Metabolic Remodeling during Long-Lasting Cultivation of the Endomyces magnusii Yeast on Oxidative and Fermentative Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Culture Conditions
2.2. Cell Viability and Vitality Assays
2.3. Cell Respiration
2.4. Potential-Dependent Staining
2.5. Transmission Electron Microscopy (TEM)
2.6. Preparation of Cellular Homogenate
2.7. Antioxidant Enzymes Activities Assay
2.8. AH, Glucose-6-Phosphate Dehydrogenase (G6PDH), and IDH Activity Assays
2.9. Cellular IDH Activity
2.10. G6PDH Activity
2.11. Assay of Enzyme Activities of Glutathione Antioxidant System
2.11.1. Cellular Glutathione Peroxidases (GPxs)
2.11.2. Cellular GR Activity
2.12. Glutathione HPLC-ECD Analysis
2.13. Detection of ROS
2.14. Assay of Diene Conjugation
2.15. Statistical Analysis
3. Results
3.1. The Growth of Yeast Cultivated on Two Substrates
3.2. Respiratory Activity of E. magnusii Yeast Cultivated on Different Substrates
3.3. Potential-Dependent Staining of Mitochondria in the E. magnusii Cells
3.4. The Survival of Yeast Cultivated on Different Substrates
3.5. ROS Generation and DC Accumulation
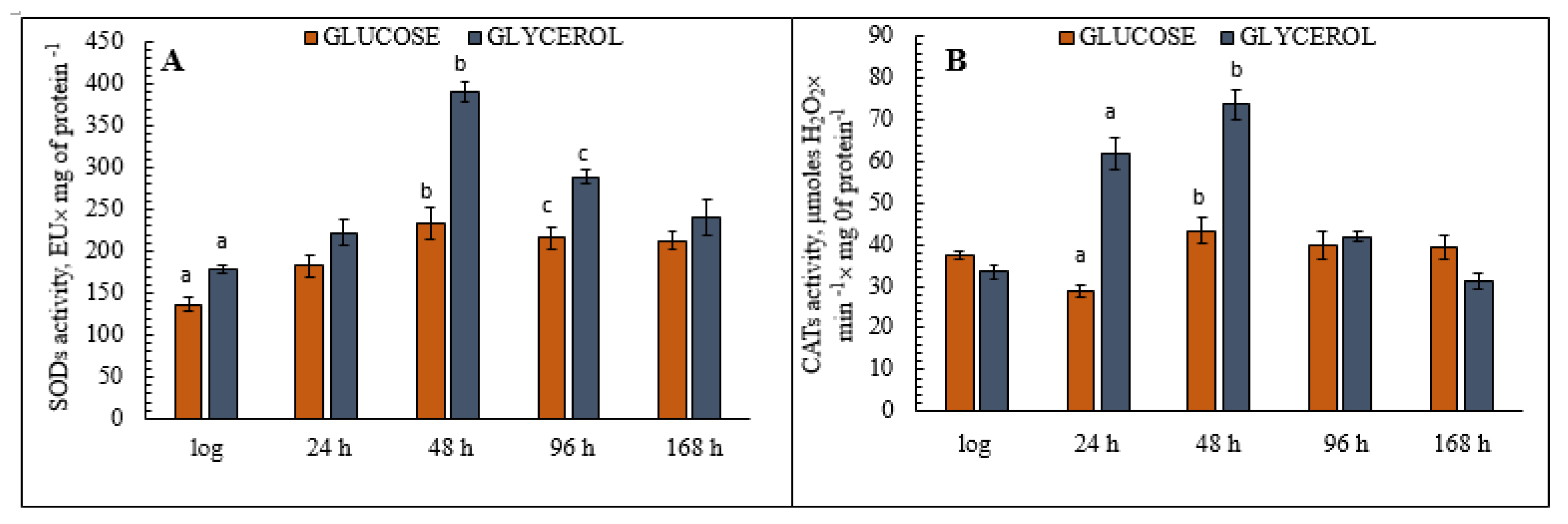
3.6. Analysis of Antioxidant Enzyme (CATs and SODs) Activity in the Yeast E. magnusii
3.7. Assay of the Activity and Level of the Glutathione System Enzymes
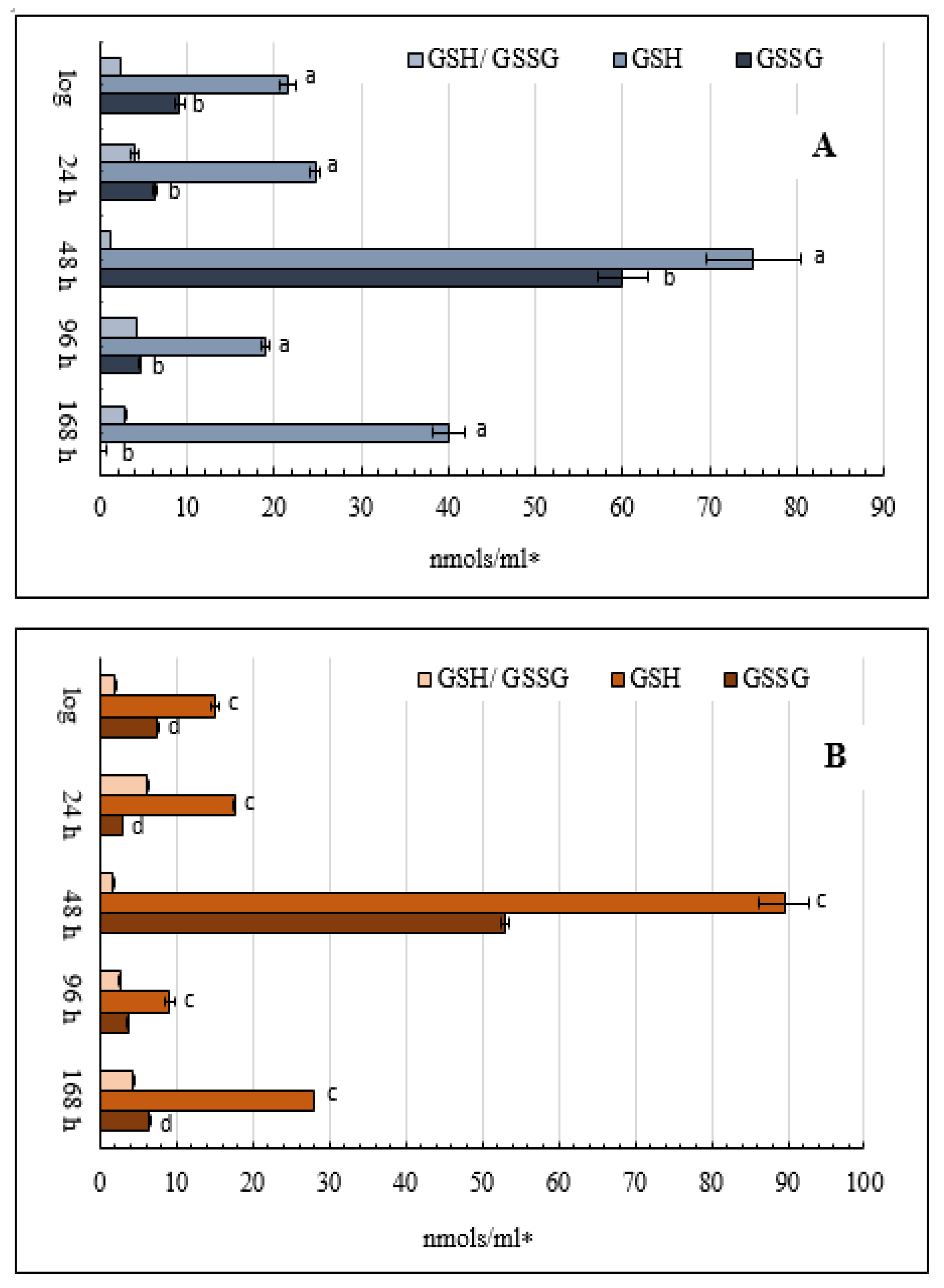
3.8. Glutathione Content in the Yeast E. magnusii
3.9. The Activity of Enzymes Serving as the Main Producers of NADPH in the Yeast Cell
3.10. Total Activity of Cellular AH
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harman, D. Aging: Theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef]
- Harman, D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.; Ros, J.; Bel, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta 2008, 1780, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants, and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Bulteau, A.L.; Ikeda-Saito, M.; Szweda, L.I. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry 2003, 42, 14846–14855. [Google Scholar] [CrossRef]
- Rebrin, I.; Kamzalov, S.; Sohal, R.S. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003, 35, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Houthoofd, K.; Braeckman, B.P.; Lenaerts, I.; Brys, K.; De Vreese, A.; Van Eygen, S.; Vanfleteren, J.R. Axenic Growth up-regulates mass-specific metabolic rate, stress resistance, and extends life span in Caenorhabditis elegans. Exp. Gerontol. 2002, 37, 1371–1378. [Google Scholar] [CrossRef]
- Herker, E.; Jungwirth, H.; Lehmann, K.A.; Maldener, C.; Fröhlich, K.U.; Wissing, S.; Büttner, S.; Fehr, M.; Sigrist, S.; Madeo, F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004, 164, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Reverter-Branchat, G.; Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae: Common targets and prevention by calorie restriction. J. Biol. Chem. 2004, 279, 31983–31989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, A.E.; Leroux, A.; Alaamery, M.A.; Hoffman, C.S.; Chartrand, P.; Ferbeyre, G.; Rokeach, L.A. Pro-aging effects of glucose signaling through a G protein-coupled glucose receptor in fission yeast. PLoS Genet. 2009, 5, e1000408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y. Mitochondria, reactive oxygen species, and chronological aging: A message from yeast. Exp. Gerontol. 2011, 46, 847–852. [Google Scholar] [CrossRef]
- Pan, Y.; Shadel Gerald, S. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging 2009, 1, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.C.; Pérez, V.I.; Song, W.; Lustgarten, M.S.; Salmon, A.B.; Mele, J.; Qi, W.; Liu, Y.; Liang, H.; Chaudhuri, A.; et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1114–1125. [Google Scholar] [CrossRef] [Green Version]
- Van Remmen, H.; Ikeno, Y.; Hamilton, M.; Pahlavani, M.; Wolf, N.; Thorpe, S.R.; Alderson, N.L.; Baynes, J.W.; Epstein, C.J.; Huang, T.T.; et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genom. 2003, 16, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Deryabina, Y.I.; Bazhenova, E.N.; Saris, N.E.; Zvyagilskaya, R.A. Ca(2+) efflux in mitochondria from the yeast Endomyces magnusii. J. Biol. Chem. 2001, 276, 47801–47806. [Google Scholar] [CrossRef] [Green Version]
- Deryabina, Y.I.; Bazhenova, E.N.; Zvyagilskaya, R.A. The Ca2+-Transport System of Yeast (Endomyces magnusii) Mitochondria: Independent Pathways for Ca2+ Uptake and Release. Biochemistry 2000, 65, 1352–1356. [Google Scholar] [PubMed]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014, 14, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Deryabina, Y.; Isakova, E.; Antipov, A.; Saris, N.E. The inhibitors of antioxidant cell enzymes induce permeability transition in yeast mitochondria. J. Bioenerg. Biomembr. 2013, 45, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar]
- Kostyuk, V.A.; Potapovich, A.I.; Kovaleva, J.V. A simple and sensitive method of determination of superoxide dismutase activity based on the reaction of quercetin oxidation. Vopr. Med. Chim. 1990, 2, 88–91. [Google Scholar]
- Henson, C.P.; Cleland, W.W. Purification and kinetic studies of beef liver cytoplasmic aconitase. J. Biol. Chem. 1967, 242, 3833–3838. [Google Scholar]
- Robinson, J.B., Jr.; Brent, L.G.; Sumegi, B.; Srere, P.A. An enzymatic approach to the study of the Krebs tricarboxylic acid cycle. In Mitochondria: A Practical Approach; Darley-Usmar, V.M., Rickwood, D., Wilson, M.T., Eds.; IRL Press: Washington, DC, USA, 1987; pp. 153–169. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.S.; LaBella, F.S. Comparison of analytical methods for monitoring autoxidation profiles of authentic lipids. J. Lipid Res. 1987, 28, 1110–1117. [Google Scholar]
- Stalnaya, I. Method for definition of dien conjugation of unsaturated higher fatty acids. In The modern Methods in Biochemistry; Medicina: Moscow, Russia, 1977; pp. 63–64. [Google Scholar]
- Klein, M.; Carrillo, M.; Xiberras, J.; Islam, Z.U.; Swinnen, S.; Nevoigt, E. Towards the exploitation of glycerol’s high reducing power in Saccharomyces cerevisiae-based bioprocesses. Metab. Eng. 2016, 38, 464–472. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on lipid and amino acid metabolism in yeast cells. Biol. Trace Elem. Res. 2019, 187, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J. Simultaneous Production of Lipids and Carotenoids by the Red Yeast Rhodotorula from Waste Glycerol Fraction and Potato Wastewater. Appl. Biochem. Biotechnol. 2019, 189, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Werner-Washburne, M.; Braun, E.; Johnston, G.C.; Singer, R. Stationary Phase in the Yeast Saccharomyces cerevisiae. Microbiol. Rev. 1993, 57, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.; Klein, M.; Carrillo, M.; McInnes, J.; Nguyen, H.T.T.; Nevoigt, E. Re-evaluation of glycerol utilization in Saccharomyces cerevisiae: Characterization of an isolate that grows on glycerol without supporting supplements. Biotechnol. Biofuels 2013, 6, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinnen, S.; Ho, P.W.; Klein, M.; Nevoigt, E. Genetic determinants for enhanced glycerol growth of Saccharomyces cerevisiae. Metab. Eng. 2016, 36, 68–79. [Google Scholar] [CrossRef]
- Ho, P.W.; Swinnen, S.; Duitama, J.; Nevoigt, E. The sole introduction of two single-point mutations establishes glycerol utilization in Saccharomyces cerevisiae CEN.PK derivatives. Biotechnol. Biofuels 2017, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Frenk, S.; Pizza, G.; Walker, R.V.; Housel, J. Aging yeast gain a competitive advantage on non-optimal carbon sources. Aging Cell 2017, 16, 602–604. [Google Scholar] [CrossRef] [Green Version]
- Heeren, G.; Jarolim, S.; Laun, P.; Rinnerthaler, M.; Stolze, K.; Perrone, G.G.; Kohlwein, S.D.; Nohl, H.; Dawes, I.W.; Breitenbach, M. The role of respiration, reactive oxygen species and oxidative stress in mother cell-specific ageing of yeast strains defective in the RAS signalling pathway. FEMS Yeast Res. 2004, 5, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Laun, P.; Büttner, S.; Rinnerthaler, M.; Burhans, W.C.; Breitenbach, M. Yeast aging and apoptosis. Subcell Biochem. 2012, 57, 207–232. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2016, 16, 1323–1367. [Google Scholar] [CrossRef]
- Schulz, E.; Wenzel, P.; Münzel, T.; Daiber, A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014, 20, 308–324. [Google Scholar] [CrossRef]
- Zhang, N.; Cao, L. Starvation signals in yeast are integrated to coordinate metabolic reprogramming and stress response to ensure longevity. Curr. Genet. 2017, 63, 839–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga, A.; Arrabaça, J.D.; Loureiro-Dias, M.C. Cyanide-resistant respiration, a very frequent metabolic pathway in yeasts. FEMS Yeast Res. 2003, 3, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.; Borovskii, G.; Panda, S.K. Alternative oxidase and plant stress tolerance. Plant Signal. Behav. 2016, 11, e1256530. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wu, S.B.; Wu, Y.T.; Wei, Y.H. Oxidative stress response elicited by mitochondrial dysfunction: Implication in the pathophysiology of aging. Exp. Biol. Med. 2013, 238, 450–460. [Google Scholar] [CrossRef]
- Martins, D.; English, A.M. Catalase activity is stimulated by H(2)O(2) in rich culture medium and is required for H(2)O(2) resistance and adaptation in yeast. Redox Biol. 2014, 2, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.B.; Delaunay-Moisan, A.; Outten, C.E.; Igbaria, A. Functions and cellular compartmentation of the thioredoxin and glutathione pathways in yeast. Antioxid. Redox Signal. 2013, 18, 1699–1711. [Google Scholar] [CrossRef] [Green Version]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Song, J.Y.; Cha, J.; Lee, J.; Roe, J.H. Glutathione reductase and a mitochondrial thioredoxin play overlapping roles in maintaining iron-sulfur enzymes in fission yeast. Eukaryot. Cell 2006, 5, 1857–1865. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, M.; Eltayb, W.A.; Yousif, A. Comparison of structures among Saccharomyces cerevisiae Grxs proteins. Genes Environ. 2018, 40, 17. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ströher, E.; Millar, A.H. The biological roles of glutaredoxins. Biochem. J. 2012, 446, 333–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliola, M.; Tramonti, A.; Lanini, C.; Cialfi, S.; De Biase, D.; Falcone, C. Intracellular NADPH level affects the oligomeric state of the glucose 6-phosphate dehydrogenase. Eukaryot. Cell 2012, 11, 1503–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, P.; Ge, Y.; Wang, W.; Abbas, A.; Zhu, G. NADP(+)-specific isocitrate dehydrogenase from oleaginous yeast Yarrowia lipolytica CLIB122: Biochemical characterization and coenzyme sites evaluation. Appl. Biochem. Biotechnol. 2013, 171, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, G.; Masullo, U.; Passarelli, S.; Filosa, S. Glucose-6-phosphate dehydrogenase deficiency: Disadvantages and possible benefits. Cardiovasc. Hematol. Disord. Drug Targets 2013, 13, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, R.; Wang, K.; Marcu, O.; Yu, Z.; Lim, T.K.; Lin, Q.; Schueler-Furman, O.; Pines, O. Yeast aconitase mitochondrial import is modulated by interactions of its C and N terminal domains and Ssa1/2 (Hsp70). Sci. Rep. 2018, 8, 5903. [Google Scholar] [CrossRef]
- Jung, S.J.; Seo, Y.; Lee, K.C.; Lee, D.; Roe, J.H. Essential function of Aco2, a fusion protein of aconitase and mitochondrial ribosomal protein bL21, in mitochondrial translation in fission yeast. FEBS Lett. 2015, 589, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Matasova, L.B.; Popova, T.N. Aconitase of the mammals under oxidative stress. Biochemistry 2008, 73, 1189–1198. [Google Scholar]
- Farooq, M.A.; Pracheil, T.M.; Dong, Z.; Xiao, F.; Liu, Z. Mitochondrial DNA instability in cells lacking aconitase correlates with iron citrate toxicity. Oxid. Med. Cell. Longev. 2013, 493–536. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.J.; Wang, X.; Butow, R.A. Yeast aconitase binds and provides metabolically coupled protection to mitochondrial DNA. Proc. Natl. Acad. Sci. USA 2007, 104, 13738–13743. [Google Scholar] [CrossRef] [Green Version]
- Galdieri, L.; Mehrotra, S.; Yu, S.; Vancura, A. Transcriptional Regulation in Yeast during Diauxic Shift and Stationary Phase. OMICS 2010, 14, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, V.D.; Fabrizio, P. Chronological aging in Saccharomyces cerevisiae. Subcell. Biochem. 2012, 57, 101–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? J. Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, E.A.; Raimundo, N.; Shadel, G.S. Epigenetic silencing mediates mitochondria stress-induced longevity. Cell Metab. 2013, 17, 954–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, N.; Youle, R.J.; Finkel, T. The mitochondrial basis of aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.; Swinnen, S.; Thevelein, J.; Nevoigt, E. Glycerol metabolism and transport in yeast and fungi: Established knowledge and ambiguities. Environ. Microbiol. 2017, 19, 878–893. [Google Scholar] [CrossRef] [Green Version]



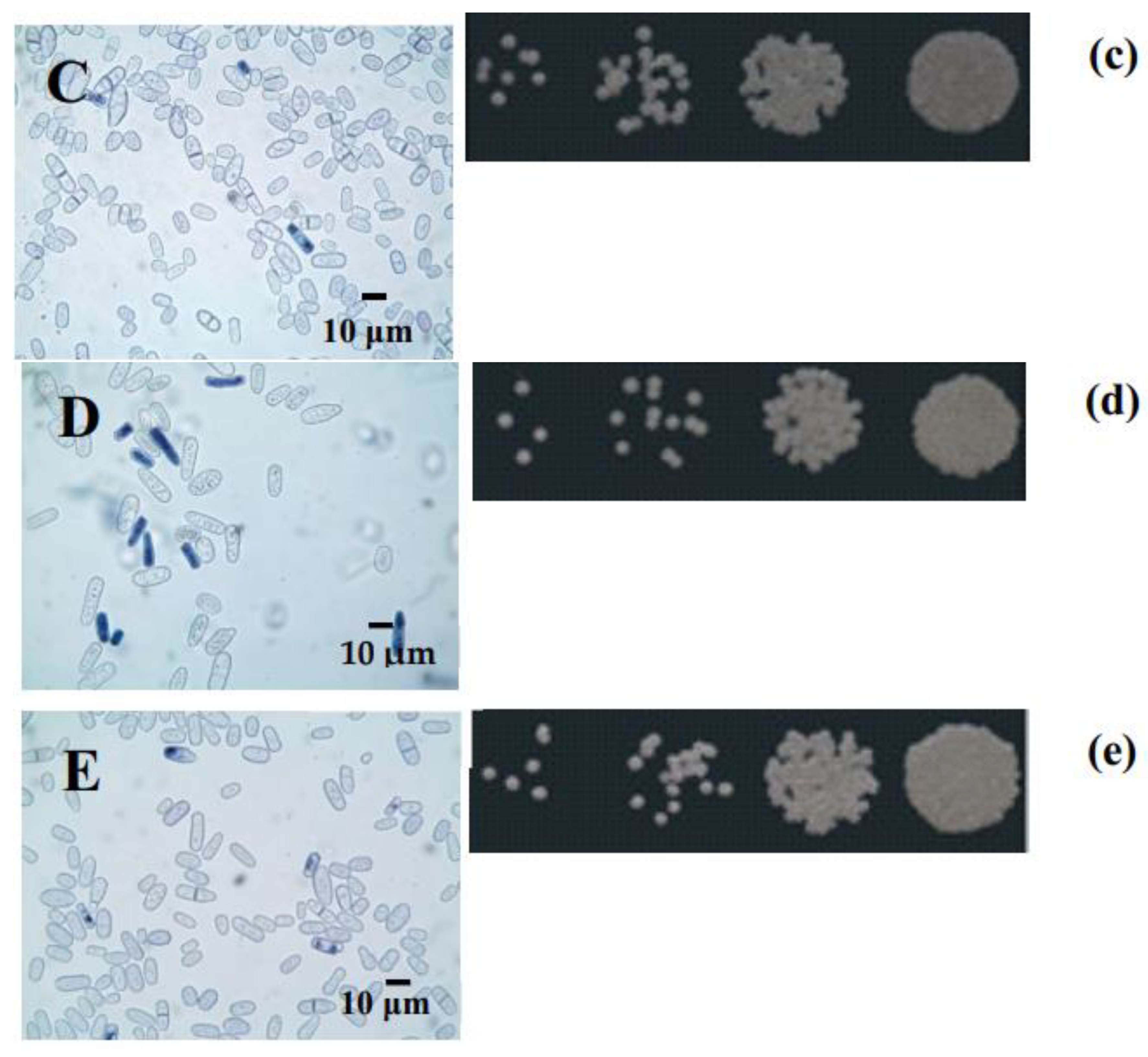



| Growth Phase | Respiration Rate, ng-Atom Consumed O per 1 mg of Dry Weight * | |||
|---|---|---|---|---|
| Control | +KCN, 4 mM | |||
| Glycerol | Glucose | Glycerol | Glucose | |
| Logarithmic phase | 43.8 ± 2.3 a | 26.24 ± 3.01 b | 0c | 0c |
| 24 h of growth | 28.85 ± 3.75 a | 35.28 ± 3.03 b | 1.71 ± 0.14 c (5.9 ± 0.48%of resistance) | 0 d |
| 48 h of growth | 27.96 ± 3.6 a | 21.37 ± 1.69 b | 13.39 ± 0.75 c (47.88 ± 2.68% of resistance) | 3.98 ± 0.2 d (14.23 ± 0.64% of resistance) |
| 96 h of growth | 25.04 ± 3.07 a | 21.71 ± 1.14 a | 13.26 ± 0.97 b (52.95 ± 3.86% of resistance) | 14.21 ± 0.72 b (65.45% ± 3.3 of resistance) |
| 168 h of growth | 7.17 ± 0.38 a | 4.38 ± 0.38 b,* | 2.29 ± 0.1 c (31.88 ± 1.43% of resistance) | 1.68 ± 0.04 d (38.36 ± 0.92% of resistance) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isakova, E.P.; Matushkina, I.N.; Popova, T.N.; Dergacheva, D.I.; Gessler, N.N.; Klein, O.I.; Semenikhina, A.V.; Deryabina, Y.I.; La Porta, N.; Saris, N.-E.L. Metabolic Remodeling during Long-Lasting Cultivation of the Endomyces magnusii Yeast on Oxidative and Fermentative Substrates. Microorganisms 2020, 8, 91. https://doi.org/10.3390/microorganisms8010091
Isakova EP, Matushkina IN, Popova TN, Dergacheva DI, Gessler NN, Klein OI, Semenikhina AV, Deryabina YI, La Porta N, Saris N-EL. Metabolic Remodeling during Long-Lasting Cultivation of the Endomyces magnusii Yeast on Oxidative and Fermentative Substrates. Microorganisms. 2020; 8(1):91. https://doi.org/10.3390/microorganisms8010091
Chicago/Turabian StyleIsakova, Elena P., Irina N. Matushkina, Tatyana N. Popova, Darya I. Dergacheva, Natalya N. Gessler, Olga I. Klein, Anastasya V. Semenikhina, Yulia I. Deryabina, Nicola La Porta, and Nils-Eric L. Saris. 2020. "Metabolic Remodeling during Long-Lasting Cultivation of the Endomyces magnusii Yeast on Oxidative and Fermentative Substrates" Microorganisms 8, no. 1: 91. https://doi.org/10.3390/microorganisms8010091
APA StyleIsakova, E. P., Matushkina, I. N., Popova, T. N., Dergacheva, D. I., Gessler, N. N., Klein, O. I., Semenikhina, A. V., Deryabina, Y. I., La Porta, N., & Saris, N.-E. L. (2020). Metabolic Remodeling during Long-Lasting Cultivation of the Endomyces magnusii Yeast on Oxidative and Fermentative Substrates. Microorganisms, 8(1), 91. https://doi.org/10.3390/microorganisms8010091






