Staph. aureus | 0.95 | 0.7 | 0.9 | |
| Supernatant | E. coli | 0.45 | 0.35 | 0.3 |
| L. monocytogenes | 0.5 | 0.25 | 0.4 | |
| Salmonella | 0.55 | 0.35 | 0.3 | |
| Staph. aureus | 0.5 | 0.4 | 0.3 | |
| Buffered Supernatant | E. coli | - | - | - |
| L. monocytogenes | - | - | - | |
| Salmonella | - | - | - | |
| Staph. aureus | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campaniello, D.; Speranza, B.; Bevilacqua, A.; Altieri, C.; Rosaria Corbo, M.; Sinigaglia, M. Industrial Validation of a Promising Functional Strain of Lactobacillus plantarum to Improve the Quality of Italian Sausages. Microorganisms 2020, 8, 116. https://doi.org/10.3390/microorganisms8010116
Campaniello D, Speranza B, Bevilacqua A, Altieri C, Rosaria Corbo M, Sinigaglia M. Industrial Validation of a Promising Functional Strain of Lactobacillus plantarum to Improve the Quality of Italian Sausages. Microorganisms. 2020; 8(1):116. https://doi.org/10.3390/microorganisms8010116
Chicago/Turabian StyleCampaniello, Daniela, Barbara Speranza, Antonio Bevilacqua, Clelia Altieri, Maria Rosaria Corbo, and Milena Sinigaglia. 2020. "Industrial Validation of a Promising Functional Strain of Lactobacillus plantarum to Improve the Quality of Italian Sausages" Microorganisms 8, no. 1: 116. https://doi.org/10.3390/microorganisms8010116
APA StyleCampaniello, D., Speranza, B., Bevilacqua, A., Altieri, C., Rosaria Corbo, M., & Sinigaglia, M. (2020). Industrial Validation of a Promising Functional Strain of Lactobacillus plantarum to Improve the Quality of Italian Sausages. Microorganisms, 8(1), 116. https://doi.org/10.3390/microorganisms8010116